Abstract
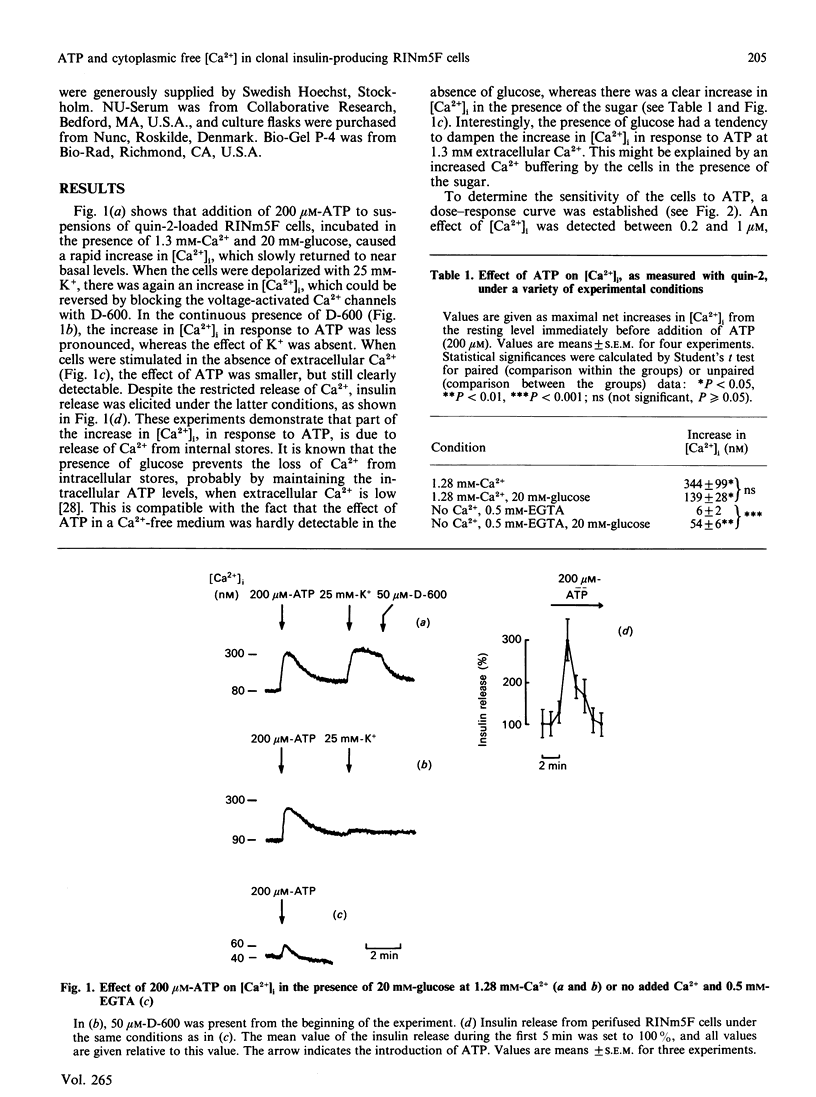
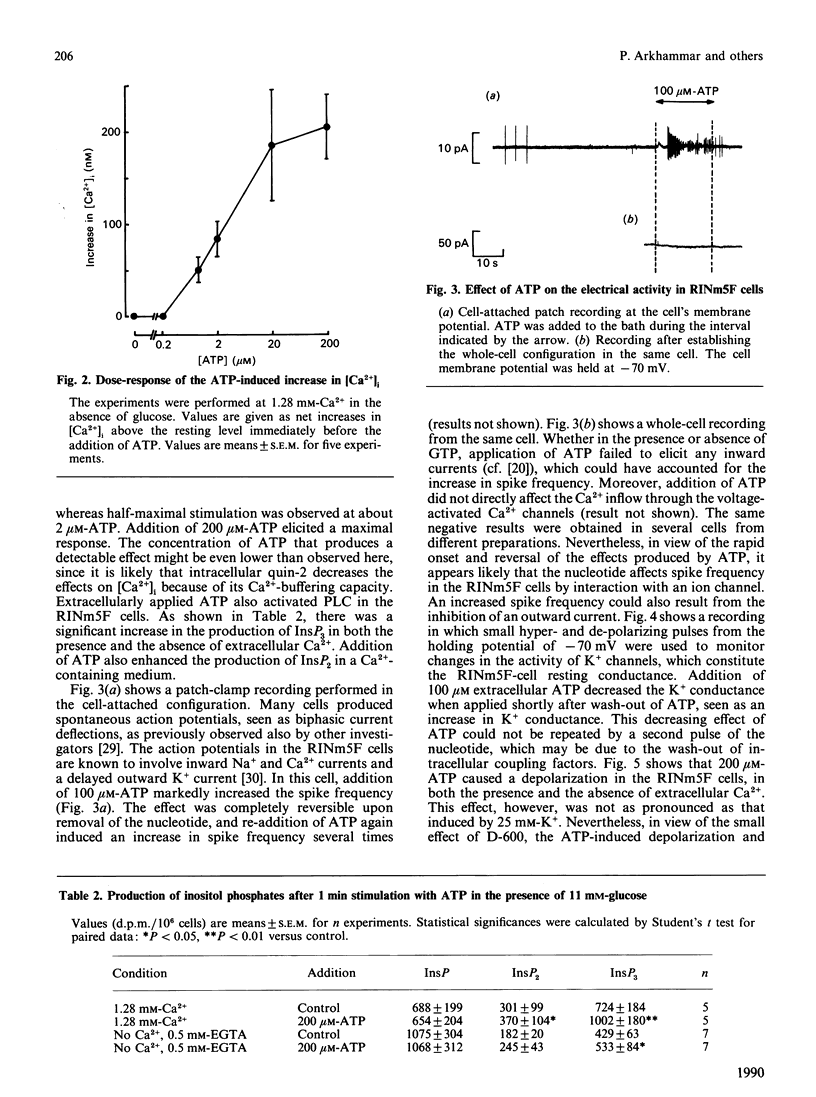
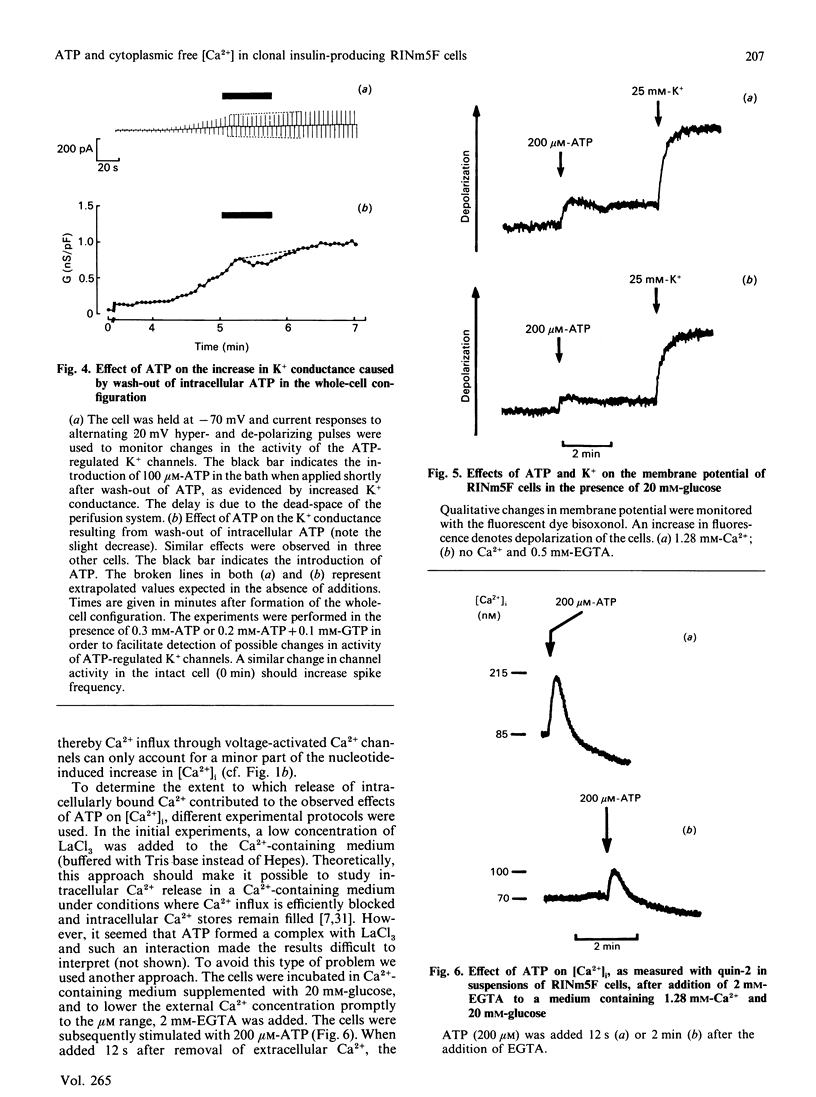
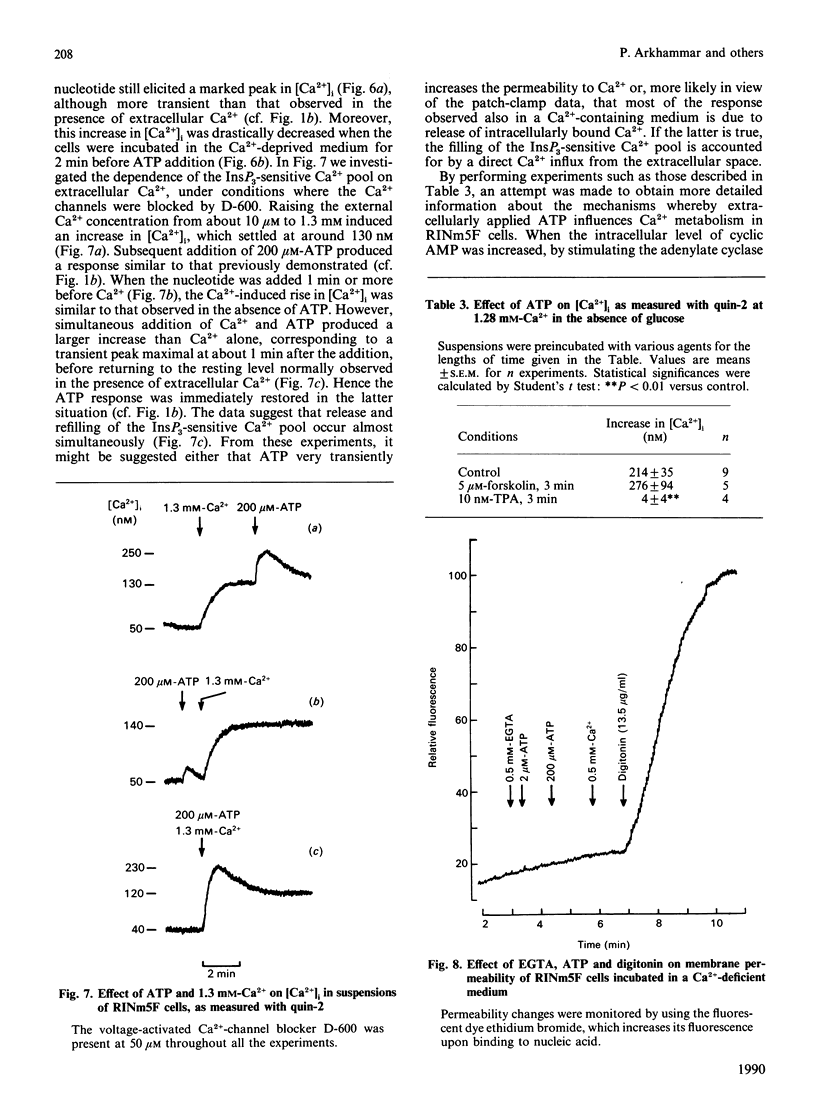
Effects of extracellularly applied ATP (added as disodium salt) on stimulus-secretion coupling were investigated in clonal insulin-producing RINm5F cells. Cytoplasmic free Ca2+ concentration [( Ca2+]i), electrical activity, membrane potential, formation of InsP3 and insulin release were measured. Addition of ATP in a Ca2(+)-containing medium promoted a rapid rise in [Ca2+]i, which was followed by a slow decline towards the basal level. In a Ca2(+)-free medium, the ATP-induced increase in [Ca2+]i was smaller, but still enough to elicit insulin secretion. Upon normalization of the extracellular Ca2+ concentration, the response to ATP recovered instantaneously. The presence of glucose in the incubation medium was a prerequisite to obtain a pronounced effect of ATP in the absence of extracellular Ca2+. However, glucose did not enhance the response to ATP in a Ca2(+)-containing medium. The effect of ATP was dose-dependent, with a clearly detectable increase in [Ca2+]i at 1 microM and a maximal response being obtained at 200 microM-ATP. The response to ATP was unaffected by activating adenylate cyclase by forskolin, but was abolished by 10 nM of the phorbol ester phorbol 12-myristate 13-acetate. The effects of ATP on [Ca2+]i could not be accounted for by a generalized increase in plasma-membrane permeability, as evident from the failure of the nucleotide to increase the fluorescence of the nuclear stain ethidium bromide. After stimulation with ATP there was an increase in membrane potential, in both the absence and the presence of extracellular Ca2+. Blockage of the voltage-activated Ca2+ channals with D-600, in a Ca2(+)-containing medium, decreased the effect of ATP on [Ca2+]i slightly. Patch-clamp measurements using the cell-attached patch configuration revealed that the RINm5F cells produce spontaneous action potentials, the frequency of which increased markedly on addition of ATP. Whole-cell recordings demonstrated that the increase in spike frequency was not associated with the development of an inward current, but was rather accountable for by a decrease in the activity of the ATP-regulated K+ channels. Addition of 200 microM-ATP stimulated phospholipase C activity, as evident from the formation of InsP3, both in the absence and in the presence of extracellular Ca2+. Thus in the absence of extracellular Ca2+ the stimulatory effect of ATP on insulin release can be explained by InsP3-induced mobilization of intracellularly bound Ca2+. Hence, in the RINm5F cells extracellular ATP acts in a manner similar to other Ca2(+)-mobilizing agents.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arkhammar P., Nilsson T., Berggren P. O. Stimulation of insulin release by the phorbol ester 12-O-tetradecanoylphorbol 13-acetate in the clonal cell line RINm5F despite a lowering of the free cytoplasmic Ca2+ concentration. Biochim Biophys Acta. 1986 Jul 11;887(2):236–241. doi: 10.1016/0167-4889(86)90060-1. [DOI] [PubMed] [Google Scholar]
- Arkhammar P., Nilsson T., Rorsman P., Berggren P. O. Inhibition of ATP-regulated K+ channels precedes depolarization-induced increase in cytoplasmic free Ca2+ concentration in pancreatic beta-cells. J Biol Chem. 1987 Apr 25;262(12):5448–5454. [PubMed] [Google Scholar]
- Ashcroft F. M., Harrison D. E., Ashcroft S. J. Glucose induces closure of single potassium channels in isolated rat pancreatic beta-cells. 1984 Nov 29-Dec 5Nature. 312(5993):446–448. doi: 10.1038/312446a0. [DOI] [PubMed] [Google Scholar]
- Barrowman M. M., Cockcroft S., Gomperts B. D. Potentiation and inhibition of secretion from neutrophils by phorbol ester. FEBS Lett. 1986 May 26;201(1):137–142. doi: 10.1016/0014-5793(86)80586-5. [DOI] [PubMed] [Google Scholar]
- Benham C. D., Tsien R. W. A novel receptor-operated Ca2+-permeable channel activated by ATP in smooth muscle. Nature. 1987 Jul 16;328(6127):275–278. doi: 10.1038/328275a0. [DOI] [PubMed] [Google Scholar]
- Bennett J. P., Cockcroft S., Gomperts B. D. Rat mast cells permeabilized with ATP secrete histamine in response to calcium ions buffered in the micromolar range. J Physiol. 1981 Aug;317:335–345. doi: 10.1113/jphysiol.1981.sp013828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Dawson R. M., Downes C. P., Heslop J. P., Irvine R. F. Changes in the levels of inositol phosphates after agonist-dependent hydrolysis of membrane phosphoinositides. Biochem J. 1983 May 15;212(2):473–482. doi: 10.1042/bj2120473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biden T. J., Vallar L., Wollheim C. B. Regulation of inositol 1,4,5-trisphosphate metabolism in insulin-secreting RINm5F cells. Biochem J. 1988 Apr 15;251(2):435–440. doi: 10.1042/bj2510435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blachier F., Malaisse W. J. Effect of exogenous ATP upon inositol phosphate production, cationic fluxes and insulin release in pancreatic islet cells. Biochim Biophys Acta. 1988 Jun 30;970(2):222–229. doi: 10.1016/0167-4889(88)90182-6. [DOI] [PubMed] [Google Scholar]
- Boynton A. L., Cooney R. V., Hill T. D., Nilsson T., Arkhammar P., Berggren P. O. Extracellular ATP mobilizes intracellular Ca2+ in T51B rat liver epithelial cells: a study involving single cell measurements. Exp Cell Res. 1989 Mar;181(1):245–255. doi: 10.1016/0014-4827(89)90198-5. [DOI] [PubMed] [Google Scholar]
- Bozem M., Henquin J. C. Glucose modulation of spike activity independently from changes in slow waves of membrane potential in mouse B-cells. Pflugers Arch. 1988 Dec;413(2):147–152. doi: 10.1007/BF00582524. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Purinergic receptors. J Theor Biol. 1976 Oct 21;62(2):491–503. doi: 10.1016/0022-5193(76)90133-8. [DOI] [PubMed] [Google Scholar]
- Chahwala S. B., Cantley L. C. Extracellular ATP induces ion fluxes and inhibits growth of Friend erythroleukemia cells. J Biol Chem. 1984 Nov 25;259(22):13717–13722. [PubMed] [Google Scholar]
- Cockcroft S., Gomperts B. D. ATP induces nucleotide permeability in rat mast cells. Nature. 1979 Jun 7;279(5713):541–542. doi: 10.1038/279541a0. [DOI] [PubMed] [Google Scholar]
- Cockcroft S., Gomperts B. D. Activation and inhibition of calcium-dependent histamine secretion by ATP ions applied to rat mast cells. J Physiol. 1979 Nov;296:229–243. doi: 10.1113/jphysiol.1979.sp013002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook D. L., Hales C. N. Intracellular ATP directly blocks K+ channels in pancreatic B-cells. Nature. 1984 Sep 20;311(5983):271–273. doi: 10.1038/311271a0. [DOI] [PubMed] [Google Scholar]
- Dahlquist R., Diamant B. Interaction of ATP and calcium on the rat mast cell: effect on histamine release. Acta Pharmacol Toxicol (Copenh) 1974 May;34(5):368–384. doi: 10.1111/j.1600-0773.1974.tb03533.x. [DOI] [PubMed] [Google Scholar]
- Dubyak G. R., De Young M. B. Intracellular Ca2+ mobilization activated by extracellular ATP in Ehrlich ascites tumor cells. J Biol Chem. 1985 Sep 5;260(19):10653–10661. [PubMed] [Google Scholar]
- Dubyak G. R. Extracellular ATP activates polyphosphoinositide breakdown and Ca2+ mobilization in Ehrlich ascites tumor cells. Arch Biochem Biophys. 1986 Feb 15;245(1):84–95. doi: 10.1016/0003-9861(86)90192-x. [DOI] [PubMed] [Google Scholar]
- Dunne M. J., Findlay I., Petersen O. H., Wollheim C. B. ATP-sensitive K+ channels in an insulin-secreting cell line are inhibited by D-glyceraldehyde and activated by membrane permeabilization. J Membr Biol. 1986;93(3):271–279. doi: 10.1007/BF01871181. [DOI] [PubMed] [Google Scholar]
- Gomperts B. D. Involvement of guanine nucleotide-binding protein in the gating of Ca2+ by receptors. Nature. 1983 Nov 3;306(5938):64–66. doi: 10.1038/306064a0. [DOI] [PubMed] [Google Scholar]
- Gordon J. L. Extracellular ATP: effects, sources and fate. Biochem J. 1986 Jan 15;233(2):309–319. doi: 10.1042/bj2330309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gylfe E., Hellman B. External ATP mimics carbachol in initiating calcium mobilization from pancreatic beta-cells conditioned by previous exposure to glucose. Br J Pharmacol. 1987 Oct;92(2):281–289. doi: 10.1111/j.1476-5381.1987.tb11322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedeskov C. J. Mechanism of glucose-induced insulin secretion. Physiol Rev. 1980 Apr;60(2):442–509. doi: 10.1152/physrev.1980.60.2.442. [DOI] [PubMed] [Google Scholar]
- Hellman B. The significance of calcium for glucose stimulation of insulin release. Endocrinology. 1975 Aug;97(2):392–398. doi: 10.1210/endo-97-2-392. [DOI] [PubMed] [Google Scholar]
- Henquin J. C. ATP-sensitive K+ channels may control glucose-induced electrical activity in pancreatic B-cells. Biochem Biophys Res Commun. 1988 Oct 31;156(2):769–775. doi: 10.1016/s0006-291x(88)80910-0. [DOI] [PubMed] [Google Scholar]
- Heppel L. A., Weisman G. A., Friedberg I. Permeabilization of transformed cells in culture by external ATP. J Membr Biol. 1985;86(3):189–196. doi: 10.1007/BF01870597. [DOI] [PubMed] [Google Scholar]
- Hoftiezer V., Berggren P. O., Hellman B. Effects of altered Ca2+ and Mg2+ concentrations on proliferation and functional differentiation of the clonal insulin-producing cells RINm5F. Cancer Lett. 1985 May;27(1):7–14. doi: 10.1016/0304-3835(85)90003-5. [DOI] [PubMed] [Google Scholar]
- Kanatsuna T., Lernmark A., Rubenstein A. H., Steiner D. F. Block in insulin release from column-perifused pancreatic beta-cells induced by islet cell surface antibodies and complement. Diabetes. 1981 Mar;30(3):231–234. doi: 10.2337/diab.30.3.231. [DOI] [PubMed] [Google Scholar]
- Loubatieres-Mariani M. M., Chapal J., Lignon F., Valette G. Structural specificity of nucleotides for insulin secretory action from the isolated perfused rat pancreas. Eur J Pharmacol. 1979 Nov 16;59(3-4):277–286. doi: 10.1016/0014-2999(79)90291-7. [DOI] [PubMed] [Google Scholar]
- Manzini S., Hoyle C. H., Burnstock G. An electrophysiological analysis of the effect of reactive blue 2, a putative P2-purinoceptor antagonist, on inhibitory junction potentials of rat caecum. Eur J Pharmacol. 1986 Aug 15;127(3):197–204. doi: 10.1016/0014-2999(86)90364-x. [DOI] [PubMed] [Google Scholar]
- Muallem S., Schoeffield M. S., Fimmel C. J., Pandol S. J. Agonist-sensitive calcium pool in the pancreatic acinar cell. I. Permeability properties. Am J Physiol. 1988 Aug;255(2 Pt 1):G221–G228. doi: 10.1152/ajpgi.1988.255.2.G221. [DOI] [PubMed] [Google Scholar]
- Nilsson T., Arkhammar P., Berggren P. O. Extracellular Ca2+ induces a rapid increase in cytoplasmic free Ca2+ in pancreatic beta-cells. Biochem Biophys Res Commun. 1987 Nov 30;149(1):152–158. doi: 10.1016/0006-291x(87)91617-2. [DOI] [PubMed] [Google Scholar]
- Nilsson T., Arkhammar P., Hallberg A., Hellman B., Berggren P. O. Characterization of the inositol 1,4,5-trisphosphate-induced Ca2+ release in pancreatic beta-cells. Biochem J. 1987 Dec 1;248(2):329–336. doi: 10.1042/bj2480329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y., Yada T., Ohno-Shosaku T., Oiki S., Ueda S., Machida K. Exogenous ATP induces electrical membrane responses in fibroblasts. Exp Cell Res. 1984 Jun;152(2):552–557. doi: 10.1016/0014-4827(84)90657-8. [DOI] [PubMed] [Google Scholar]
- Orellana S. A., Solski P. A., Brown J. H. Phorbol ester inhibits phosphoinositide hydrolysis and calcium mobilization in cultured astrocytoma cells. J Biol Chem. 1985 May 10;260(9):5236–5239. [PubMed] [Google Scholar]
- Pace C. S., Goldsmith K. T. Action of a phorbol ester on B-cells: potentiation of stimulant-induced electrical activity. Am J Physiol. 1985 May;248(5 Pt 1):C527–C534. doi: 10.1152/ajpcell.1985.248.5.C527. [DOI] [PubMed] [Google Scholar]
- Phaneuf S., Berta P., Casanova J., Cavadore J. C. ATP stimulates inositol phosphates accumulation and calcium mobilization in a primary culture of rat aortic myocytes. Biochem Biophys Res Commun. 1987 Mar 13;143(2):454–460. doi: 10.1016/0006-291x(87)91375-1. [DOI] [PubMed] [Google Scholar]
- Praz G. A., Halban P. A., Wollheim C. B., Blondel B., Strauss A. J., Renold A. E. Regulation of immunoreactive-insulin release from a rat cell line (RINm5F). Biochem J. 1983 Feb 15;210(2):345–352. doi: 10.1042/bj2100345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentki M., Corkey B. E., Matschinsky F. M. Inositol 1,4,5-trisphosphate and the endoplasmic reticulum Ca2+ cycle of a rat insulinoma cell line. J Biol Chem. 1985 Aug 5;260(16):9185–9190. [PubMed] [Google Scholar]
- Putney J. W., Jr A model for receptor-regulated calcium entry. Cell Calcium. 1986 Feb;7(1):1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- Rasmussen H., Barrett P. Q. Calcium messenger system: an integrated view. Physiol Rev. 1984 Jul;64(3):938–984. doi: 10.1152/physrev.1984.64.3.938. [DOI] [PubMed] [Google Scholar]
- Rink T. J., Montecucco C., Hesketh T. R., Tsien R. Y. Lymphocyte membrane potential assessed with fluorescent probes. Biochim Biophys Acta. 1980;595(1):15–30. doi: 10.1016/0005-2736(80)90243-6. [DOI] [PubMed] [Google Scholar]
- Rorsman P., Arkhammar P., Berggren P. O. Voltage-activated Na+ currents and their suppression by phorbol ester in clonal insulin-producing RINm5F cells. Am J Physiol. 1986 Dec;251(6 Pt 1):C912–C919. doi: 10.1152/ajpcell.1986.251.6.C912. [DOI] [PubMed] [Google Scholar]
- Sistare F. D., Picking R. A., Haynes R. C., Jr Sensitivity of the response of cytosolic calcium in Quin-2-loaded rat hepatocytes to glucagon, adenine nucleosides, and adenine nucleotides. J Biol Chem. 1985 Oct 15;260(23):12744–12747. [PubMed] [Google Scholar]
- Supattapone S., Danoff S. K., Theibert A., Joseph S. K., Steiner J., Snyder S. H. Cyclic AMP-dependent phosphorylation of a brain inositol trisphosphate receptor decreases its release of calcium. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8747–8750. doi: 10.1073/pnas.85.22.8747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trams E. G. Evidence for ATP action on the cell surface. Nature. 1974 Dec 6;252(5483):480–482. doi: 10.1038/252480a0. [DOI] [PubMed] [Google Scholar]
- Wiener E., Dubyak G., Scarpa A. Na+/H+ exchange in Ehrlich ascites tumor cells. Regulation by extracellular ATP and 12-O-tetradecanoylphorbol 13-acetate. J Biol Chem. 1986 Apr 5;261(10):4529–4534. [PubMed] [Google Scholar]
- Wollheim C. B., Biden T. J. Signal transduction in insulin secretion: comparison between fuel stimuli and receptor agonists. Ann N Y Acad Sci. 1986;488:317–333. doi: 10.1111/j.1749-6632.1986.tb46568.x. [DOI] [PubMed] [Google Scholar]
- Wollheim C. B., Dunne M. J., Peter-Riesch B., Bruzzone R., Pozzan T., Petersen O. H. Activators of protein kinase C depolarize insulin-secreting cells by closing K+ channels. EMBO J. 1988 Aug;7(8):2443–2449. doi: 10.1002/j.1460-2075.1988.tb03090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollheim C. B., Ullrich S., Pozzan T. Glyceraldehyde, but not cyclic AMP-stimulated insulin release is preceded by a rise in cytosolic free Ca2+. FEBS Lett. 1984 Nov 5;177(1):17–22. doi: 10.1016/0014-5793(84)80972-2. [DOI] [PubMed] [Google Scholar]
- de Weille J., Schmid-Antomarchi H., Fosset M., Lazdunski M. ATP-sensitive K+ channels that are blocked by hypoglycemia-inducing sulfonylureas in insulin-secreting cells are activated by galanin, a hyperglycemia-inducing hormone. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1312–1316. doi: 10.1073/pnas.85.4.1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Weille J., Schmid-Antomarchi H., Fosset M., Lazdunski M. ATP-sensitive K+ channels that are blocked by hypoglycemia-inducing sulfonylureas in insulin-secreting cells are activated by galanin, a hyperglycemia-inducing hormone. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1312–1316. doi: 10.1073/pnas.85.4.1312. [DOI] [PMC free article] [PubMed] [Google Scholar]



