Abstract
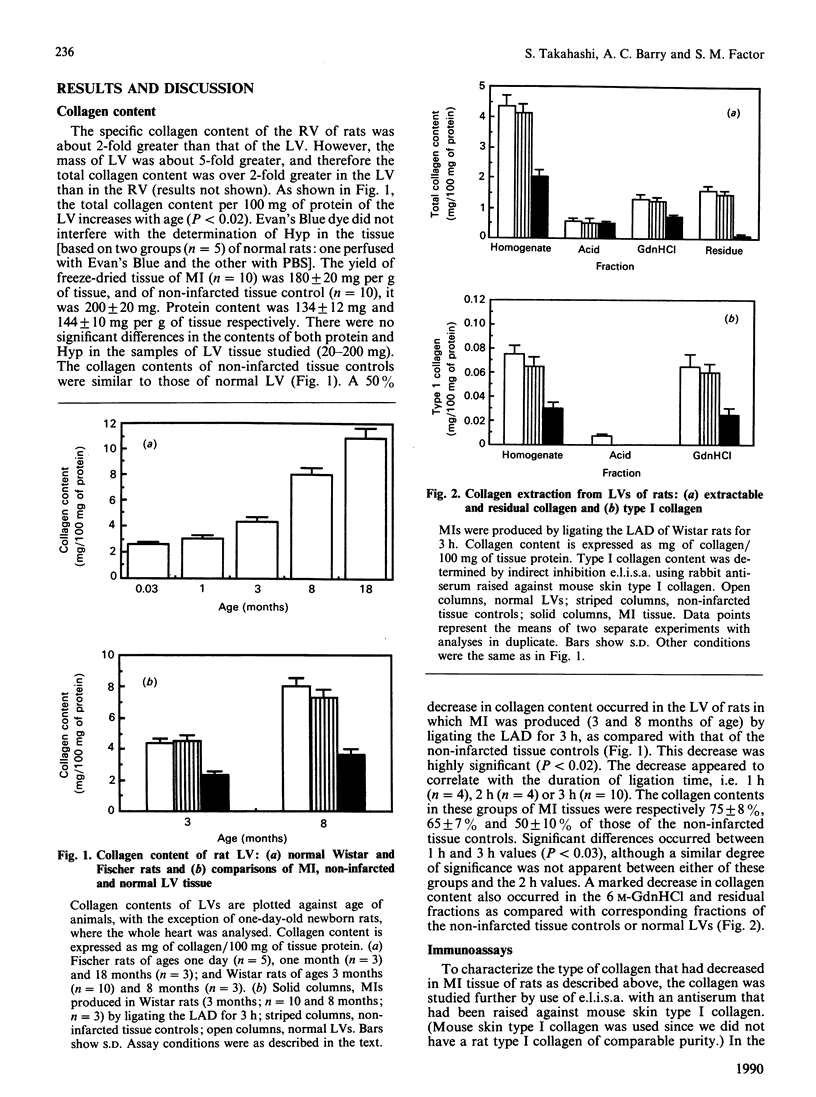
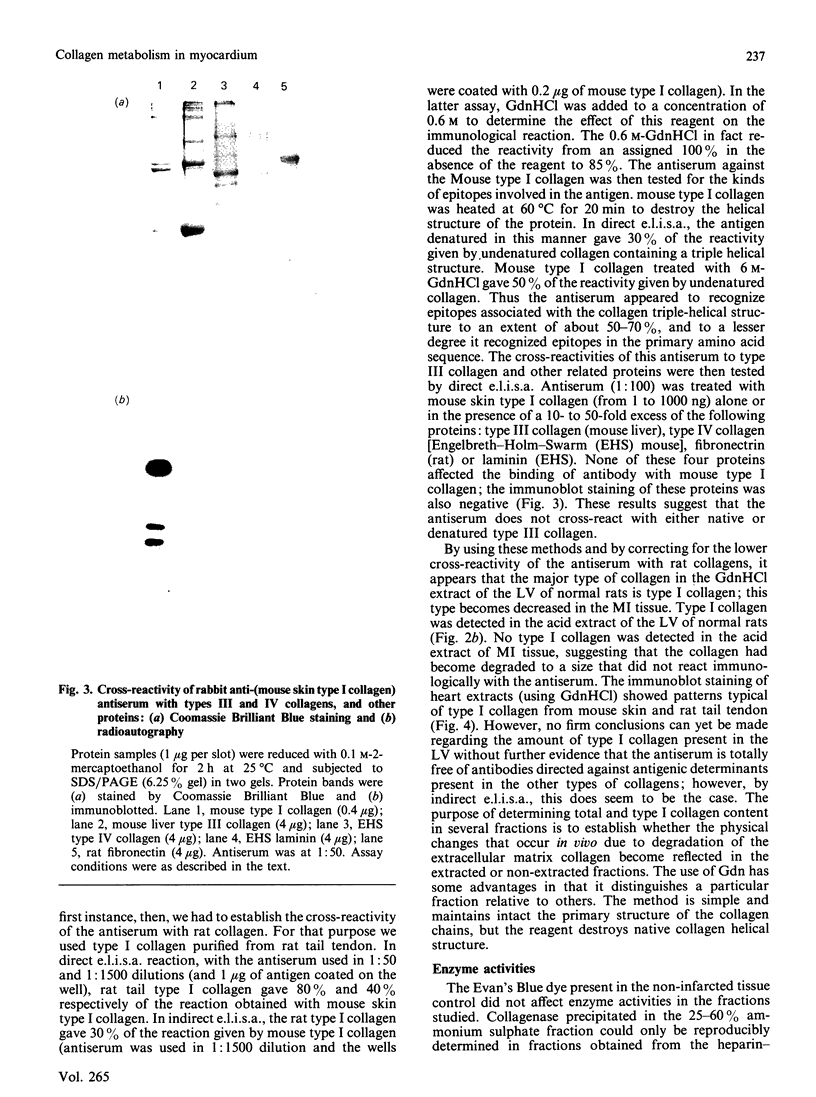
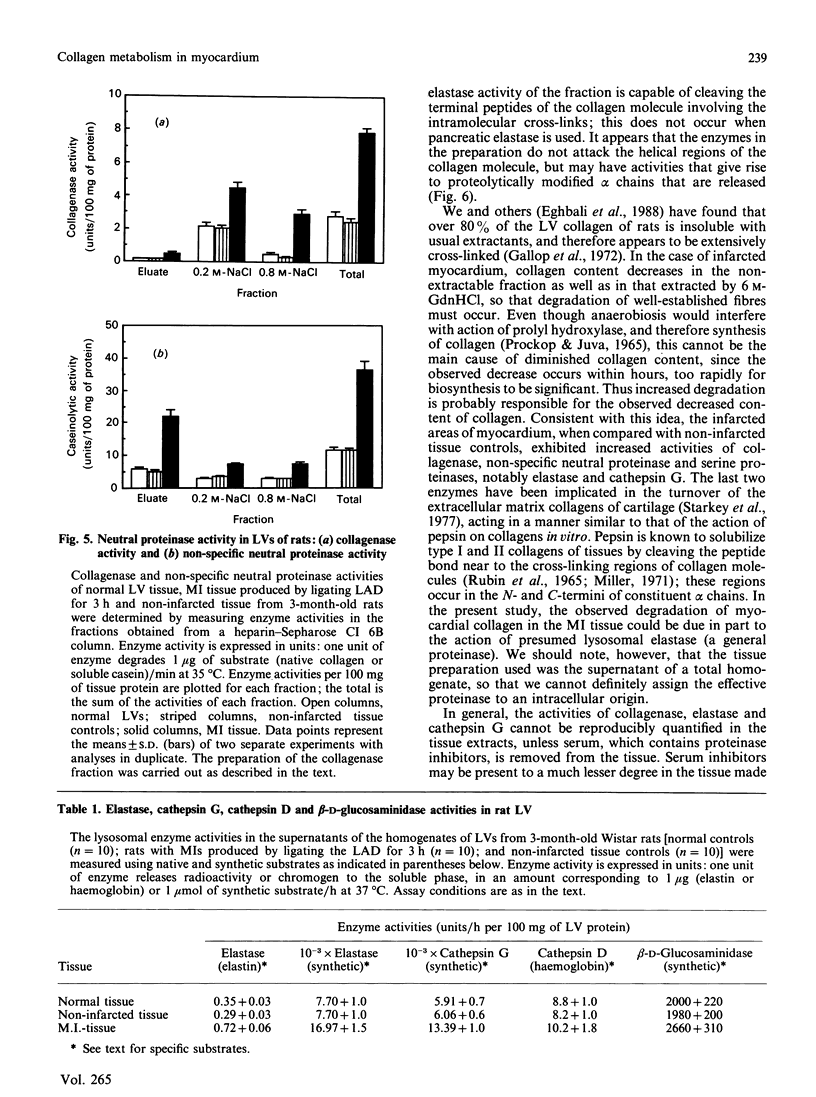
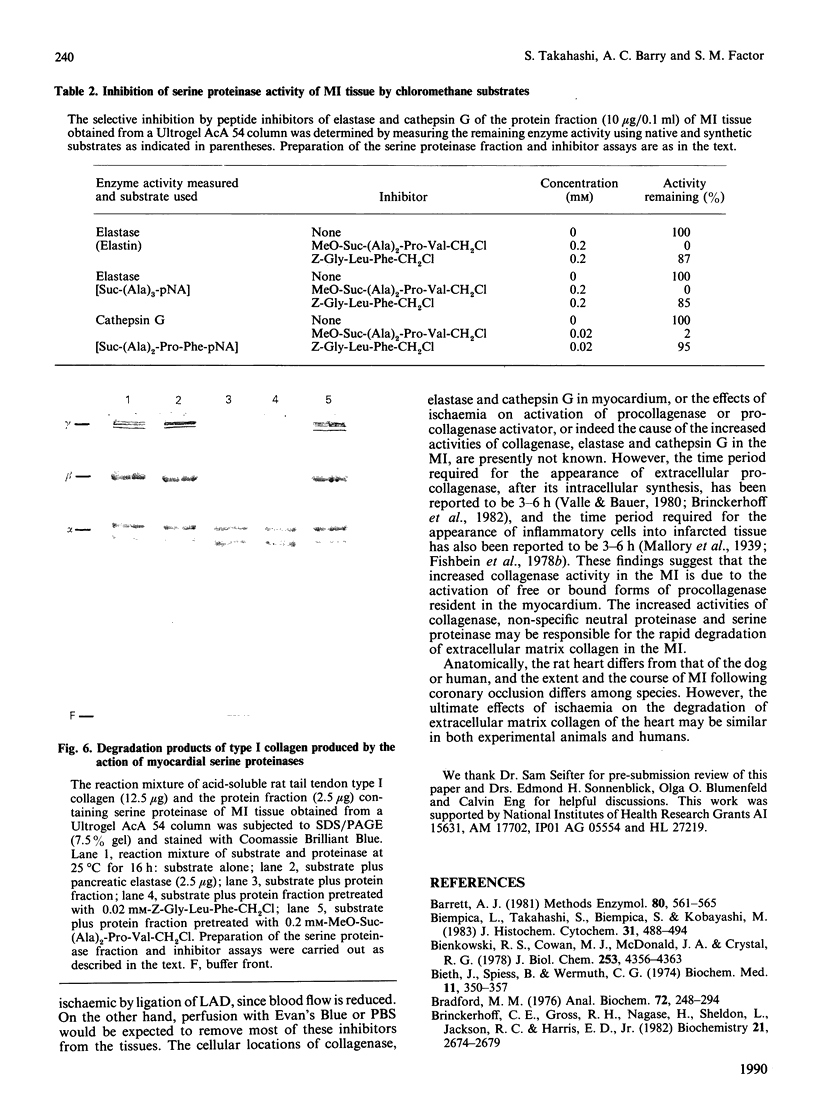
Myocardial extracellular matrix is organized into a complex arrangement of intercellular and pericellular fibres and fibrils that serves as a supporting framework for contracting cells. Recent evidence suggests that changes in ventricular shape and function occurring after ischaemic injury may be related to alterations of this matrix. In this report we describe the rapid and extensive loss of collagen in myocardial infarction produced by ligating the left anterior descending coronary artery of the rat for 1-3 h. The total collagen content in the myocardial infarct zones after 1, 2 and 3 h of ligation was 75 +/- 8%, 65 +/- 7% and 50 +/- 10% respectively (mean +/- S.D.) of that of either the non-infarcted tissue controls or of the same regions in sex- and age-matched normal left ventricles. A marked decrease also occurred in the residual collagens which were not extractable with 6 M-guanidine hydrochloride, suggesting that rapid degradation of insoluble collagen fibres may also occur. The decreased collagen content in the 3 h myocardial infarct coincided with the appearance of several enzyme activities. Collagenase, other neutral proteinase and presumed lysosomal serine proteinase activities were increased by 3, 3 and 2 times the control values respectively. These results suggest that the increased activities of collagenase and other neutral proteinases may be responsible for the rapid degradation of extracellular matrix collagen in myocardial infarct.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett A. J. Cathepsin G. Methods Enzymol. 1981;80(Pt 100):561–565. doi: 10.1016/s0076-6879(81)80044-4. [DOI] [PubMed] [Google Scholar]
- Biempica L., Takahashi S., Biempica S., Kobayashi M. Immunohistochemical localization of collagenase in hepatic murine schistosomiasis. J Histochem Cytochem. 1983 Apr;31(4):488–494. doi: 10.1177/31.4.6298308. [DOI] [PubMed] [Google Scholar]
- Bienkowski R. S., Cowan M. J., McDonald J. A., Crystal R. G. Degradation of newly synthesized collagen. J Biol Chem. 1978 Jun 25;253(12):4356–4363. [PubMed] [Google Scholar]
- Bieth J., Spiess B., Wermuth C. G. The synthesis and analytical use of a highly sensitive and convenient substrate of elastase. Biochem Med. 1974 Dec;11(4):350–357. doi: 10.1016/0006-2944(74)90134-3. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brinckerhoff C. E., Gross R. H., Nagase H., Sheldon L., Jackson R. C., Harris E. D., Jr Increased level of translatable collagenase messenger ribonucleic acid in rabbit synovial fibroblasts treated with phorbol myristate acetate or crystals of monosodium urate monohydrate. Biochemistry. 1982 May 25;21(11):2674–2679. doi: 10.1021/bi00540a015. [DOI] [PubMed] [Google Scholar]
- Burleigh M. C., Barrett A. J., Lazarus G. S. Cathepsin B1. A lysosomal enzyme that degrades native collagen. Biochem J. 1974 Feb;137(2):387–398. doi: 10.1042/bj1370387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHN Z. A., WIENER E. THE PARTICULATE HYDROLASES OF MACROPHAGES. II. BIOCHEMICAL AND MORPHOLOGICAL RESPONSE TO PARTICLE INGESTION. J Exp Med. 1963 Dec 1;118:1009–1020. doi: 10.1084/jem.118.6.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon R. O., 3rd, Butany J. W., McManus B. M., Speir E., Kravitz A. B., Bolli R., Ferrans V. J. Early degradation of collagen after acute myocardial infarction in the rat. Am J Cardiol. 1983 Aug;52(3):390–395. doi: 10.1016/0002-9149(83)90145-5. [DOI] [PubMed] [Google Scholar]
- Cawston T. E., Galloway W. A., Mercer E., Murphy G., Reynolds J. J. Purification of rabbit bone inhibitor of collagenase. Biochem J. 1981 Apr 1;195(1):159–165. doi: 10.1042/bj1950159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton L. W., Weiss J. L., Bulkley B. H., Garrison J. B., Weisfeldt M. L. Regional cardiac dilatation after acute myocardial infarction: recognition by two-dimensional echocardiography. N Engl J Med. 1979 Jan 11;300(2):57–62. doi: 10.1056/NEJM197901113000202. [DOI] [PubMed] [Google Scholar]
- Eghbali M., Czaja M. J., Zeydel M., Weiner F. R., Zern M. A., Seifter S., Blumenfeld O. O. Collagen chain mRNAs in isolated heart cells from young and adult rats. J Mol Cell Cardiol. 1988 Mar;20(3):267–276. doi: 10.1016/s0022-2828(88)80059-2. [DOI] [PubMed] [Google Scholar]
- Factor S. M., Robinson T. F., Dominitz R., Cho S. H. Alterations of the myocardial skeletal framework in acute myocardial infarction with and without ventricular rupture. A preliminary report. Am J Cardiovasc Pathol. 1987 Jan;1(1):91–97. [PubMed] [Google Scholar]
- Fishbein M. C., Maclean D., Maroko P. R. Experimental myocardial infarction in the rat: qualitative and quantitative changes during pathologic evolution. Am J Pathol. 1978 Jan;90(1):57–70. [PMC free article] [PubMed] [Google Scholar]
- GROSS J., LAPIERE C. M. Collagenolytic activity in amphibian tissues: a tissue culture assay. Proc Natl Acad Sci U S A. 1962 Jun 15;48:1014–1022. doi: 10.1073/pnas.48.6.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallop P. M., Blumenfeld O. O., Seifter S. Structure and metabolism of connective 801 tissue proteins. Annu Rev Biochem. 1972;41:617–672. doi: 10.1146/annurev.bi.41.070172.003153. [DOI] [PubMed] [Google Scholar]
- Galloway W. A., Murphy G., Sandy J. D., Gavrilovic J., Cawston T. E., Reynolds J. J. Purification and characterization of a rabbit bone metalloproteinase that degrades proteoglycan and other connective-tissue components. Biochem J. 1983 Mar 1;209(3):741–752. doi: 10.1042/bj2090741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilfillan R. F. Phagocytosis and degradation of elastin by normal and tumor cells in culture. Cancer Res. 1968 Jan;28(1):137–147. [PubMed] [Google Scholar]
- Goldstein I. M., Weissmann G. Generation of C5-derived lysosomal enzyme-releasing activity (C5a) by lysates of leukocyte lysosomes. J Immunol. 1974 Nov;113(5):1583–1588. [PubMed] [Google Scholar]
- HURYCH J., CHVAPIL M. Conversion of proline into hydroxyproline and their incorporation into hydroxyproline-containing compounds in skin slices of chicken embryos. Biochim Biophys Acta. 1962 Nov 19;65:170–172. doi: 10.1016/0006-3002(62)90170-1. [DOI] [PubMed] [Google Scholar]
- Harper E., Bloch K. J., Gross J. The zymogen of tadpole collagenase. Biochemistry. 1971 Aug 3;10(16):3035–3041. doi: 10.1021/bi00792a008. [DOI] [PubMed] [Google Scholar]
- Harper E. Collagenases. Annu Rev Biochem. 1980;49:1063–1078. doi: 10.1146/annurev.bi.49.070180.005215. [DOI] [PubMed] [Google Scholar]
- Harris E. D., Jr, Farrell M. E. Resistance to collagenase: a characteristic of collagen fibrils cross-linked by formaldehyde. Biochim Biophys Acta. 1972 Aug 31;278(1):133–141. doi: 10.1016/0005-2795(72)90114-6. [DOI] [PubMed] [Google Scholar]
- Kuettner K. E., Hiti J., Eisenstein R., Harper E. Collagenase inhibition by cationic proteins derived from cartilage and aorta. Biochem Biophys Res Commun. 1976 Sep 7;72(1):40–46. doi: 10.1016/0006-291x(76)90957-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lazarus G. S., Brown R. S., Daniels J. R., Fullmer H. M. Human granulocyte collagenase. Science. 1968 Mar 29;159(3822):1483–1485. doi: 10.1126/science.159.3822.1483. [DOI] [PubMed] [Google Scholar]
- Leibovich S. J., Weiss J. B. Failure of human rheumatoid synovial collagenase to degrade either normal or rheumatoid arthritic polymeric collagen. Biochim Biophys Acta. 1971 Oct;251(1):109–118. doi: 10.1016/0005-2795(71)90067-5. [DOI] [PubMed] [Google Scholar]
- Lerman R. H., Apstein C. S., Kagan H. M., Osmers E. L., Chichester C. O., Vogel W. M., Connelly C. M., Steffee W. P. Myocardial healing and repair after experimental infarction in the rabbit. Circ Res. 1983 Sep;53(3):378–388. doi: 10.1161/01.res.53.3.378. [DOI] [PubMed] [Google Scholar]
- Miller E. J., Finch J. E., Jr, Chung E., Butler W. T., Robertson P. B. Specific cleavage of the native type III collagen molecule with trypsin. Similarity of the cleavage products to collagenase-produced fragments and primary structure at the cleavage site. Arch Biochem Biophys. 1976 Apr;173(2):631–637. doi: 10.1016/0003-9861(76)90300-3. [DOI] [PubMed] [Google Scholar]
- Miller E. J. Isolation and characterization of a collagen from chick cartilage containing three identical alpha chains. Biochemistry. 1971 Apr 27;10(9):1652–1659. doi: 10.1021/bi00785a024. [DOI] [PubMed] [Google Scholar]
- PROCKOP D. J., JUVA K. SYNTHESIS OF HYDROXYPROLINE IN VITRO BY THE HYDROXYLATION OF PROLINE IN A PRECURSOR OF COLLAGEN. Proc Natl Acad Sci U S A. 1965 Mar;53:661–668. doi: 10.1073/pnas.53.3.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers J. C., Gupton B. F., Harley A. D., Nishino N., Whitley R. J. Specificity of porcine pancreatic elastase, human leukocyte elastase and cathepsin G. Inhibition with peptide chloromethyl ketones. Biochim Biophys Acta. 1977 Nov 23;485(1):156–166. doi: 10.1016/0005-2744(77)90203-0. [DOI] [PubMed] [Google Scholar]
- Rauterberg J. The C-terminal non-helical portion of the collagen molecule. Clin Orthop Relat Res. 1973 Nov-Dec;(97):196–212. doi: 10.1097/00003086-197311000-00027. [DOI] [PubMed] [Google Scholar]
- Rennard S. I., Berg R., Martin G. R., Foidart J. M., Robey P. G. Enzyme-linked immunoassay (ELISA) for connective tissue components. Anal Biochem. 1980 May 1;104(1):205–214. doi: 10.1016/0003-2697(80)90300-0. [DOI] [PubMed] [Google Scholar]
- Robinson T. F., Cohen-Gould L., Remily R. M., Capasso J. M., Factor S. M. Extracellular structures in heart muscle. Adv Myocardiol. 1985;5:243–255. doi: 10.1007/978-1-4757-1287-2_19. [DOI] [PubMed] [Google Scholar]
- Roughley P. J., Barrett A. J. The degradation of cartilage proteoglycans by tissue proteinases. Proteoglycan structure and its susceptibility to proteolysis. Biochem J. 1977 Dec 1;167(3):629–637. doi: 10.1042/bj1670629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel R. C. Lysyl oxidase. Int Rev Connect Tissue Res. 1979;8:73–118. doi: 10.1016/b978-0-12-363708-6.50009-6. [DOI] [PubMed] [Google Scholar]
- Starkey P. M., Barrett A. J., Burleigh M. C. The degradation of articular collagen by neutrophil proteinases. Biochim Biophys Acta. 1977 Aug 11;483(2):386–397. doi: 10.1016/0005-2744(77)90066-3. [DOI] [PubMed] [Google Scholar]
- Starkey P. M., Barrett A. J. Human cathepsin G. Catalytic and immunological properties. Biochem J. 1976 May 1;155(2):273–278. doi: 10.1042/bj1550273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkey P. M., Barrett A. J. Human lysosomal elastase. Catalytic and immunological properties. Biochem J. 1976 May 1;155(2):265–271. doi: 10.1042/bj1550265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S., Biempica L. Effects of vitamin A and dexamethasone on collagen degradation in mouse mammary adenocarcinoma. Cancer Res. 1985 Jul;45(7):3311–3321. [PubMed] [Google Scholar]
- Takahashi S., Dunn M. A., Seifter S. Liver collagenase in murine schistosomiasis. Gastroenterology. 1980 Jun;78(6):1425–1431. [PubMed] [Google Scholar]
- Takahashi S., Koda K. Radioimmunoassay of soluble and insoluble collagenases in fibrotic liver. Biochem J. 1984 May 15;220(1):157–164. doi: 10.1042/bj2200157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S., Lee M. J. Quantitative study of tissue collagen metabolism. Anal Biochem. 1987 May 1;162(2):553–561. doi: 10.1016/0003-2697(87)90433-7. [DOI] [PubMed] [Google Scholar]
- Takahashi S., Nakagawa S., Zeydel M., Bhargava G. Ammonia production by IMR-90 fibroblast cultures: effects of ammonia on glutathione, gamma-glutamyl transpeptidase, lysosomal enzymes, and cell division. J Cell Physiol. 1985 Oct;125(1):107–114. doi: 10.1002/jcp.1041250114. [DOI] [PubMed] [Google Scholar]
- Takahashi S., Seifter S., Rifas L. gamma-Glutamyltransferase in human diploid fibroblasts and other mammalian cells. In Vitro. 1978 Mar;14(3):282–289. doi: 10.1007/BF02616037. [DOI] [PubMed] [Google Scholar]
- Takahashi S., Seifter S., Yang F. C. A new radioactive assay for enzymes with elastolytic activity using reduced tritiated elastin. The effect of sodium dodecyl sulfate on elastolysis. Biochim Biophys Acta. 1973 Nov 15;327(1):138–145. doi: 10.1016/0005-2744(73)90111-3. [DOI] [PubMed] [Google Scholar]
- Takahashi S., Shefer A. Effects of vitamin A and dexamethasone on capsule collagen metabolism in mouse mammary adenocarcinoma. J Natl Cancer Inst. 1987 Mar;78(3):497–507. [PubMed] [Google Scholar]
- Takahashi S., Simpser E. Granuloma collagenase and EDTA-sensitive neutral protease production in hepatic murine schistosomiasis. Hepatology. 1981 May-Jun;1(3):211–220. doi: 10.1002/hep.1840010304. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valle K. J., Bauer E. A. Enhanced biosynthesis of human skin collagenase in fibroblast cultures from recessive dystrophic epidermolysis bullosa. J Clin Invest. 1980 Aug;66(2):176–187. doi: 10.1172/JCI109842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vater C. A., Harris E. D., Jr, Siegel R. C. Native cross-links in collagen fibrils induce resistance to human synovial collagenase. Biochem J. 1979 Sep 1;181(3):639–645. doi: 10.1042/bj1810639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werb Z., Gordon S. Elastase secretion by stimulated macrophages. Characterization and regulation. J Exp Med. 1975 Aug 1;142(2):361–377. doi: 10.1084/jem.142.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley D. E., Glanville R. W., Crossley M. J., Evanson J. M. Purification of rheumatoid synovial collagenase and its action on soluble and insoluble collagen. Eur J Biochem. 1975 Jun;54(2):611–622. doi: 10.1111/j.1432-1033.1975.tb04173.x. [DOI] [PubMed] [Google Scholar]
- Woolley D. E., Glanville R. W., Roberts D. R., Evanson J. M. Purification, characterization and inhibition of human skin collagenase. Biochem J. 1978 Feb 1;169(2):265–276. doi: 10.1042/bj1690265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley D. E., Roberts D. R., Evanson J. M. Small molecular weight beta 1 serum protein which specifically inhibits human collagenases. Nature. 1976 May 27;261(5558):325–327. doi: 10.1038/261325a0. [DOI] [PubMed] [Google Scholar]
- Zeydel M., Nakagawa S., Biempica L., Takahashi S. Collagenase and elastase production by mouse mammary adenocarcinoma primary cultures and cloned cells. Cancer Res. 1986 Dec;46(12 Pt 1):6438–6445. [PubMed] [Google Scholar]
- Zhao M. J., Zhang H., Robinson T. F., Factor S. M., Sonnenblick E. H., Eng C. Profound structural alterations of the extracellular collagen matrix in postischemic dysfunctional ("stunned") but viable myocardium. J Am Coll Cardiol. 1987 Dec;10(6):1322–1334. doi: 10.1016/s0735-1097(87)80137-7. [DOI] [PubMed] [Google Scholar]