Abstract
Background
Endometriosis is a chronic, recurring condition that can develop during the reproductive years. It is characterised by the development of endometrial tissue outside the uterine cavity. It is the most common cause of pelvic pain in women. This endometrial tissue development is dependent on oestrogen produced primarily by the ovaries and, therefore, traditional management has focused on suppression of ovarian function. Mounting evidence shows that altered immune function plays a crucial role in the genesis and development of endometriosis. In this review we considered modulation of the inflammation as an alternative approach.
Objectives
To determine the effectiveness and safety of anti‐tumour necrosis factor‐α (anti‐TNF‐α) treatment in the management of endometriosis in premenopausal women.
Search methods
For the first publication of this review, we searched for trials in the following databases (from their inception to August 2009): Cochrane Menstrual Disorders and Subfertility Group Specialised Register, Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library), MEDLINE, EMBASE, CINAHL, and PsycINFO. In addition, we searched all reference lists of included trials and contacted experts in the field in an attempt to locate trials. We reran this search to 3 September 2012 for this update.
Selection criteria
Randomised controlled trials (RCTs) comparing anti‐TNF‐α drugs with placebo, no treatment, medical treatment, or surgery for pelvic pain associated with endometriosis were included.
Data collection and analysis
Two review authors independently selected trials for inclusion, assessed trial quality, and extracted data using data extraction forms. The domains assessed for risk of bias were sequence generation, allocation concealment, blinding, incomplete outcome data, and selective outcome reporting. We used risk ratios (RR) for reporting dichotomous data with 95% confidence intervals (CI), whilst we expressed continuous data as mean differences (MD). We assessed statistical heterogeneity using the I2 statistic.
Main results
Only one trial involving 21 participants was included. The results showed no evidence of an effect of infliximab, one of the known anti‐TNF‐α drugs, on pelvic pain reduction using the Biberoglu‐Behrman (BB) score (0 to 3 scale) for participants (MD ‐0.14, 95% CI ‐0.43 to 0.15), the BB score for clinicians (MD ‐0.14, 95% CI ‐0.39 to 0.11), or a visual analogue pain score (VAS, 100 mm scale) (MD ‐5.60, 95% CI ‐16.10 to 4.90), or on the use of pain killers (ibuprofen, g/day) (MD ‐0.10, 95% CI ‐0.30 to 0.10). There was no evidence of an increase in adverse events in the infliximab group compared with placebo (RR 3.73, 95% CI 0.22 to 63.66). We found no evidence of clinical benefits of infliximab for endometriotic lesions, dysmenorrhoea, dyspareunia, or pelvic tenderness. To date, there is no trial that has reported a cost‐effectiveness analysis of anti‐TNF‐α drugs, or the odds of recurrence.
Authors' conclusions
This review was updated in 2012. The results of the original review published in 2010 remain unchanged. There is still not enough evidence to support the use of anti‐TNF‐α drugs in the management of women with endometriosis for the relief of pelvic pain.
Keywords: Female; Humans; Anti‐Inflammatory Agents; Anti‐Inflammatory Agents/therapeutic use; Antibodies, Monoclonal; Antibodies, Monoclonal/therapeutic use; Endometriosis; Endometriosis/complications; Infliximab; Pelvic Pain; Pelvic Pain/drug therapy; Premenopause; Randomized Controlled Trials as Topic; Tumor Necrosis Factor‐alpha; Tumor Necrosis Factor‐alpha/antagonists & inhibitors
Plain language summary
Anti‐TNF‐α drugs for women with endometriosis‐related pelvic pain
Endometriosis is a painful condition in which endometrial tissue grows outside the uterus. It potentially affects a woman's ability to conceive. Recent studies support the contributing role of inflammation in endometriosis‐related pain. Since anti‐TNF‐α drugs can inhibit the inflammation process, they may relieve the symptoms of the disease without inhibiting ovulation. However, this systematic review included one randomised controlled trial and found that there was not enough evidence from which to draw conclusions about the effectiveness and safety of anti‐TNF‐α drugs in relieving pain in women with endometriosis. There was no evidence of an increase in adverse events in the anti‐TNF‐α drugs group compared with the placebo group.
Background
Description of the condition
Endometriosis is a chronic, recurrent disease characterised by the presence and proliferation of functional endometrial glands and stroma (the supportive framework of an organ, gland, or other structure) outside the uterine cavity (Child 2001). The prevalence of endometriosis among asymptomatic women ranges from 2% to 22%, while in women with dysmenorrhoea the prevalence range is 40% to 60% (Farquhar 2007). Endometriosis is the most common cause of pelvic pain in women (D'Hooghe 2003). The pain may occur at the same time as menstrual bleeding (dysmenorrhoea), during or after sexual intercourse (dyspareunia and post‐coital pain), or as a pelvic pain occurring in a cyclical or non‐cyclical pattern. The extent of the pain is influenced primarily by the location and depth of the endometriotic implant, with deep implants in highly innervated areas being most consistently associated with pain. Definitive diagnosis is usually made through laparoscopic investigation, although some research suggests that the symptoms may have a greater positive predictive value (Ling 1999; Winkel 2003).
Previous management of endometriosis focused on the relief of symptoms, for example pain, and mainly included medical and surgical interventions. Endometriotic cysts were treated by surgery, as the gold standard. Surgery, alone or in combination with medical therapy, remains a common treatment for all stages of endometriosis (Viganò 2003). Surgical treatment aims to remove visible areas of endometriosis and restore anatomy by division of adhesions (abnormal union of body tissues). In some cases, destruction of the nerve pathways thought to be responsible for carrying pain fibres from the pelvis is used (uterine nerve ablation and presacral neurectomy). Although data directly comparing the results of surgical and medical treatment are scarce, the available evidence indicates that surgery does not render any greater relief of painful symptoms than medical therapy (Winkel 2000).
Currently available medical therapies for endometriosis are designed to suppress oestrogen synthesis, induce atrophy of ectopic (displaced or malpositioned) endometrial implants, or interrupt the cycle of stimulation and bleeding (Olive 2003). Oral contraceptives, androgenic agents, progestins, and gonadotropin‐releasing hormone (GnRH) analogues (that is, leuprolide acetate, goserelin acetate, nafarelin acetate) have all been used successfully in the treatment of endometriosis (Davis 2007; Prentice 1999; Prentice 2000; Selak 2007). At present, GnRH analogues are the most popular medical therapy for endometriosis. They strongly reduce the estrogenic pattern in women with endometriosis; however, these drugs cannot cure the disease. Substantial side effects limit their long‐term use yet the effects of GnRH analogues are short‐term because endometriotic foci recur at resumption of ovulation (Prentice 2000). For these reasons, treatment of endometriosis is required to act locally on the lesions rather than by inhibiting ovarian function. Thus more satisfactory approaches should be explored for the management of endometriosis.
Although the disease is one of the most investigated disorders of gynaecology, endometriosis is still one of the most enigmatic diseases. Retrograde menstruation, postulated in the early 1920s by Dr Sampson, was thought to be a key factor in the pathogenesis of endometriosis (D'Hooghe 2002). It operates in up to 90% of women (Halme 1984), however endometriosis affects 5% to 10% of the female population of child‐bearing age (Bullimore 2003). The current consensus is that the local pelvic inflammatory process, with altered function of immune‐related cells in the peritoneal environment, plays a crucial role in the genesis and development of endometriosis (Siristatidis 2006). Development of endometriosis appears to be a complex process. Some of the suggested cytokine mechanisms include defective immunosurveillance (Christodoulakos 2007; Taylor 1997), resistance of the endometrial cells to apoptosis and phagocytosis (Braun 1998; Lebovic 2001), and an associated decreased natural killer cell activity and cytotoxicity against the endometrial cells (Berkkanoglu 2003; Oosterlynck 1991). Since immunological factors are thought to be significantly involved in endometriosis, therapeutic manipulation of the immune system may play a beneficial role in its treatment. The potential immunomodulatory agents, including pentoxifylline (Balasch 1997), loxoribine (Keenan 2000), interferon alpha 2b (Ingelmo 1999), interleukin‐12 (Somigliana 1999), and tumour necrosis factor‐α (TNF‐α) inhibitors (Bullimore 2003), provide an alternative approach to the combat of this disease and the alleviation of pain and infertility without inhibition of ovulation.
Description of the intervention
Anti‐TNF‐α therapy blocks the effects of TNF‐α on target tissues. Anti‐TNF‐α therapy may include both a monoclonal antibody (that is infliximab) and soluble TNF‐α receptors (that is etanercept, recombinant human TNF binding protein‐1 (r‐hTBP‐I)). The commercially available anti‐TNF‐α drugs include certolizumab pegol (Cimzia), etanercept (Enbrel), adalimumab (Humira), and infliximab (Remicade). To date, clinical trials and laboratory studies are scarce in this field.
Infliximab is a chimeric (composed of parts that are of different origin) monoclonal antibody that binds to both soluble and membrane forms of TNF‐α to neutralise its biologic effects. It has been approved by the US Food and Drug Administration (FDA) for the treatment of psoriasis, Crohn's disease, ankylosing spondylitis, psoriatic arthritis, rheumatoid arthritis, and ulcerative colitis. Infliximab blocks the action of TNF‐α by preventing it binding to its receptor in the cell. The safety of infliximab is of major concern. The most frequent severe adverse events are related to serious infections and reactivation of tuberculosis. Non life‐threatening infusion reactions occur frequently and seem to be related to the formation of antibodies (Ridder 2007). Infliximab is usually administered at a clinic or hospital by intravenous infusion, typically at six to eight‐week intervals. However, it is very expensive. It can cost USD 19,000 to USD 22,000 a year per woman wholesale and is covered by very few insurance programmes (according to the drug developer, Centocor Inc).
Etanercept is a fusion protein consisting of human recombinant soluble TNF receptor‐2 (p75) conjugated to a human Fc antibody subunit. Thus it is not, strictly speaking, a monoclonal antibody although it binds to and inhibits the action of TNF‐α. Etanercept lessens TNF‐α activity and is currently used to reduce the signs and symptoms of ankylosing spondylitis, juvenile rheumatoid arthritis, psoriasis, psoriatic arthritis, and rheumatoid arthritis. It has the potential to treat a variety of other disorders mediated by excess TNF‐α. Etanercept needs to be injected subcutaneously, typically by the woman at home. The FDA approved dose is 25 mg twice weekly or 50 mg once weekly. The most frequent adverse reactions are headaches, injection site reactions, and respiratory tract infection. The most serious adverse effects are allergic reactions and reactivation of tuberculosis. Braun et al (Braun 2002) demonstrated that etanercept blocks TNF‐α in peritoneal fluid obtained from women with endometriosis. Thus TNF‐α could enhance the proliferation of eutopic or ectopic endometrial cells. Barrier confirmed this finding in a baboon model, showing that etanercept effectively reduced the extent of endometriotic lesions and the surface area of red lesions (Barrier 2004).
D'Antonio et al (D'Antonio 2000) showed that r‐hTBP‐I, the soluble form of TNF receptor type I, can inhibit the development of endometriotic lesions in rats. In addition, D'Hooghe et al (D'Hooghe 2001) confirmed this finding in a prospective, randomised controlled study in a baboon model, in which r‐hTBP‐I resulted in inhibition of the development and growth of endometriosis. More recently, Falconer et al (Falconer 2004) showed that the anti‐TNF‐α monoclonal antibody c5N significantly reduced both the area and number of red endometriotic lesions in the baboon, but there was no increase in pregnancy rates (Falconer 2008). However, Kyama et al (Kyama 2008) reported that mRNA expression of TNF‐α was not affected in endometrial and endometriosis biopsies from baboons with endometriosis treated with r‐hTBP‐I.
How the intervention might work
The pathophysiology for the association between endometriosis and pain remains elusive. Several hypotheses have been put forward. These include that menstrual bleeding (Brosens 1997) causes pain because of pelvic irritation by the blood, an inflammatory reaction, or by tension in the micro‐cysts of the lesions. Inflammatory reactions and neo angiogenesis (Oosterlynck 1993) around endometriotic lesions have often been observed during laparoscopy and in pathology investigations. In addition, the observed endometriotic invasion of nerve fibres associated with mast cells (Anaf 2006) or sclerotic compression of ureters and of nerve fibres caused by deep lesions might explain the severe pain associated with deep lesions. In some cases (Possover 2007; Robert 1998) the excision of endometriosis surrounding the sciatic nerve in menstrual sciatalgia (neuralgia of the sciatic nerve characterised by pain radiating down through the buttocks and the back of the thigh) or the pudendal nerve can alleviate the deep pelvic pain. Therefore, inflammatory reactions may play a role in the pelvic pain of mild to moderate endometriosis or deep endometriosis after surgical clearance of lesions.
TNF‐α, which is thought to have an essential role in the inflammatory process, is a pro‐inflammatory cytokine mainly produced by activated macrophages. It activates inflammatory leukocytes and promotes the production of other pro‐inflammatory cytokines such as interleukin 1, interleukin 6 and additional TNF‐α. The primary function of TNF‐α is its ability to initiate the cascade of cytokines and other factors associated with inflammatory responses. TNF‐α is a factor in the normal physiology of endometrial proliferation and shedding. However, peritoneal fluid TNF‐α concentrations are elevated in women with endometriosis and some studies show that higher concentrations correlate with the stage of disease (Eisermann 1988). Some in vitro studies suggest that peritoneal macrophages and peripheral blood monocytes from these women have up‐regulated TNF‐α protein secretion (Keenan 1995). The secreted TNF‐α may play a central role in the local and systematic manifestations of endometriosis, on the basis of evidence showing that it promotes the growth of endometriotic cells (Iwabe 2000). Furthermore, some studies (Lee 2008; Teramoto 2004) have explored the association of TNF‐α polymorphisms with endometriosis; to clarify the polymorphic markers in the TNF promoter region and determine the genetic susceptibility to endometriosis. Polymorphisms are implicated in the pathogenesis of endometriosis; therefore, balancing or counteracting TNF‐α production might have a beneficial effect on endometriosis.
In brief, TNF‐α mediated inflammation may be a causal factor in the pain associated with endometriosis. Blocking TNF‐α appears to inhibit the development of the disease in animal models. Therefore, it is timely to determine whether anti‐TNF‐α therapy provides a satisfactory management option for women with endometriosis.
Why it is important to do this review
Traditionally, endometriosis has been managed using hormonal suppression. However, previous Cochrane systematic reviews (Davis 2007; Prentice 1999; Prentice 2000; Selak 2007) show that current treatments are all associated with high rates of recurrence and their short‐term benefits have to be balanced by concerns about immediate and longer‐term side effects. Thus, there is an increasing demand for non‐hormonal drugs, probably acting by modulating immune function, in order to combat the disease and alleviate the associated pain and infertility without inhibiting ovulation. This systematic review aimed to fully assess the effectiveness and safety of anti‐TNF‐α therapy for endometriosis and to explore this unique approach to the management of endometriosis, with immunomodulation.
Objectives
To determine the effectiveness and safety of anti‐tumour necrosis factor‐α (anti‐TNF‐α) treatment in the management of endometriosis in premenopausal women.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials (RCTs) of anti‐TNF‐α drugs in the treatment of endometriosis. Quasi‐RCTs and cross‐over studies which did not have pre‐cross‐over data available were excluded.
Types of participants
Women with visually diagnosed endometriosis by laparoscopy or on the basis of international guidelines (Kennedy 2005; RCOG 2006) used to diagnose endometriosis. Trials where medical treatment was administered after surgical treatment for endometriosis were included.
Types of interventions
Anti‐TNF‐α drug versus placebo
Anti‐TNF‐α drug versus no treatment
Anti‐TNF‐α drug versus medical treatment
Anti‐TNF‐α drug versus surgery
The commercially available anti‐TNF‐α drugs include certolizumab pegol (Cimzia), etanercept (Enbrel), adalimumab (Humira), and infliximab (Remicade). Additional interventions were permitted provided they were common to both groups of a study.
Types of outcome measures
Primary outcomes
Relief of pelvic pain (pain scores, days off work, use of pain killers)
Adverse events resulting from anti‐TNF‐α intervention
Secondary outcomes
Evaluation of improvement in endometriotic lesions
Rate of reoccurrence
Improvement of endometriosis‐related symptoms (apart from pelvic pain), such as dyspareunia (pain during sexual intercourse), post‐coital pain (pain following sexual intercourse), and dyschezia (pain on defecation)
Economic evaluations (the cost of drug, and the time taking for treatment)
We assessed measures of subjective pain relief. These include quantitative measures (such as visual analogue scales, or any other recognised scoring system) or qualitative measures (such as the terms cured, better, same, or worse). A significant number of rating scales are used to assess pain. Scales vary in quality and many are poorly validated. Pelvic pain measured using unpublished rating scales or scales with no established reliability or validity were excluded from the review. We considered outcome measures for each pain symptom at the end of treatment.
Objective evaluations of improvements in endometriotic implants were assessed by transvaginal ultrasound, where possible, using measures of the volume of the endometriotic lesions and their thickness.
We defined recurrent disease as new cyst formation confirmed by endovaginal ultrasound or laparoscopy, or recurrence of symptoms after they had subsided. Recurrence could occur both during treatment and after the end of treatment.
Based on studies and reports, we considered the following adverse events to relate to anti‐TNF‐α therapy: serious blood disorders, infections, headaches, injection site reactions, allergic reactions, serious liver injury, congestive heart failure, lymphoma, and solid tissue cancers, drug‐induced lupus and demyelinating central nervous system disorders.
Search methods for identification of studies
We developed a comprehensive and exhaustive search strategy in collaboration with the Menstrual Disorders and Subfertility Group Trials Search Co‐ordinator. We used the strategy in an attempt to identify all relevant studies regardless of language or publication status (published, unpublished, in press, and in progress). This review drew on the search strategy developed for the Cochrane Menstrual Disorders and Subfertility Group as a whole. We identified relevant trials from both electronic databases and other resources. There was no language restriction in these searches.
We plan to conduct a further update of this review should new studies be published investigating anti‐TNF‐α drugs for endometriosis.
Electronic searches
We searched the following electronic databases (from inception to 3 September 2012).
Cochrane Menstrual Disorders and Subfertility Group (MDSG) Trials Register.
Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library latest issue) (see Appendix 1).
English language electronic databases: MEDLINE, EMBASE, PsycINFO, and CINAHL (see Appendix 2; Appendix 3; Appendix 4).
Chinese language electronic databases: Chinese Biomedical Literature Database (CBM) and Chinese Medical Current Contents (CMCC) (using the corresponding Chinese terms for: "endometriosis" AND "TNF‐α", "Anti‐TNF‐alpha", "infliximab", "etanercept").
Using comparable search terms, the databases:
Current Controlled Trials (www.controlled‐trials.com/);
ClinicalTrials.gov (http://clinicaltrials.gov/ct2/home);
World Health Organization International Trials Registry Platform search portal (www.who.int/trialsearch/Default.aspx);
Citation indexes (http://scientific.thomson.com/products/sci/);
Conference abstracts on the ISI Web of Knowledge (http://isiwebofknowledge.com/);
LILACS (Latin American and Caribbean Health Science Literature) (http://bases.bireme.br/cgi‐bin/wxislind.exe/iah/online/?IsisScript=iah/iah.xis&base=LILACS&lang=i&form=F);
OpenSIGLE (grey Literature in Europe) (http://opensigle.inist.fr/).
For the MEDLINE and EMBASE search strategies, we used different filters for identifying randomised trials.
MEDLINE search: combined with the Cochrane highly sensitive search strategy for identifying randomised trials, which appears in the Cochrane Handbook of Systematic Reviews of Interventions (Version 5.1.0 Chapter 6, 6.4.11) (Higgins 2011).
EMBASE search: combined with trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN) (www.sign.ac.uk/methodology/filters.html#random).
Searching other resources
We also:
searched the references lists of all included studies and relevant reviews to identify further relevant articles;
contacted the authors and experts in the relevant field for potential studies;
handsearched the following relevant Chinese journals:
Chinese Journal of Obstetrics and Gynecology (from 1953 to September 2012);
Chinese Journal of Practical Gynecology and Obstetrics (from 1985 to September 2012);
Journal of Practical Obstetrics and Gynecology (from 1985 to September 2012);
Maternal and Child Health Care of China (from 1986 to September 2012).
Data collection and analysis
We performed statistical analysis in accordance with the Cochrane Handbook for Systematic Reviews of Interventions 5.1.0 (Higgins 2011). We used Review Manager 5 to analyse the data (RevMan 2011).
Selection of studies
HS (Huan Song) and DL (Donghao Lu) independently scrutinised the title, abstract, and keywords of each record retrieved to determine which studies required further assessment. We retrieved the full text when the information given in the titles, abstracts and keywords suggested that the study:
used an anti‐TNF‐α drug as an intervention; and
had a prospective design and a control group.
If there was any doubt regarding these criteria, from scanning the titles and abstracts, we retrieved the full article for clarification. Disagreements were resolved by discussion with a third review author (Gang Shi).
Data extraction and management
We extracted the following information from the studies included in the review. It is presented in the table 'Characteristics of included studies'.
Trial characteristics
(a) Randomisation (b) Allocation concealment (c) Trial design: multicentre or single centre; single phase or cross‐over design (d) Number of women randomised, excluded, and analysed (e) Duration, timing, and location of the trial (f) Source of funding
Baseline characteristics of the studied groups
(a) Definition and duration of pre‐existing infertility (b) Age of the women (c) Investigative work‐up (d) Other causes of infertility (e) Previously administered treatment(s)
Intervention
(a) Type of intervention and control (b) Dose regimen
Outcomes
(a) Outcomes reported (b) How outcomes were defined (c) How outcomes were measured (d) Timing of outcome measurement
Two of the authors (HS, DL) extracted all data independently using forms designed according to Cochrane guidelines. We sought additional information from the study authors on trial methodology and data for trials that appeared to meet the eligibility criteria but had aspects of methodology that were unclear or data that were in an unsuitable form for meta‐analysis. Differences of opinion were to be noted and resolved by consensus.
For dichotomous outcomes (for example, recurrence and adverse events) we recorded the number of participants experiencing the event in each trial group. For continuous outcomes (for example, multidimensional pain scores) we extracted the final value and standard deviation of the outcome of interest and the number of women assessed at the endpoint in each treatment arm at the end of follow‐up. From these data we estimated the mean difference between treatment arms and its standard error. We planned to extract any relevant data reporting medians and ranges and report them in tables.
Assessment of risk of bias in included studies
Two review authors (HS, DL) considered sources of bias. The two authors made 'Risk of bias' assessments independently (HS, DL) according to a modification of the quality criteria specified by the Cochrane Handbook for Systematic Reviews of Interventions 5.1.0 (Higgins 2011). We assessed the following risk of bias domains.
Sequence generation: adequate method described (for example, by computer, random number tables or drawing lots) or not clear (for example, stated but not further described).
Allocation concealment: adequate (for example, by third party, sealed opaque envelopes); inadequate (for example, open list of allocation codes); not clear (for example, not stated, or 'envelopes' stated without further description).
Blinding of participants, personnel, and outcome assessors.
Attrition bias: whether an intention‐to‐treat analysis was performed.
Incomplete outcome data.
Selective outcome reporting.
Other sources of bias.
Disagreements in the assessment were noted and resolved by a third review author (GS). The 'Risk of bias' table is included in the table 'Characteristics of included studies'.
Measures of treatment effect
We analysed different comparisons separately. We used risk ratios (RR) with 95% confidence intervals (CI) for reporting dichotomous data. We expressed continuous data as mean differences (MD) with 95% CI. In the case of outcomes with continuous data in different scales, we planned to use standardised mean difference (SMD) with 95% CI.
Outcomes measured using unpublished rating scales or scales with no established reliability or validity were excluded from the review.
Unit of analysis issues
We only considered RCTs. We planned to assess any non‐standard designs, such as cluster‐randomised trials, in order to avoid unit of analysis errors including: (i) recruitment bias, (ii) baseline imbalance, (iii) loss of clusters, (iv) incorrect analysis, and (v) comparability with individually randomised trials.
Dealing with missing data
We contacted the authors of a primary study to source missing data and to resolve queries. The unpublished means and standard deviations (SD) in Koninckx 2008 were provided by the contact author.
Where possible, we extracted data to allow an intention‐to‐treat analysis (this analysis includes all the participants in the groups to which they were originally randomly assigned). If the numbers randomised were inconsistent with the number analysed, we planned to calculate the percentage loss to follow‐up and report it in an additional table.
Assessment of heterogeneity
Since only one study was included in this review, heterogeneity assessment was not necessary.
If further eligible studies are identified in the future, we will carry out tests for heterogeneity using the Chi2 test, with significance set at P < 0.1. In addition, we will use the I2 statistic to estimate the total variation across studies that is due to heterogeneity, where < 25% is considered as low‐level, 25% to 50% as moderate‐level, and > 50% as high‐level heterogeneity (Higgins 2011). If high levels of heterogeneity are apparent for the primary outcomes, we will explore possible sources of heterogeneity using sensitivity and subgroup analyses as described below.
Assessment of reporting biases
As there was only one included study, potential publication bias could not be assessed using a funnel plot or other corrective analytical methods (Egger 1997).
Data synthesis
If there had been data suitable for pooling, we planned that for any dichotomous outcomes (for example, adverse events) we would calculate risk ratios using the inverse‐variance, random‐effects method.
For continuous outcomes (for example, pain relief measures), if all trials measured the outcome on the same scale we planned to calculate the pooled mean difference between treatment arms at the end of follow‐up; otherwise we would calculate the standardised mean difference.
If in the future any trials have multiple treatment groups, we planned that the 'shared' comparison group would be divided into the number of treatment groups and comparisons between each treatment group and the split comparison group would be treated as independent comparisons.
Subgroup analysis and investigation of heterogeneity
We planned subgroup analyses as follows, had there been sufficient studies:
Different types of control groups (placebo, no treatment, different medical treatment, or different surgical procedures).
Duration of therapy (less than six months, six to 12 months, at least 12 months).
Classification of severity of endometriosis based on the American Fertility Society score (ASRM 1997) (I through IV).
Age (under 30 years, 30 to 40 years, at least 40 years).
If applicable in future updates of this review, we will explore the following potential sources of heterogeneity using subgroup analyses: dosage levels (infliximab ≤ 5 mg/kg, > 5 mg/kg), and duration of treatment.
Sensitivity analysis
A sensitivity analysis was not warranted in this version of the review. If required in future updates, we will perform sensitivity analyses by repeating the analysis in order to explore the influence of the following factors on effect size:
Study publication status, restricting analysis to published studies.
Study quality, restricting analysis to studies with satisfactory methods of allocation concealment and blinding, and low risk of attrition bias.
Very long or large studies to establish how much they dominated the results.
Use of different rating scales to assess symptom relief.
The robustness of the results by repeating the analysis with a different (fixed‐effect) statistical model.
Overall quality of the body of evidence: Summary of Findings Table
We planned to generate a Summary of Findings Table using GRADEPRO software. This table would evaluate the overall quality of the body of evidence for main review outcomes, using GRADE criteria (study limitations (i.e. risk of bias), consistency of effect, imprecision, indirectness and publication bias). Judgements about evidence quality (high, moderate or low) would be justified, documented, and incorporated into reporting of results for each outcome. If future searches identify studies suitable for inclusion such that this review is updated, we will produce such a table for the updated review.
Results
Description of studies
The characteristics of identified studies have been briefly summarised in tables (Characteristics of included studies; Characteristics of excluded studies). See also Figure 1.
1.
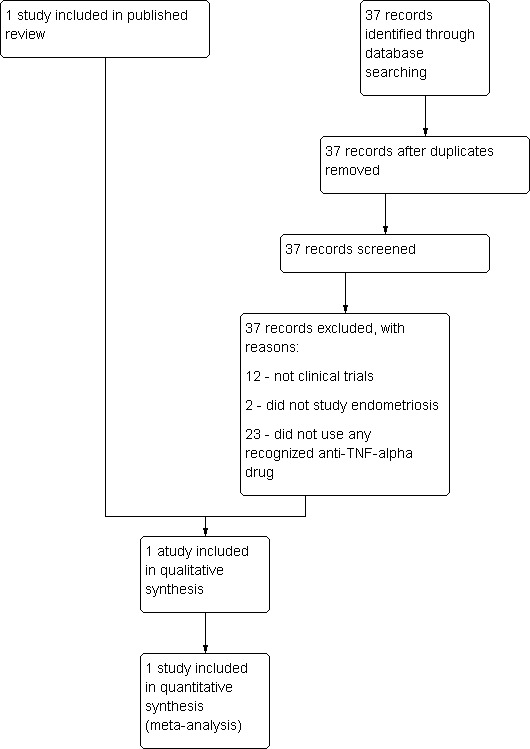
Study flow diagram.
Results of the search
A total of 61 citations (excluding duplications) were obtained from the electronic search strategy, in late 2009. Handsearching of reference lists and journals resulted in retrieval of a further two reports. Of this total of 63 reports, 16 were review articles, 25 were articles not involving anti‐TNF‐alpha drugs, nine were irrelevant articles not involving endometriosis, and 11 were experimental studies. The remaining two reports are listed under included or excluded studies. We sought their full texts for detailed assessment. Only one RCT of anti‐TNF‐alpha drugs versus placebo in women suffering from endometriosis was identified. At this update in 2012, the new search found 37 citations, but all of them were irrelevant to this topic, which meant that no additional studies were identified for inclusion in this update.
Included studies
Study design
The only included trial was published in 2008 and used a two‐arm parallel‐group design in a single centre. A total of 21 participants, distributed 2:1 between active and placebo treatment groups, were included in this trial.
Participants
The included trial specified that women with moderate to severe pelvic pain and a retrovaginal nodule of at least 1 cm in diameter were eligible for inclusion, and used surgery for confirmation during the study. Participant age ranged from 18 to 50 years. The severity of endometriosis was not reported, since in the investigators' view the revised American Fertility Society (rAFS) classification system poorly describes the severity of deep endometriosis.
Interventions
The infusion of infliximab was given at 5 mg/kg and a placebo control was used in the included trial. After the 12‐week treatment period, all participants underwent surgery. The extent of the surgery was not described.
We did not identify any studies that assessed the effectiveness of anti‐TNF‐alpha agents other than infliximab, or that compared anti‐TNF‐α drugs with other medical drugs or surgery.
Outcome measures
Outcomes reported were pain (dysmenorrhoea, deep dyspareunia, and non‐menstrual pain) rated by a visual analogue pain scale (VAS, 100 mm scale) and the Biberoglu‐Behrman (BB) scale. BB scale scored from 0 to 3 for five items which included the former three outcomes recorded both by the gynaecologist and participants, and also pelvic tenderness and induration which were only evaluated by the gynaecologist. Adverse events were described but without severity grade details.
Excluded studies
The study Lian 2009 was excluded because no evidence was shown that Quyu Jiedu granule is a specific anti‐TNF‐α drug.
Risk of bias in included studies
The risk of bias in the included study has been summarised in Figure 2 and Figure 3.
2.
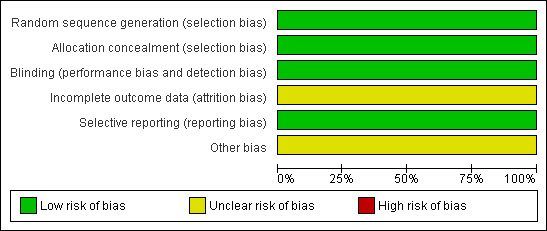
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
3.
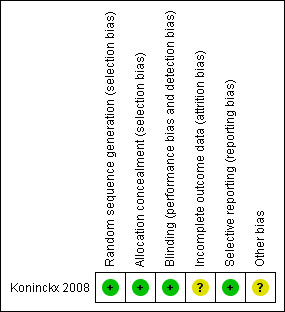
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Allocation
The method of randomisation was not specified in Koninckx 2008, however the information provided by the primary author was: "computer generated randomisation prepared by Centocor Paris". Therefore, we assessed the sequence generation as 'low risk' for randomisation. With respect to allocation concealment, we judged the included study as at low risk of bias because sealed envelopes were prepared and opened consecutively by the pharmacist prior to the preparation of the intervention.
Blinding
Koninckx 2008 stated that all investigators, research nurses, and participants were blinded throughout the study and the randomisation code was broken only after the database had been locked. Thus, we rated the risk of performance and detection bias as low.
Incomplete outcome data
Intention‐to‐treat (ITT) analyses were not performed in Koninckx 2008. The exclusion rate after randomisation was 4.8% (1/21, the infliximab group) since no endometriotic nodule was found during surgery. We rated the risk of attrition bias as unclear.
Selective reporting
The study protocol for Koninckx 2008 was available at ClinicalTrials.gov (http://clinicaltrials.gov/ct2/home). The trial investigators reported data on all the expected outcomes. We rated the risk of selective reporting bias as low.
Other potential sources of bias
The trial Koninckx 2008 was funded by Centocor, manufacturer of infliximab, without any declaration of conflict of interest. We rated the risk of other potential bias as unclear.
Effects of interventions
We extracted summary data from the one included study. Infliximab was used as a 12‐week preoperative treatment. All outcomes were measured at week 12. Since means and SD were not reported, we approximated these values from the published tables and figures in order to assess relief of pelvic pain and related symptoms, and these were confirmed by subsequent information and data from the study author.
Primary outcomes
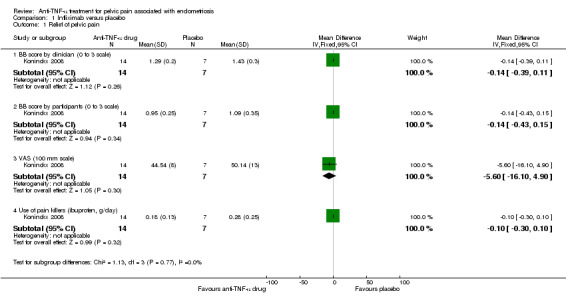
(1) Relief of pelvic pain
For pain scores, both the BB score (0 to 3 scale) and VAS (100 mm scale) were used in this study. The analysis found no relationship between infliximab use and reduction of pelvic pain when the total BB score was determined either by the clinician (21 participants, one trial, Analysis 1.1.1) or by the women (21 participants, one trial, Analysis 1.1.2). In addition, relief of pelvic pain as evaluated by the VAS showed no statistically significant difference between the infliximab group and the placebo group (21 participants, one trial, Analysis 1.1.3).
Regarding the use of pain killers (ibuprofen, g/day), 100 mg tablets of ibuprofen were given in this study and participants recorded their daily intake. No difference in intake of ibuprofen (g/day) was found between the infliximab group and the placebo group (21 participants, one trial, Analysis 1.1.4).
See also Figure 4.
4.
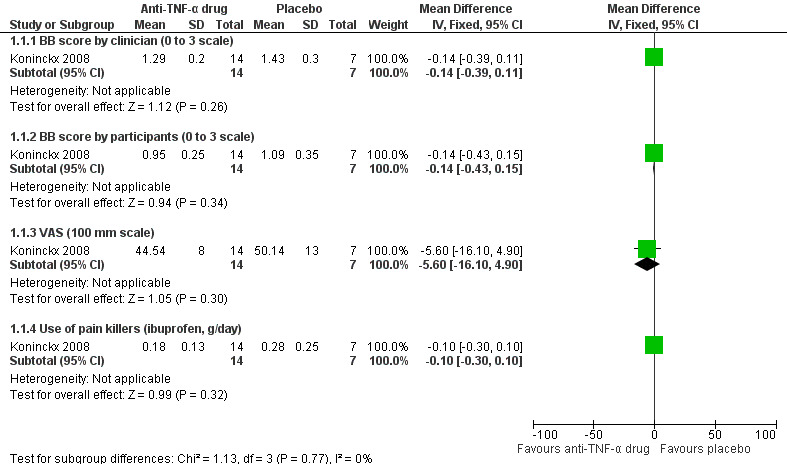
Forest plot of comparison: 1 Infliximab versus placebo, outcome: 1.1 Relief of pelvic pain.
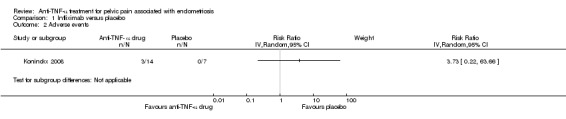
(2) Adverse events
Koninckx 2008 reported three adverse events in the infliximab group: one case of acute tonsillitis, one case of mild infusion reaction, and one case of acute leukaemia. The analysis showed no statistically significant difference in the odds of adverse events in the infliximab group as compared with the placebo group (21 participants, one trial, Analysis 1.2). See also Figure 5.
5.

Forest plot of comparison: 1 Infliximab versus placebo, outcome: 1.2 Adverse events.
Secondary outcomes
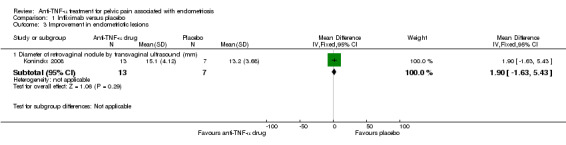
(1) Evaluation of improvement in endometriotic lesions
The diameter of retrovaginal nodules (mm) was measured by transvaginal ultrasound at week 12. This trial yielded a mean difference of 1.90 cm (21 participants, one trial, Analysis 1.3), a statistically non‐significant finding in favour of placebo.
(2) Rate of recurrence
This was not reported in the included study.
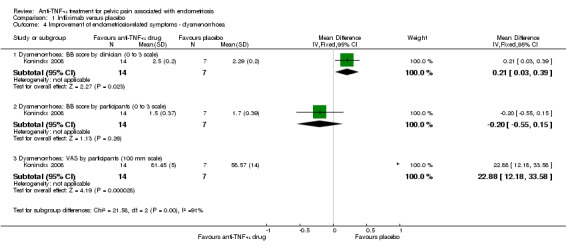
(3) Improvement of other endometriosis‐related symptoms
With regard to dysmenorrhoea, both the BB score and VAS were used. The analysis found no relationship between infliximab use and dysmenorrhoea relief when the BB score was assessed either by the clinician (21 participants, one trial, Analysis 1.4.1) or by the women (21 participants, one trial, Analysis 1.4.2) . However, the analysis found a statistically significant reduction in dysmenorrhoea based on the VAS in the placebo group as compared with the infliximab group (MD 22.88, 95% CI 12.18 to 33.58; 21 participants, one trial, Analysis 1.4.3).
With regard to dyspareunia, the BB score was recorded by both women and clinician. No differences were found between the two intervention groups using either measurement (by clinician: 21 participants, one trial, Analysis 1.5.1; by women: 21 participants, one trial, Analysis 1.5.2).
With regard to pelvic tenderness evaluated by the clinician, the BB score showed no difference between the two groups (21 participants, one trial, Analysis 1.6).
(4) Economic evaluations
This was not reported in the included study.
Discussion
There is not enough evidence to support the use of anti‐TNF‐α drugs in the treatment of pelvic pain associated with endometriosis.
Summary of main results
This systematic review has shown that there is limited evidence of relief or deterioration in pelvic pain associated with endometriosis when treated with anti‐TNF‐α drugs. The only RCT (Koninckx 2008) addressed a highly selected group of women with advanced endometriosis that involved retrovaginal nodules, without reporting rAFS stages. Therefore, this finding needs to be interpreted with caution since inconsistent disease types and unknown grades were included in the analysis. Thus, there is no reliable evidence to draw any conclusions about the effectiveness of anti‐TNF‐α drugs in the treatment of pelvic pain associated with endometriosis. Based on the available evidence, infliximab appears not to affect lesion volume or other endometriosis‐related symptoms, for example dysmenorrhoea, dyspareunia, and pelvic tenderness. Interestingly, the women in the placebo group had a greater reduction in the VAS scores than women in the treatment group. Thus, we note the strong placebo effect in participants, which further emphasises the need for well‐conducted, double‐blind RCTs for pain evaluation.
With respect to safety, infliximab did not increase the odds of adverse events for participants with endometriosis in the short term. However, we draw this conclusion with caution due to the small sample included. In addition, there is no evidence to support the effectiveness of anti‐TNF‐α drugs in raising or lowering the odds of recurrence of endometriosis, since no relevant data could be extracted. Finally, since no study conducted an economic evaluation, it is hard to draw any conclusion about the cost‐effectiveness of anti‐TNF‐α drugs in the treatment of pelvic pain associated with endometriosis.
Overall completeness and applicability of evidence
The identified study only partially addressed the objectives of the review. Firstly, Koninckx 2008 was restricted to a highly selected group of women with advanced endometriosis, the kind involving rectovaginal nodules. That may decrease the generalisability of the results regarding the effectiveness of anti‐TNF‐α drugs for women with endometriosis in general. Secondly, the included study gave infliximab as a preoperative therapy. Thus other types of interventions, such as infliximab after surgery, infliximab versus medical treatment or surgery, and other anti‐TNF‐α drugs, should be examined in future studies. Finally, the design of the included study overlooked some important outcomes, for example rate of recurrence and economic outcomes. These outcomes should be explored in further studies, which would allow more complete assessment of anti‐TNF‐α drugs for treating endometriosis.
Quality of the evidence
This systematic review has reported on one RCT that included only 21 women for the comparison of infliximab versus placebo. Therefore, the major quality issue that needs to be addressed relates to the small sample of the included RCT. Chance could be a reasonable explanation for the reported findings and this study is underpowered to determine any treatment effect.
Another limitation of this review relates to the methodological quality of the included study. Koninckx 2008 did not specify details relating to the randomisation method in the written report, although we obtained sufficient information later from the primary author. There was also a 4.8% loss to follow‐up without application of the intention‐to‐treat principle.
Potential biases in the review process
In order to prevent bias in the review process, the search was guided and developed by the Cochrane Menstrual Disorders and Subfertility Group. No limitations (such as a language restriction) were applied to the search. The study selection, 'Risk of bias' assessment, and data collection were conducted independently by two review authors without blinding. Any disagreement was resolved by discussion with the third review author. The included study has an extremely small sample from which any potential treatment effect could not be determined. There were no conflicts of interest with the review authors.
Agreements and disagreements with other studies or reviews
There are still no clinical trials on anti‐TNF‐α drugs for endometriosis other than Koninckx 2008. Further, well‐designed large‐sample RCTs are warranted to confirm or refute the current evidence. There is no other systematic review on anti‐TNF‐α drugs for endometriosis at present.
Authors' conclusions
Implications for practice.
At this update in 2012, there still appears to be no evidence at this time to support using anti‐TNF‐α drugs as treatment for pelvic pain associated with endometriosis. This review will be further updated should new studies be published investigating anti‐TNF‐α drugs for endometriosis.
Implications for research.
Future research should expand the disease types and grades for enrolling women. Well‐designed and well‐conducted RCTs with double‐blinding should be conducted in order to reduce the placebo effect when evaluating novel treatments for pain. The current study compared infliximab with placebo before surgery, which highlights the need for more types of comparison, such as postoperative use of infliximab, infliximab compared with other medical treatments or surgery, and other types of anti‐TNF‐α drugs. The lack of data on recurrence rates and cost‐effectiveness highlights the need for any future research to incorporate these outcomes.
What's new
| Date | Event | Description |
|---|---|---|
| 31 May 2013 | Review declared as stable | As no further studies are expected, this review will no longer be updated. |
History
Protocol first published: Issue 4, 2009 Review first published: Issue 3, 2010
| Date | Event | Description |
|---|---|---|
| 5 February 2013 | New search has been performed | We conducted a new search, but identified no new studies. |
| 5 February 2013 | New citation required but conclusions have not changed | There were no new studies to include in this update. |
| 13 May 2010 | Amended | Data obtained and included from authors of the included study |
Acknowledgements
We wish to thank Dr Armando C Rodriguez for his language support in the preparation of this manuscript. We thank Philippe R Koninckx and Cindy Farquhar for providing additional information and unpublished data about Koninckx 2008. We thank Jane Clarke (previous Managing Editor), Marian Showell (Trials Search Co‐ordinator), and the editorial board of the Cochrane Menstrual Disorders and Subfertility Group (MDSG) for their invaluable assistance in developing this review.
Appendices
Appendix 1. CENTRAL search strategy
1 Endometriosis/ (370) 2 (pelv* adj2 pain).tw. (348) 3 adenomyosis.tw. (23) 4 Endometrio*.tw. (677) 5 dyspareunia.tw. (152) 6 dyschezia.tw. (5) 7 or/1‐6 (1067) 8 exp Tumor Necrosis Factor‐alpha/ (1409) 9 Tumor Necrosis Factor‐alpha.tw. (1027) 10 Tumour Necrosis Factor‐alpha.tw. (285) 11 (anti tumour necrosis factor or anti tumor necrosis factor).tw. (125) 12 (tumour necrosis factor antibod* or tumor necrosis factor).tw. (1576) 13 (anti tumour necrosis factor antibod* or anti tumor necrosis factor antibod*).tw. (7) 14 (anti TNF or anti TNF alpha).tw. (129) 15 (TNF antibod* or TNF alpha antibod*).tw. (45) 16 (anti TNF antibod* or anti TNF alpha antibod*).tw. (36) 17 (infliximab* or monoclonal antibody cA2 or Remicade*).tw. (281) 18 CDP571.tw. (15) 19 etanercept*.tw. (244) 20 (adalimumab* or d2e7).tw. (87) 21 onercept*.tw. (5) 22 cachectin.tw. (4) 23 tnf superfamily.tw. (0) 24 or/8‐23 (2692) 25 24 and 7 (6) 26 from 25 keep 1‐6 (6)
Appendix 2. MEDLINE search strategy
1 Endometriosis/ (13267) 2 (pelv* adj2 pain).tw. (4200) 3 adenomyosis.tw. (1195) 4 Endometrio*.tw. (14589) 5 dyspareunia.tw. (1669) 6 dyschezia.tw. (112) 7 or/1‐6 (22315) 8 exp Tumor Necrosis Factor‐alpha/ (71189) 9 Tumor Necrosis Factor‐alpha.tw. (36991) 10 Tumour Necrosis Factor‐alpha.tw. (7965) 11 (anti tumour necrosis factor or anti tumor necrosis factor).tw. (1595) 12 (tumour necrosis factor antibod* or tumor necrosis factor).tw. (58679) 13 (anti tumour necrosis factor antibod* or anti tumor necrosis factor antibod*).tw. (72) 14 (anti TNF or anti TNF alpha).tw. (3754) 15 (TNF antibod* or TNF alpha antibod*).tw. (1469) 16 (anti TNF antibod* or anti TNF alpha antibod*).tw. (1129) 17 (infliximab* or monoclonal antibody cA2 or Remicade*).tw. (3924) 18 CDP571.tw. (39) 19 etanercept*.tw. (2156) 20 (adalimumab* or d2e7).tw. (941) 21 onercept*.tw. (21) 22 cachectin.tw. (433) 23 tnf superfamily.tw. (392) 24 or/8‐23 (97728) 25 24 and 7 (217) 26 randomized controlled trial.pt. (278136) 27 controlled clinical trial.pt. (80231) 28 randomized.ab. (187362) 29 placebo.tw. (117986) 30 clinical trials as topic.sh. (145561) 31 randomly.ab. (135743) 32 trial.ti. (81574) 33 (crossover or cross‐over or cross over).tw. (43620) 34 or/26‐33 (658983) 35 (animals not (humans and animals)).sh. (3335965) 36 34 not 35 (609881) 37 25 and 36 (6) 38 from 37 keep 1‐6 (6)
Appendix 3. EMBASE search strategy
1 Endometriosis/ (11245) 2 (pelv* adj2 pain).tw. (3980) 3 adenomyosis.tw. (1043) 4 Endometrio*.tw. (12428) 5 dyspareunia.tw. (1571) 6 dyschezia.tw. (83) 7 or/1‐6 (19531) 8 exp Tumor Necrosis Factor‐alpha/ (80752) 9 Tumor Necrosis Factor‐alpha.tw. (34417) 10 Tumour Necrosis Factor‐alpha.tw. (7601) 11 (anti tumour necrosis factor or anti tumor necrosis factor).tw. (1538) 12 (tumour necrosis factor antibod* or tumor necrosis factor).tw. (54853) 13 (anti tumour necrosis factor antibod* or anti tumor necrosis factor antibod*).tw. (72) 14 (anti TNF or anti TNF alpha).tw. (3463) 15 (TNF antibod* or TNF alpha antibod*).tw. (1280) 16 (anti TNF antibod* or anti TNF alpha antibod*).tw. (983) 17 (infliximab* or monoclonal antibody cA2 or Remicade*).tw. (5711) 18 CDP571.tw. (41) 19 etanercept*.tw. (2267) 20 (adalimumab* or d2e7).tw. (1012) 21 onercept*.tw. (32) 22 cachectin.tw. (371) 23 tnf superfamily.tw. (348) 24 or/8‐23 (108553) 25 24 and 7 (317) 26 limit 25 to yr="2008 ‐Current" (56) 27 from 26 keep 1‐56 (56)
Appendix 4. PsycINFO search strategy
1 Endometriosis/ (0) 2 (pelv* adj2 pain).tw. (277) 3 adenomyosis.tw. (4) 4 Endometrio*.tw. (106) 5 dyspareunia.tw. (283) 6 dyschezia.tw. (3) 7 or/1‐6 (627) 8 exp Tumor Necrosis Factor‐alpha/ (0) 9 Tumor Necrosis Factor‐alpha.tw. (610) 10 Tumour Necrosis Factor‐alpha.tw. (58) 11 (anti tumour necrosis factor or anti tumor necrosis factor).tw. (9) 12 (tumour necrosis factor antibod* or tumor necrosis factor).tw. (900) 13 (anti tumour necrosis factor antibod* or anti tumor necrosis factor antibod*).tw. (1) 14 (anti TNF or anti TNF alpha).tw. (20) 15 (TNF antibod* or TNF alpha antibod*).tw. (7) 16 (anti TNF antibod* or anti TNF alpha antibod*).tw. (5) 17 (infliximab* or monoclonal antibody cA2 or Remicade*).tw. (21) 18 CDP571.tw. (0) 19 etanercept*.tw. (22) 20 (adalimumab* or d2e7).tw. (6) 21 onercept*.tw. (0) 22 cachectin.tw. (0) 23 tnf superfamily.tw. (4) 24 or/8‐23 (964) 25 24 and 7 (1) 26 from 25 keep 1 (1)
Data and analyses
Comparison 1. Infliximab versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Relief of pelvic pain | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 BB score by clinician (0 to 3 scale) | 1 | 21 | Mean Difference (IV, Fixed, 95% CI) | ‐0.14 [‐0.39, 0.11] |
| 1.2 BB score by participants (0 to 3 scale) | 1 | 21 | Mean Difference (IV, Fixed, 95% CI) | ‐0.14 [‐0.43, 0.15] |
| 1.3 VAS (100 mm scale) | 1 | 21 | Mean Difference (IV, Fixed, 95% CI) | ‐5.60 [‐16.10, 4.90] |
| 1.4 Use of pain killers (ibuprofen, g/day) | 1 | 21 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.30, 0.10] |
| 2 Adverse events | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 3 Improvement in endometriotic lesions | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Diameter of retrovaginal nodule by transvaginal ultrasound (mm) | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 1.90 [‐1.63, 5.43] |
| 4 Improvement of endometriosis‐related symptoms ‐ dysmenorrhoea | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 Dysmenorrhoea: BB score by clinician (0 to 3 scale) | 1 | 21 | Mean Difference (IV, Fixed, 95% CI) | 0.21 [0.03, 0.39] |
| 4.2 Dysmenorrhoea: BB score by participants (0 to 3 scale) | 1 | 21 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.55, 0.15] |
| 4.3 Dysmenorrhoea: VAS by participants (100 mm scale) | 1 | 21 | Mean Difference (IV, Fixed, 95% CI) | 22.88 [12.18, 33.58] |
| 5 Improvement of endometriosis‐related symptoms ‐ dyspareunia | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | ‐0.24 [‐0.46, ‐0.01] |
| 5.1 Dyspareunia: BB score by clinician (0 to 3 scale) | 1 | 21 | Mean Difference (IV, Fixed, 95% CI) | ‐0.14 [‐0.41, 0.13] |
| 5.2 Dyspareunia: BB score by participants (0 to 3 scale) | 1 | 21 | Mean Difference (IV, Fixed, 95% CI) | ‐0.44 [‐0.84, ‐0.04] |
| 6 Improvement of endometriosis‐related symptoms ‐ pelvic tenderness | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 Pelvic tenderness: BB score by clinician (0 to 3 scale) | 1 | 21 | Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.20, 0.34] |
1.1. Analysis.
Comparison 1 Infliximab versus placebo, Outcome 1 Relief of pelvic pain.
1.2. Analysis.
Comparison 1 Infliximab versus placebo, Outcome 2 Adverse events.
1.3. Analysis.
Comparison 1 Infliximab versus placebo, Outcome 3 Improvement in endometriotic lesions.
1.4. Analysis.
Comparison 1 Infliximab versus placebo, Outcome 4 Improvement of endometriosis‐related symptoms ‐ dysmenorrhoea.
1.5. Analysis.
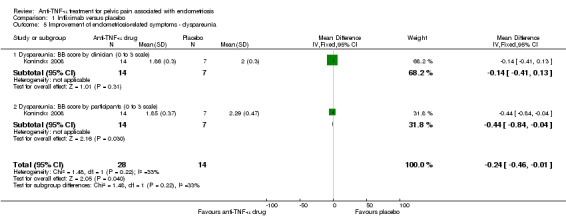
Comparison 1 Infliximab versus placebo, Outcome 5 Improvement of endometriosis‐related symptoms ‐ dyspareunia.
1.6. Analysis.
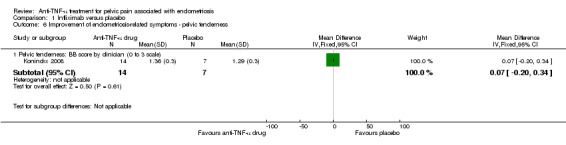
Comparison 1 Infliximab versus placebo, Outcome 6 Improvement of endometriosis‐related symptoms ‐ pelvic tenderness.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Koninckx 2008.
| Methods | RCT duration 40 weeks, single centre, 21 participants | |
| Participants | 21 enrolled: active = 14, placebo = 7 Evaluated: active = 13, placebo = 7 Age (years): active 28.4 ± 4.5, placebo 30.7 ± 5.5 Weight (kg): active 62.5 ± 7.4, placebo 52.7 ± 5.4 Diameters of nodules (mm): active 15.2 ± 4.6, placebo 13.6 ± 3.2 Diagnosis: endometriosis with severe pelvic pain Inclusion criteria: 20 to 45 years olds with a deep endometriosis nodule of at least 1 cm in diameter, severe pain (at least 1 severe pain score on Biberoglu‐Behrman scale), no previous surgery for deep endometriosis, no hormonal medication for at least 3 months prior to enrolment in the study Exclusion criteria: evidence of infection in the previous 3 months, previous transplant surgery, previous anti‐TNF‐α treatment |
|
| Interventions | Active group: infliximab (5 mg/kg) administered as infusion at weeks 0, 2, and 6. Surgery was performed 3 months later and follow‐up continued for a further 6 months Placebo group: placebo was administered as infusion at weeks 0, 2, and 6. Surgery was performed 3 months later and follow‐up continued for a further 6 months |
|
| Outcomes | 1. Reduction of pain based on VAS and Biberoglu‐Behrman scale 2. Intake of pain killers 3. Improvement of endometriotic lesions |
|
| Notes | Belgium Katholieke Universiteit Leuven Drug company: Centocor BV | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes were prepared and opened consecutively by the pharmacist prior to the preparation of the intervention |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No ITT |
| Selective reporting (reporting bias) | Low risk | Consistent with protocol |
| Other bias | Unclear risk | Funded by the manufacturer |
Abbreviations: anti‐TNF‐α: anti‐tumour necrosis factor‐α; ITT: intention‐to‐treat; RCT: randomised controlled trial; VAS: visual analogue scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Lian 2009 | No evidence that this Chinese herbal medicine, Quyu Jiedu granule, is a specific anti‐TNF‐α drug |
Contributions of authors
Donghao Lu: drafting of protocol and review; search for trials; data entry into RevMan; extraction of data; selection of trials for inclusion or exclusion. Huan Song: extraction of data; selection of trials for inclusion or exclusion; obtaining copies of trial reports; extraction of data. Gang Shi: all correspondence; drafting of protocol and review; interpretation of results.
Sources of support
Internal sources
Dept of Obstetrics and Gynaecology, Sichuan University, Chengdu, China.
Dept of Obstetrics and Gynaecology, University of Auckland, New Zealand.
External sources
No sources of support supplied
Declarations of interest
The authors declare that all the analysis and interpretation reflected the opinions of the authors; no conflict of interest was involved in the analysis or interpretation of data, or in the writing of the systematic review.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Koninckx 2008 {published data only}
- Koninckx PR, Craessaerts M, Timmerman D, Cornillie F, Kennedy S. Anti‐TNF‐alpha treatment for deep endometriosis‐associated pain: a randomized placebo‐controlled trial. Human Reproduction 2008;23(9):2017‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Lian 2009 {published data only}
- Lian F, Li XL, Sun ZG, Zhang JW, Liu YH, Ma FM. Effect of Quyu Jiedu granule on microenvironment of ova in patients with endometriosis. Chinese Journal of Integrative Medicine 2009;15(1):42‐6. [DOI] [PubMed] [Google Scholar]
Additional references
Anaf 2006
- Anaf V, Chapron C, Nakadi I, Moor V, Simonart T, Noel JC. Pain, mast cells, and nerves in peritoneal, ovarian, and deep infiltrating endometriosis. Fertility and Sterility 2006;86:1336‐43. [DOI] [PubMed] [Google Scholar]
ASRM 1997
- American Society for Reproductive Medicine. Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertility and Sterility 1997;67:817‐21. [DOI] [PubMed] [Google Scholar]
Balasch 1997
- Balasch J, Creus M, Fabregues F, Carmona F, Martinez‐Roman S, Manau D, et al. Pentoxifylline versus placebo in the treatment of infertility associated with minimal or mild endometriosis: a pilot randomized clinical trial. Human Reproduction 1997;12:2046‐50. [DOI] [PubMed] [Google Scholar]
Barrier 2004
- Barrier BF, Bates GW, Leland MM, Leach DA, Robinson RD, Propst MA. Efficacy of anti‐tumor necrosis factor therapy in the treatment of spontaneous endometriosis in baboons. Fertility and Sterility 2004;81 Suppl 1:775‐9. [DOI] [PubMed] [Google Scholar]
Berkkanoglu 2003
- Berkkanoglu M, Arici A. Immunology and endometriosis. American Journal of Reproductive Immunology 2003;50(1):48‐59. [DOI] [PubMed] [Google Scholar]
Braun 1998
- Braun DP, Dmowski WP. Endometriosis: abnormal endometrium and dysfunctional immune response. Current Opinion in Obstetrics and Gynecology 1998;10:365‐9. [DOI] [PubMed] [Google Scholar]
Braun 2002
- Braun DP, Ding J, Dmowski WP. Peritoneal fluid‐mediated enhancement of eutopic and ectopic endometrial cell proliferation is dependent on tumor necrosis factor‐alpha in women with endometriosis. Fertility and Sterility 2002;78:727‐32. [DOI] [PubMed] [Google Scholar]
Brosens 1997
- Brosens IA. Endometriosis ‐ a disease because it is characterized by bleeding. American Journal of Obstetrics and Gynecology 1997;176:263‐7. [DOI] [PubMed] [Google Scholar]
Bullimore 2003
- Bullimore DW. Endometriosis is sustained by tumour necrosis factor‐alpha. Medical Hypotheses 2003;60(1):84‐8. [DOI] [PubMed] [Google Scholar]
Child 2001
- Child TJ, Tan SL. Endometriosis: aetiology, pathogenesis and treatment. Drugs 2001;61:1735‐50. [DOI] [PubMed] [Google Scholar]
Christodoulakos 2007
- Christodoulakos G, Augoulea A, Lambrinoudaki I, Sioulas V, Creatsas G. Pathogenesis of endometriosis: the role of defective 'immunosurveillance'. European Journal of Contraception and Reproductive Health Care 2007;12:194‐202. [DOI] [PubMed] [Google Scholar]
D'Antonio 2000
- D'Antonio M, Martelli F, Peano S, Papoian R, Borrelli F. Ability of recombinant human TNF binding protein‐1 (r‐hTBP‐1) to inhibit the development of experimentally induced endometriosis in rats. Journal of Reproductive Immunology 2000;48:81‐98. [DOI] [PubMed] [Google Scholar]
D'Hooghe 2001
- D'Hooghe TM, Cuneo S, Nugent N, Chai D, Deer F, Mwenda J. Recombinant human TNF binding protein‐1 (r‐hTBP‐1) inhibits the development of endometriosis in baboons: a prospective, randomized, placebo and drug‐controlled study. Fertility and Sterility 2001;76 Suppl 1:1. [Google Scholar]
D'Hooghe 2002
- D'Hooghe TM, Debrock S. Endometriosis, retrograde menstruation and peritoneal inflammation in women and in baboons. Human Reproduction Update 2002;8(1):84‐8. [DOI] [PubMed] [Google Scholar]
D'Hooghe 2003
- D'Hooghe TM, Debrock S, Hill JA, Meuleman C. Endometriosis and subfertility: is the relationship resolved?. Seminars in Reproductive Medicine 2003;21:243‐54. [DOI] [PubMed] [Google Scholar]
Davis 2007
- Davis LJ, Kennedy SS, Moore J, Prentice A. Oral contraceptives for pain associated with endometriosis. Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI: 10.1002/14651858.CD001019.pub2] [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Smith GD, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eisermann 1988
- Eisermann J, Gast MJ, Pineda J, Odem RR, Collins JL. Tumor necrosis factor in peritoneal fluid of women undergoing laparoscopic surgery. Fertility and Sterility 1988;50:573‐9. [DOI] [PubMed] [Google Scholar]
Falconer 2004
- Falconer H, Mwenda JM, Chai DC, Wagner C, Cornillie FJ, D'Hooghe TM. Treatment with anti‐TNF antibody c5N reduces the extent of induced endometriosis in the baboon. Fertility and Sterility 2004;82 Suppl 2:54‐5. [DOI] [PubMed] [Google Scholar]
Falconer 2008
- Falconer H, Mwenda JM, Chai DC, Song XR, Cornillie FJ, Bergqvist A, et al. Effects of anti‐TNF‐mAb treatment on pregnancy in baboons with induced endometriosis. Fertility and Sterility 2008;89(5):1537‐45. [DOI] [PubMed] [Google Scholar]
Farquhar 2007
- Farquhar C. Endometriosis. BMJ 2007;334:249‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Halme 1984
- Halme J, Becker S, Wing R. Accentuated cyclic activation of peritoneal macrophages in patients with endometriosis. American Journal of Obstetrics and Gynecology 1984;148:85‐90. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration 2011. Available from www.cochrane‐handbook.org.
Ingelmo 1999
- Ingelmo JM, Quereda F, Acien P. Intraperitoneal and subcutaneous treatment of experimental endometriosis with recombinant human interferon‐alpha‐2b in murine model. Fertility and Sterility 1999;71:907‐11. [DOI] [PubMed] [Google Scholar]
Iwabe 2000
- Iwabe T, Harada T, Tsudo T, Nagano Y, Yoshida S, Tanikawa M, et al. Tumor necrosis factor‐alpha promote proliferation of endometriotic stromal cells by inducing interleukin‐8 gene and protein expression. Journal of Clinical Endocrinology and Metabolism 2000;85:824‐9. [DOI] [PubMed] [Google Scholar]
Keenan 1995
- Keenan JA, Chen TT, Chadwell NL, Torry DS, Caudle MR. IL‐1 beta, TNF‐alpha, and IL‐2 in peritoneal fluid and macrophage‐conditioned media of women with endometriosis. American Journal of Reproductive Immunology 1995;34:381‐5. [DOI] [PubMed] [Google Scholar]
Keenan 2000
- Keenan JA, Williams‐Boyce PK, Massey PJ, Chen TT, Caudle MR, Bukovsky A. Regression of endometrial explants in a rat model of endometriosis treated with immune modulators loxoribine and levamisole. Fertility and Sterility 2000;72:135‐41. [DOI] [PubMed] [Google Scholar]
Kennedy 2005
- Kennedy S, Bergqvist A, Chapron C, D'Hooghe T, Duselman G, Greb R, et al. ESHRE guideline for the diagnosis and treatment of endometriosis. Human Reproduction 2005;20(10):2698‐704. [DOI] [PubMed] [Google Scholar]
Kyama 2008
- Kyama CM, Overbergh L, Mihalyi A, Cuneo S, Chai D, Debrock S, et al. Effect of recombinant human TNF‐binding protein‐1 and GnRH antagonist on mRNA expression of inflammatory cytokines and adhesion and growth factors in endometrium and endometriosis tissues in baboons. Fertility and Sterility 2008;89 Suppl 5:1306‐13. [DOI] [PubMed] [Google Scholar]
Lebovic 2001
- Lebovic DI, Mueller MD, Taylor RN. Immunobiology of endometriosis. Fertility and Sterility 2001;75(1):1‐10. [DOI] [PubMed] [Google Scholar]
Lee 2008
- Lee GH, Choi YM, Kim SH, Hong MA, Oh ST, Lim YT, et al. Association of tumor necrosis factor‐a gene polymorphisms with advanced stage endometriosis. Human Reproduction 2008;23(4):977‐81. [DOI] [PubMed] [Google Scholar]
Ling 1999
- Ling FW. Randomized controlled trial of depot leuprolide in patients with chronic pelvic pain and clinically suspected endometriosis. Obstetrics and Gynecology 1999;37:51‐8. [DOI] [PubMed] [Google Scholar]
Olive 2003
- Olive DL, Pritts EA. Treatment of endometriosis. New England Journal of Medicine 2003;345(4):266‐75. [DOI] [PubMed] [Google Scholar]
Oosterlynck 1991
- Oosterlynck DJ, Cornillie FJ, Waer M, Vandeputte M, Koninckx PR. Women with endometriosis show a defect in natural killer activity resulting in a decreased cytotoxicity to autologous endometrium. Fertility and Sterility 1991;56:45‐51. [DOI] [PubMed] [Google Scholar]
Oosterlynck 1993
- Oosterlynck DJ, Meuleman C, Sobis H, Vandeputte M, Koninckx PR. Angiogenic activity of peritoneal fluid from women with endometriosis. Fertility and Sterility 1993;59:778‐82. [DOI] [PubMed] [Google Scholar]
Possover 2007
- Possover M, Chiantera V. Isolated infiltrative endometriosis of the sciatic nerve: a report of three patients. Fertility and Sterility 2007;87:417‐9. [DOI] [PubMed] [Google Scholar]
Prentice 1999
- Prentice A, Deary A, Goldbeck‐Wood S, Farquhar CM, Smith S. Gonadotrophin‐releasing hormone analogues for pain associated with endometriosis. Cochrane Database of Systematic Reviews 1999, Issue 2. [DOI: 10.1002/14651858.CD000346.pub2] [DOI] [Google Scholar]
Prentice 2000
- Prentice A, Deary AJ, Bland E. Progestagens and anti‐progestagens for pain associated with endometriosis. Cochrane Database of Systematic Reviews 2000, Issue 2. [DOI: 10.1002/14651858.CD002122] [DOI] [PubMed] [Google Scholar]
RCOG 2006
- Royal College of Obstetricians and Gynaecologists (RCOG). The investigation and management of endometriosis. London (UK): Royal College of Obstetricians and Gynaecologists (RCOG) 2006.
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Ridder 2007
- Ridder LD, Benninga MA, Tamminiau JA, Hommes DW, Deventer SJ. Infliximab use in children and adolescents with inflammatory bowel disease. Journal of Pediatric Gastroenterology and Nutrition 2007;45:3‐14. [DOI] [PubMed] [Google Scholar]
Robert 1998
- Robert R, Prat‐Pradal D, Labat JJ, Bensignor M, Raoul S, Rebai R, et al. Anatomic basis of chronic perineal pain: role of the pudendal nerve. Radiologic Anatomy 1998;20:93‐8. [DOI] [PubMed] [Google Scholar]
Selak 2007
- Selak V, Farquhar C, Prentice A, Singla A. Danazol for pelvic pain associated with endometriosis. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD000068.pub2] [DOI] [PubMed] [Google Scholar]
Siristatidis 2006
- Siristatidis C, Nissotakis C, Chrelias C, Iacovidou H, Salamalekis E. Immunological factors and their role in the genesis and development of endometriosis. Journal of Obstetrics and Gynaecology Research 2006;32(2):162‐70. [DOI] [PubMed] [Google Scholar]
Somigliana 1999
- Somigliana E, Vigano P, Rossi G, Carinelli S, Vignali M, Panina‐Bordignon P. Endometrial ability to implant in ectopic sites can be prevented by interleukin‐12 ina murine model of endometriosis. Human Reproduction 1999;14:2944‐50. [DOI] [PubMed] [Google Scholar]
Taylor 1997
- Taylor RN, Ryan IP, Moore ES, Hornung D, Shifren JL, Tseng JF. Angiogenesis and macrophage activation in endometriosis. Annals of the New York Academy of Sciences 1997;828:194‐207. [DOI] [PubMed] [Google Scholar]
Teramoto 2004
- Teramoto M, Kitawaki J, Koshiba H, Kitaoka Y, Obayashi H, Hasegawa G, et al. Genetic contribution of tumor necrosis factor (TNF)‐alpha gene promoter (‐1031,‐863 and ‐857) and TNF receptor 2 gene polymorphisms in endometriosis susceptibility. American Journal of Reproductive Immunology 2004;51:352‐7. [DOI] [PubMed] [Google Scholar]
Viganò 2003
- Viganò P, Mangioni S, Odorizzi MP, Chiodini A, Rocca S, Chiodo I. Use of estrogen antagonists and aromatase inhibitors in endometriosis. Current Opinion in Investigational Drugs 2003;4:1209‐12. [PubMed] [Google Scholar]
Winkel 2000
- Winkel CA. A cost‐effective approach to the management of endometriosis. Current Opinion in Obstetrics and Gynecology 2000;12:317‐20. [DOI] [PubMed] [Google Scholar]
Winkel 2003
- Winkel CA. Evaluation and management of women with endometriosis. Obstetrics and Gynecology 2003;102:397‐408. [DOI] [PubMed] [Google Scholar]


