Abstract
Background
High rates of HIV infection among women of reproductive age have dramatic consequences for personal and public health. Prophylaxis during sexual intercourse in the form of condoms has been the most effective way to prevent both STI and HIV transmission among people living with HIV.
Objectives
To investigate the effectiveness of behavioral interventions in promoting condom use among women living with HIV.
Search methods
We conducted a comprehensive literature search in several scientific databases, clinical trials databases, conference proceedings, and conference websites to identify studies produced between 1980 and May 2010 that met our selection criteria.
Selection criteria
Studies were included in the analysis if they conducted a randomized controlled trial that examined the effects of behavioral interventions on condom use among HIV‐positive women; considered at least one HIV‐related behavioral outcome (e.g., reported protected anal, vaginal, or oral sex) or biological outcome (e.g., acquisition of STIs); and one follow‐up assessment three months or more after the intervention. Studies were assessed irregardless of language or publication status.
Data collection and analysis
We used random effects models to summarize odds ratios (ORs) that compared intervention and control groups with respect to a dichotomous outcome (consistent versus inconsistent condom use). We used funnel plots to examine publication bias and a χ2 statistic to test for heterogeneity. The methodological and evidence quality was evaluated through risk of bias criteria and the GRADE system, respectively.
Main results
Five primary studies that collectively researched a total of 725 women living with HIV were analysed. When compared to standard care or minimal HIV support intervention, meta‐analysis showed that behavioral interventions had no effect on increasing condom use among HIV‐positive women. This finding was consistent at 3 (OR= 0.72; 95% CI 0.43‐1.20; p=0.21), 6 (OR= 0.96; 95% CI 0.66‐1.40; p=0.83) and 12‐months follow‐up meetings (OR= 0.75; 95% CI 0.51‐1.11; p=0.15). Only one study presented adequate data to analyze the relationship between behavioral interventions and STI incidence. Studies included in this analysis demonstrated low risk of bias based on the risk of bias criteria. However, sample size was considered inadequate across all studies.
Authors' conclusions
Meta‐analysis shows that behavioral interventions have little effect on increasing condom use among HIV‐positive women. However, these findings should be used with caution since results were based on a few small trials that were targeted specifically towards HIV‐positive women. To decrease sexual transmission of HIV among this population, we recommend interventions that combine condom promotion, family planning provision and counselling, and efforts to reduce viral loads among HIV‐positive women and their partners (e.g., HAART treatment provision). New research is needed to address the needs of HIV‐positive women, including an assessment of the impact of interventions that combine safer sexual behavior and harm reduction approaches.
Keywords: Adult, Female, Humans, Risk Reduction Behavior, Condoms, Condoms/statistics & numerical data, HIV Infections, HIV Infections/prevention & control, HIV Infections/transmission, HIV Seropositivity, HIV Seropositivity/psychology, Randomized Controlled Trials as Topic, Sexually Transmitted Diseases, Sexually Transmitted Diseases/prevention & control, Sexually Transmitted Diseases/transmission, Standard of Care
Plain language summary
Behavioral interventions to promote condom use among women living with HIV
Behavioral interventions to promote condom use and/or to modify HIV sexual risk behaviours include individual counseling, skills training, coping strategies, peer education, and social and educational support. This systematic review of randomized controlled trials assessed the effects of behavioral interventions on promoting condom use among women living with HIV, a population at higher risk to other sexually transmitted infections (STIs). Based on five eligible studies, we found that behavioral interventions promoting consistent condom use in HIV‐positive women did not have a significant impact on outcomes, when compared to standard care or minimal HIV‐related support. However, these findings should be used with caution since they are based on a few small trials that were targeted specifically towards HIV‐positive women. New research is needed to assess the potential personal and public health gains that could arise from a combination of interventions that promote safe sexual behavior and adopt a harm reduction approach, particularly in developing countries, where HIV infection rates among women remain high.
Summary of findings
Summary of findings for the main comparison. Behavioral intervention to promote condom use for women living with HIV.
| Behavioral intervention to promote condom use for women living with HIV | ||||||
| Patient or population: patients with women living with HIV Settings: Health Care Settings Intervention: Behavioral intervention to promote condom use | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Behavioral intervention to promote condom use | |||||
| Consistent condom use ‐ 3 months Self‐report Follow‐up: mean 3 months | Study population | OR 0.72 (0.43 to 1.2) | 272 (3 studies) | ⊕⊕⊝⊝ low1,2 | ||
| 431 per 1000 | 353 per 1000 (245 to 476) | |||||
| Moderate | ||||||
| 426 per 1000 | 348 per 1000 (242 to 471) | |||||
| Consistent condom use ‐ 6 months Self‐report Follow‐up: mean 6 months | Study population | OR 0.96 (0.66 to 1.4) | 637 (4 studies) | ⊕⊕⊝⊝ low1,2 | ||
| 673 per 1000 | 664 per 1000 (576 to 743) | |||||
| Moderate | ||||||
| 632 per 1000 | 622 per 1000 (531 to 706) | |||||
| Consistent condom use ‐ 12 months Self‐report Follow‐up: mean 12 months | Study population | OR 0.75 (0.51 to 1.11) | 487 (2 studies) | ⊕⊕⊝⊝ low1,2 | ||
| 684 per 1000 | 618 per 1000 (524 to 706) | |||||
| Moderate | ||||||
| 626 per 1000 | 557 per 1000 (461 to 650) | |||||
| Incidence of STI3 ‐ not reported | See comment | See comment | Not estimable3 | ‐ | See comment | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Generalization can not be reached to diverse population of women with HIV. 2 Trials are underpowered due to inadequate sample size. 3 Results from two studies could not be pooled due to different follow‐up assessments and insuficient data.
Background
HIV infection rates among women greatly increased in the 1990s, and have remained stable since then. In 2009, women accounted for half of the 33.3 million global total cases of infection, primarily as a result of heterosexual transmission (UNAIDS 2010).
The large number of HIV infection among women in reproductive age has dramatic consequences for both their own health and public health. HIV‐positive women are at increased risk for sexually transmitted infections (STIs), such as Treponema pallidum, Trichomonas vaginalis, Chlamydia trachomatis, human papillomavirus (Landes 2007; McClelland 2005), and for cervical dysplasia and genital cancer (Jong 2008; Lehtovirta 2008; Oliveira 2010). As an example, in a European sample of 1,050 HIV pregnant women, 25% had a diagnosis with at least one bacterial or viral STI (Landes 2007). In addition to HIV infection, sexual risk behaviours (e.g., inconsistent condom use, large number of sexual partners and high‐risk sexual behavior of the partner) significantly increase the risk of STI among women (Almonte 2008; Oliveira 2010).
In the context of pregnancy, the presence of STIs can also increase the risk for mother‐to‐child HIV transmission. High maternal viral RNA level and cervical or vaginal ulcers are significantly associated with infant infection (John 2001). The presence of maternal syphilis is also associated with mother‐to‐child transmission (Mwapasa 2006). Finally, infants can be at increased risk for adverse outcomes, including congenital infection, due to the strong association between STIs and HIV in seropositive pregnant women (Landes 2007).
Although women are approximately twice as likely as a men to contract HIV infection during vaginal intercourse (Nicolosi 1994), female‐to‐male transmission rates increase in the presence of some factors such as: high viral loads; concurrent STI; genital trauma to the uninfected partner; and poor immune responses from the uninfected partner (Galvin 2004; O’Farrell 2001;McClelland 2005; Quinn 2000).
Protected sexual intercourse through consistent condom use has been described as the most effective way to prevent both STI and HIV transmission among people living with HIV. While the prevalence of sexual risk behavior generally declines following HIV diagnosis, a substantial group of HIV‐positive people continue to engage in unprotected intercourse (Marks 2005; Wilson 2004). For example, in an American sample, 36.5% of HIV‐positive women have engaged in any unprotected sexual intercourse during the last 3‐months (Weinhardt 2004).
Many reasons may account for unprotected sexual practices among HIV‐positive women, including difficulties in negotiating condom use, domestic violence, alcohol abuse, and reproductive intentions (Finocchario‐Kessler 2010; Murphy 1998; Peretti‐Watel 2006). HIV‐positive women are also less likely to use a condom when they have a HIV‐positive partner. Findings showed that 51% of unprotected sexual intercourse among HIV‐positive women in the past 3 months involved a HIV‐positive partner (30% of unprotected sexual intercourse was reportedly with HIV‐negative partner, and 26% with a partner of unknown serostatus) (Weinhardt 2004). Beliefs regarding lower levels of infectivity under antiretroviral therapy also are associated with less condom use. Studies reported higher levels of unprotected sex among women after antiretroviral treatment initiation, which not vary with the therapeutic response (Wilson 2004). Moreover, social determinants (e.g., precarious socioeconomic conditions, low educational level, and gendered power imbalances) are associated with lower likelihood of women using condoms (Ghosh 2009; Santos 2009).
Reducing sexual risk behaviours, as well as coping with other challenges from living with HIV, have been the focus of many behavioral interventions (Faria 2010;Crepaz 2006). Interventions to increase antiretroviral adherence (Johnson 2007), disclosure of HIV diagnosis to sexual partner (Serovich 2009), and reductions in anxiety and depression (Balfour 2006; Blanch 2002) also have been successfully addressed by behavioral interventions.
Regarding sexual risk behaviours, studies showed that behavioral interventions tailored specifically to HIV risk groups can reduce unprotected sexual practices among people living with HIV (Kalichman 2001; Gore‐Felton 2005; The Healthy Living 2007). This effectiveness is also attested by meta‐analysis studies, especially when an intervention includes skills training (Crepaz 2006, Johnson 2006). Those interventions that were guided by behavioral theories, were more intensive, and were undertaken with longer duration were found to be more effective at promoting protected sexual intercourse. Those interventions delivered by health‐care providers, on a one‐to‐one basis, and in medical care settings were also associated with reductions in unprotected sex (Crepaz 2006). Meta‐analysis also found that behavioral interventions were more successful in increasing condom use if younger participants and fewer men who have sex with men were included in the sample (Johnson 2006).
Behavioral interventions appear to hold public health promise; however, to date, few intervention studies have comprehensively addressed the effects of gender, culture, and power imbalances on HIV risk behavior (Quadagno 1996; Wingood 2004). Interventions designed specifically for HIV‐positive women demonstrated divergent results on reducing sexual risk behavior. While two studies reported increases in condom use among HIV‐positive women in the intervention group compared with a control group (Wingood 2004; Wyatt 2004), the same result was not found in a third study with a similar design and purpose (Saleh‐Onoya 2009).
Although an emerging body of evidences indicates that behavioral interventions can be effective, the absence of a solid evidence base in this area presents barriers for designing and implementing effective interventions that can increase condom use among HIV‐positive women. This is a missed opportunity to promote the health of these women and to reduce the spread of HIV. The current review addresses a gap in the existing literature because no systematic review has been published evaluating the empirical evidence on behavioral interventions among HIV‐positive women.
Objectives
To investigate the effectiveness of behavioral interventions in promoting condom use among women living with HIV.
Methods
Criteria for considering studies for this review
Types of studies
Studies included in this analysis were randomized controlled trials (RCTs) that investigated HIV or STI behavioral interventions designed for people living with HIV. Trials had to include women, have outcomes presented by gender, and have sufficient data to calculate effect sizes. Authors were contacted for additional information if their study performed analysis by gender but did not publish results by gender.
We excluded studies that lacked a control group, used a pre‐ and post‐intervention design, or allowed participants to self‐select into the intervention.
Types of participants
All studies included adult women living with HIV who know their HIV diagnosis at baseline. Studies targeting women at risk of but not infected by HIV were excluded. Interventions could be carried out in a variety of settings (e.g., clinic, home, community).
Types of interventions
Only studies concerned with behavioral interventions that promote condom use and/or modify HIV sexual risk behaviours among people living with HIV were included. Interventions could focus on providing information, counseling, individual cognition, emotional well‐being, skills training, coping strategies, or peer education related to HIV risk behaviours. There were no restrictions as to the intervention theoretical approach, setting, frequency, or duration. Studies that focused on biomedical interventions (e.g., vaccines, HIV testing, or administering HAART) were excluded unless behavioral intervention effects were described separately.
Types of outcome measures
Studies included in the analysis had to include data on at least one HIV‐related behavioral outcome (e.g., reported unprotected/protected anal, vaginal, or oral sex) or biological outcome (e.g., acquisition of STIs, including hepatitis B) and at least one follow‐up assessment at three months or more post‐intervention. Protected sexual intercourse (or consistent condom use) was described as use of condoms in all vaginal, anal or oral sexual relationships with casual and/or steady partners. All other situations were considered inconsistent condom use (e.g. "almost always" or "sometimes" condom use).
Search methods for identification of studies
We searched the following electronic databases irregardless of language or publication status using the optimal sensitive search strategy developed by The Cochrane Collaboration (Higgins 2008):
Cochrane Central Register of Controlled Trials (CENTRAL)
MEDLINE (PubMed)
EMBASE
Latin American and Caribbean Health Sciences Literature (LILACS)
PsycInfo
Social Science Citation Index (SocINDEX and CINAHL through EBSCO)
We used specific search terms to identify relevant studies from January 1980 to May 2010. Appendix 1describes the search strategy applied to CENTRAL, EMBASE and PubMed which was also applied to other search engines without substantial modifications.
In addition, we examined reference lists of all pertinent reviews and studies for published studies; the WHO International Clinical Trials Registry Platform and the Clinicaltrials.gov database for unpublished studies; and the International AIDS Conferences, National HIV Prevention Conference (through Meeting Abstracts Database ‐ NLM Gateway), the International Society for Sexually Transmitted Disease Research, and the Conference on HIV Pathogenesis and Treatment (through Conferences’ website) for conference proceedings. Finally, experts in the field were contacted for recommendations about additional intervention research reports and unpublished sources.
Data collection and analysis
The search was completed by one author (TG) with the assistance of the Cochrane HIV/AIDS Group (San Francisco). Citations retrieved from electronic searches were inspected by two authors (FC and EF) who independently screened studies for inclusion. Any uncertainties were resolved by consensus.
Selection of studies
Following an initial screening, all potentially eligible studies were independently read by at least two authors (EF, FC, or TG) who assessed in detail the study design, types of participants, types of interventions, and outcome measures. The Kappa coefficient indicated a good agreement across those rating the study (K=0.76).
Data extraction and management
Using a standardized data extraction form, EF, FC, and TG extracted the following characteristics from each study that met the inclusion criteria:
Description of study participants (e.g., sample size, demographic characteristics, country where study was performed);
Eligibility criteria for enrolment (e.g., HIV+ diagnosis);
Details about the intervention (e.g., length of intervention and follow‐up, individual or group modality, behavioral techniques);
Assessment of risk of bias (e.g., study design, generation of allocation sequence, allocation concealment, blinding, loss to follow‐up, inclusion of all randomized participants, incomplete outcome data addressed, and sample size calculation);
Outcome measures (e.g., acquisition of STI or hepatitis B, self‐reported protected anal, vaginal, or oral sexual intercourse) and data analysis strategy.
Methodological quality was assessed through RevMan5 and in accordance with the recommendations of the Cochrane Handbook of Systematic Reviews of Interventions (Higgins 2008). Quality was categorized as either "Low risk”, "High risk”, or "Unclear", and was listed in risk of bias tables broken down by trial. Sample size was calculated by the authors according to Hulley 2001 using the earliest study that met the inclusion criteria (Wingood 2004). From this calculation it was concluded that a sample size of 1,133 participants per group was necessary to adequately compare proportions of dichotomous variables. See Appendix 2 for further information on the sample size calculation. In the case of missing data, authors were contacted directly.
Finally, the GRADE system was used to evaluate the overall evidence quality for the outcome.
Measure of treatment effect
Statistical analysis was conducted according to Cochrane guidelines and compared the impact of distinct treatments (Higgins 2008). For the dichotomous outcome (consistent versus inconsistent condom use), the absolute numbers of participants reporting consistent condom use in each group (intervention and control) was extracted. Results for the effect of each intervention were expressed as odds ratios (ORs) with 95% confidence intervals and were combined for meta‐analysis using a random‐effects model in the RevMan 5.0 software. This strategy accounts for any potential heterogeneity that may occur following unique intervention approaches developed in various study settings.
Statistical heterogeneity between results of different studies was examined by χ2 tests. A P value for a χ2 test of less than 0.10 indicated heterogeneity. An alternative approach to quantify the effect of heterogeneity is assessing the inconsistency among the results of studies with 95% uncertainty intervals. A value of 0% indicates no observed heterogeneity and a value greater than 50% indicates the presence of substantial heterogeneity. Condom use was estimated using an intention‐to‐treat analysis and included all subjects who had undergone randomization, regardless of their baseline condom use behavior. Reporting bias was assessed by examining a funnel plot graphic which can detect small trial effects even those resulting from publication bias.
Subgroup and sensitivity analyses were not feasible given the small number of trials included in the assessment.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies.
The study selection process is summarized in Figure 1. Out of 3,046 citations, 35 potentially relevant studies and their full‐text version were extracted. After assessment, the following 30 studies were excluded: 10 that did not consider gender and/or age in their outcomes analyses (Kalichman 2005; Lightfoot 2007; Naar‐King 2006; Olley 2006; Patterson 2003; Purcell 2007; Rotheram‐Borus 2001; Rotheram‐Borus 2004; Rotheram‐Borus 2009; The Healthy Living 2007); 3 that included strategies to promote condom use in control groups (Cosio 2010; Fogarty 2001; Jones 2005); 4 that were not RCT studies (Bunnell 2006; Fisher 2006; Jones 2006; Magnano San Lio 2009); 2 that only described formative analyses of interventions (Holstad 2006; NIMH Multisite Group 2008); 2 that did not describe outcomes and/or analysis by HIV status (Belcher 1998; Bhave 1995); 2 that did not assess the target outcomes (Burman 2008; Carrico 2009); 2 that only included participants at risk for HIV (Champion 2001; Dilley 2008); 1 that assessed the baseline assessment before HIV diagnosis (Cleary 1995); 1 that conducted a control group assessment only in immediate post‐intervention follow‐up (Wyatt 2004). Even after contact with authors by e‐mail, 3 studies could not be included due to lack of information from the authors about absolute number of HIV‐positive women engaged in safer sex (Jones 2001; MacNeil 1999) and lack of information on intervention effects by gender (Kalichman 2001).
1.
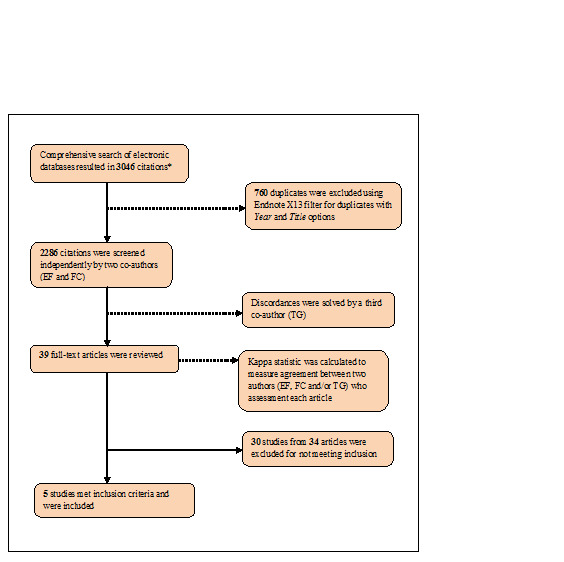
*Note: Figure shows an integrated view of the screening process considering the first comprehensive searches (performed 26‐28 May, 2009) were subsequently updated on 18‐21 May, 2010 to complete the review.
Data from five studies (Figure 2) encompassing a total of 725 female respondents living with HIV were analysed. Of these studies, three were carried out in the United States(Gilbert 2008; Sikkema 2008; Wingood 2004) and two in South Africa (Cornman 2008; Saleh‐Onoya 2009). Two interventions were developed exclusively for women living with HIV (Saleh‐Onoya 2009; Wingood 2004), while the other three targeted both women and men living with HIV (Cornman 2008; Gilbert 2008; Sikkema 2008). All interventions followed the initiation of HAART. The Characteristics of included studies tables show details about methodology, participants, interventions, and outcomes for each study.
2.
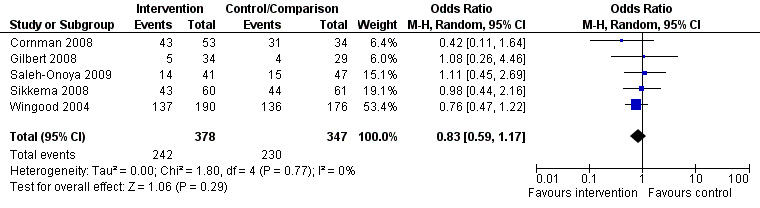
Forest plot of comparison: 1 Protected sex among women living with HIV in the baseline.
Risk of bias in included studies
Evidence of selective reporting and other biases were limited across all studies. All authors who were contacted responded with explanations for participant drop out. One study did not include attrition analysis (Saleh‐Onoya 2009), and another one (Sikkema 2008) inferred that attrition analysis was made however no further information was provided. All studies performed an intention‐to treat analysis except one, (Saleh‐Onoya 2009), However the authors of this study did describe the absolute number of participants for each outcome assessed. According to an estimated sample size performed by authors (Appendix 2), sample size was considered inadequate for all studies. A summary of each reviewer's assessment of the studies' methodological qualities is presented in Figure 3 and Figure 4.
3.
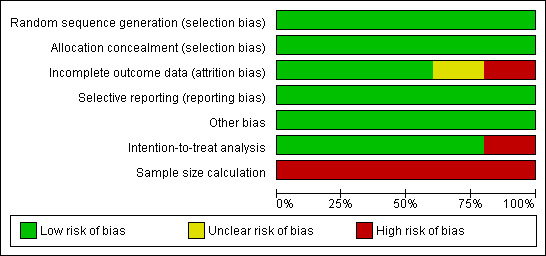
Risk of bias graph: review authors' judgments about each risk of bias item presented as percentages across all included studies.
4.
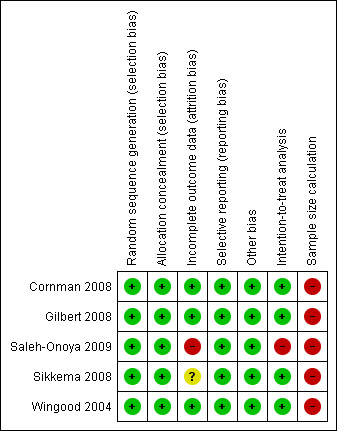
Risk of bias summary: review authors' judgments about each risk of bias item for each included study.
Publication Bias
Meta‐analysis may be vulnerable to publication bias if studies with less favorable results are excluded. A useful test for publication bias is based on the funnel plot, which compares intervention effects estimated from individual studies against a measure of study size. In the absence of bias, the plot resembles a symmetrical inverted funnel (Sterne 2001). In the current review, there was no clear evidence of funnel plot asymmetry (Figure 5).
5.
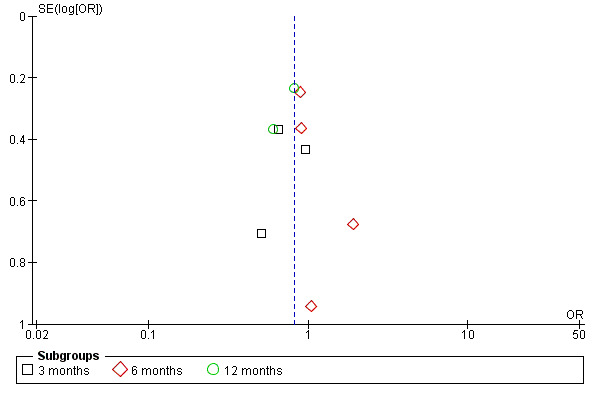
Funnel plot of comparison: 2 Increasing in protected sex among women living with HIV after intervention, outcome: 2.1 Time intervention.
Quality of Evidence
We performed a GRADE evaluation on the quality of evidence for all interventions included in this review (GRADE Working Group 2004).This classification indicated low evidence quality for the specific outcome in the target population (See Table 1). We only included randomized controlled trials in this portion of the review. The main methodological limitation among studies was inadequate sample sizes, which may reduce the power of analyses.
Effects of interventions
See: Table 1
Meta‐analysis conducted on the five studies showed no effect of behavioral interventions on condom use promotion among HIV‐positive women when compared to standard care or minimal HIV support interventions. No intervention effects on consistent condom use promotion were noted at the 3 (OR= 0.72; 95% CI 0.43‐1.20; p=0.21), 6 (OR= 0.96; 95% CI 0.66‐1.40; p=0.83), or 12‐month follow up (OR= 0.75; 95% CI 0.51‐1.11; p=0.15), nor over the full 12‐month follow‐up period (OR=0.82; 95% CI 0.65‐1.04; p=0.11). Conversely to what was expected, we could also observe a slight trend towards interventions effects being favorable to control groups (condom use being lower among women in intervention groups). Even so, four studies found positive results to increase condom use on their published articles (Cornman 2008; Gilbert 2008; Sikkema 2008; Wingood 2004). Although, three of these studies have considered a combined number of sexual events in which condom were used as their primary outcome instead of consistency on condom use (Cornman 2008; Sikkema 2008; Wingood 2004). Also, other three studies did not originally analyse interventions results by gender (Cornman 2008; Gilbert 2008; Sikkema 2008).
No evidence of heterogeneity was found among studies (Tau2=0.00; Chi2=3.56, df=8, p=0.89, I2=0%), meaning each study contributed results for the meta‐analysis in a similar way. These results are shown in Figure 6. We were not able to conduct subgroup analyses due to the small number of studies included in the analysis. For this reason, it was not possible to distinguish effects from different participant characteristics or intervention designs.
6.
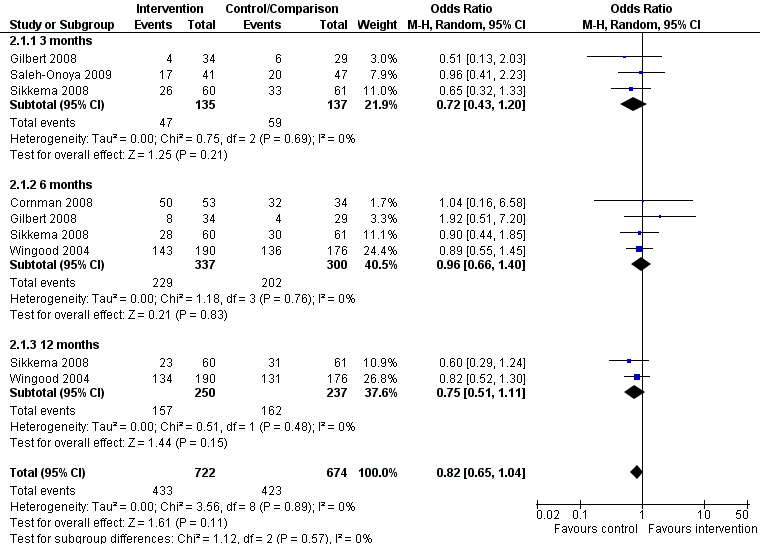
Forest plot of comparison: 2 Increase in protected sex among women living with HIV after intervention, outcome: 2.1 Time intervention.
The current review also aimed to assess the effect of behavioral interventions on STI incidence, but only two studies (Saleh‐Onoya 2009; Wingood 2004) assessed this outcome. Nevertheless, both studies had different time of follow‐up assessments, and only one (Wingood 2004) presented adequate data for grouping by results. Despite limitations, these positive studies results are individually described for the systematic review purpose. One study found significantly higher incidences of Chlamydia Trachomatis (CT), Neisseria Gonorrhoea (NG) and Trichomona vaginalis (TV) in the control group than in the intervention condition at 3‐month follow‐up assessment (Saleh‐Onoya 2009). No significant difference was found for Bacterial Vaginosis (BV) incidence. Wingood 2004 reported that participants in the intervention group were not significantly less likely to have an incident Trichomonas infections, bacterial infection of Chlamydia or gonorrhea at any follow‐up assessment, but were significantly less likely to have an incident bacterial STD at the 12‐month assessment and over the entire 12‐month. These findings indicate that behavior interventions could be a promising strategy to reduce STI incidence. However, these positive results cannot be confirmed through meta‐analysis.
Discussion
Previous meta‐analyses reported that behavioral interventions are effective in reducing sexual risk behavior among adults living with HIV, however these analysis concentrated primarily on male participants (Crepaz 2006; Johnson 2006). After assessing data on interventions among female participants, different results arose. Our meta‐analysis identified no increase in consistent condom use among HIV‐positive women following participation in behavioral interventions.
Some issues that may be influencing these findings deserve further mention. First, we found few randomized controlled trials evaluating the effect of interventions on condom use by gender. Second, there is a gap in research on behavioral interventions for HIV‐positive women that promote prophylaxis during sexual intercourse; only two interventions were found to be tailored specifically toward HIV‐positive women. Despite continued emphasis on the challenges faced by women living with HIV, the research literature has yet to address how various gender‐linked factors (e.g., capacity to negotiate condom use, gender power imbalances regarding resources) can impact the ability of behavioral interventions to successfully increase condom use (Finocchario‐Kessler 2010; Murphy 1998; Peretti‐Watel 2006; Santos 2009). Moreover, none of the included studies explicitly reported the promotion or use of the female condom, leading us to assume that the male condom was the main focus of these interventions. This oversight represents a remarkable limitation in these interventions since the male condom is not a female initiated contraceptive method. To that end, we recommend and advocate for more female condom interventions that can empower women to independently make decisions in sexual situations, especially among women living with HIV.
Also, it would be useful for future research to better account for the diversity across HIV‐positive women, focusing on subgroup analyses that highlight varying intervention effects across heterogeneous samples of women living with HIV (e.g., women planning to become pregnant; women with casual versus steady partners; women in a relationship with seronegative versus seropositive partner). These analyses would be helpful in identifying specific groups of women who could benefit from these interventions as well as identifying new strategies for tailoring interventions.
The present systematic review holds some limitations which must be considered. The results themselves could be affected by gender‐based factors since studies in the analysis described if and how male partners were involved in the intervention strategies; only two of the included studies were focused exclusively on women living with HIV. Taking this into consideration, it would be interesting to systematically review interventions targeted at heterosexual couples where at least the woman lives with HIV. This type of investigation could be used to explore how these interventions encourage condom negotiation, woman's empowerment, or the involvement of men. It is also important to highlight the possibility of self‐selection bias in this analysis. All studies included were clinic‐based and their participants were already receiving HIV‐related care therefore the population does not accurately reflect the general population of HIV positive women.
Another shortcoming of our study results is posed by our outcome – consistent condom use – which was measured as a dichotomous variable. The majority of favorable intervention results were observed among studies that considered frequencies of risk behaviours as the main outcome (Crepaz 2006; Johnson 2006). It implies that HIV‐positive people who had never used a condom before and began using one soon after the intervention may have increased the frequency of condom use, however their use is still considered inconsistent. To this end, the condom use outcome measure adopted in our review is so rigorous that positive results could be considered less attainable and it also can be related to the contrary effects (condom use being higher in the control group) found for some behavioral interventions in the meta‐analysis. For this reason, significant increasing on frequency of condom use among HIV‐positive women reported by some of our included studies (Cornman 2008; Sikkema 2008; Wingood 2004) does not match with our results.
Finally, only two revised studies individually reported positive effects of behavioral intervention on STI incidence among HIV+ women, but their results could not be combined through meta‐analysis. STI assessment as a critical outcome to trials on behavioral interventions since strong evidences supports biological mechanisms through which STIs facilitate HIV transmission and the synergistic negative effects of multiple sexually transmitted infections, especially among women (Galvin 2004; McClelland 2005; Landes 2007). Then, we endorse that even small reductions in STI incidence could yield critical reductions in HIV morbidity and its associates treatment cost and more studies are needed to examine behavioral interventions effects.
Authors' conclusions
Implications for practice.
Our meta‐analysis showed behavioral interventions to have little effect on promoting condom use among HIV‐positive women. These findings are in contrast to previous analyses which found these interventions to be effective among all adults living with HIV. Since our findings are based on only five studies, we are hesitant to discourage behavioral interventions all together, but instead recommend that behavioral interventions be conducted in conjunction with other strategies such as family planning and contraceptive counseling or biomedical interventions to reduce viral load. We also recommend that future interventions more effectively address gender‐linked strategies to promote female condom use and the inclusion of male partners.
Implications for research.
None of the studies included in our review had an adequate number of female participants warranting further research using larger female sample sizes. Future studies should also consider assessing STI prevalence outcomes (an important public health indicator), since few studies included in the current review contained data related to STIs. Furthermore, to establish definitively the efficacy of behavioral interventions on condom use among HIV‐positive women, more randomized controlled trials designed specifically for this population are needed. Other important intervention research innovations include the ability to address the social context within which this population lives (Finocchario‐Kessler 2010; Ghosh 2009; Murphy 1998; Peretti‐Watel 2006; Santos 2009), including their position within society and the potential to work with sexual partners where appropriate and feasible.
Feedback
Response to feedback, 16 January 2012
Summary
Date of Submission: 06‐Jan‐2012
Feedback: From David Sinclair and Paul Garner
We are writing to highlight several concerns about the above Cochrane review.
This review is obviously an important topic within HIV and makes what would be very important conclusions: ‘Meta‐analysis shows that behavioral interventions have little effect on increasing condom use among HIV‐positive women’. The summary of findings table states that this is ‘High’ quality evidence meaning that we can have full confidence in the result and further research is unnecessary.
However there are some major errors in the way the data has been handled:
The numbers given in the SoF table for the primary outcome (Consistent condom use: OR 0.83, 95% CI 0.59 to 1.17) are actually the data for the use of condoms at baseline, before the intervention is given (shown in figure 2)
The numbers given for the same outcome in the abstract are also incorrect (OR 0.82, 95% CI 0.65 to 1.04). These figures are taken from the meta‐analysis in figure 6 which combines data at 3 months, 6 months, and 12 months. It is incorrect to perform this meta‐analysis as it triple counts some data.
The actual data which should be used in both instances is from 2 trials: OR 0.75, 95% CI 0.51 to 1.11)
There are then some important deficiencies in how the data has been interpreted:
This figure suggests that there is a trend towards behavioural interventions actually being harmful (condom use being lower in the intervention group) but this is not adequately discussed.
The evidence from 2 small trials (or even 5 small trials) is unlikely to be of high quality, due to concerns about imprecision (the trials are underpowered), and indirectness (can we really generalise this data to all women with HIV?). The authors themselves note several limitations in the data such as ‘None of the studies included in our review had an adequate number of female participants warranting further research using larger female sample sizes’ and ‘ Since our findings are based on only five studies, we are hesitant to discourage behavioral interventions all together’. These comments are not consistent with a quality GRADE of high.
In addition the results section appear very short. Some positive benefits on the incidence of STI are noted but inadequately reported and inadequately discussed. These are predefined outcomes and should be included in the Summary of findings tables.
We hope this helps in improving the review.
Best wishes,
Paul and Dave
Submitter agrees with default conflict of interest statement:
I certify that I have no affiliations with or involvement in any organization or entity with a financial interest in the subject matter of my feedback.
Reply
We thank Dr. Garner and Dr. Sinclair for their helpful comments and we have corrected their primary points of concern. We plan to update this review in May 2012, when additional modification will be made.
Contributors
Tonantzin Ribeiro Gonçalves
What's new
| Date | Event | Description |
|---|---|---|
| 18 January 2012 | Feedback has been incorporated | New feedback, and response to feedback. |
Acknowledgements
The authors wish to thank the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and the Brazilian Health Ministry for financial support. Thanks also to Tara Horvath at the Cochrane HIV/AIDS Group for editorial and administrative support.
Appendices
Appendix 1. MEDLINE (PubMed) search strategy queries
Date range: 1 January 1980 18 May 2010
HIV/AIDS terms
#1 “HIV Infections”[MeSH]
#2 “HIV”[MeSH]
#3 hiv[tw]
#4 hiv‐1*[tw]
#5 hiv‐2*[tw]
#6 hiv1[tw]
#7 hiv2[tw]
#8 hiv infect*[tw]
#9 human immunodeficiency virus[tw]
#10 human immunedeficiency virus[tw]
#11 human immuno‐deficiency virus[tw]
#12 human immune‐deficiency virus[tw]
#13 ((human immun*) AND (deficiency virus[tw]))
#14 acquired immunodeficiency syndrome[tw]
#15 acquired immunedeficiency syndrome[tw]
#16 acquired immuno‐deficiency syndrome[tw]
#17 acquired immune‐deficiency syndrome[tw]
#18 ((acquired immun*) AND (deficiency syndrome[tw]))
#19 “sexually transmitted diseases, viral” [MESH:NoExp]
#20 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7OR #8 OR #9 #10 #11 OR #12 OR #13 OR #14 OR #14 OR #15 OR #16 OR #17OR #18 OR #19
RCT and CCTs terms
#21 randomized controlled trial[pt]
#22 controlled clinical trial[pt]
#23 randomized[tiab]
#24 placebo[tiab]
#25 drug therapy[sh]
#26 randomly[tiab]
#27 trial[tiab]
#28 groups[tiab]
# 29 #21OR #22 OR #23 #24 OR #25 OR #26 OR #27 OR #28
#30 #29 AND humans[mh]
Sexual behavior terms
#31 sexual[tiab] AND (behavior[tiab] OR behavior[tiab])
#32 sexual[tiab] AND (risk[tiab] OR risk‐taking[tiab] OR risk behavior[tiab] OR risk behaviour[tiab] OR risk practice[tiab] OR risky behavior[tiab] OR risky behaviour[tiab] OR risky practice[tiab] OR risky activity[tiab])
#33 unsafe[tiab] AND (sex[tiab] OR intercourse[tiab])
#34 unprotected[tiab] AND (vaginal sex[tiab] OR anal sex[tiab] OR vaginal intercourse[tiab] OR anal intercourse[tiab] OR sexual practice[tiab])
#35 condom*[tiab]
#36 #31OR #32 OR #33 #34 OR#35
Behavioral interventions terms
#37 "Behavior Therapy"[Mesh]
#38 "Cognitive Therapy"[Mesh]
#39 "Imagery (Psychotherapy)"[Mesh]
#40 "Psychotherapy, Rational‐Emotive"[Mesh]
#41 cognitive*[tiab] AND (therap*[tiab] OR train*[tiab] OR techni*[tiab] OR question*[tiab] OR approach*[tiab] OR intervention[tiab])
#42 ((behavior*[tiab] OR behaviour*[tiab]) AND (therap*[tiab] OR train*[tiab] OR modif*[tiab] OR experiment*[tiab] OR intervention[tiab] OR coping[tiab]))
#43 ((educat*[tiab]) AND (intervention[tiab] OR therap*[tiab] OR counsel*[tiab] OR program[tiab] OR train*[tiab] OR client[tiab] OR patient[tiab] OR health[tiab]))
#44 ((patient[tiab]) AND (counsel*[tiab] OR compliance[tiab] OR educ*[tiab] OR teach*[tiab]))
#45 ((safer‐sex[tiab] OR risk reduction[tiab]) AND (counsel*[tiab] OR intervention[tiab] OR prevention[tiab]))
#46 ((problem solving[tiab] OR self control[tiab]) AND (therap*[tiab] OR intervention[tiab] OR train*[tiab]))
#47 motivation*[tiab] AND (debriefing[tiab] OR interview[tiab])
#48 brief[tiab] AND (psychotherap*[tiab] OR therap*[tiab])
#49 group[tiab] AND (psychotherap*[tiab] OR therap*[tiab])
#50 rational*[tiab] AND emotive*[tiab]
#51 cbt[tiab]
#52 psychoeducation[tiab]
#53 peer‐led intervention[tiab]
#54 peer‐mentoring intervention[tiab]
#55 social skills train*[tiab]
#56 health promotion[tiab]
#57 HIV prevention intervention[tiab]
#58 #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 OR #46 OR #47 OR #48 OR #49 OR #50 OR #51 OR #52 OR #53 OR #54 OR #55 OR #56 OR #57
Terms combination
#59 #20 AND #29 AND #36 AND #58
Appendix 2. Sample size calculation for assessment of risk of bias
Based on the results of the first published article in order to determine the efficacy of a behavioral intervention on condom use among women living with HIV (Wingood 2004).
P1 expected proportion in the control group 136/176= 0.77
P2 expected proportion in the treated group 143/190=0.75
Lowest p= 0.75
Difference between P1 and P2 = 0.02
Table 6.B.1 (pg. 86, Hulley 2001): alpha and beta 0.05 and 0.20 (middle column) 15th line (0.75) and first column (0.05) the middle column in each group = 1.133
Data and analyses
Comparison 1. Consistent condom use among women living with HIV.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Baseline | 5 | 725 | Odds Ratio (M‐H, Random, 95% CI) | 0.83 [0.59, 1.17] |
1.1. Analysis.
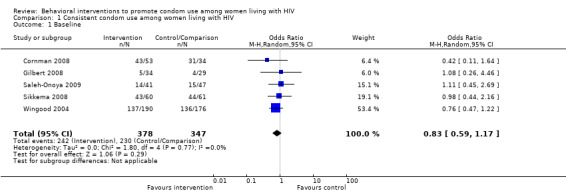
Comparison 1 Consistent condom use among women living with HIV, Outcome 1 Baseline.
Comparison 2. Increasing in consistent condom use among women living with HIV after intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Time intervention | 5 | 1396 | Odds Ratio (M‐H, Random, 95% CI) | 0.82 [0.65, 1.04] |
| 1.1 3 months | 3 | 272 | Odds Ratio (M‐H, Random, 95% CI) | 0.72 [0.43, 1.20] |
| 1.2 6 months | 4 | 637 | Odds Ratio (M‐H, Random, 95% CI) | 0.96 [0.66, 1.40] |
| 1.3 12 months | 2 | 487 | Odds Ratio (M‐H, Random, 95% CI) | 0.75 [0.51, 1.11] |
2.1. Analysis.
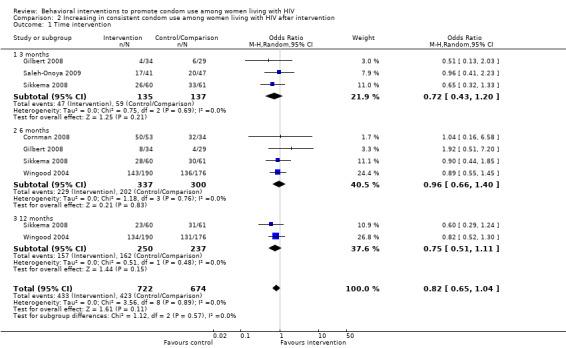
Comparison 2 Increasing in consistent condom use among women living with HIV after intervention, Outcome 1 Time intervention.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Cornman 2008.
| Methods | Randomized controlled trial | |
| Participants | 87 women (from a total of 152 HIV‐positive patients) receiving clinical care at an urban hospital in South Africa. Intervention condition: 53 HIV‐positive women; mean age was 33 years old (SD=8.63); 91% were Zulu; 47% had high school; 76% were unemployed; 81% were single; 28% had learned of their diagnosis within the past 3 years, with 47% diagnosed in the past year. Control condition: 34 HIV‐positive women; mean age was 33.7 years old (SD=5.42); 91% were Zulu; 41% had high school; 82% were unemployed; 80% were single; 47% had learned of their diagnosis within the past 3 years, with 35% diagnosed in the past year. |
|
| Interventions |
Intervention condition: The Izindlela Zokuphila/Options for Health intervention consisted of brief (15‐minute) patient‐centered discussions between a counselor and a patient during regular clinical visits (every 3 months), and these discussions were repeated at each visit over an interval of approximately 6 months. The intervention consisted of an 8‐step framework used by the counselor to tailor the discussions to a specific patient’s HIV risk reduction (or maintenance of safer behavior) needs. The counselor used motivational interviewing techniques to (1) introduce the discussion of safer sex, (2) assess the patient’s risk behavior, (3) determine how important it is to the patient to change her risk behavior, (4) determine how confident the patient is that she can change her risk behavior, (5) identify information, motivation, behavioral skills, and other barriers to consistently practicing safer behavior, (6) discuss specific strategies for overcoming these barriers, and (7) negotiate a risk reduction action plan with the patient. The final intervention step was to document the agreed upon goal on the ‘‘Action Plan’’ form, which was handed to the patient. Upon completion of the Options for Health visit, the counselor documented what transpired during the discussion (intervention steps completed, risk behaviours identified, agreed upon goal, etc.) on the
‘‘Patient Record Form,’’ which was then stored in the patient’s medical file. Control condition: Standard HIV counseling about infection, ARVs, medication adherence, and nutrition during regularly clinic visits. Standard HIV counseling did not include systematic discussion of HIV prevention, but such discussions were not prohibited and occurred on an ad hoc basis. |
|
| Outcomes | Total number of unprotected sex events (vaginal and anal sexual events without condoms) in previous 3 months; number of times condoms were used for each type of sex; number and perceived HIV serostatus of their partners. Outcome assessments were conducted at baseline and 6‐month follow‐up through self‐report questionnaires. |
|
| Notes | Participants were not tested to STIs. Estimated means reported in the article. After request, authors provided absolute number of female participants who reported unprotected sexual behavior at baseline and 6‐month follow‐up. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Information provided by e‐mail: "Since we had two intervention participants to every one control participant 152 small cards were created; 49 with the word "control" and 103 with the word "intervention." The cards were folded so the text could not be seen and placed in a box. A participant would draw one card out of the box which would determine the randomly assigned study condition. Once drawn cards were not replaced." |
| Allocation concealment (selection bias) | Low risk | As described in the item above. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | |
| Intention‐to‐treat analysis | Low risk | |
| Sample size calculation | High risk | Authors did not perform a sample size calculation because it was a preliminary study (information provided by e‐mail). |
Gilbert 2008.
| Methods | Randomized controlled trial | |
| Participants | 101 women (from a total of 471 HIV‐positive adults) reporting substance use or sexual risk behaviours from Positive Choice trial, attending on outpatient HIV clinics in the San Francisco Bay Area. Considering the entire sample, 236 were Black or African‐American, and 266 had High school diploma or GED. The number of women in intervention and control conditions was 56 and 45, respectively. | |
| Interventions | Intervention condition: Participants received a computer based intervention (The Video Doctor intervention). Interactive risk reduction messages, based on principles of Motivational Interviewing, were delivered by an actor‐portrayed Video Doctor, whose tone was respectful and non‐judgmental. These messages simulated an ideal discussion where the health care provider expressed reflexive understanding of the patient’s concerns, showed compassion for the patient, and provided nonjudgmental counseling. Using a library of digital video clips, extensive branching logic, and participant input, the program tailored the video clips to the participant’s gender, risk profile, and readiness to change. At the conclusion of each session, the program printed 2 documents: 1) an ‘‘Educational Worksheet’’ for participants with questions for self‐reflection, harm reduction tips, and local resources; and 2) a ‘‘Cueing Sheet’’ for providers, which offered an at‐a‐glance summary of the patient’s risk profile and readiness to change, and suggested risk‐reduction counseling statements. Intervention participants received ‘‘booster’’ Video Doctor counseling at 3‐month follow‐up, including feedback reflecting changes made since baseline, and updated Cueing Sheets and Educational Worksheets. Control condition: Participants did not interact with the Video Doctor and did not receive the Educational Worksheets or the Cueing Sheets. Following completion of the risk assessment they received the clinic’s regular care. | |
| Outcomes | Number of drinks per week, number of binge drinking episodes, total days of all drug use, absolute percent change in self‐reported condom use with steady and casual partners, and number of casual sex partners. Outcome assessments were conducted at baseline, 3, and 6‐month follow‐up through self‐reported information. |
|
| Notes | Participants were not tested to STIs. Absolute number of female participants who reported unprotected sexual behavior at baseline and each follow‐up assessment was provided by e‐mail. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Low risk | "The Video Doctor program was programmed to access the secure file and select the next randomization assignment within that stratum. Thus, stratified randomization was completely concealed from the participants and the study staff. Only the programming staff would have been able to access the program in case of a problem." (Information provided by e‐mail). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Postrandomization exclusion due to no risk event reported. |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | |
| Intention‐to‐treat analysis | Low risk | |
| Sample size calculation | High risk | |
Saleh‐Onoya 2009.
| Methods | Randomized controlled trial | |
| Participants | 102 HIV‐positive women between 18 and 50 years old, black, isiXhosa speaking, sexually active in the prior 12 months. They were attending primary health clinics in Western Cape Province, South Africa. Participants were on average 29 years old. The number of women in intervention and control conditions was 53 and 49, respectively. The demographic characteristics of the intervention group did not significantly differ from the control group. | |
| Interventions | Each group session comprised of 8 to 10 participants and was implemented by a black, isiXhosa speaking, female health educator and a black isiXhosa speaking HIV‐positive woman co‐facilitator. Intervention condition: Consisted of four four‐hour sessions of sexual risk reduction and coping training, implemented weekly. Session 1 focused on enhancing ethnic and gender pride, and self‐esteem. The group discussed ways of expanding support networks and maintaining social support. In session 2 participants discussed communication styles and potential outcomes of each option. Role plays were used to demonstrate and reinforce assertive communication skills. Session 3 focused on reinforcing HIV and STI infection and re‐infection knowledge, and highlighted personal HIV risk associated to unsafe sexual behavior. Participants discussed and role‐played strategies for negotiating condom use with sex partners. They learnt skills for correct condom use by observing demonstrations by health educators and practicing condom application on penis models. In session 4 participants differentiated healthy and unhealthy relationships, and discussed abuse in relationships (emotional, sexual, or physical) and methods for safely resolving relationship problems. Most intervention activities were adopted from the original WiLLOW program which was culturally adapted through previous focus group discussions. Control condition: Consisted of one four‐hour session focusing on reiterating motivational messages about developing a positive outlook on life despite the challenges of living with HIV. |
|
| Outcomes | STI prevalence and incidence (vaginal swabs tested for CT, NG, TV and BV); sexual behavior (self‐reported information about condom use in the last sexual intercourse, and in the last month); psychosocial determinants of condom use (scales focusing HIV Knowledge, attitude towards condom use, self‐efficacy for negotiating and correct condom use, control in relationships, self‐esteem, and coping with HIV). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | High risk | |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | |
| Intention‐to‐treat analysis | High risk | An ITT analysis was not performed but, in the article, authors reported absolute number of participants for each outcome. |
| Sample size calculation | High risk | Authors did not perform a power size calculation (information provided by e‐mail). |
Sikkema 2008.
| Methods | Randomized controlled trial | |
| Participants | 130 women (from a total of 247 HIV‐positive adults) with extensive sexual trauma histories, attending AIDS service organizations and community health care clinics in New York City. Considering the entire sample, participants had a mean age of 42.3 years (SD = 6.8) and 12.2 years (SD = 2.4) of education. On average, participants were diagnosed with HIV for 10.0 years (SD =5.8). Authors did not report demographic characteristics by gender but they reported no statistically significant differences by study condition. | |
| Interventions | In both group conditions, co‐therapists delivered the interventions in a community health center over a course of 15 weekly 90‐minutes sessions. Intervention condition: The intervention model integrated the cognitive theory of stress and coping and effective cognitive‐behavioral treatment strategies for sexual trauma within a transactional framework for understanding sexual abuse outcomes. Participants identified stressors related to sexual abuse and HIV. Parallels between these traumatic experiences in terms of stress response and coping strategies were addressed. Other therapeutic activities included identification of individual triggers, selection of attainable goals, skill‐building exercises, and exposure. Risk reduction skills were addressed in the context of elements necessary for healthy relationships (e.g., safety, intimacy, power, self‐esteem), including sexual relations after sexual abuse, re‐victimization, and HIV infection. Participants shared experiences and offered mutual support and feedback. Control condition: The comparison group paralleled a standard therapeutic support group and was led by experienced co‐therapists not trained on the coping intervention model. The purpose of the group was to provide a supportive environment for participants to address issues of HIV and trauma. Because group leaders were skilled clinicians with substantial experience, this treatment condition resembled an interpersonal process group model more than a standard community‐based support group. Despite the open format, the group content had a predominant focus on the connections between sexual abuse, HIV/AIDS, current relationships, and life events. |
|
| Outcomes | Participants reported the number of times they had engaged in anal and vaginal intercourse in the past 4 months at baseline, 3, 6 and 12‐month follow ups. Condom use and partner serostatus were assessed specific to intercourse occasions. | |
| Notes | Absolute number of female participants who reported unprotected sexual behavior at baseline and each follow‐up assessment was provided by e‐mail. Participants were not tested to STIs. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | After contact by e‐mail, authors clarified that they used block randomization by gender, so each wave or block of approximately 30 female or male participants was randomized independently, with previously prepared sealed envelopes presented to participants following the baseline interview. Both participants and interviewers were blind to condition until after baseline. |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Article has referred attrition analysis in the discussion section (p. 511) but did not provide complete information on that. |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | |
| Intention‐to‐treat analysis | Low risk | |
| Sample size calculation | High risk | Considering power sample calculation, authors answered our request as follow: "For original proposal, a sample size of 240 HIV‐positive women and men to participate in the study; 120 participants randomly assigned to each condition." |
Wingood 2004.
| Methods | Randomized controlled trial | |
| Participants | 366 women receiving medical care for HIV/AIDS in Alabama and Georgia, USA. They were sexually active in the previous 6 months, had been living with HIV for an average of 5 years (SD = 3.8), and had a mean age of 34.7 years (SD = 7.6). 190 were randomized to the WiLLOW intervention, and 176 to the comparison condition. Authors reported no significant group differences on demographic characteristics. | |
| Interventions |
Intervention condition: The WiLLOW intervention consisted of 4 4‐hour interactive group sessions that were implemented over consecutive weeks. Each session included 8 to 10 participants, was implemented by a trained female health educator, and was co‐facilitated by an HIV‐positive female peer educator.
The social cognitive theory and the theory of gender and power were used as theoretic frameworks for the development and implementation of the WiLLOW intervention. Session 1 emphasized gender pride by discussing the joys and challenges of being a woman and by acknowledging the accomplishments of women in society. This session also sought to assist women in identifying people in their social network who have provided social support and in recognizing the essential qualities of supportive network members. Session 2 discussed ways of maintaining supportive network members, encouraged women to seek new network members, and informed participants about how to disengage from network members who were not supportive. Peer educators emphasized that social support could be requested without having to disclose serostatus. Session 3 enhanced awareness of HIV transmission risk behaviours and debunked common myths regarding HIV prevention for people living with HIV (e.g., “If both partners are HIV‐positive, it is okay to have unprotected sex”). This session also taught participants communication skills for negotiating safer sex and reinforced the benefits of using condoms consistently, and peer educators modeled proper condom use skills. Session 4 taught women to distinguish between healthy and unhealthy relationships, discussed the impact of abusive partners on safer sex, and informed women of local shelters for women in abusive relationships. Control condition: Participants from a health promotion comparison received 4 4‐hour interactive group sessions administered over consecutive weeks. These sessions addressed medication adherence, nutrition, and provider interaction skills. Two peer educators co‐facilitated implementing the comparison condition. |
|
| Outcomes | Sexual behavior (self‐reported frequency of unprotected vaginal intercourse in the 30 days and 6 months preceding assessments); psychosocial mediators of condom use (scales focusing HIV transmission risk knowledge, partner communication, perceived partner‐related barriers to condom use, beliefs that condoms interfere with sex, and condom use self‐efficacy; and STI prevalence and incidence (vaginal swabs tested for CT, NG, TV‐entire 12‐month follow‐up period). Assessment at baseline, 6 and 12‐month follow‐ups. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | |
| Intention‐to‐treat analysis | Low risk | |
| Sample size calculation | High risk | "Sample size calculations were based on preliminary research with this population. We estimated a moderate effect size, a 35% difference between the study conditions in the number of unprotected vaginal sex acts in the 30 days preceding assessment. Estimating 20% attrition over the 12‐month follow‐up period and setting the type I error rate at 0.05 for a 2‐tailed test (power = 0.80) required a total sample of 185 participants in each study condition to detect the specified effect size." (p. S61) |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Belcher 1998 | Intervention did not target HIV population. Outcomes not reported by HIV status (only 18 HIV‐positive women were included) |
| Bhave 1995 | Not randomized study. Outcomes not reported by HIV status. Participants aged between 15‐25 ‐ outcomes not reported by age |
| Bunnell 2006 | Not randomized study |
| Burman 2008 | Outcome is not behavioral |
| Carrico 2009 | Outcome does not involve condom use but psychosocial adjustment |
| Champion 2001 | Descriptive study. Participants not HIV‐positive |
| Cleary 1995 | Baseline assessment was conducted when participants did not have their HIV diagnosis yet |
| Cosio 2010 | Both groups received intervention for increasing condom use |
| Dilley 2008 | Participants were homosexual men not HIV‐infected |
| Fisher 2006 | Not randomized study |
| Fogarty 2001 | Both groups received intervention for increasing condom use. Participants are the same reported in Gielen 2001 (Outcome reported refers to condom use with main partners only) |
| Holstad 2006 | Study in recruitment phase |
| Jones 2001 | Authors were contacted and did not have available data on absolute number of female participants who reported unprotected sexual intercourse in baseline and each follow‐up assessment in both groups |
| Jones 2005 | Both groups received intervention for condom use |
| Jones 2006 | Study not controlled |
| Kalichman 2001 | We have tried insistently to contact authors and we had no answer. In spite of the study presents results by gender it does not show absolute results of consistent condom use |
| Kalichman 2005 | Outcomes not reported by gender. Study includes men and women |
| Lightfoot 2007 | Study includes adolescents. Outcomes not reported by age and gender |
| MacNeil 1999 | Authors were contacted and did not have available data on absolute number of female participants who reported unprotected sexual intercourse in baseline and each follow‐up assessment in both groups. |
| Magnano San Lio 2009 | Intervention not focused in condom use. Not a clinical trial design |
| Naar‐King 2006 | Study includes adolescents. Outcomes not reported by age and gender |
| NIMH Multisite Group 2008 | No outcomes reported. |
| Olley 2006 | Outcomes not reported by gender. Intervention not clearly focused in condom use |
| Patterson 2003 | Outcomes not reported by gender or age. Study includes men and women |
| Purcell 2007 | Outcomes not reported by gender. Study includes men and women |
| Rotheram‐Borus 2001 | Study includes adolescents. Outcomes not reported by age and gender |
| Rotheram‐Borus 2004 | Outcomes not reported by gender or age. Study includes adolescents |
| Rotheram‐Borus 2009 | Outcomes not reported by gender. Study does not examine gender differences. |
| The Healthy Living 2007 | Outcomes not reported by gender |
| Wyatt 2004 | Control group assessment was obtained only in an immediately post‐intervention follow‐up but not at 3 or 6 months follow‐up |
Differences between protocol and review
In the review protocol, we had planned to include CCTs in the review but we identified only one controlled clinical trial (Fisher 2006) that was excluded to avoid potential confounders in the meta‐analysis. Moreover, STI outcome could not be considered in the meta‐analysis because the outcomes reported in two studies (Saleh‐Onoya 2009; Wingood 2004) were not comparable due to distinct follow‐up periods, and insufficient data in one case (Saleh‐Onoya 2009).
Contributions of authors
FC, TG, EF, JS, CP, and MR were involved in the study design and concept. TG conducted the trials search and, together with FC and EF, worked on selection of studies and data extraction. LM collaborated with data extraction and management, and performed the analyses. TG and EF drafted the manuscript. All authors critically revised the manuscript and approved the final version.
Sources of support
Internal sources
No sources of support supplied
External sources
Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brazil.
Health Ministry, Brazil.
Declarations of interest
None
Edited (no change to conclusions), comment added to review
References
References to studies included in this review
Cornman 2008 {published data only}
- Cornman DH, Kiene SM, Christie S, Fisher WA, Shuper PA, Pillay S, et al. Clinic‐based intervention reduces unprotected sexual behavior among HIV‐infected patients in KwaZulu‐Natal, South Africa: Results of a pilot study. J Acquir Immune Defic Syndr 2008;48:553‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gilbert 2008 {published data only}
- Gilbert P, Ciccarone D, Gansky SA, Bangsberg DR, Clanon K, McPhee SJ, et al. Interactive "Video Doctor" counseling reduces drug and sexual risk behaviors among HIV‐positive patients in diverse outpatient settings. PLoS ONE 2008;3:e1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
Saleh‐Onoya 2009 {published data only}
- Saleh‐Onoya D, Reddy PS, Ruiter RA, Sifunda S, Wingood G, Borne B. Condom use promotion among isiXhosa speaking women living with HIV in the Western Cape Province, South Africa: a pilot study. Aids Care 2009;21:817‐825. [DOI] [PubMed] [Google Scholar]
Sikkema 2008 {published data only}
- Sikkema KJ, Wilson PA, Hansen NB, Kochman A, Neufeld S, Ghebremichael MS, et al. Effects of a coping intervention on transmission risk behavior among people living with HIV/AIDS and a history of childhood sexual abuse. J Acquir Immune Defic Syndr 2008;47:506‐513. [DOI] [PubMed] [Google Scholar]
Wingood 2004 {published data only}
- Wingood GM, DiClemente RJ, Mikhail I, Lang DL, McCree DH, Davies SL, et al. A randomized controlled trial to reduce HIV transmission risk behaviors and sexually transmitted diseases among women living with HIV: The WiLLOW Program. J Acquir Immune Defic Syndr 2004;37:S58‐67. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Belcher 1998 {published data only}
- Belcher L, Kalichman S, Topping M, Smith S, Emshoff J, Norris F, et al. A randomized trial of a brief HIV risk reduction counseling intervention for women. Journal of Consulting and Clinical Psychology 1998;66:856‐861. [DOI] [PubMed] [Google Scholar]
Bhave 1995 {published data only}
- Bhave G, Lindan CP, Hudes ES, Desai S, Wagle U, Tripathi SP, et al. Impact of an intervention on HIV, sexually transmitted diseases, and condom use among sex workers in Bombay, India. AIDS (London, England) 1995;9(Suppl 1):S21‐30. [PubMed] [Google Scholar]
Bunnell 2006 {published data only}
- Bunnell R, Ekwaru JP, Solberg P, Wamai N, Bikaako‐Kajura W, Were W, et al. Changes in sexual behavior and risk of HIV transmission after antiretroviral therapy and prevention interventions in rural Uganda. Aids 2006;20:85‐92. [DOI] [PubMed] [Google Scholar]
Burman 2008 {published data only}
- Burman W, Grund B, Neuhaus J, Douglas J Jr, Friedland G, Telzak E, et al. Episodic antiretroviral therapy increases HIV transmission risk compared with continuous therapy: Results of a randomized controlled trial. J Acquir Immune Defic Syndr 2008;49:142‐150. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carrico 2009 {published data only}
- Carrico A W, Chesney M A, Johnson M O, Morin S F, Neilands T B, Remien R H, et al. Randomized controlled trial of a cognitive‐behavioral intervention for HIV‐positive persons: an investigation of treatment effects on psychosocial adjustment. AIDS and Behaviour 2009;13:555‐563. [DOI] [PMC free article] [PubMed] [Google Scholar]
Champion 2001 {published data only}
- Champion JD, Shain RN, Piper J, Perdue ST. Sexual abuse and sexual risk behaviors of minority women with sexually transmitted diseases. Western Journal of Nursing Research 2001;23:241‐254. [DOI] [PubMed] [Google Scholar]
Cleary 1995 {published data only}
- Cleary PD, Devanter N, Steilen M, Stuart A, Shipton‐Levy R, McMullen W, et al. A randomized trial of an education and support program for HIV‐infected individuals. AIDS (London, England) 1995;9:1271‐8. [DOI] [PubMed] [Google Scholar]
Cosio 2010 {published data only}
- Cosio D, Heckman T G, Anderson T, Heckman BD, Garske J, McCarthy J. Telephone‐administered motivational interviewing to reduce risky sexual behavior in HIV‐infected rural persons: A pilot randomized clinical trial. Dissertation Abstracts International, The Sciences and Engineering, ProQuest Information & Learning: US Guilford Publications 2010;37:140‐146. [DOI] [PubMed] [Google Scholar]
Dilley 2008 {published data only}
- Dilley, J. W, Loeb, L, Marson, K, Chen, S, Schwarcz, S, Paul, J, McFarland, W. Sexual compulsiveness and change in unprotected anal intercourse: unexpected results from a randomized controlled HIV counseling intervention study. Journal of Acquired Immune Deficiency Syndromes 2008;48(1):113‐114. [DOI] [PubMed] [Google Scholar]
Fisher 2006 {published data only}
- Fisher JD, Fisher WA, Cornman DH, Amico RK, Bryan A, & Friedland GH. Clinician‐delivered intervention during routine clinical care reduces unprotected sexual behavior among HIV‐infected patients. Journal of Acquired Immune Deficiency Syndrome 2006;41(1):44‐52. [DOI] [PubMed] [Google Scholar]
Fogarty 2001 {published data only}
- Fogarty LA, Heilig CM, Armstrong K, Cabral R, Galavotti C, Gielen AC, et al. Long‐term effectiveness of a peer‐based intervention to promote condom and contraceptive use among HIV‐positive and at‐risk women. Public Health Reports 2001;116:103‐119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gielen A C, Fogarty, L A, Armstrong K, Green B M, Cabral R, Milstein B, Galavotti C, Heilig C M. Promoting condom use with main partners: A behavioral intervention trial for women. AIDS & Behavior 2001;5(3):193‐204. [Google Scholar]
Holstad 2006 {published data only}
- Holstad MM, DiIorio C, Magowe MK. Motivating HIV positive women to adhere to antiretroviral therapy and risk reduction behavior: The KHARMA Project. Online J Issues Nurs 2006;11:5. [PubMed] [Google Scholar]
Jones 2001 {published data only}
- Jones D L, Weiss S M, Malow R, Ishii M, Devieux J, Stanley H, et al. A brief sexual barrier intervention for women living with AIDS: acceptability, use, and ethnicity. Journal of Urban Health 2001;78:593‐604. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jones 2005 {published data only}
- Jones DL, Ross D, Weiss SM, Bhat G, Chitalu N. Influence of partner participation on sexual risk behavior reduction among HIV‐positive Zambian women. Journal of Urban Health: Bulletin of the New York Academy of Medicine 2005;82:92‐100. [DOI] [PubMed] [Google Scholar]
Jones 2006 {published data only}
- Jones DL, Weiss SM, Bhat GJ, Bwalya V. Influencing sexual practices among HIV‐positive Zambian women. AIDS Care 2006;18:629‐634. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kalichman 2001 {published data only}
- Kalichman SC, Rompa D, Cage M, DiFonzo K, Simpson D, Austin J, et al. Effectiveness of an intervention to reduce HIV transmission risks in HIV‐positive people. American Journal of Preventive Medicine 2001;21:84‐92. [DOI] [PubMed] [Google Scholar]
Kalichman 2005 {published data only}
- Kalichman SC, Rompa D, Cage M. Group intervention to reduce HIV transmission risk behavior among persons living with HIV/AIDS. Behavior Modification 2005;29:256‐285. [DOI] [PubMed] [Google Scholar]
- Kalichman, S.C. The other side of the healthy relationships intervention: mental health outcomes and correlates of sexual risk behavior change. AIDS Education and Prevention 2005;17(1 Suppl A):66‐75. [DOI] [PubMed] [Google Scholar]
Lightfoot 2007 {published data only}
- Lightfoot M A, Kasirye R, Comulada W S, Rotheram‐Borus M J. Efficacy of a culturally adapted intervention for youth living with HIV in Uganda. Prevention Science 2007;8:271‐273. [DOI] [PMC free article] [PubMed] [Google Scholar]
MacNeil 1999 {published data only}
- MacNeil JM, Mberesero F, Kilonzo G. Is care and support associated with preventive behaviour among people with HIV?. Aids Care 1999;11:537‐546. [DOI] [PubMed] [Google Scholar]
Magnano San Lio 2009 {published data only}
- Magnano San Lio M, Mancinelli S, Palombi L, Buonomo E, Altan AD, Germano P, et al. The DREAM model's effectiveness in health promotion of AIDS patients in Africa. Health Promot Int 2009;24:6‐15. [DOI] [PubMed] [Google Scholar]
Naar‐King 2006 {published data only}
- Naar‐King S, Lam P, Wang B, Wright K, Parsons J T, Frey M A. Brief report: maintenance of effects of motivational enhancement therapy to improve risk behaviors and HIV‐related Health in a randomized controlled trial of youth living with HIV. Journal of Pediatric Psychology 2008;33(4):441‐445. [DOI] [PubMed] [Google Scholar]
- Naar‐King S, Wright K, Parsons JT, Frey M, Templin T, Lam P, et al. Healthy Choices: Motivational enhancement therapy for health risk behaviors in HIV‐positive youth. AIDS Education and Prevention 2006;18:1‐11. [DOI] [PubMed] [Google Scholar]
NIMH Multisite Group 2008 {published data only}
- NIMH Multisite HIV/STD Prevention Trial for African American Couples Group. Methodological overview of an African American couple‐based HIV/STD prevention trial. J Acquir Immune Defic Syndr 2008;49:S3‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Olley 2006 {published data only}
- Olley BO. Improving well‐being through psycho‐education among voluntary counseling and testing seekers in Nigeria: A controlled outcome study. Aids Care 2006;18:1025‐31. [DOI] [PubMed] [Google Scholar]
Patterson 2003 {published data only}
- Patterson TL, Shaw WS, Semple SJ. Reducing the sexual risk behaviors of HIV+ individuals: outcome of a randomized controlled trial. Annals of Behavioral Medicine 2003;25:137‐145. [DOI] [PubMed] [Google Scholar]
Purcell 2007 {published data only}
- Purcell DW, Latka MH, Metsch LR, Latkin CA, Gomez CA, Mizuno Y, et al. Results from a randomized controlled trial of a peer‐mentoring intervention to reduce HIV transmission and increase access to care and adherence to HIV medications among HIV‐seropositive injection drug users. J Acquir Immune Defic Syndr 2007;46:S35‐47. [DOI] [PubMed] [Google Scholar]
Rotheram‐Borus 2001 {published data only}
- Lightfoot, M, Lee, M, Rotheram‐Borus, M. J, et al. Interventions for positive youth: are groups the right modality?. 152nd Annual Meeting of the American Psychiatric Association. May15‐20, 1999.
- Rotheram‐Borus M J, Lee M B, Murphy D A, Futterman D, Duan N, Birnbaum J M, et al. Efficacy of a preventive intervention for youths living with HIV. American Journal of Public Health 2001;91:400‐405. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rotheram‐Borus 2004 {published data only}
- Rotheram‐Borus MJ, Swendeman D, Comulada WS, Weiss RE, Lee M, Lightfoot M. Prevention for substance‐using HIV‐positive young people: Telephone and in‐person delivery. J Acquir Immune Defic Syndr 2004;37:S68‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rotheram‐Borus 2009 {published data only}
- Rotheram‐Borus MJ, Desmond K, Comulada WS, Arnold EM, Johnson M. Reducing risky sexual behavior and substance use among currently and formerly homeless adults living with HIV. Am J Public Health 2009;99:1100‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
The Healthy Living 2007 {published data only}
- The Healthy Living Project Team. Effects of a behavioral intervention to reduce risk of transmission among people living with HIV: The Healthy Living Project randomized controlled study. J Acquir Immune Defic Syndr 2007;44:213‐221. [DOI] [PubMed] [Google Scholar]
Wyatt 2004 {published data only}
- Wyatt GE, Longshore D, Chin D, Carmona JV, Loeb TB, Myers HF, et al. The efficacy of an integrated risk reduction intervention for HIV‐positive women with child sexual abuse histories. AIDS and Behavior 2004;8:453‐462. [DOI] [PubMed] [Google Scholar]
Additional references
Almonte 2008
- Almonte M, Albero G, Molano M, et al. Risk factors for human papillomavirus exposure and co‐factors for cervical cancer in Latin America and the Caribbean. Vaccine 2008;26(Suppl 11):L16‐36. [DOI] [PubMed] [Google Scholar]
Balfour 2006
- Balfour L, Kowal J, Silverman A, Tasca GA, Angel JB, Macpherson PA, Garber G, Cooper CL, Cameron DW. A randomized controlled psycho‐education intervention trial: Improving psychological readiness for successful HIV medication adherence and reducing depression before initiating HAART. AIDS Care 2006;18(7):830‐838. [DOI] [PubMed] [Google Scholar]
Blanch 2002
- Blanch J, Rousaud A, Hautzinger M, Martinez E, Peri JM, Andres S, Cirera E, Gatell JM, Gasto C. Assessment of the efficacy of a cognitive‐behavioural group psychotherapy programme for HIV‐infected patients referred to a consultation‐liaison psychiatry department. Psychotherapy and Psychosomatics 2002;71(2):77‐84. [DOI] [PubMed] [Google Scholar]
Crepaz 2006
- Crepaz N, Lyles CM, Wolitski RJ, Passin WF, Rama SM, Herbst JH, Purcell DW, Malow RM, Stall R. Do prevention interventions reduce HIV risk behaviours among people living with HIV? A meta‐analytic review of controlled trials. AIDS 2006;20:143‐157. [DOI] [PubMed] [Google Scholar]
Faria 2010
- Faria ER, Carvalho FT, Gonçalves TR, Moskovics JM, Piccinini CA. Psychological interventions for people living with HIV/Aids: Models, results and gaps [Intervenções psicológicas para pessoas vivendo com HIV/Aids: Modelos, resultados e lacunas]. Interamerican Journal of Psychology 2010 (In press);44(3):xx‐xx. [Google Scholar]
Finocchario‐Kessler 2010
- Finocchario‐Kessler S, Sweat MD, Dariotis JK, Trent ME, Kerrigan DL, Keller JM, Anderson JR. Understanding high fertility desires and Intentions among a sample of urban women living with HIV in the United States. AIDS and Behavior 2010;14(5):1106‐1114. [DOI] [PubMed] [Google Scholar]
Galvin 2004
- Galvin SR, Cohen MS. The role of sexually transmitted diseases in HIV transmission. Nat Review Microbiol 2004;2:33‐42. [DOI] [PubMed] [Google Scholar]
Ghosh 2009
- Ghosh J, Wadhwa V, Kalipeni E. Vulnerability to HIV/AIDS among women of reproductive age in the slums of Delhi and Hyderabad, India. Social Science & Medicine 2009;68(4):638‐642. [DOI] [PubMed] [Google Scholar]
Gore‐Felton 2005
- Gore‐Felton C, Rotheram‐Borus MJ, Weinhardt LS, Kelly JA, Lightfoot M, Kirshenbaum SB, Johnson MO, Chesney MA, Catz SL, Ehrhardt A, Remien RH, Morin SF, NIMH Healthy Living Project Team. The Healthy Living Project: An individually tailored, multidimensional intervention for HIV‐infected persons. AIDS Education and Prevention 2005;17(Suppl A):21‐39. [DOI] [PubMed] [Google Scholar]
GRADE Working Group 2004
- Grades of Recommendation, Assessment, Development, and Evaluation (GRADE) Working Group. Grading quality of evidence and strength of recommendations. British Medical Journal 2004;328:1490‐7. [Google Scholar]
Higgins 2008
- Higgins Julian P T, Green Sally Prof. Cochrane handbook for systematic reviews of interventions Version 5.0.0. Chichester: John Wiley, 2008. [Google Scholar]
Hulley 2001
- Hulley SB, Cummings SR, Browner WS, Grady D, Hearst N, Newman TB. Designing Clinical Research. 2. Lippincott Williams & Wilkins, 2001. [Google Scholar]
John 2001
- John GC, Nduati RW, Mbori‐Ngacha DA, Richardson BA, Panteleeff D, Mwatha A, et al. Correlates of mother‐to‐child human immunodeficiency virus type 1 (HIV‐1) transmission: Association with maternal plasma HIV‐1 RNA load, genital HIV‐1 DNA shedding, and breast infections. J Infect Dis 2001;183:206–212. [DOI] [PubMed] [Google Scholar]
Johnson 2006
- Johnson BT, Carey MP, Chaudoir SR, Reid AE. Sexual Risk Reduction for Persons Living with HIV: Research Synthesis of Randomized Controlled Trials, 1993–2004. J Acquir Immune Defic Syndr 2006;41(5):642–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
Johnson 2007
- Johnson MO, Charlebois E, Morin SF, Remien RH, Chesney MA, The NIMH Healthy Living Project Team. Effects of a behavioral intervention on antiretroviral medication adherence among people living with HIV: The Healthy Living Project randomized controlled study. J Acquir Immune Defic Syndr 2007;46(5):574–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jong 2008
- Jong E, Mulder JW, Gorp EC, Wagenaar JK, Derksen J, Westerga J, Tol A, Smits PH. The prevalence of human papillomavirus (HPV) infection in paired urine and cervical smear samples of HIV‐infected women. J Clin Virol 2008;41(2):111‐115. [DOI] [PubMed] [Google Scholar]
Landes 2007
- Landes M, Thorne C, Barlow P, Fiore S, Malyuta R, Martinelli P, Posokhova S, Savasi V, Semenenko I, Stelmah A, Tibaldi C, Newell ML. Prevalence of sexually transmitted infections in HIV‐1 infected pregnant women in Europe. Eur J Epidemiol 2007;22(12):925‐936. [DOI] [PubMed] [Google Scholar]
Lehtovirta 2008
- Lehtovirta P, Paavonen J, Heikinheimo O. Risk factors, diagnosis and prognosis of cervical intraepithelial neoplasia among HIV‐infected women. Int J STD AIDS 2008;19(1):37‐41. [DOI] [PubMed] [Google Scholar]
Marks 2005
- Marks G, Crepaz N, Senterfitt JW, Janssen RS. Meta‐analysis of high‐risk sexual behavior in persons aware and unaware they are infected with HIV in the United States: Implications for HIV prevention programs. Journal of Acquired Immune Deficiency Syndromes 2005;39(4):446‐453. [DOI] [PubMed] [Google Scholar]
McClelland 2005
- McClelland RS, Lavreys L, Katingima C, et al. Contribution of HIV‐1 infection to acquisition of sexually transmitted disease: a 10‐year prospective study. J Infect Dis 2005;191:333–8. [DOI] [PubMed] [Google Scholar]
Murphy 1998
- Murphy DA, Mann T, O'Keefe Z, Rotheram‐Borus MJ. Number of pregnancies, outcome expectancies, and social norms among HIV‐infected young women. Health Psychology 1998;17(5):470‐475. [DOI] [PubMed] [Google Scholar]
Mwapasa 2006
- Mwapasa V, Rogerson SJ, Kwiek JJ, Wilson PE, Milner D, Molyneux ME, Kamwendo DD, Tadesse E, Chaluluka E, Meshnick SR. Maternal syphilis infection is associated with increased risk of mother‐to‐child transmission of HIV in Malawi. AIDS 2006;20:1869–1877. [DOI] [PubMed] [Google Scholar]
Nicolosi 1994
- Nicolosi A, Leite MLC, Musicco M, Arici C, Gavazzeni G, Lazzarin A. The Efficiency of Male‐to‐Female and Female‐to‐Male Sexual Transmission of the Human Immunodeficiency Virus: A Study of 730 Stable Couples. Epidemiology 1994;5(6):570‐575. [DOI] [PubMed] [Google Scholar]
Oliveira 2010
- Oliveira PM, Oliveira RPC, Travessa IEM, Gomes MVC, Santos MLJ, Grassi MFR. Prevalence and risk factors for cervical intraepithelial neoplasia in HIV‐infected women in Salvador, Bahia, Brazil. Sao Paulo Med J 2010;128(4):197‐201. [DOI] [PMC free article] [PubMed] [Google Scholar]
O’Farrell 2001
- O’Farrell N. Enhanced Efficiency of Female‐to‐Male HIV Transmission in Core Groups in Developing Countries. The Need to Target Men. Sexually Transmitted Diseases 2001;28(2):84‐91. [DOI] [PubMed] [Google Scholar]
Peretti‐Watel 2006
- Peretti‐Watel P, Spire B, Schiltz MA, et al. Vulnerability, unsafe sex and non‐adherence to HAART: Evidence from a large sample of French HIV/AIDS outpatients. Social Science & Medicine 2006;62(10):2420‐2433. [DOI] [PubMed] [Google Scholar]
Quadagno 1996
- Quadagno D, Harrison DF, Eberstein IW, Sly DF, Yoshioka M, Soler H. The development and implementation of a cognitive‐based intervention aimed at culturally diverse women at risk for HIV/AIDS. Quarterly of community health education 1996‐97;16(3):271‐285. [DOI] [PubMed] [Google Scholar]
Quinn 2000
- Quinn TC, Wawer MJ, Sewankambo N, Serwadda D, Li C, Wabwire‐Mangen F, Meehan MO, Lutalo T, Gray RH. Viral Load and Heterosexual Transmission of Human Immunodeficiency Virus Type 1. N Engl J Med 2000;342:921‐929. [DOI] [PubMed] [Google Scholar]
Santos 2009
- Santos NJ, Barbosa RM, Pinho AA, Villela WV, Aidar T, Filipe EM. Contexts of HIV vulnerability among Brazilian women [Contextos de vulnerabilidade para o HIV entre mulheres brasileiras]. Cadernos de Saúde Pública 2009;25(Suppl 2):S321‐333. [DOI] [PubMed] [Google Scholar]
Serovich 2009
- Serovich JM, Reed S, Grafsky EL, Andrist D. An intervention to assist men who have sex with men disclose their serostatus to casual sex partners: Results from a pilot study. AIDS Education and Prevention 2009;21(3):207‐219. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sterne 2001
- Sterne JAC, Egger M, Smith JD. Investigating and dealing with publication and otherbiases in metaanalysis. British Medicasl Journal 2001;323:101‐5. [Google Scholar]
UNAIDS 2010
- Joint United Nations Programme on HIV/AIDS (UNAIDS). Global report: UNAIDS report on the global AIDS epidemic 2010. World Health Organization (WHO) Library 2010.
Weinhardt 2004
- Weinhardt LS, Kelly JA, Brondino MJ, et al. HIV transmission risk behavior among men and women living with HIV in 4 cities in the United States. J Acquir Immune Defic Syndr 2004;36:1057‐66. [DOI] [PubMed] [Google Scholar]
Wilson 2004
- Wilson TE, Gore ME, Greenblatt R, et al. Changes in sexual behavior among HIV‐infected women after initiation of HAART. American Journal of Public Health 2004;94(7):1141‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]


