Abstract
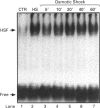
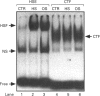
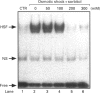
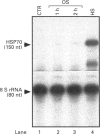
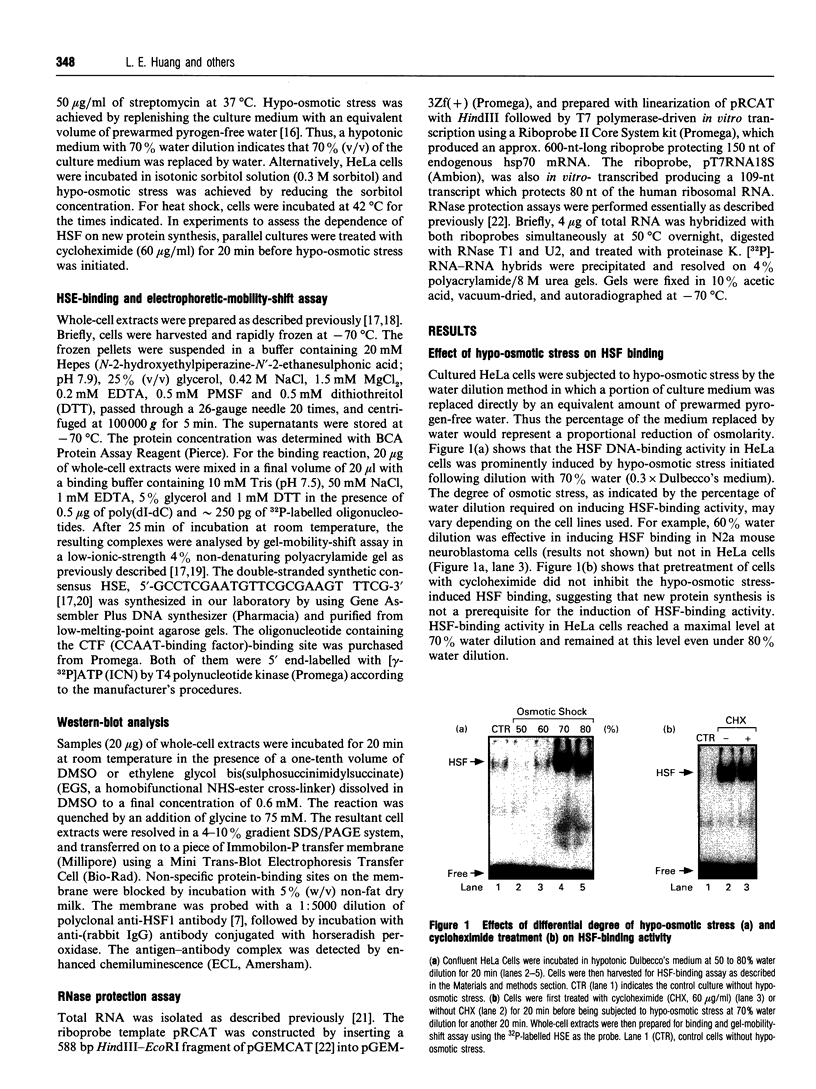
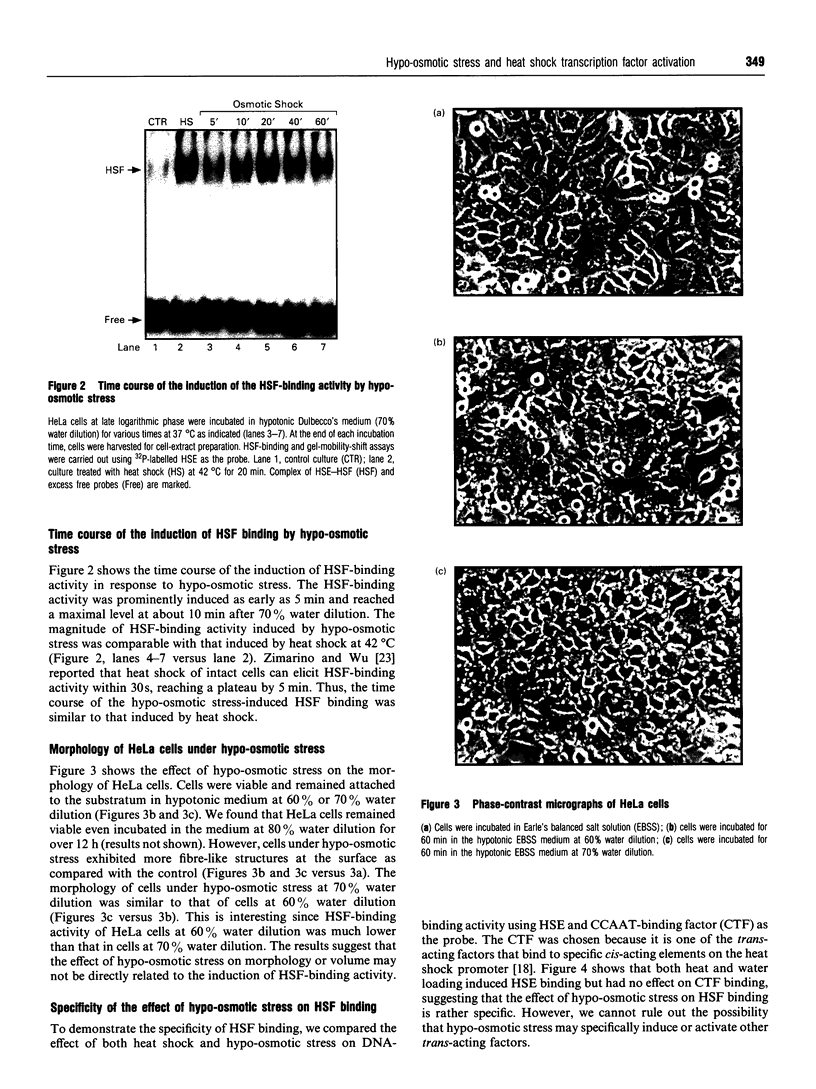
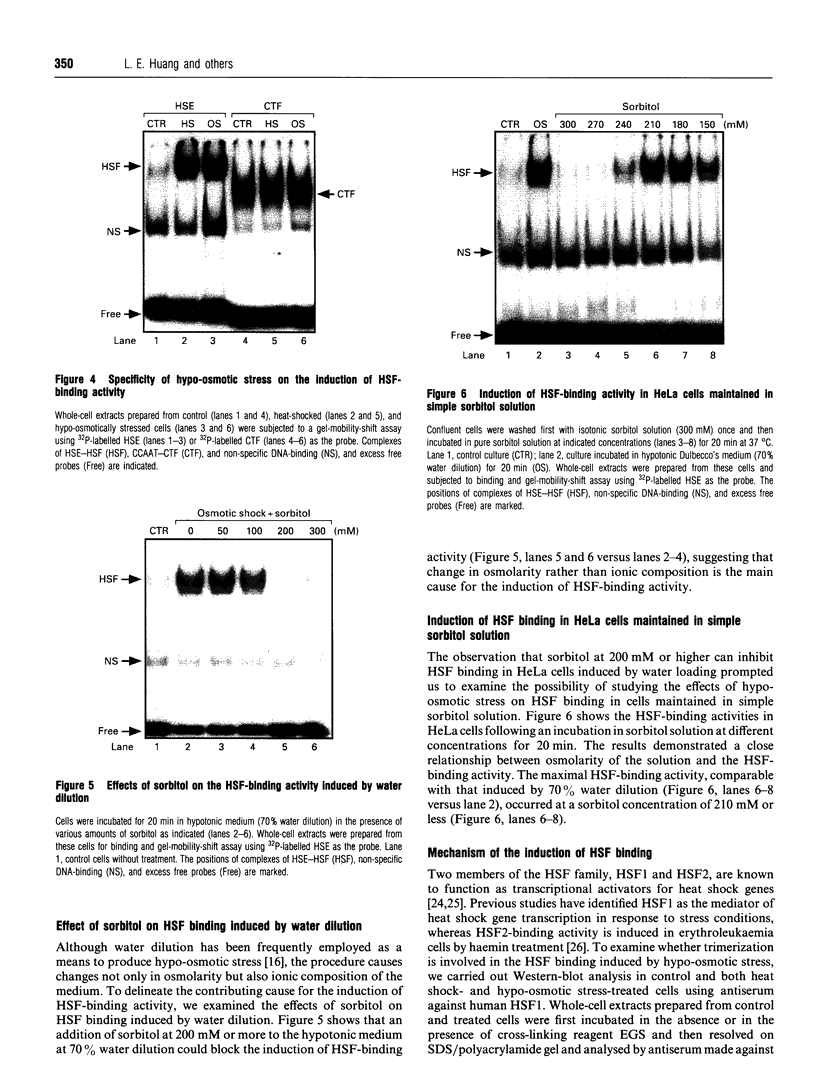
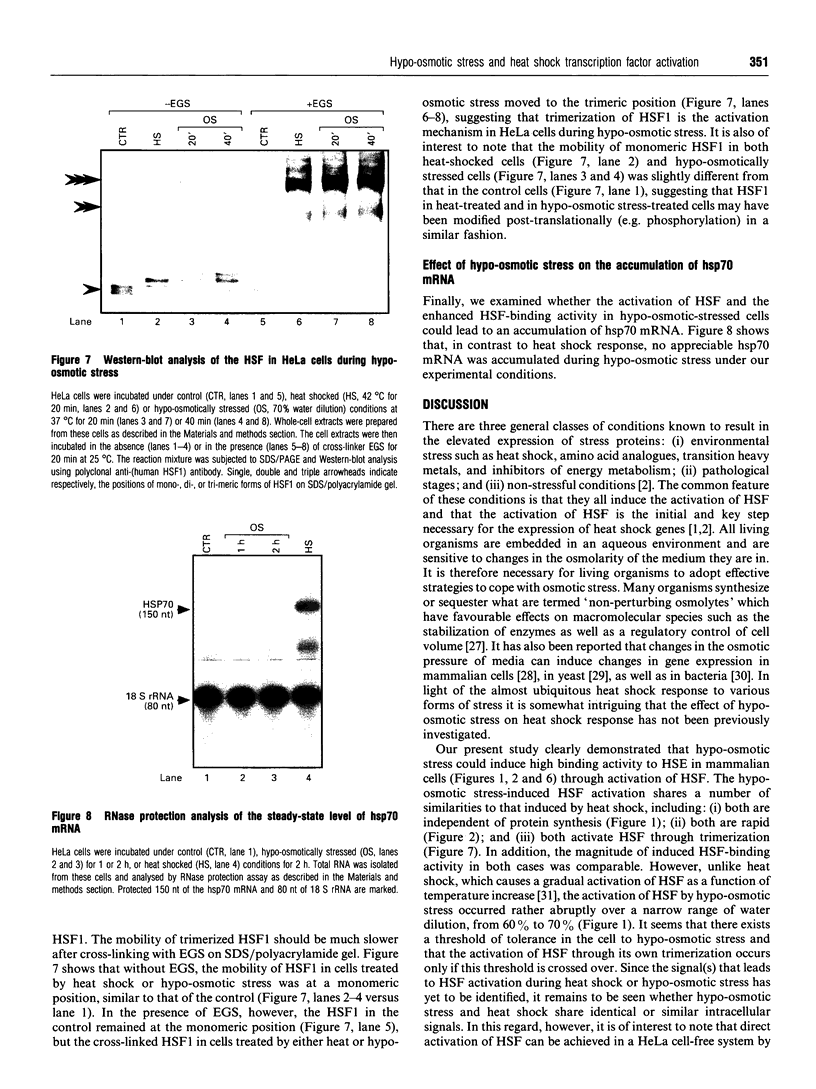
Osmoregulation is important to living organisms for survival in responding to environmental changes of water and ionic strength. We demonstrated here for the first time that exposure of HeLa cells to a hypotonic medium (30% growth medium and 70% water) prominently induced the binding activity of the heat shock transcription factor (HSF). Pretreatment of cells with cycloheximide did not inhibit the induction of HSF-binding activity, indicating that the mechanisms of induction are independent of new protein synthesis. The magnitude of hypo-osmotic stress-induced HSF-binding activity was comparable with that induced by heat shock. The induction, as monitored by gel-mobility-shift assay, occurred within 5 min of hypo-osmotic stress and persisted at least up to 4 h in HeLa cells under the hypotonic conditions. Addition of sorbitol to the hypotonic medium abolished HSF activation. Hypo-osmotic stress-induced HSF binding could also be demonstrated in HeLa cells maintained in simple sorbitol solution by decreasing the sorbitol concentration from 300 mM to 200 mM or less. Competition analysis suggests that the effects of hypo-osmotic stress on HSF-binding activity was specific. Cross-linking experiments and Western-blot analysis demonstrated that hypo-osmotic stress induced trimerization of human heat shock factor 1 (HSF1) in intact HeLa cells, suggesting that trimer formation of HSF1 was responsible for inducing HSF-binding activity in hypo-osmotically stressed cells. However, unlike heat shock response, the activation of HSF by hypo-osmotic stress did not lead to accumulation of hsp70 mRNA in HeLa cells.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chamberlin M. E., Strange K. Anisosmotic cell volume regulation: a comparative view. Am J Physiol. 1989 Aug;257(2 Pt 1):C159–C173. doi: 10.1152/ajpcell.1989.257.2.C159. [DOI] [PubMed] [Google Scholar]
- Choi H. S., Lin Z., Li B. S., Liu A. Y. Age-dependent decrease in the heat-inducible DNA sequence-specific binding activity in human diploid fibroblasts. J Biol Chem. 1990 Oct 15;265(29):18005–18011. [PubMed] [Google Scholar]
- Finkenzeller G., Newsome W., Lang F., Häussinger D. Increase of c-jun mRNA upon hypo-osmotic cell swelling of rat hepatoma cells. FEBS Lett. 1994 Mar 7;340(3):163–166. doi: 10.1016/0014-5793(94)80129-0. [DOI] [PubMed] [Google Scholar]
- Goldenberg C. J., Luo Y., Fenna M., Baler R., Weinmann R., Voellmy R. Purified human factor activates heat shock promoter in a HeLa cell-free transcription system. J Biol Chem. 1988 Dec 25;263(36):19734–19739. [PubMed] [Google Scholar]
- Le Rudulier D., Strom A. R., Dandekar A. M., Smith L. T., Valentine R. C. Molecular biology of osmoregulation. Science. 1984 Jun 8;224(4653):1064–1068. doi: 10.1126/science.224.4653.1064. [DOI] [PubMed] [Google Scholar]
- Liu R. Y., Kim D., Yang S. H., Li G. C. Dual control of heat shock response: involvement of a constitutive heat shock element-binding factor. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):3078–3082. doi: 10.1073/pnas.90.7.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundgren D. W. Effect of hypotonic stress on ornithine decarboxylase mRNA expression in cultured cells. J Biol Chem. 1992 Apr 5;267(10):6841–6847. [PubMed] [Google Scholar]
- Margalit A., Sofer Y., Grossman S., Reynaud D., Pace-Asciak C. R., Livne A. A. Hepoxilin A3 is the endogenous lipid mediator opposing hypotonic swelling of intact human platelets. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):2589–2592. doi: 10.1073/pnas.90.7.2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno T., Chou M. Y., Inouye M. A comparative study on the genes for three porins of the Escherichia coli outer membrane. DNA sequence of the osmoregulated ompC gene. J Biol Chem. 1983 Jun 10;258(11):6932–6940. [PubMed] [Google Scholar]
- Morimoto R. I. Cells in stress: transcriptional activation of heat shock genes. Science. 1993 Mar 5;259(5100):1409–1410. doi: 10.1126/science.8451637. [DOI] [PubMed] [Google Scholar]
- Mosser D. D., Kotzbauer P. T., Sarge K. D., Morimoto R. I. In vitro activation of heat shock transcription factor DNA-binding by calcium and biochemical conditions that affect protein conformation. Proc Natl Acad Sci U S A. 1990 May;87(10):3748–3752. doi: 10.1073/pnas.87.10.3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosser D. D., Theodorakis N. G., Morimoto R. I. Coordinate changes in heat shock element-binding activity and HSP70 gene transcription rates in human cells. Mol Cell Biol. 1988 Nov;8(11):4736–4744. doi: 10.1128/mcb.8.11.4736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nover L. HSFs and HSPs--a stressful program on transcription factors and chaperones. Stress Proteins and the Heat Shock Response, sponsored by Cold Spring Harbor Laboratory, Cold Spring Harbor, NY USA, April 29-May 2, 1991. New Biol. 1991 Sep;3(9):855–859. [PubMed] [Google Scholar]
- Pang J. H., Chen K. Y. A specific CCAAT-binding protein, CBP/tk, may be involved in the regulation of thymidine kinase gene expression in human IMR-90 diploid fibroblasts during senescence. J Biol Chem. 1993 Feb 5;268(4):2909–2916. [PubMed] [Google Scholar]
- Peppel K., Baglioni C. A simple and fast method to extract RNA from tissue culture cells. Biotechniques. 1990 Dec;9(6):711–713. [PubMed] [Google Scholar]
- Poulin R., Pegg A. E. Regulation of ornithine decarboxylase expression by anisosmotic shock in alpha-difluoromethylornithine-resistant L1210 cells. J Biol Chem. 1990 Mar 5;265(7):4025–4032. [PubMed] [Google Scholar]
- Prince W. S., Villarejo M. R. Osmotic control of proU transcription is mediated through direct action of potassium glutamate on the transcription complex. J Biol Chem. 1990 Oct 15;265(29):17673–17679. [PubMed] [Google Scholar]
- Rabindran S. K., Giorgi G., Clos J., Wu C. Molecular cloning and expression of a human heat shock factor, HSF1. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):6906–6910. doi: 10.1073/pnas.88.16.6906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabindran S. K., Haroun R. I., Clos J., Wisniewski J., Wu C. Regulation of heat shock factor trimer formation: role of a conserved leucine zipper. Science. 1993 Jan 8;259(5092):230–234. doi: 10.1126/science.8421783. [DOI] [PubMed] [Google Scholar]
- Schlesinger M. J. Heat shock proteins. J Biol Chem. 1990 Jul 25;265(21):12111–12114. [PubMed] [Google Scholar]
- Schuetz T. J., Gallo G. J., Sheldon L., Tempst P., Kingston R. E. Isolation of a cDNA for HSF2: evidence for two heat shock factor genes in humans. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):6911–6915. doi: 10.1073/pnas.88.16.6911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sistonen L., Sarge K. D., Phillips B., Abravaya K., Morimoto R. I. Activation of heat shock factor 2 during hemin-induced differentiation of human erythroleukemia cells. Mol Cell Biol. 1992 Sep;12(9):4104–4111. doi: 10.1128/mcb.12.9.4104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorger P. K. Heat shock factor and the heat shock response. Cell. 1991 May 3;65(3):363–366. doi: 10.1016/0092-8674(91)90452-5. [DOI] [PubMed] [Google Scholar]
- Varela J. C., van Beekvelt C., Planta R. J., Mager W. H. Osmostress-induced changes in yeast gene expression. Mol Microbiol. 1992 Aug;6(15):2183–2190. doi: 10.1111/j.1365-2958.1992.tb01392.x. [DOI] [PubMed] [Google Scholar]
- Williams G. T., McClanahan T. K., Morimoto R. I. E1a transactivation of the human HSP70 promoter is mediated through the basal transcriptional complex. Mol Cell Biol. 1989 Jun;9(6):2574–2587. doi: 10.1128/mcb.9.6.2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao H., Lis J. T. Germline transformation used to define key features of heat-shock response elements. Science. 1988 Mar 4;239(4844):1139–1142. doi: 10.1126/science.3125608. [DOI] [PubMed] [Google Scholar]
- Xiao H., Perisic O., Lis J. T. Cooperative binding of Drosophila heat shock factor to arrays of a conserved 5 bp unit. Cell. 1991 Feb 8;64(3):585–593. doi: 10.1016/0092-8674(91)90242-q. [DOI] [PubMed] [Google Scholar]
- Yancey P. H., Clark M. E., Hand S. C., Bowlus R. D., Somero G. N. Living with water stress: evolution of osmolyte systems. Science. 1982 Sep 24;217(4566):1214–1222. doi: 10.1126/science.7112124. [DOI] [PubMed] [Google Scholar]
- Yang Y. C., Bastos M., Chen K. Y. Effects of osmotic stress and growth stage on cellular pH and polyphosphate metabolism in Neurospora crassa as studied by 31P nuclear magnetic resonance spectroscopy. Biochim Biophys Acta. 1993 Nov 7;1179(2):141–147. doi: 10.1016/0167-4889(93)90135-c. [DOI] [PubMed] [Google Scholar]
- Zimarino V., Wu C. Induction of sequence-specific binding of Drosophila heat shock activator protein without protein synthesis. 1987 Jun 25-Jul 1Nature. 327(6124):727–730. doi: 10.1038/327727a0. [DOI] [PubMed] [Google Scholar]