Abstract
Study Design
Retrospective cohort study.
Purpose
This study aimed to compare data from patients who received intradiscal condoliase (chondroitin sulfate ABC endolyase) injection for primary lumbar disc herniation (LDH) and recurrent LDH.
Overview of Literature
Chemonucleolysis with condoliase for LDH is a treatment with relatively good results and a high safety profile; however, few studies have reported recurrence after LDH surgery.
Methods
The study participants were 249 patients who underwent intradiscal condoliase injection for LDH at nine participating institutions, including 241 patients with initial LDH (group C) and eight with recurrent LDH (group R). Patient characteristics including age, sex, body mass index, disease duration, intervertebral LDH level, smoking history, and diabetes history were evaluated. Low back pain/leg pain Numerical Rating Scale (NRS) scores and the Oswestry Disability Index (ODI) were used to evaluate clinical symptoms before treatment and at 6 months and 1 year after treatment.
Results
Low back pain NRS scores (before treatment and at 6 months and 1 year after treatment, respectively) in group C (4.9 → 2.6 → 1.8) showed significant improvement until 1 year after treatment. Although a tendency for improvement was observed in group R (3.5 → 2.8 → 2.2), no significant difference was noted. Groups C (6.6 → 2.4 → 1.4) and R (7.0 → 3.1 → 3.2) showed significant improvement in the leg pain NRS scores after treatment. Group C (41.4 → 19.5 → 13.7) demonstrated significant improvement in the ODI up to 1 year after treatment; however, no significant difference was found in group R (35.7 → 31.7 → 26.4).
Conclusions
Although intradiscal condoliase injection is less effective for LDH recurrence than for initial cases, it is useful for improving leg pain and can be considered a minimally invasive and safe treatment method.
Keywords: Intervertebral disc displacement, Intervertebral disc chemolysis, Leg pain, Back pain
Introduction
Lumbar disc herniation (LDH) is a disease in which the nerve roots are compressed by a degenerated nucleus pulposus and occurs frequently in patients aged 20–40 years. Conservative treatment is initially recommended for these patients; however, surgery is required for cases that are resistant to conservative treatment for an extended period or when motor paralysis developed. Although the therapeutic efficacy of discectomy for LDH is generally good, LDH recurrence remains a challenging problem because of the high surgical risks. Conservative treatment is initially recommended for patients with recurrent LDH; however, if conservative treatment fails, reoperation is often needed.
In a longitudinal observational study of 34,639 patients who underwent surgery for LDH, surgical complications occurred in 2.7%, and the reoperation rate within 90 days was 2.1% [1,2]. In addition, the incidence of recurrent LDH within 2 years after surgery ranges from 0% to 23%, and the reoperation rate varies from 0% to 13% [3]. However, surgical resection of recurrent LDH surgery is extremely difficult because of epidural adhesions and scarring, and clinical symptom improvement is usually poorer after this surgery than after initial surgery [4–8].
Until now, treatments for LDH have been broadly divided into conservative, such as drug therapy, exercise therapy, and nerve root infiltration, which are expected to have a temporary effect, and surgical, such as hernia removal.
Chemonucleolysis, which was developed as a new treatment for LDH, is considered an intermediate method between conservative and surgical approaches, and a treatment using chondroitin sulfate ABC endolyase (condoliase) was developed. Condoliase is a pure mucopolysaccharidase derived from the Gram-negative rod Proteus vulgaris that has high specificity for its substrates chondroitin sulfate and hyaluronic acid, glycosaminoglycans, which are abundant in the proteoglycans of the nucleus pulposus of the intervertebral discs. Unlike chymopapain, which has traditionally been used in Western countries, condoliase does not have protease activity; thus, it can induce chemonucleolysis without damaging surrounding nerves or ligament tissues [9–11].
Clinical trials for the contained type of LDH (protrusion and subligamentous extrusion types of LDH) in L4–L5 and L5–S1 demonstrated the safety and efficacy of condoliase. Condoliase was approved as an intradiscal treatment for LDH by the Japanese regulatory authority in 2018 [12,13]. Subsequently, LDH treatment using condoliase was covered by insurance in 2019 and is frequently used in general practice.
Condoliase was also reported to be effective against transligamentous extrusion type of LDH [14]. Intradiscal condoliase injection therapy (CD therapy) for LDH achieves relatively good results and is highly safe; however, few studies have reported cases of LDH recurrence [14–17]. Thus, this study aimed to compare data from patients receiving CD therapy for primary and recurrent LDH and evaluate its effectiveness in recurrent LDH.
Materials and Methods
Study design
This multicenter retrospective cohort study was conducted to evaluate the outcomes of CD therapy in patients with primary and recurrent LDH. The study was approved by the ethical review boards of all participating institutions before the start of the study. We declare that all protocols involving humans were approved by the Ethics Committee of the Japanese Society for Spine Surgery and Related Research (IRB approval no., #16) and were performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. All participants provided documentation of their written informed consent before their inclusion in this study.
A total of 249 patients (average age, 45.8±17.1 years; 157 males) who received CD therapy for LDH at nine participating institutions between August 2018 and October 2020 were selected. Moreover, 241 patients with newly diagnosed LDH without a history of LDH treatment (regardless of conservative or surgical treatment) (group C) were compared with eight patients with relapsed LDH with a history of treatment (group R). Treatment is indicated for patients with unilateral lower limb pain regardless of the presence or absence of low back pain, for whom magnetic resonance imaging (MRI) reveals nerve root compression caused by LDH and whose symptoms do not improve with conservative treatments such as drug therapy, nerve root infiltration, or physical therapy. Patients with a history of lower limb muscle weakness or severe allergies and patients with foraminal or sequestrated LDH were excluded.
Intradiscal injection technique
All intradiscal injections were performed by a board-certified orthopedic spine surgeon. With the patient lying in the prone position, the affected disc was penetrated using a conventional disc puncture needle under fluoroscopy. Once fluoroscopic frontal and lateral views confirmed that the tip of the disc puncture needle was located at the center of the disc, condoliase (Hernicore; Seikagaku Corp., Tokyo, Japan; Kaken Pharmaceutical Co., Tokyo, Japan) was administered. A solution of 1.25 U/mL was prepared by dissolving the condoliase preparation in 1.2 mL of physiological saline, which was injected into the intervertebral disc. All patients were carefully observed for at least 1.5 hours after the injection and allowed to return home after confirming that no adverse events occurred.
Clinical outcomes
Patient characteristics, including age, sex, body mass index (BMI), disease duration, intervertebral LDH level, smoking history, and diabetes history, were evaluated in both groups. For group R, the time to recurrence and time from symptom recurrence to treatment were also evaluated. The Numerical Rating Scale (NRS) score and Oswestry Disability Index (ODI) for low back pain and leg pain were evaluated before treatment and at 6 months and 1 year after treatment.
Radiologic evaluation
LDH was diagnosed in all patients using standard T1- and T2-weighted MRI in sagittal and axial views. In group R, LDH classification, Pfirrmann classification [18], presence or absence of high-intensity signal changes in the LDH region on T2-weighted MRI, and changes in the LDH size after treatment were also evaluated. MRI was performed before treatment and 1 year after treatment.
Statistical analysis
Continuous variables were compared between groups using the Student t-test, and categorical variables were compared using the chi-square test. Changes in the NRS scores and ODI over time (before treatment and at 6 months and 1 year after treatment) were analyzed using the one-way repeated-measures analysis of variance, followed by Dunnett’s post hoc test; p<0.05 was considered significant. All statistical analyses were conducted using JMP ver. 17.0 (SAS Institute Inc., Cary, NC, USA).
Results
Patient background characteristics
Regarding patient characteristics (group C, group R, p-value), age (45.7±17.0 years, 48.8±19.2 years, p=0.622), male sex (n=152 [63.1%], n=5 [62.5%], p=0.974), BMI (kg/m2) (24.6±4.6, 20.8±2.1, p=0.109), disease duration (months) (9.9±16.4, 6.7±6.9, p=0.607), smoking history (n=28 [11.6%], n=1 [12.5%], p=0.234), and diabetes complication rate (n=13 [5.4%], n=0 [0%], p=0.528) were analyzed, and no significant difference in these baseline characteristics was found between the two groups (Table 1). The LDH levels were as follows: group C, L1–L2 (n=4, 1.7%), L2–L3 (n=12, 5.0%), L3–L4 (n=11, 4.6%), L4–L5 (n=105, 43.6%), and L5–S1 (n=109, 45.2%); group R, L3–L4 (n=1, 12.5%), L4–L5 (n=1, 12.5%), and L5–S1 (n=6, 75%). In group R, the mean time from the initial treatment to LDH recurrence was 58.4 months (range, 4–168 months). The average time from LDH recurrence to CD therapy was 6.0 months (range, 1–21 months). The recurrent LDH was subligamentous in four patients (50%) and transligamentous in four patients (50%), and four patients (50%) had a Pfirrmann grade III, 3 (37.5%) had grade IV, and 1 (12.5%) had grade V. Two patients (25%) exhibited high-intensity signal changes in the LDH region on T2-weighted MRI (Table 2).
Table 1.
Patient demographic and clinical characteristics
| Characteristic | Primary LDH (group C, n=241) | Recurrent LDH (group R, n=8) | p-value |
|---|---|---|---|
| Age (yr) | 45.7±17.0 | 48.8±19.2 | 0.622 |
| Sex (male) | 152 (63.1) | 5 (62.5) | 0.974 |
| Body mass index (kg/m2) | 24.6±4.6 | 20.8±2.1 | 0.109 |
| Duration of symptoms (mo) | 9.9±16.4 | 6.7±6.9 | 0.607 |
| Herniated disc level | |||
| L1–2 | 4 (1.7) | 0 | |
| L2–3 | 12 (5.0) | 0 | |
| L3–4 | 11 (4.6) | 1 (12.5) | |
| L4–5 | 105 (43.6) | 1 (12.5) | |
| L5–S1 | 109 (45.2) | 6 (75.0) | |
| Smoking status | 28 (11.6) | 1 (12.5) | 0.234 |
| Diabetes mellitus | 13 (5.4) | 0 | 0.528 |
| NRS for low back pain | 4.9±2.9 | 3.5±2.3 | 0.168 |
| NRS for leg pain | 6.6±2.6 | 7.0±3.1 | 0.628 |
| Oswestry Disability Index (%) | 41.5±18.1 | 35.7±13.8 | 0.408 |
Values are presented as mean±standard deviation or number (%).
LDH, lumbar disc herniation; NRS, Numerical Rating Scale.
Table 2.
Summary of findings for the eight patients with recurrent LDH (group R)
| Variable | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Patient 8 |
|---|---|---|---|---|---|---|---|---|
| Age (yr) | 64 | 25 | 44 | 43 | 63 | 38 | 82 | 31 |
| Sex | Male | Male | Female | Female | Male | Male | Female | Male |
| Body mass index (kg/m2) | 23.9 | 20.1 | 19 | 20.3 | - | - | - | - |
| Previous treatments | Conservative treatment | Discectomy (MED) | Discectomy (MED) | Discectomy (MED) | Discectomy (LOVE) | Discectomy (LOVE) | Discectomy (LOVE) | Discectomy (LOVE) |
| Herniated disc level | L5–S1 | L4–5 | L5–S1 | L5–S1 | L5–S1 | L5–S1 | L3–4 | L5–S1 |
| Duration of recurrence (mo) | 60 | 132 | 39 | 4 | 36 | 15 | 13 | 168 |
| D_uration of from onset of symptoms to treatment (mo) | 9 | 1 | 1 | 1 | 4 | 7 | 21 | 4 |
| NRS for low back pain | ||||||||
| Preoperative | 1 | 5 | 0 | 3 | 7 | 5 | 3 | 4 |
| Final observation | 1 | 1 | 0 | 0 | 3 | 1 | 3 | 6 |
| NRS for leg pain | ||||||||
| Preoperative | 1 | 10 | 10 | 10 | 7 | 5 | 7 | 5 |
| Final observation | 0 | 1 | 0 | 2 | 5 | 2 | 7 | 3 |
| Oswestry Disability Index (%) | ||||||||
| Preoperative | 20 | 56 | - | 18 | 34 | 34 | 40 | 48 |
| Final observation | - | - | 10 | 28 | 16 | 18 | 52 | 36 |
| Pfirrmann grade | V | IV | IV | III | III | III | IV | III |
| H_igh-intensity signal change at herniation site on T2-weighted MRI | - | + | - | - | - | - | - | + |
| Type of disc herniation | Transligamentous | Subligamentous | Transligamentous | Transligamentous | Transligamentous | Subligamentous | Subligamentous | Subligamentous |
| Location disc herniation | Paracentral | Paracentral | Paracentral | Paracentral | Paracentral | Paracentral | Paracentral | Paracentral |
| Size of disc herniation | Reduced | Reduced | Reduced | Reduced | Reduced | Reduced | No change | Reduced |
LDH, lumbar disc herniation; MED, microendoscopic discectomy; LOVE, fenestration discectomy (LOVE surgery); NRS, Numerical Rating Scale; MRI, magnetic resonance imaging.
Clinical outcomes
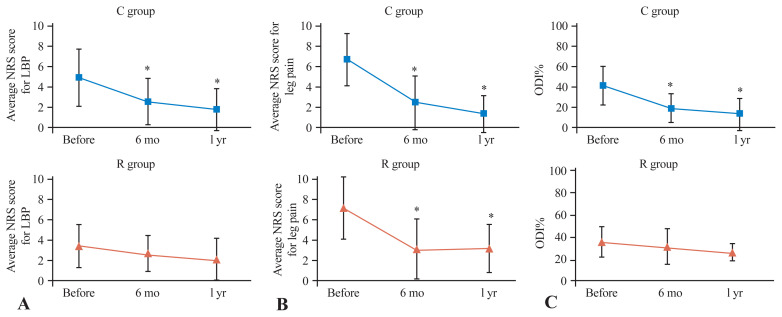
The mean NRS scores for low back pain (before treatment and at 6 months and 1 year after treatment) were 4.9 → 2.6 → 1.8 in group C and were significantly greater at both 6 months and 1 year after treatment than before treatment. Although group R showed an improvement trend of 3.5 → 2.8 → 2.2, no significant difference was found at any time after treatment (Fig. 1A).
Fig. 1.
Line graph of the Numeric Rating Scale (NRS) score for low back pain (LBP) (A) and leg pain (B), and Oswestry Disability Index (ODI) % (C) before treatment and at 3–6 months, and 1 year after treatment. *p<0.05; Significantly better than the pretreatment score.
The mean NRS score for lower limb pain improved significantly in group C at 6.6 → 2.4 → 1.4 and in group R at 7.0 → 3.1 → 3.2 both 6 months and 1 year after treatment compared with before treatment (Fig. 1B). Similarly, compared with those before treatment, the ODI (%) in group C significantly improved at 41.4 → 19.5 → 13.7 both 6 months and 1 year after treatment. Although group R showed an improvement trend at 35.7 → 31.7 → 26.4, no significant difference was found (Fig. 1C).
No significant difference in the NRS scores for low back pain was found before, 6 months after, or 1 year after treatment. In addition, no significant difference in the NRS scores for leg pain was noted before and at 6 months after treatment; however, after 1 year, the NRS scores for leg pain were significantly greater in group C, with 1.4 in group R, and 3.2 in group R (p=0.006).
Moreover, no significant difference in the ODI was noted before treatment or at 1 year after treatment; however, at 6 months after treatment, the ODI was significantly greater in group C (19.5 in group R, 31.7 in group R) (p=0.045) (Table 3).
Table 3.
Comparison of NRS score and ODI between groups
| Variable | Primary LDH (group C, n=241) | Recurrent LDH (group R, n=8) | p-value |
|---|---|---|---|
| NRS score for low back pain | |||
| Preoperative | 4.9±2.9 | 3.5±2.3 | 0.168 |
| 3–6 mo | 2.6±2.3 | 2.8±1.9 | 0.824 |
| 1 yr | 1.8±2.1 | 2.2±2.3 | 0.654 |
| NRS score for leg pain | |||
| Preoperative | 6.6±2.6 | 7.0±3.1 | 0.628 |
| 3–6 mo | 2.4±2.6 | 3.1±3.0 | 0.259 |
| 1 yr | 1.4±1.8 | 3.2±2.5 | 0.006 |
| ODI (%) | |||
| Preoperative | 41.5±18.1 | 35.7±13.8 | 0.408 |
| 3–6 mo | 19.5±14.2 | 31.7±16.2 | 0.045 |
| 1 yr | 13.7±15.6 | 26.4±17.3 | 0.08 |
Values are presented as mean±standard deviation.
NRS, Numerical Rating Scale; ODI, Oswestry Disability Index; LDH, lumbar disc herniation.
Representative case presentation
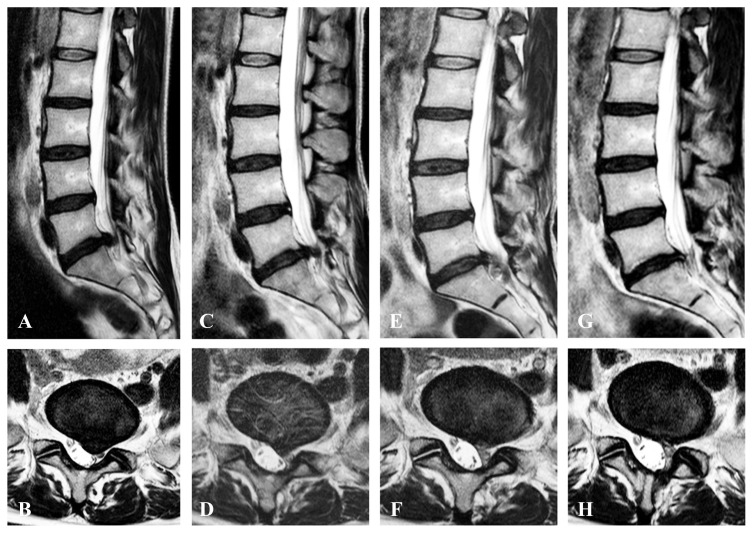
A 44-year-old woman underwent microendoscopic discectomy for L5–S1 left LDH (Fig. 2A, B) at Shimoshizu National Hospital when she was 41 years old; however, 39 months after the surgery, she complained of severe pain radiating to her left lower limb and was rushed to our hospital. The chief complaint was pain from the left buttock to the back of the thigh and back of the lower leg, which worsened when sitting, and she could not stand.
Fig. 2.
T2-weighted magnetic resonance images of representative case presentation (a 44-year-old female patient with recurrence). (A, C, E, G) Sagittal image. (B, D, F, H) Axial image. (A, B) Initial left L5–S1 transligamentous lumbar disc herniation before microendoscopic lumbar discectomy at age 41 years. (C, D) Recurrent left L5–S1 transligamentous lumbar disc herniation. (E, F) After 1.5 months of intradiscal condoliase injection therapy, the L5–S1 disc herniation had shrunk. (G, H) 6 months after intradiscal condoliase injection therapy, the L5–S1 disc herniation disappeared.
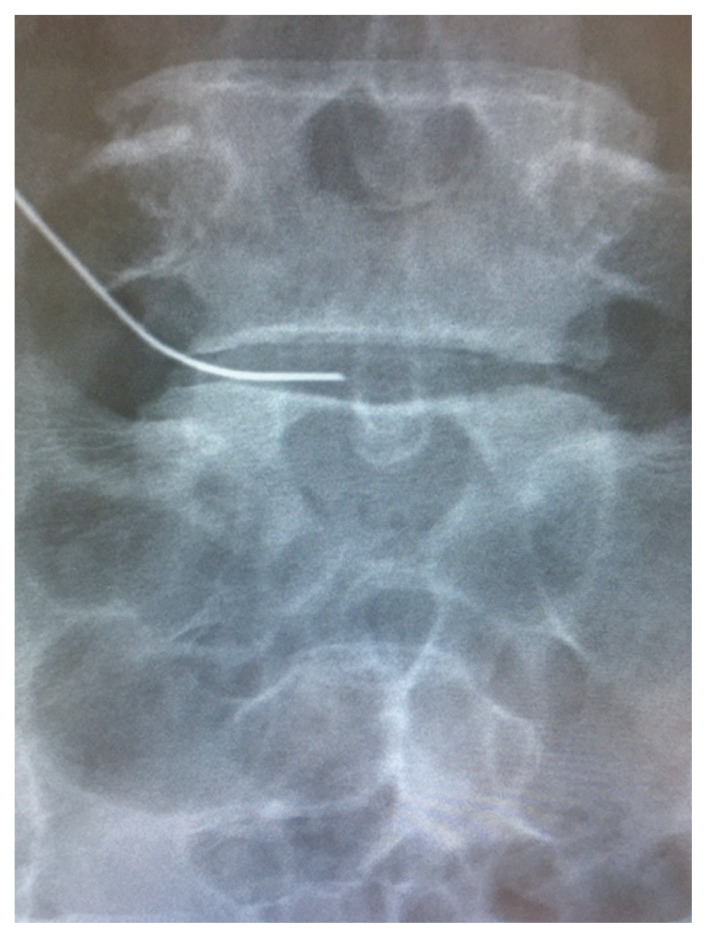
On physical examination, lower limb muscle strength did not decrease, and lower limb sensation was as normally expected; however, a straight leg-raising test was limited to 30° on the left due to pain and was positive. The Japanese Orthopedic Association (JOA) score was 13 of 29, and the NRS scores for low back pain, leg pain, and leg numbness were 0, 10, and 3, respectively. T2-weighted MRI of the lumbar spine revealed a transligamentous disc herniation protruding to the left at L5–S1 (Fig. 2C, D). The disc degeneration was Pfirrmann grade IV (Fig. 2C, D). Selective left S1 nerve root infiltration was performed, and the leg pain disappeared; however, it recurred several hours later and was resistant to conservative treatment. Because the patient had recurrent LDH and surgical risk was high, CD therapy was selected (Fig. 3). After treatment, no adverse events were observed, and the pain radiating to her left leg improved to an NRS score of 3 points 1.5 months after surgery. T2-weighted MRI of the lumbar spine 1.5 months after treatment showed that the L5–S1 disc herniation had shrunk (Fig. 2E, F), and at 6 months after treatment, the disc herniation had disappeared, indicating the disc degeneration did not progress (Fig. 2G, H). The NRS scores for low back pain, leg pain, and leg numbness were 0, 0, and 3 points, respectively, indicating significant improvement.
Fig. 3.
Fluoroscopic images of L5–S1 intradiscal condoliase injection therapy (frontal view).
Discussion
In this study, intradiscal condoliase injection was administered for primary and recurrent LDH, and NRS scores and ODI for low back pain and leg pain were recorded before treatment and at 6 months and 1 year after treatment. MRI was also performed. Intradiscal condoliase injection for primary LDH improved low back pain, leg pain, the NRS score, and the ODI. Although intradiscal condoliase injection was less effective for recurrent LDH than for primary LDH, leg pain improved significantly for up to 1 year after treatment.
Many studies have reported the effectiveness and safety of CD therapy for newly diagnosed LDH. In patients with LDH (those with protrusion and subligamentous extrusion and transligamentous extrusion type), CD therapy improves leg pain [14,19,20], low back pain, and quality of life [12].
In a previous randomized study, adverse events resulting from CD therapy (Hernicore versus placebo) included back pain (36.6% versus 33.3%), Modic type I changes (26.6% versus 17.3%), and disc height reduction of ≥30% (8.5%) [12]. However, severe complications such as death, anaphylactic shock, or neurological sequelae were not recorded.
Regarding long-term results evaluated in clinical trials, only 2% of patients needed surgery 5 years after administration [13], which is approximately 6%–24% of patients who underwent surgery for hernia removal [21–24], suggesting that LDH recurrence after CD therapy is lower than that after surgery.
Furthermore, recent studies on CD therapy have shown good results for lateral LDH [25]. Other studies have that cited that CD therapy was effective in transligamentous extrusion type of LDH and LDH showing high signal intensity on T2-weighted MRI [14,26]. In the present study, as in the previous study, CD therapy for primary LDH also improved low back pain, leg pain, and the ODI.
Few studies have reported CD therapy for patients with LDH recurrence. In a report on CD therapy for recurrent LDH, conservative treatments with oral analgesics and selective nerve root infiltration were not successful. Nevertheless, low back pain, leg pain, and leg numbness improved immediately after intradiscal condoliase injection [15], which was as effective as reoperation [27].
A study reported that half of the patients stated that their leg pain alleviated by >50%; however, improvements in low back pain and JOA scores were not significant [17]; moreover, no improvement in the visual analog scale score for leg pain was reported in the relapsed LDH group in a previous study [14]. Although we found no significant improvement in low back pain or ODI score, leg pain was significantly improved after surgery in four/eight patients (50%), and the results were good in six/eight patients (75%) 1 year after surgery.
Until now, short-term results (up to 3–6 months) on recurrent LDH have been reported [14–17], and to our knowledge, this is the first long-term follow-up study of more than 1 year on the efficacy of condoliase injection for recurrent and primary LDH.
Banno et al. [14] reported that a history of surgery at the same intervertebral level contributed to the poor results of CD therapy; however, the result was based on a short-term follow-up of 3 months in only three patients with recurrent LDH. We compared eight cases of recurrent LDH with 241 cases of primary LDH (Table 3) and found no significant difference in leg pain between the groups after 6 months (p>0.05); however, the intensity of leg pain was significantly higher in recurrent LDH than in primary LDH after 1 year (p<0.05), suggesting that CD therapy is also effective in improving leg pain in recurrent LDH, although it is less effective than in primary LDH. However, it is less effective at improving low back pain and the ODI in patients with recurrent LDH than in those with primary LDH, similar to the 6-month follow-up of six cases of recurrent LDH by Fukui et al. [17].
In the present study, as in previous studies, no adverse events from CD therapy were observed, and it appears that condoliase injection for postoperative LDH recurrence is safe [14–17]. CD therapy is also more cost-effective than surgical treatment [28] and is considered useful as a minimally invasive, safe, and cost-effective alternative to surgical or conservative treatment.
This study has several limitations. First, sufficient statistical power was lacking because of the small number of recurrent cases analyzed; thus, additional studies in a larger population are required to confirm our findings. Previous reports on recurrent LDH included a small number of cases: three cases by Banno et al. [14], one by Funayama et al. [15], eight by Nakajima et al. [16], and six by Fukui et al. [17], and they reported short-term results of 3–6 months. Thus, to our knowledge, this is the first study to compare 241 cases of primary LDH and eight cases of recurrent LDH.
Second, although the presence or absence of recurrent disc herniation regression was evaluated, a quantitative evaluation was not performed. Finally, the eight recurrent cases showed heterogeneity in the duration of relapse (range, 4–168 months) and the duration from symptom onset to treatment (range, 1–21 months). In one case (patient 7) in which the duration from the onset of symptoms to treatment was 21 months, the improvement in leg pain was poor at NRS 7 at the final observation. Thus, longer treatment durations with CD therapy may lead to worse clinical outcomes.
Conclusions
Intradiscal condoliase injection for primary LDH improved low back pain and leg pain, as measured by the NRS score and ODI. Although intradiscal condoliase injection was less effective for recurrent LDH than for primary LDH, leg pain improved significantly for up to 1 year after treatment. In addition, although intradiscal condoliase injection is less effective for LDH recurrence than for primary LDH, it improves leg pain in patients with recurrent LDH and can be considered a minimally invasive and safe treatment method.
Key Points.
Patients with primary lumbar disc herniation (LDH) showed significant improvement in low back pain, leg pain, and Oswestry Disability Index (ODI) scores at both 6 months and 1 year after intradiscal condoliase injection.
Although patients with recurrent LDH demonstrated improved leg pain, the intradiscal condoliase injection was less effective for recurrent LDH than primary LDH, with no significant improvement in low back pain or ODI scores.
The study confirmed the high safety profile of intradiscal condoliase injection, with no severe complications reported. It highlighted the treatment as a minimally invasive and cost-effective alternative to surgical intervention.
The study noted that while the injection was beneficial for leg pain in recurrent LDH, it was less effective for improving low back pain and disability scores, indicating a potential need for alternative or adjunctive treatments for recurrent cases.
Footnotes
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Author Contributions
NS conducted data collection and data entry, performed the statistical analysis, and wrote the manuscript. All authors contributed to and approved the final manuscript.
References
- 1.Lurie JD, Tosteson TD, Tosteson AN, et al. Surgical versus nonoperative treatment for lumbar disc herniation: eight-year results for the spine patient outcomes research trial. Spine (Phila Pa 1976) 2014;39:3–16. doi: 10.1097/BRS.0000000000000088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fjeld OR, Grovle L, Helgeland J, et al. Complications, reoperations, readmissions, and length of hospital stay in 34 639 surgical cases of lumbar disc herniation. Bone Joint J. 2019;101-B:470–7. doi: 10.1302/0301-620X.101B4.BJJ-2018-1184.R1. [DOI] [PubMed] [Google Scholar]
- 3.Parker SL, Mendenhall SK, Godil SS, et al. Incidence of low back pain after lumbar discectomy for herniated disc and its effect on patient-reported outcomes. Clin Orthop Relat Res. 2015;473:1988–99. doi: 10.1007/s11999-015-4193-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Swartz KR, Trost GR. Recurrent lumbar disc herniation. Neurosurg Focus. 2003;15:E10. doi: 10.3171/foc.2003.15.3.10. [DOI] [PubMed] [Google Scholar]
- 5.Yuce I, Kahyaoglu O, Cavusoglu H, Aydın Y. Surgical outcome and efficacy of lumbar microdiscectomy technique with preserving of ligamentum flavum for recurrent lumbar disc herniations. J Clin Neurosci. 2019;63:43–7. doi: 10.1016/j.jocn.2019.02.010. [DOI] [PubMed] [Google Scholar]
- 6.Dai LY, Zhou Q, Yao WF, Shen L. Recurrent lumbar disc herniation after discectomy: outcome of repeat discectomy. Surg Neurol. 2005;64:226–31. doi: 10.1016/j.surneu.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 7.Nolte MT, Basques BA, Louie PK, et al. Patients undergoing revision microdiskectomy for recurrent lumbar disk herniation experience worse clinical outcomes and more revision surgeries compared with patients undergoing a primary microdiskectomy. J Am Acad Orthop Surg. 2019;27:e796–803. doi: 10.5435/JAAOS-D-18-00366. [DOI] [PubMed] [Google Scholar]
- 8.Miwa S, Yokogawa A, Kobayashi T, et al. Risk factors of recurrent lumbar disk herniation: a single center study and review of the literature. J Spinal Disord Tech. 2015;28:E265–9. doi: 10.1097/BSD.0b013e31828215b3. [DOI] [PubMed] [Google Scholar]
- 9.Kato F, Iwata H, Mimatsu K, Miura T. Experimental chemonucleolysis with chondroitinase ABC. Clin Orthop Relat Res. 1990;253:301–8. [PubMed] [Google Scholar]
- 10.Sugimura T, Kato F, Mimatsu K, Takenaka O, Iwata H. Experimental chemonucleolysis with chondroitinase ABC in monkeys. Spine (Phila Pa 1976) 1996;21:161–5. doi: 10.1097/00007632-199601150-00001. [DOI] [PubMed] [Google Scholar]
- 11.Hamai A, Hashimoto N, Mochizuki H, et al. Two distinct chondroitin sulfate ABC lyases: an endoeliminase yielding tetrasaccharides and an exoeliminase preferentially acting on oligosaccharides. J Biol Chem. 1997;272:9123–30. doi: 10.1074/jbc.272.14.9123. [DOI] [PubMed] [Google Scholar]
- 12.Chiba K, Matsuyama Y, Seo T, Toyama Y. Condoliase for the treatment of lumbar disc herniation: a randomized controlled trial. Spine (Phila Pa 1976) 2018;43:E869–76. doi: 10.1097/BRS.0000000000002528. [DOI] [PubMed] [Google Scholar]
- 13.Matsuyama Y, Chiba K, Iwata H, Seo T, Toyama Y. A multicenter, randomized, double-blind, dose-finding study of condoliase in patients with lumbar disc herniation. J Neurosurg Spine. 2018;28:499–511. doi: 10.3171/2017.7.SPINE161327. [DOI] [PubMed] [Google Scholar]
- 14.Banno T, Hasegawa T, Yamato Y, et al. Clinical outcome of condoliase injection treatment for lumbar disc herniation: indications for condoliase therapy. J Orthop Sci. 2021;26:79–85. doi: 10.1016/j.jos.2020.02.002. [DOI] [PubMed] [Google Scholar]
- 15.Funayama T, Setojima Y, Shibao Y, et al. A case of postoperative recurrent lumbar disc herniation conservatively treated with novel intradiscal condoliase injection. Case Rep Orthop. 2022;2022:3656753. doi: 10.1155/2022/3656753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakajima H, Kubota A, Maezawa Y, et al. Short-term outcome and predictors of therapeutic effects of intradiscal condoliase injection for patients with lumbar disc herniation. Spine Surg Relat Res. 2020;5:264–71. doi: 10.22603/ssrr.2020-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fukui H, Kamei N, Fujiwara Y, et al. Intradiscal condoliase injection therapy for recurrent lumbar disc herniation: case series and literature review. Medicina (Kaunas) 2023;59:1561. doi: 10.3390/medicina59091561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pfirrmann CW, Metzdorf A, Zanetti M, Hodler J, Boos N. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine (Phila Pa 1976) 2001;26:1873–8. doi: 10.1097/00007632-200109010-00011. [DOI] [PubMed] [Google Scholar]
- 19.Okada E, Suzuki S, Nori S, et al. The effectiveness of chemonucleolysis with condoliase for treatment of painful lumbar disc herniation. J Orthop Sci. 2021;26:548–54. doi: 10.1016/j.jos.2020.06.004. [DOI] [PubMed] [Google Scholar]
- 20.Hirai T, Takahashi T, Tanaka T, et al. Intradiscal injection with condoliase (chondroitin sulfate ABC endolyase) for painful radiculopathy caused by lumbar disc herniation. Spine Surg Relat Res. 2021;6:252–60. doi: 10.22603/ssrr.2021-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yorimitsu E, Chiba K, Toyama Y, Hirabayashi K. Long-term outcomes of standard discectomy for lumbar disc herniation: a follow-up study of more than 10 years. Spine (Phila Pa 1976) 2001;26:652–7. doi: 10.1097/00007632-200103150-00019. [DOI] [PubMed] [Google Scholar]
- 22.Vik A, Zwart JA, Hulleberg G, Nygaard OP. Eight year outcome after surgery for lumbar disc herniation: a comparison of reoperated and not reoperated patients. Acta Neurochir (Wien) 2001;143:607–11. doi: 10.1007/s007010170066. [DOI] [PubMed] [Google Scholar]
- 23.Davis RA. A long-term outcome analysis of 984 surgically treated herniated lumbar discs. J Neurosurg. 1994;80:415–21. doi: 10.3171/jns.1994.80.3.0415. [DOI] [PubMed] [Google Scholar]
- 24.Lewis PJ, Weir BK, Broad RW, Grace MG. Long-term prospective study of lumbosacral discectomy. J Neurosurg. 1987;67:49–53. doi: 10.3171/jns.1987.67.1.0049. [DOI] [PubMed] [Google Scholar]
- 25.Funayama T, Mataki K, Murakami K, et al. Two cases of lateral lumbar disc herniation successfully treated with intradiscal condoliase injection. Spine Surg Relat Res. 2020;5:437–41. doi: 10.22603/ssrr.2020-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ishibashi K, Fujita M, Takano Y, Iwai H, Inanami H, Koga H. Chemonucleolysis with chondroitin sulfate ABC endolyase for treating lumbar disc herniation: exploration of prognostic factors for good or poor clinical outcomes. Medicina (Kaunas) 2020;56:627. doi: 10.3390/medicina56110627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yao Y, Zhang H, Wu J, et al. Comparison of three minimally invasive spine surgery methods for revision surgery for recurrent herniation after percutaneous endoscopic lumbar discectomy. World Neurosurg. 2017;100:641–7. doi: 10.1016/j.wneu.2017.01.089. [DOI] [PubMed] [Google Scholar]
- 28.Takaki S, Miyama H, Iwasaki M. Cost-effectiveness analysis of intradiscal condoliase injection vs. surgical or conservative treatment for lumbar disc herniation. J Med Econ. 2023;26:233–42. doi: 10.1080/13696998.2023.2173465. [DOI] [PubMed] [Google Scholar]