Abstract
Multiple sclerosis (MS) is a devastating immune-mediated disorder of the central nervous system resulting in progressive disability accumulation. As there is no cure available yet for MS, the primary therapeutic objective is to reduce relapses and to slow down disability progression as early as possible during the disease to maintain and/or improve health-related quality of life. However, optimizing treatment for people with MS (pwMS) is complex and challenging due to the many factors involved and in particular, the high degree of clinical and sub-clinical heterogeneity in disease progression among pwMS. In this paper, we discuss these many different challenges complicating treatment optimization for pwMS as well as how a shift towards a more pro-active, data-driven and personalized medicine approach could potentially improve patient outcomes for pwMS. We describe how the ‘Clinical Impact through AI-assisted MS Care’ (CLAIMS) project serves as a recent example of how to realize such a shift towards personalized treatment optimization for pwMS through the development of a platform that offers a holistic view of all relevant patient data and biomarkers, and then using this data to enable AI-supported prognostic modelling.
Keywords: multiple sclerosis, personalized medicine, disease progression, prognosis, diagnosis, AI, data
1. The heterogeneous disease course of multiple sclerosis
Multiple sclerosis (MS) is a devastating immune-mediated disorder of the central nervous system (CNS) resulting in progressive disability accumulation in most individuals affected (1, 2). MS imposes a significant burden on patients, affecting all aspects of their life, and additionally, it poses a significant challenge to society as with growing disability, indirect expenses (productivity losses associated with sick absence, inability to work, and early retirement) and care costs rise substantially (3).
The classical view on MS describes different clinical subtypes, with relapsing-remitting MS (RRMS) being the most common form, occurring in 85% of patients (National MS Society). Patients with RRMS experience neurological exacerbation (relapses) as well as intermittent periods of remission in which they remain clinically stable. Relapses can either recover completely or leave persistent clinical disability, referred to as Relapse Associated Worsening (RAW). Among these patients, approximately two-thirds progress to secondary-progressive MS (SPMS) (4). In contrast to RRMS, the disease course of patients with SPMS or primary-progressive MS (PPMS, 15% of MS patients) is mainly driven by a gradual worsening of disability in the absence of relapse activity (5).
Recent research has challenged this classical view of distinct MS subtypes, as they may not sufficiently account for the large spectrum of multifaceted clinical phenotypes and disease courses as well as sub-clinical disease variability (6). This disease heterogeneity is further complicated by a high prevalence of comorbidities and multi-pharmacy in MS. Data from the NARCOMS registry suggested that, at the time of MS diagnosis, 35% of MS patients suffer physical comorbidities while 18% reported a psychiatric comorbidity (7, 8). Additionally, accumulation of clinical disability independent of acute inflammatory relapses - commonly referred to as Progression Independent of Relapse Activity (PIRA) (9) - was found to occur in any of the classical MS subtypes, including RRMS, and at any stage of the disease (10, 11). Most importantly, in a substantial proportion of people with MS (pwMS), PIRA occurs already very early on, and this is associated with worse long-term outcomes (2). Recent studies have also shown that PIRA gradually becomes the dominant driver of disability worsening as the disease progresses (9).
While new insights into PIRA continue to be unraveled, exact criteria of how to define, assess, and monitor PIRA are still lacking. Several definitions have been put forward, but these focus mainly only on measuring disability worsening by means of the Expanded Disability Status Scale (EDSS) and Confirmed Disability Worsening (CDW) (2). Relying solely on EDSS or CDW to describe PIRA, however, seems to be insufficient as (i) there are heterogeneous symptoms and disease aspects contributing to disability worsening and MS severity, and (ii) this omits sub-clinical processes such as compartmentalized inflammation, chronically active (smouldering) lesions, diffuse normal-appearing matter damage (12, 13), as well as brain (14) and spinal cord atrophy (15, 16). Such processes seem to represent relevant substrates of (silent/smouldering) disease progression even during early stages and to contribute to enhanced long-term disability worsening in pwMS (17). In this regard, the topographical disease model proposed by Krieger et al. may facilitate the interpretation of the clinical course revision, providing a unified visualization across phenotypes, while providing insights in the interplay between the distinct processes of relapse activity and progression, and accounting for latent variables such as relapse localization, frequency, severity, recovery and progression rate (18). Additionally, this model was recently validated in terms of brain MRI markers (19). Aligning with this model, individuals deemed neurologically normal in early MS (e.g., with an EDSS score of 0) demonstrated subtle deficits in high-challenging motor tasks (20) and often have fatigue (21) and cognitive impairments (22). The former was also shown to correlate with imaging markers of disease burden and brain reserve, challenging traditional severity definitions and underscoring the importance of looking beyond standard clinical measures such as the EDSS (20).
2. A changing landscape in treatment strategies
The heterogeneity in disease progression among individuals with MS (both clinically and sub-clinically) contributes to a high diversity in treatment responses across pwMS (23). As there is no cure available yet for MS, the primary therapeutic objective is to slow down disability progression and to reduce relapses as early as possible during the disease to maintain and/or improve the health-related quality of life (24).
To this end, all regulatory-approved disease-modifying treatments (DMT) have shown their worth in preventing relapses during the few years of the clinical trial in which their efficacy was evaluated. However, the impact on the long-term accumulation of disability and chronic subtle disease processes was often limited as even the most effective DMTs available were only able to mitigate the short-term risk of disability progression by 30-42% (25). A recent review from Gasperini et al. emphasizes how dire the situation really is, indicating that only 30- 40% of patients receiving a DMT remain stable over a period of 5 to 7 years, and only up to 10% over a period of 7 to 10 years after initiating DMT (26).
Despite the approval of ±20 different DMTs by the European Medicines Agency (EMA) and the US Food and Drug Administration (FDA) (27, 28), concerns about side effects and efficacy might discourage many pwMS from initiating a high-efficacy DMT therapy (29, 30), an issue further aggravated by therapeutic inertia (31). Additionally, those who do receive a DMT usually start with one of the less effective but well-established therapies due to their minimal side effects (32). Traditionally, it’s only when these well-established DMTs fail to prevent relapses and disability progression, that the treatment is escalated to a higher-efficacy treatment, which usually is more expensive, might have more pronounced side effects, and is potentially more challenging to administer (oral and injectables versus infusions) (33). However, multiple studies support the observation that reducing the accrual of neurological damage in the initial stages of the disease potentially improves overall clinical outcomes throughout the patient’s lifespan when employing early intervention with higher efficacy DMT (34–38). Additionally, DMTs were shown to be more efficacious, and side effects less likely to occur in younger patients (39). Taken together, these studies question the traditional treatment escalation paradigm which is therefore nowadays considered outdated by most physicians. Instead, current thinking emphasizes the potential advantages of early initiation of high-efficacy DMTs, indicating the need for and the significance of an early MS diagnosis, proactive monitoring to detect disease activity early, and shared decision-making as crucial elements in patient care (32, 40).
Additionally, given the shortcomings of current DMTs to halt long-term disability accumulation, a next generation of DMTs might focus more on the silent progression of the disease. A first novel category of DMTs in this regard are potentially the Bruton tyrosine kinase inhibitors. This new class of drugs might become the first to target both acute inflammatory relapses as well chronic inflammatory processes in the CNS thought to drive disability accumulation (41). In this context, especially the early recognition of individuals prone to developing PIRA will be essential. A better understanding of PIRA and RAW as well as their interplay, combined with data-driven prognosis, will enhance the selection of current and future DMTs and allow to treat patients beyond just relapse activity. Nevertheless, certain variables pose challenges to the trajectory of precision medicine and treatment optimization on an individual level. While there are guidelines on the use of DMTs in MS (24), these are all based on expert judgment and differ across countries, even within the EU (28, 37). This variance extends to therapy selection post-diagnosis or during follow-ups, driven by perceived levels of clinical and subclinical disease activity and progression.
3. Precision medicine enables treatment optimization
Accumulating evidence suggests that the reactive treatment of lesion activity is insufficient, negatively impacting long-term patient outcomes (42). In the complex landscape of MS treatment, an increasing acknowledgment of disease heterogeneity and underlying disease mechanisms underscores the imperative for a paradigm shift toward proactive, data-driven precision medicine (43). However, despite its promise, such data-driven approaches come hand in hand with substantial challenges.
The understanding of the complex and heterogeneous underlying neuropathology of MS is still limited. The adoption of precision medicine in MS is further complicated by the chronic nature of the disease, exhibiting variable courses over time. Consequently, given the longitudinal disease aspect, one must account for the fact that data might be incomplete at times, particularly in routine practice. In addition, the influence of comorbidities adds another layer of complexity (44). Various biomarkers are deemed relevant for their role in identifying diverse MS aspects and patterns of progression in MS, aiding diagnosis, prognosis, and treatment selection (45). However, they might not capture the full complexity of MS and their interpretation requires a nuanced understanding of the disease context. Moreover, the heterogeneous nature of MS challenges the development of universally applicable biomarkers and complicates the tracking of different treatment effects on an individual basis (46).
Notably, with a variety of treatment options being available (27, 28), emerging biomarkers, including liquid and imaging markers, have shown potential in monitoring treatment efficacy (45, 47). However, the validation, availability, and implementation of biomarker assessments in real-world clinical practice is often still missing as this differs significantly from their application in clinical trials. Moreover, biomarkers that demonstrate both sensitivity and specificity in the context of progressive MS are still lacking (47). While early diagnosis and prognosis modelling are pivotal for timely and effective treatment initiation, the ability to clearly define and disentangle disability accumulation attributed to RAW or PIRA will be key to optimizing individual treatment over the course of the disease.
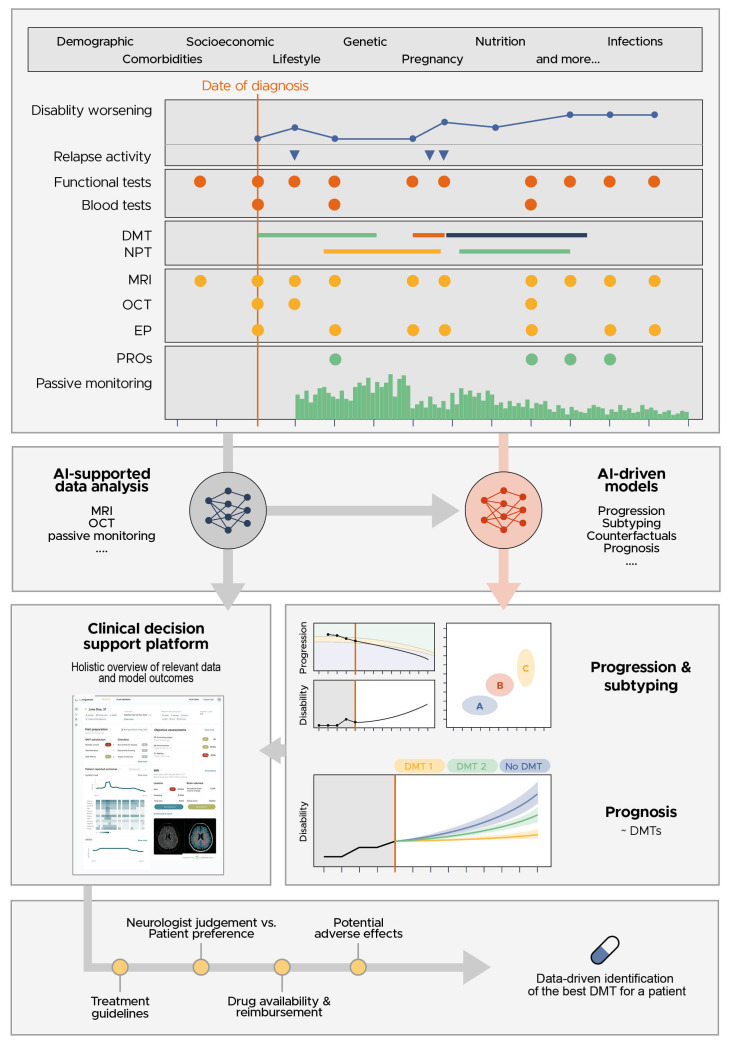
Advancements in artificial intelligence (AI) can offer enhanced and data-driven support by considering longitudinal data on multiple biomarkers simultaneously and subtyping patients more accurately. In particular, this can include biomarkers more related to PIRA such as motor dysfunction beyond EDSS (2, 48), optical coherence tomography (49–51), magnetic resonance imaging markers predictive of disability worsening such as brain atrophy (14), slowly expanding lesions and paramagnetic rim lesions (52–54) and cognitive impairment (55–57), as well as subjective markers [i.e. patient-reported outcomes (PROs) such as quality of live (58, 59)]. We believe that a holistic overview of the patient will be crucial to avoid overlooking relevant information, including both existing and new biomarkers as our disease understanding evolves further ( Figure 1 ).
Figure 1.
A clinical decision support tool should be capable of visualizing the very heterogenous MS patient data, the AI-supported analysis of this data and the outcome of prognostic models using this data, enabling a data-driven discussion between the neurologist and patient to identify the best DMT for the patient.
Such transformative approaches hold the potential to significantly enhance treatment strategies and extend the adjusted quality of life years for individuals with MS. Nevertheless, the current landscape is still fragmented, often focusing on singular aspects or biomarkers rather than adopting a more holistic and comprehensive approach. Data strategies to reduce the level of heterogeneity, particularly improving data harmonization by means of a common data model, are wishful to guarantee standardization in clinical decision making (60). However, the implementation of such initiatives is still in the early stages. Care pathways for pwMS are also not commonly standardized and while some diagnostic and treatment guidelines and recommendations are available (1, 61, 62), the assessment of relevant outcomes may not always be sufficiently covered and integrated into the routine clinical workflow (63). A modular-integrative framework of digital patient pathways for MS management and treatment is needed, which should incorporate AI, data harmonization and review relevant research concerning the use of pathways in healthcare (64, 65). Although initial evidence of acting upon AI-driven MRI biomarkers has indicated to improve patient outcome (66), the evaluation of impact in real-world practice and evidence on whether acting upon data-driven models and biomarkers truly improves the quality of life for patients with MS are crucial components that demand more attention in the pursuit of effective precision medicine strategies for MS.
4. Clinical impact through AI-assisted MS care
A data-driven and personalized clinical decision support tool is urgently needed for MS, to prevent and slow down disease progression more efficiently via optimizing treatment. The EU-funded ‘Clinical Impact through AI-assisted MS Care’ (CLAIMS, www.claims.ms) project aims to address this need. The project will develop, validate and seek regulatory approval for an AI-driven clinical decision-support platform, which offers the MS care team a holistic view of the patient through the visualization of all relevant patient data and the prognosis on the expected disease trajectories under different treatment regimens.
Initially, the project focusses on the development and optimization of these prognostic models via the use of retrospectively collected clinical routine data in combination with clinical trial data. A detailed description of this retrospective multi-center observational study (called RECLAIM) is accessible via ClincialTrials.gov. This study aims to collect and harmonize both clinical and subclinical data and store it in a central database on a secure cloud environment. Data harmonization will be following the common data model proposed in Parciak et al. (67), but kept to the minimum necessary as we aim to stay as close as possible to the real-world clinical setting and to ensure the clinical relevance.
The combination of real-world with clinical trial data is an important aspect of the study. Clinical trial data is very homogeneous and highly curated, making it an ideal dataset to develop AI-driven prognostic models. For instance, MRI scans obtained in clinical trials adhere to a standardized protocol, include all necessary sequences, and ensure follow-up scans within a specific timeframe. In contrast, MRI scans acquired in a real-world setting frequently don’t meet these requirements (68, 69). As the CLAIMS project aims to create AI-based prediction models applicable in real-world clinical settings, it is crucial to also incorporate routine care data in the development and validation phases. By combining both types of data, we aim to achieve an extensive dataset that leverages the strengths of both types of data ensuring applicability in a routine clinical care setting where confounding factors (e.g., comorbidities), low quality data and missing data are common (70, 71).
The focus will be on modelling disease progression. Disease progression models often have strong assumptions about the monotonicity of disease progression processes, the missingness model and associated completeness of the data, the longitudinal regularity of the observations, and homoscedastic noise characteristics of the measurements. Due to the different MS subtypes, and relapse and recurrence events, many of these assumptions do not hold in a MS setting. Furthermore, when using clinical observational data, data points are missing-not-at-random, both because patients often miss their appointments, but also because certain examinations (clinical assessments, MRI, etc) are performed as a function of patient presentation. Tackling this requires us to explore applicability of advanced and appropriate models of data imputation, and from generative models that explicitly model the causal relationships of the observations.
Contrary to clinical research trials where patients are assigned to a treatment or placebo arm at random, in an observational setting, DMTs are given to patients according to guideline recommendations and patient presentation. Observational data is thus biased by these guidelines, and appropriate measures are needed to control for this bias. Causal inference mechanisms via counterfactuals allows one to model such observational data and predict what the potential outcome would have been under a counterfactual treatment. By disentangling causes and effects, one gains a clearer understanding of the underlying biological or pathological markers that are predictive of the observed effect and outcome. This enables a more grounded clustering of patients (e.g., what are the patient characteristics that predict drug efficacy), providing an explanation of the optimal therapeutic inference (e.g., what is the biological reason why a certain drug is optimal for a specific patient). While some of these challenges have been addressed in highly controlled randomized clinical research environments, solving them using an observational experimental setup would allow one to exploit large amounts of data while ensuring the models remain accurate when deployed in a real-world environment where the aforementioned problems exist. Observational studies using real-world data allow for more heterogeneous and comprehensive cohorts, thereby elevating external validity and supplying valuable insights to guide treatment approaches (69).
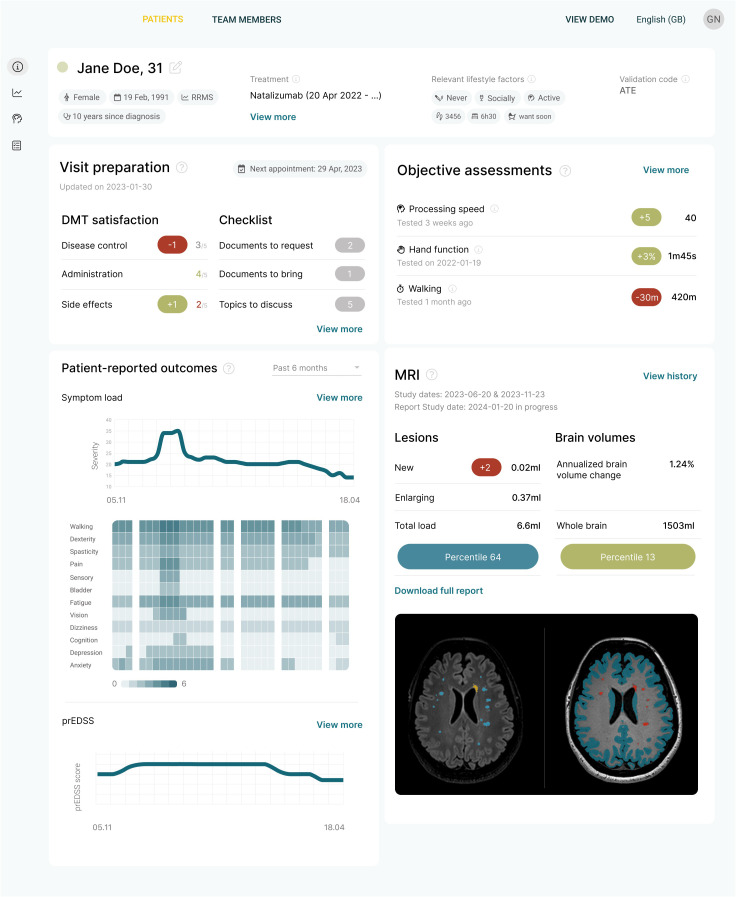
At the time of writing this paper, the first version of the CLAIMS platform was already available, building upon a regulatory cleared AI solution for brain MRI quantification, a patient app for pwMS and a regulatory cleared AI solution for optical coherence tomography (OCT) quantification (72–75), but without the prognostic models ( Figure 2 ). The complete clinical decision support platform, including the prognostic models, will be included in prospective clinical trial (called PROCLAIM), designed to obtain regulatory approval, and bringing it to the market as soon as possible. Meanwhile, the platform will be iteratively improved as new biomarker data becomes available and models are further refined. This iterative approach ensures that the CLAIMS project achieves true clinical impact for patients sooner rather than later.
Figure 2.
The first iteration of the clinical decision support platform being developed in the CLAIMS project. It offers a concise overview of the most important data for making a clinical decision.
5. Digital health and how this support prognosis
The CLAIMS project is exploring an additional avenue for the identification of promising markers of disease progression by capturing digital biomarkers using digital health tools. A first set of digital health tools includes AI solutions tailored for the quantification of brain MRI scans (74, 75). Notable advancement of these tools’ accuracy, in combination with rigorous technological, workflow, clinical and even initial health economic validation makes that this solution steadily gains recognition as standard of care. In the United States, this trend towards embracing AI-based brain MRI quantification is further exemplified by the recent provision of two new Current Procedural Terminology (CPT) codes. Evidence has shown that by using such a solution, disease activity can be detected up to 3 years earlier with a potentially significant impact on treatment decisions (66).
Patient apps, another major trend in the digital health tools, could enhance the early detection of disease progression in pwMS and allow monitoring disease progression in between visits with their treating physician. This can be achieved by monitoring symptoms and disability progression through capturing patient-reported outcomes (PROs), through passive monitoring of various markers (activity, sleep, vital signs, …) or through the digital administration of tests assessing for example cognition, vision, mobility, etc. (76, 77). In addition, these tools can play an important role in increasing and monitoring medication adherence, improving a patient’s lifestyle through creating awareness, and to educate and empower patients in managing their disease better. As such, disease monitoring via digital health tools provides a dynamic, more continuous, and more nuanced understanding of disease progression.
Development of such tools poses a socio-technical challenge. Any tool which aims to obtain regulatory clearance for use in a clinical setting will need to obtain sufficient technical and clinical evidence, which is often a long and laborious process. A bigger challenge, however, is patient adoption and thereafter adherence in using the tools. Concerns on data security and privacy need to be adequately addressed and simultaneously, it needs to be very clear to patients that they will benefit from enhanced care and personalized interventions driven by a more holistic understanding and monitoring of their health status and disease progression. CLAIMS aims to address this by empowering and educating patients on the need to better monitor their disease. In this light, the patient app used in CLAIMS is positioned as a companion app, available to support the patient as needed, focusing on topics of interest to the patient, rather than mandating the app usage. Actively involving patients and capturing their feedback on the app utilization, whether via real-world usage or within a clinical study setting, will contribute valuable insights, allowing to further refine the tools and ultimately, the clinical decision support platform.
Besides patient adherence, integration into routine clinical workflows poses another challenge. To address this, the clinical decision support platform in CLAIMS aims to keep the steps of platform adoption to a bare minimum. It aggregates all of a patient’s data, including data from the patient app, from the AI-driven MRI analysis and from the AI-driven OCT-analysis. While the full datasets and analyses will be available via this platform, the main dashboard focusses on providing a holistic overview of all clinically actionable measures and markers. While this is rather straightforward for subjective and episodic data such as with questionnaires or simple tests captured via the patient app, this will be harder to achieve for data from passive monitoring. The latter is known to generate large longitudinal datasets where AI algorithms are needed to identify subtle patterns and disease subtypes, and to predict trajectories.
Patient-reported outcomes (PROs) represent a unique occasion to involve patients using digital health tools and measure the impact of health care on outcomes that hold utmost significance to pwMS. However, the variety of PRO measures available and the absence of standards across different healthcare centers and countries present a considerable challenge (58). The recently established initiative ‘Patient-Reported Outcomes for Multiple Sclerosis’ (PROMS), consisting of an interdisciplinary, international network of different stakeholders, addresses the challenge of creating PRO measures that meet the diverse needs of all parties involved to enhance the influence of both scientific research and patient perspectives on the lives of pwMS (59). In this context, digital health tools enable meaningful assessments, but patient satisfaction can influence assessment compliance and indirectly affect outcome measures. To assess patient satisfaction with digital tools, patient-reported and expert-reported experience measures (PREM) should be collected in parallel (78).
6. The road ahead
As our understanding of MS increases, it becomes evident that we should go beyond making treatment decisions solely based on relapses, EDSS progression and lesion activity and move towards proactively treating pwMS for the best possible prognostic outcome. A focus on maintaining/improving health-related quality of life and slowing down disease progression and disability worsening - also independent of relapse activity - has sprouted a clear need for data-driven and personalized clinical decision support tools in MS. Such tools are crucial to administer the right drug to the right patient at the right time to preserve long-term neurological function while minimizing side effects. However, such solutions require well validated biomarkers and models that clearly link to the specificity of the disease course and outcome at individual patient level and can be easily implemented along the clinical care path of the patient.
The CLAIMS project aims to develop such a data-driven and personalized clinical decision support tool while addressing the posed challenges. Biomarker validation and model building will be performed in the retrospective RECLAIM study using both real world data and data from clinical trials. Subsequently, the prospective PROCLAIM study will evaluate the envisioned platform in daily clinical routine, evaluating feasibility and impact on patient care pathways and patient outcome. As such the project will generate a platform for daily clinical routine that provides a holistic view of each patient including existing and novel biomarker assessments to better monitor relapse related disability worsening and progression independent of relapse activity. Driven by deep-learning-based disease subtyping and progression models, the platform will allow the estimation of individual disease trajectories and as such contribute to the urgent need of a more pro-active and data-driven precision medicine in MS care.
Funding Statement
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The CLAIMS project is supported by the Innovative Health Initiative Joint Undertaking (JU) under grant agreement No 101112153. The JU receives support from the European Union’s Horizon Europe research and innovation program and COCIR, EFPIA, EuropaBio, MedTech Europe, Vaccines Europe, AB Science SA and Icometrix NV. This work was partially supported by an ITEA grant (20030 HeKDisco, HBC.2021.0500) from Flanders Innovation and Entrepreneurship. DH has received support from the Charles University Cooperation Program in Neuroscience, from the National Institute for Neurological Research (Program EXCELES, ID Project No. LX22NPO5107), from the European Union –Next Generation EU, and from the General University Hospital in Prague (project MH CZ-RVO-VFN64165).
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
JP: Conceptualization, Funding acquisition, Project administration, Visualization, Writing – original draft, Writing – review & editing. LA: Conceptualization, Project administration, Writing – original draft, Writing – review & editing. GC: Conceptualization, Writing – review & editing. DH: Conceptualization, Writing – review & editing. TZ: Conceptualization, Writing – review & editing. PV: Conceptualization, Writing – review & editing. CL: Conceptualization, Writing – review & editing. KV: Conceptualization, Writing – review & editing. MS: Conceptualization, Writing – review & editing. CA: Conceptualization, Funding acquisition, Project administration, Writing – review & editing. EK: Conceptualization, Writing – review & editing. AB: Conceptualization, Writing – review & editing. JV: Conceptualization, Writing – review & editing. ED: Conceptualization, Writing – review & editing. VZ: Conceptualization, Writing – review & editing. DS: Conceptualization, Funding acquisition, Writing – review & editing. AR: Conceptualization, Funding acquisition, Project administration, Visualization, Writing – original draft, Writing – review & editing. FP: Conceptualization, Funding acquisition, Project administration, Visualization, Writing – original draft, Writing – review & editing.
Conflict of interest
JP is a shareholder of icometrix NV. AR is a shareholder of icometrix NV. DS is a shareholder of icometrix NV. MS has no relevant or material financial interests that relate to the research described in this paper. However, she is employed as Operational Director of The European Charcot Foundation. TZ reports scientific advisory board and/or consulting for Biogen, Roche, Novartis, Celgene, and Merck; compensation for serving on speaker’s bureaus for Roche, Novartis, Merck, Sanofi, Celgene, and Biogen; and research support from Biogen, Novartis, Merck, and Sanofi. VZ is a shareholder of F. Hoffmann-La Roche Ltd PV reports honorarium, contributions to meeting from Biogen, Sanofi-Genzyme, Novartis, Teva, Merck, Roche, Imcyse, AB Science, Janssen, Ad Scientiam and BMS-Celgene. Research support from Novartis, Sanofi-Genzyme and Merck. EK is a shareholder of Nocturne GmbH. DH received compensation for travel, speaker honoraria and consultant fees from Biogen Idec, Novartis, Merck, Bayer, Sanofi Genzyme, Roche, and Teva, as well as support for research activities from Biogen Idec. JR is a shareholder of Imcyse SA. GC has received consulting and speaking fees from Novartis, Sanofi, Janssen, Bristol Myers Squibb, Roche and Rewind. FP provided research support to Neurosciences Clinical Research Center, German Ministry for Education and Research (BMBF), Deutsche Forschungsgemeinschaft (DFG), Einstein Foundation, Guthy Jackson Charitable Foundation, EU FP7 Framework Program, Biogen, Genzyme, Merck Serono, Novartis, Bayer, Roche, Parexel and Almirall, received honoraria for lectures, presentations, speakers from Guthy Jackson Foundation, Bayer, Biogen, Merck Serono, Sanofi Genzyme, Novartis, Viela Bio, Roche, UCB, Mitsubishi Tanabe and Celgene, in addition, received compensation for serving on a scientific advisory board of Celgene, Roche, UCB and Merck, and is an Academic Editor of PLos One and Associate Editor of Neurology® Neuroimmunology & Neuroinflammation, all unrelated to this work.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, Comi G, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. (2018) 17:162–73. doi: 10.1016/s1474-4422(17)30470-2 [DOI] [PubMed] [Google Scholar]
- 2. Tur C, Carbonell-Mirabent P, Cobo-Calvo A, Otero-Romero S, Arrambide G, Midaglia L, et al. Association of early progression independent of relapse activity with long-term disability after a first demyelinating event in multiple sclerosis. JAMA Neurol. (2023) 80:151. doi: 10.1001/jamaneurol.2022.4655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kobelt G, Berg J, Lindgren P, Jönsson B. Costs and quality of life in multiple sclerosis in Europe: method of assessment and analysis. Eur J Health Econ. (2006) 7:5–13. doi: 10.1007/s10198-006-0365-y [DOI] [PubMed] [Google Scholar]
- 4. Tutuncu M, Tang J, Zeid NA, Kale N, Crusan DJ, Atkinson EJ, et al. Onset of progressive phase is an age-dependent clinical milestone in multiple sclerosis. Multiple Sclerosis. (2013) 19:188–98. doi: 10.1177/1352458512451510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lublin FD, Reingold SC, Cohen JA, Cutter GR, Sørensen PS, Thompson AJ, et al. Defining the clinical course of multiple sclerosis. Neurology. (2014) 83:278–86. doi: 10.1212/wnl.0000000000000560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kuhlmann T, Moccia M, Coetzee T, Cohen JA, Correale J, Graves J, et al. Multiple sclerosis progression: time for a new mechanism-driven framework. Lancet Neurol. (2023) 22:78–88. doi: 10.1016/s1474-4422(22)00289-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Marrie RA, Horwitz RI, Cutter G, Tyry T, Vollmer T. Association between comorbidity and clinical characteristics of MS. Acta Neurol Scand. (2011) 124:135–41. doi: 10.1111/j.1600-0404.2010.01436.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Marrie RA, Cohen JA, Stuve O, Trojano M, Sørensen PS, Reingold S, et al. A systematic review of the incidence and prevalence of comorbidity in multiple sclerosis: Overview. Multiple Sclerosis. (2015) 21:263–81. doi: 10.1177/1352458514564491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lublin FD, Häring DA, Ganjgahi H, Ocampo A, Hatami F, Čuklina E, et al. How patients with multiple sclerosis acquire disability. Brain. (2022) 145:3147–61. doi: 10.1093/brain/awac016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kappos L, Wolinsky JS, Giovannoni G, Arnold DL, Wang Q, Bernasconi C, et al. Contribution of relapse-independent progression vs relapse-associated worsening to overall confirmed disability accumulation in typical relapsing multiple sclerosis in a pooled analysis of 2 randomized clinical trials. JAMA Neurol. (2020) 77:1132. doi: 10.1001/jamaneurol.2020.1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Portaccio E, Bellinvia A, Fonderico M, Pastò L, Razzolini L, Totaro R, et al. Progression is independent of relapse activity in early multiple sclerosis: a real-life cohort study. Brain. (2022) 145:2796–805. doi: 10.1093/brain/awac111 [DOI] [PubMed] [Google Scholar]
- 12. Lassmann H. Targets of therapy in progressive MS. Multiple Sclerosis. (2017) 23:1593–9. doi: 10.1177/1352458517729455 [DOI] [PubMed] [Google Scholar]
- 13. Lassmann H. Pathogenic mechanisms associated with different clinical courses of multiple sclerosis. Front Immunol. (2019) 9:3116. doi: 10.3389/fimmu.2018.03116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cagol A, Schaedelin S, Barakovic M, Benkert P, Todea RA, Rahmanzadeh R, et al. Association of brain atrophy with disease progression independent of relapse activity in patients with relapsing multiple sclerosis. JAMA Neurol. (2022) 79:682. doi: 10.1001/jamaneurol.2022.1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bischof A, Papinutto N, Keshavan A, Rajesh A, Kirkish G, Zhang X, et al. Spinal cord atrophy predicts progressive disease in relapsing multiple sclerosis. Ann Neurol. (2022) 91:268–81. doi: 10.1002/ana.26281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cagol A, Benkert P, Melie-Garcia L, Schaedelin SA, Leber S, Tsagkas C, et al. Association of spinal cord atrophy and brain paramagnetic rim lesions with progression independent of relapse activity in people with MS. Neurology. (2024) 102:e207768. doi: 10.1212/wnl.0000000000207768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. UCA San Francisco MS-EPIC Team. Bruce ACC, Hollenbach JA, Bove R, Kirkish G, Sacco S, et al. Silent progression in disease activity–free relapsing multiple sclerosis. Ann Neurol. (2019) 85:653–66. doi: 10.1002/ana.25463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Krieger SC, Cook K, De Nino S, Fletcher M. The topographical model of multiple sclerosis. Neurology® Neuroimmunol Neuroinflamm. (2016) 3:e279. doi: 10.1212/nxi.0000000000000279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Krieger SC, Billiet T, Maes C, Barros N, Ribbens A, Wang C, et al. MSMilan2023 – paper poster - session 1' (2023). Multiple Sclerosis. (2023) 29:137–393. doi: 10.1177/13524585231196192 [DOI] [Google Scholar]
- 20. Krieger SC, Antoine A, Sumowski JF. EDSS 0 is not normal: Multiple sclerosis disease burden below the clinical threshold. Multiple Sclerosis. (2022) 28:2299–303. doi: 10.1177/13524585221108297 [DOI] [PubMed] [Google Scholar]
- 21. Runia TF, Jafari N, Siepman DAM, Hintzen RQ. Fatigue at time of CIS is an independent predictor of a subsequent diagnosis of multiple sclerosis. J Neurol Neurosurg Psychiatry. (2015) 86:543–6. doi: 10.1136/jnnp-2014-308374 [DOI] [PubMed] [Google Scholar]
- 22. Paul F. Pathology and MRI: exploring cognitive impairment in MS. Acta Neurol Scand. (2016) 134:24–33. doi: 10.1111/ane.12649 [DOI] [PubMed] [Google Scholar]
- 23. Kalincik T, Manouchehrinia A, Sobisek L, Jokubaitis V, Spelman T, Horakova D, et al. Towards personalized therapy for multiple sclerosis: prediction of individual treatment response. Brain. (2017) 140:2426–43. doi: 10.1093/brain/awx185 [DOI] [PubMed] [Google Scholar]
- 24. Montalban X, Gold R, Thompson AJ, Otero-Romero S, Amato MP, Chandraratna D, et al. ECTRIMS/EAN Guideline on the pharmacological treatment of people with multiple sclerosis. Multiple Sclerosis J. (2018) 24:96–120. doi: 10.1177/1352458517751049 [DOI] [PubMed] [Google Scholar]
- 25. Wingerchuk DM, Carter JL. Multiple sclerosis: current and emerging disease-modifying therapies and treatment strategies. Mayo Clin Proc. (2014) 89:225–40. doi: 10.1016/j.mayocp.2013.11.002 [DOI] [PubMed] [Google Scholar]
- 26. Gasperini C, Prosperini L, Tintoré M, Sormani MP, Filippi M, Rio J, et al. Unraveling treatment response in multiple sclerosis. Neurology. (2019) 92:180–92. doi: 10.1212/wnl.0000000000006810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Amin M, Hersh CM. Updates and advances in multiple sclerosis neurotherapeutics. Neurodegenerative Dis Manage. (2023) 13:47–70. doi: 10.2217/nmt-2021-0058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bayas A, Christ M, Faissner S, Klehmet J, Pul R, Skripuletz T, et al. Disease-modifying therapies for relapsing/active secondary progressive multiple sclerosis – a review of population-specific evidence from randomized clinical trials. Ther Adv Neurol Disord. (2023) 16:175628642211468. doi: 10.1177/17562864221146836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Visser LH, Van Der Zande A. Reasons patients give to use or not to use immunomodulating agents for multiple sclerosis. Eur J Neurol. (2011) 18:1343–9. doi: 10.1111/j.1468-1331.2011.03411.x [DOI] [PubMed] [Google Scholar]
- 30. Jokubaitis VG, Spelman T, Lechner-Scott J, Barnett M, Shaw C, Vucic S, et al. The Australian Multiple Sclerosis (MS) Immunotherapy Study: A prospective, multicentre study of drug utilisation using the MSBase platform. PloS One. (2013) 8:e59694. doi: 10.1371/journal.pone.0059694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Saposnik G, Andhavarapu S, de la Maza SS, Castillo-Triviño T, Borges M, Barón BP, et al. Delayed cognitive processing and treatment status quo bias in early-stage multiple sclerosis. Multiple Sclerosis Related Disord. (2022) 68:104138. doi: 10.1016/j.msard.2022.104138 [DOI] [PubMed] [Google Scholar]
- 32. Giovannoni G, Butzkueven H, Dhib-Jalbut S, Hobart J, Kobelt G, Pepper G, et al. Brain health: time matters in multiple sclerosis. Multiple Sclerosis Related Disord. (2016) 9:S5–S48. doi: 10.1016/j.msard.2016.07.003 [DOI] [PubMed] [Google Scholar]
- 33. Inojosa H, Proschmann U, Akgün K, Ziemssen T. The need for a strategic therapeutic approach: multiple sclerosis in check. Ther Adv Chronic Dis. (2022) 13:204062232110630. doi: 10.1177/20406223211063032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Harding K, Williams O, Willis M, Hrastelj J, Rimmer A, Joseph F, et al. Clinical outcomes of escalation vs early intensive disease-modifying therapy in patients with multiple sclerosis. JAMA Neurol. (2019) 76:536. doi: 10.1001/jamaneurol.2018.4905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Buron MD, Chalmer TA, Sellebjerg F, Barzinji I, Bech D, Christensen JR, et al. Initial high-efficacy disease-modifying therapy in multiple sclerosis. Neurology. (2020) 95:e1041–51. doi: 10.1212/wnl.0000000000010135 [DOI] [PubMed] [Google Scholar]
- 36. Simpson A, Mowry EM, Newsome SD. Early aggressive treatment approaches for multiple sclerosis. Curr Treat Options Neurol. (2021) 23:19. doi: 10.1007/s11940-021-00677-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Spelman T, Magyari M, Piehl F, Svenningsson A, Rasmussen PV, Kant M, et al. Treatment escalation vs immediate initiation of highly effective treatment for patients with relapsing-remitting multiple sclerosis. JAMA Neurol. (2021) 78:1197. doi: 10.1001/jamaneurol.2021.2738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Freeman L, Longbrake EE, Coyle PK, Hendin B, Vollmer T. High-efficacy therapies for treatment-naïve individuals with relapsing–remitting multiple sclerosis. CNS Drugs. (2022) 36:1285–99. doi: 10.1007/s40263-022-00965-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Weideman AM, Tapia-Maltos MA, Johnson K, Greenwood M, Bielekova B. Meta-analysis of the age-dependent efficacy of multiple sclerosis treatments. Front Neurol. (2017) 8:577. doi: 10.3389/fneur.2017.00577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. He A, Merkel B, Brown JWL, Ryerson LZ, Kister I, Malpas CB, et al. Timing of high-efficacy therapy for multiple sclerosis: a retrospective observational cohort study. Lancet Neurol. (2020) 19:307–16. doi: 10.1016/s1474-4422(20)30067-3 [DOI] [PubMed] [Google Scholar]
- 41. Krämer J, Bar-Or A, Turner TJ, Wiendl H. Bruton tyrosine kinase inhibitors for multiple sclerosis. Nat Rev Neurol. (2023) 19:289–304. doi: 10.1038/s41582-023-00800-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hult KJ. Measuring the potential health impact of personalized medicine: evidence from multiple sclerosis treatments. In: Economic Dimensions of Personalized and Precision Medicine (2019) (Chicago, USA: University of Chicago Press; ). p. 185–216. doi: 10.7208/chicago/9780226611235.003.0008 [DOI] [Google Scholar]
- 43. Van Wijmeersch B, Hartung HP, Vermersch P, Pugliatti M, Pozzilli C, Grigoriadis N, et al. Using personalized prognosis in the treatment of relapsing multiple sclerosis: A practical guide. Front Immunol. (2022) 13:991291. doi: 10.3389/fimmu.2022.991291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Marrie RA, Fisk JD, Fitzgerald K, Kowalec K, Maxwell C, Rotstein D, et al. Etiology, effects and management of comorbidities in multiple sclerosis: recent advances. Front Immunol. (2023) 14:1197195. doi: 10.3389/fimmu.2023.1197195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yang J, Hamade M, Wu Q, Wang Q, Axtell R, Giri S, et al. Current and future biomarkers in multiple sclerosis. Int J Mol Sci. (2022) 23:5877. doi: 10.3390/ijms23115877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Voigt I, Inojosa H, Wenk J, Akgün K, Ziemssen T. Building a monitoring matrix for the management of multiple sclerosis. Autoimmun Rev. (2023) 22:103358. doi: 10.1016/j.autrev.2023.103358 [DOI] [PubMed] [Google Scholar]
- 47. Gill AJ, Schorr EM, Gadani SP, Calabresi PA. Emerging imaging and liquid biomarkers in multiple sclerosis. Eur J Immunol. (2023) 53:2250228. doi: 10.1002/eji.202250228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Trentzsch K, Schumann P, Śliwiński G, Bartscht P, Haase R, Schriefer D, et al. Using machine learning algorithms for identifying gait parameters suitable to evaluate subtle changes in gait in people with multiple sclerosis. Brain Sci. (2021) 11:1049. doi: 10.3390/brainsci11081049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Guerrieri S, Comi G, Leocani L. Optical coherence tomography and visual evoked potentials as prognostic and monitoring tools in progressive multiple sclerosis. Front Neurosci. (2021) 15:692599. doi: 10.3389/fnins.2021.692599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Paul F, Calabresi PA, Barkhof F, Green AJ, Kardon R, Sastre-Garriga J, et al. Optical coherence tomography in multiple sclerosis: A 3-year prospective multicenter study. Ann Clin Trans Neurol. (2021) 8:2235–51. doi: 10.1002/acn3.51473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Graves JS. Identifying multiple sclerosis activity. Neurology. (2022) 99:269–70. doi: 10.1212/wnl.0000000000200903 [DOI] [PubMed] [Google Scholar]
- 52. Absinta M, Sati P, Masuzzo F, Nair G, Sethi V, Kolb H, et al. Association of chronic active multiple sclerosis lesions with disability in vivo . JAMA Neurol. (2019) 76:1474. doi: 10.1001/jamaneurol.2019.2399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Blindenbacher N, Brunner E, Asseyer S, Scheel M, Siebert N, Rasche L, et al. Evaluation of the ‘ring sign’ and the ‘core sign’ as a magnetic resonance imaging marker of disease activity and progression in clinically isolated syndrome and early multiple sclerosis. Multiple Sclerosis J - Exp Trans Clin. (2020) 6:205521732091548. doi: 10.1177/2055217320915480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Preziosa P, Pagani E, Meani A, Moiola L, Rodegher M, Filippi M, et al. Slowly expanding lesions predict 9-Year multiple sclerosis disease progression. Neurology® Neuroimmunol Neuroinflamm. (2022) 9:e1139. doi: 10.1212/nxi.0000000000001139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Oreja-Guevara C, Blanco TA, Ruiz LB, Pérez MAH, Meca-Lallana V, Ramió-Torrentà L. Cognitive dysfunctions and assessments in multiple sclerosis. Front Neurol. (2019) 10:581. doi: 10.3389/fneur.2019.00581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Podda J, Ponzio M, Pedullà L, Bragadin MM, Battaglia MA, Zaratin P, et al. Predominant cognitive phenotypes in multiple sclerosis: Insights from patient-centered outcomes. Multiple Sclerosis Related Disord. (2021) 51:102919. doi: 10.1016/j.msard.2021.102919 [DOI] [PubMed] [Google Scholar]
- 57. Carotenuto A, Costabile T, Pontillo G, Moccia M, Falco F, Petracca M, et al. Cognitive trajectories in multiple sclerosis: a long-term follow-up study. Neurol Sci. (2021) 43:1215–22. doi: 10.1007/s10072-021-05356-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Brichetto G, Zaratin P. Measuring outcomes that matter most to people with multiple sclerosis: the role of patient-reported outcomes. ' Curr Opin Neurol. (2020) 33:295–9. doi: 10.1097/wco.0000000000000821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zaratin P, Vermersch P, Amato MP, Brichetto G, Coetzee T, Cutter G, et al. The agenda of the global patient reported outcomes for multiple sclerosis (PROMS) initiative: Progresses and open questions. Multiple Sclerosis Related Disord. (2022) 61:103757. doi: 10.1016/j.msard.2022.103757 [DOI] [PubMed] [Google Scholar]
- 60. Peeters LM, Parciak T, Kalra D, Moreau Y, Kasilingam E, van Galen P, et al. Multiple Sclerosis Data Alliance – A global multi-stakeholder collaboration to scale-up real world data research. Multiple Sclerosis Related Disord. (2021) 47:102634. doi: 10.1016/j.msard.2020.102634 [DOI] [PubMed] [Google Scholar]
- 61. Yamout B, Sahraian M, Bohlega S, Al-Jumah M, Goueider R, Dahdaleh M, et al. Consensus recommendations for the diagnosis and treatment of multiple sclerosis: 2019 revisions to the MENACTRIMS guidelines. Multiple Sclerosis Related Disord. (2020) 37:101459. doi: 10.1016/j.msard.2019.101459 [DOI] [PubMed] [Google Scholar]
- 62. Wattjes MP, Ciccarelli O, Reich DS, Banwell B, de Stefano N, Enzinger C, et al. 2021 MAGNIMS–CMSC–NAIMS consensus recommendations on the use of MRI in patients with multiple sclerosis. Lancet Neurol. (2021) 20:653–70. doi: 10.1016/s1474-4422(21)00095-8 [DOI] [PubMed] [Google Scholar]
- 63. Tur C, Moccia M, Barkhof F, Chataway J, Sastre-Garriga J, Thompson AJ, et al. Assessing treatment outcomes in multiple sclerosis trials and in the clinical setting. Nat Rev Neurol. (2018) 14:75–93. doi: 10.1038/nrneurol.2017.171 [DOI] [PubMed] [Google Scholar]
- 64. Voigt I, Benedict M, Susky M, Scheplitz T, Frankowitz S, Kern R, et al. A digital patient portal for patients with multiple sclerosis. Front Neurol. (2020) 11:400. doi: 10.3389/fneur.2020.00400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wenk J, Voigt I, Inojosa H, Schlieter H, Ziemssen T. Building digital patient pathways for the management and treatment of multiple sclerosis. Front Immunol. (2024) 15:1356436. doi: 10.3389/fimmu.2024.1356436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Sima DM, Esposito G, Van Hecke W, Ribbens A, Nagels G, Smeets D. Health economic impact of software-assisted brain MRI on therapeutic decision-making and outcomes of relapsing-remitting multiple sclerosis patients—A microsimulation study. Brain Sci. (2021) 11:1570. doi: 10.3390/brainsci11121570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Parciak T, Geys L, Helme A, van der Mei I, Hillert J, Schmidt H, et al. Introducing a core dataset for real-world data in multiple sclerosis registries and cohorts: Recommendations from a global task force. Multiple Sclerosis. (2023) 30:396–418. doi: 10.1177/13524585231216004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Sastre-Garriga J, Pareto D, Battaglini M, Rocca MA, Ciccarelli O, Enzinger C, et al. MAGNIMS consensus recommendations on the use of brain and spinal cord atrophy measures in clinical practice. Nat Rev Neurol. (2020) 16:171–82. doi: 10.1038/s41582-020-0314-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Aboseif A, Roos I, Krieger SC, Kalincik T, Hersh CM. Leveraging Real-World evidence and observational studies in treating multiple sclerosis. Neurol Clinics. (2024) 42:203–27. doi: 10.1016/j.ncl.2023.06.003 [DOI] [PubMed] [Google Scholar]
- 70. Katkade VB, Sanders KN, Zou KH. Real world data: an opportunity to supplement existing evidence for the use of long-established medicines in health care decision making. J Multidiscip Healthcare. (2018) 11:295–304. doi: 10.2147/jmdh.s160029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Vercruyssen S, Brys A, Verheijen M, Steach B, Van Vlierberge E, Sima DM, et al. Abstracts from the 34th annual meeting of the consortium of multiple sclerosis centers. Int J MS Care. (2020) 22:1–116. doi: 10.7224/1537-2073-22.s2.1 32123522 [DOI] [Google Scholar]
- 72. Yadav SK, Motamedi S, Oberwahrenbrock T, Oertel FC, Polthier K, Paul F, et al. CuBe: parametric modeling of 3D foveal shape using cubic Bézier. Biomed Optics Express. (2017) 8:4181. doi: 10.1364/boe.8.004181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Yadav SK, Kadas EM. Optic nerve head three-dimensional shape analysis. J Biomed Optics. (2018) 23:1. doi: 10.1117/1.jbo.23.10.106004 [DOI] [PubMed] [Google Scholar]
- 74. Rakić M, Vercruyssen S, Van Eyndhoven S, de la Rosa E, Jain S, Van Huffel S, et al. 'icobrain ms 5.1: Combining unsupervised and supervised approaches for improving the detection of multiple sclerosis lesions. NeuroImage Clin. (2021) 31:102707. doi: 10.1016/j.nicl.2021.102707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Van Hecke W, Costers L, Descamps A, Ribbens A, Nagels G, Smeets D, et al. A novel digital care management platform to monitor clinical and subclinical disease activity in multiple sclerosis. Brain Sci. (2021) 11:1171. doi: 10.3390/brainsci11091171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Dillenseger A, Weidemann ML, Trentzsch K, Inojosa H, Haase R, Schriefer D, et al. Digital biomarkers in multiple sclerosis. Brain Sci. (2021) 11:1519. doi: 10.3390/brainsci11111519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Voigt I, Inojosa H, Dillenseger A, Haase R, Akgün K, Ziemssen T. Digital twins for multiple sclerosis. Front Immunol. (2021) 12:669811. doi: 10.3389/fimmu.2021.669811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Scholz M, Haase R, Trentzsch K, Stölzer-Hutsch H, Ziemssen T. Improving digital patient care: lessons learned from patient-reported and expert-reported experience measures for the clinical practice of multidimensional walking assessment. Brain Sci. (2021) 11:786. doi: 10.3390/brainsci11060786 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.