Abstract
Background
The relationship between atherogenic index of plasma (AIP) and triglyceride glucose-body mass index (TyG-BMI) and sarcopenia has not been studied in the United States (US) population.
Methods
This research included 4,835 people from the National Health and Nutrition Examination Survey (NHANES) conducted between 2011 and 2018. The relationship between sarcopenia and TyG-BMI, as well as the AIP index, was examined through the utilization of restricted cubic spline (RCS) analysis, subgroup analysis, and multivariate logistic regression analysis. Diagnostic value of AIP and TyG-BMI for sarcopenia was compared by receiver operating characteristic (ROC) curves.
Results
In this research, 428 people with sarcopenia were identified among the 4,835 subjects that were included in the experiment. AIP and sarcopenia were positively associated with an odds ratio (OR) of 1.58 and a 95% confidence interval (CI) of (1.07, 2.34) on fully adjusted multivariate logistic regression analysis. Similarly, TyG-BMI and sarcopenia were positively associated with an OR of 8.83 and a 95% CI of (5.46, 14.26). AIP and sarcopenia had a non-linear positive connection (P-value<0.001, P-Nonlinear=0.010), while TyG-BMI and sarcopenia had a linear positive correlation (P-value<0.001, P-Nonlinear=0.064), according to RCS analysis. Subgroup analyses showed a significant interaction between TyG-BMI and sarcopenia due to gender (P = 0.023). ROC curves showed that TyG-BMI (AUC:0.738, 95% CI: 0.714 - 0.761) was more useful than AIP (AUC:0.648, 95% CI: 0.622 - 0.673) in diagnosing sarcopenia.
Conclusion
In US adults aged 20–59 years, our study revealed a correlation between elevated AIP and TyG-BMI levels and heightened sarcopenia risk. Moreover, TyG-BMI has better diagnostic validity than AIP.
Keywords: atherogenic index of plasma, triglyceride glucose-body mass index, sarcopenia, NHANES, cross-sectional studies
1. Introduction
The International Classification of Diseases, which was published in 2016, formally recognizes sarcopenia as a separate disorder (1). Sarcopenia is defined by decreased muscle mass, reduced strength, and progressive loss of function (2). It is estimated that 10% to 16% of older adults worldwide suffer from sarcopenia (3). Although commonly affecting the elderly, this condition can also onset in mid-life (2) and become prevalent among specific populations, including patients with cancer (4), kidney dysfunction (5), liver disease (6), and metabolic disorders (7). Compared to people without sarcopenia, those with sarcopenia have a markedly increased risk of undesirable results such as falls, disability, hospitalization, and even death (8). One of the main mechanisms of sarcopenia is the chronic low-grade inflammatory state associated with aging, commonly referred to as “inflammaging” (9). Several studies have demonstrated that inflammation initiates proteolytic metabolism and impedes anabolic reactions in skeletal muscle, resulting in muscle wasting and contributing to the development of sarcopenia (10, 11). Given the lack of effective pharmacological treatments for sarcopenia, identifying and implementing effective methods for prediction and prevention are paramount.
Atherogenic index of plasma (AIP), which was originally employed as a biomarker for atherosclerosis, is increasingly recognized as a significant forecaster of various disorders. Research indicates a robust correlation between AIP and cardiovascular risk, making it a reliable predictor of cardiovascular events and related mortality (12). Additionally, AIP has demonstrated utility in predicting hypertension, diabetes, and non-alcoholic fatty liver disease (13–15). Triglyceride glucose-body mass index (TyG-BMI) derived from the triglyceride-glucose (TyG) index, demonstrates superior predictive efficacy for insulin resistance in contrast to both the TyG index alone, as well as conventional metrics such as body mass index (BMI) and visceral fat index (16). Furthermore, a heightened risk of atherosclerotic cardiovascular disease and the onset of heart failure have been linked to higher levels of TyG-BMI (16, 17).
Prior research has linked various biomarkers to sarcopenia. For instance, Sung-Ho Ahn et al. identified a negative relationship between muscle mass and TyG in a Korean population (18). Bokun Kim proposed TyG as a potential predictor of obesity sarcopenia in the elderly (19). Additionally, Diao et al.’s meta-analysis revealed a correlation between a higher incidence of sarcopenia and greater inflammatory indices (20). It has been demonstrated that AIP can serve as a marker for assessing inflammatory status (21). The connection between AIP and TyG-BMI and sarcopenia has not yet been studied. Thus, this study uses NHANES data from 2011 to 2018 better understand the correlation between AIP and TyG-BMI and sarcopenia development in the US population.
By doing so, this research contributes to a better understanding of sarcopenia and the identification of effective predictive indicators for the disease.
2. Methods
2.1. The study’s format and the participants
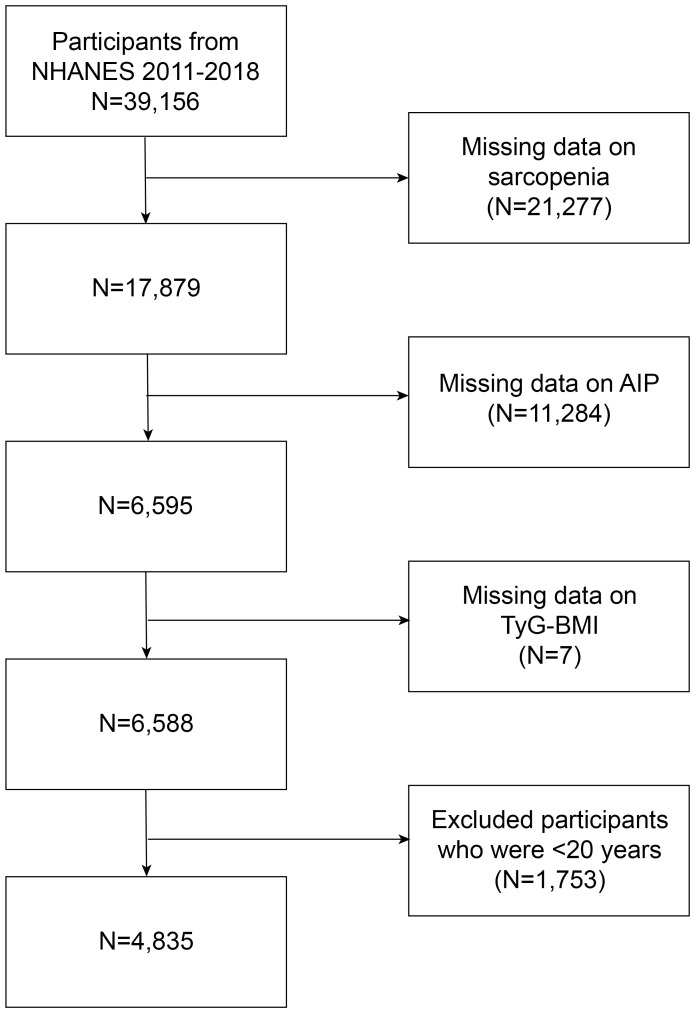
The NHANES is a program for research that aims to assess the nutritional and health status of both adults and children in the United States. It consists of both physical exams and interviews and is administered by the Centers for Disease Control and Prevention. Every participant granted written permission, and the ethical review board of the National Center for Health Statistics approved all procedures [NHANES - NCHS Research Ethics Review Board Approval (cdc.gov)]. A cross-sectional examination of NHANES data from 2011 to 2018 was conducted for this study. It focused on individuals aged 20 to 59 years who underwent measurements for dual-energy X-ray absorptiometry (DXA), triglyceride, fasting glucose, and high-density lipoprotein cholesterol. Exclusions comprised individuals without DXA testing (N=21,277), AIP index (N=11,284), TyG-BMI index (N=7), and those under 20 years of age (N=1,753). Ultimately, 4,835 participants were analyzed, as depicted in Figure 1 .
Figure 1.
Flowchart of participants selection from NHANES 2011–2018.
2.2. Definition of DIP and TyG-BMI
AIP was calculated using the logarithmic transformation of the triglycerides (TG) to high-density lipoprotein cholesterol (HDL-C) ratio with triglycerides and high-density lipoprotein cholesterol expressed in mmol/L (22). TyG-BMI was computed in this way: Weight (kg)/height squared(m2) equals BMI, and the TyG index is equal to Ln [1/2 fasting glucose (mg/dL) × fasting triglycerides (mg/dL)]; TyG index×BMI = TyG-BMI (23). It is important to emphasize that within the study’s framework, AIP and TyG-BMI were regarded as key exposure variables.
2.3. Sarcopenia
Sarcopenia, as defined by the Foundation for the National Institutes of Health (FNIH) guidelines, is described as appendicular skeletal muscle mass adjusted by body mass index (ASMBMI) of < 0.512 for female and < 0.789 for male (24). Appendicular skeletal muscle mass was determined by DXA measurements of arm and leg muscle mass. Safety precautions were implemented by excluding pregnant women and individuals weighing over 136 kg or taller than 196 cm from DXA measurements.
2.4. Ascertainment of covariates
Gender, age, education level, ethnicity, ratio of family income to poverty (PIR), alcohol consumption, smoking status, total calcium, potassium, sodium, total cholesterol, low density lipoprotein cholesterol (LDL-C), uric acid, red blood cell count, white blood cell count, presence of cancer, stroke status, hypertension and diabetes were all included as covariates in the statistical analysis. Stroke status was determined by the “Ever told you had a stroke” questionnaire, with a yes response indicating that the participant had a previous or current stroke. The presence of cancer was ascertained using the question, “ Ever told you had cancer or malignancy” with a positive response indicated a history of cancer for the participant. Diabetes status was ascertained through the administration of the “Doctors told you have diabetes” questionnaire, wherein positive answers indicated the presence of diabetes. Likewise, participants who responded positively on the questionnaire that “Your doctor informed you that you have high blood pressure” were categorized as having hypertension. Participants completed a questionnaire to ascertain if they had ever smoked more than 100 cigarettes. Individuals who replied in the affirmative were categorized as having previously smoked. Additionally, the questionnaire that “How often drink alcohol over past 12 months” was utilized to inquire about participants’ alcohol consumption frequency over the past year, with responses of 0 defined as never, 1–12 as sometimes, and >12 as often.
2.5. Statistical analyses
Mean ± standard deviation was used to express continuous variables, while frequencies (percentages) were used to express categorical variables. In order to determine the OR and corresponding 95% CI between each quartile of TyG-BMI and AIP and the prevalence of sarcopenia, multivariate logistic regression analysis was performed. The analysis comprised three logistic regression models. It was not included any covariate adjustments in Model 1, while it was included gender, age, education level, ethnicity, and PIR in Model 2. Model 3, after making complete adjustments, encompassed factors like age, gender, PIR, ethnicity, education level, total calcium, potassium, sodium, uric acid, red blood cell count, white blood cell count, total cholesterol, LDL-C, alcohol consumption, smoking status, presence of cancer, stroke status, hypertension, and diabetes. The relationship between AIP and TyG-BMI and the risk of sarcopenia development was examined through RCS analysis. Covariates including age, gender, ethnicity, education level, and PIR were considered when modifying the model. Subgroup analyses were conducted to look at any variations in the link between TyG-BMI and AIP and sarcopenia occurrence among subgroups defined by hypertension, diabetes, smoking status, alcohol consumption, gender, and age. Missing values were filled in using a random forest model. For diagnostic value analysis, ROC curves were utilized. The prognostic capacity of AIP and TyG-BMI was determined by calculating the area under the curve (AUC), as indicated by the C-statistic. And the Youden index (i.e., sensitivity + specificity-1) was utilized to find the cutoff values of AIP and TyG-BMI. The statistical studies were conducted utilizing EmpowerStats and R 4.2.2 software (https://www.empowerstats.com).
3. Results
3.1. Baseline characteristics of the population based on sarcopenia
Table 1 displays the demographic attributes and additional variables of the study subjects, delineated by the presence of sarcopenia and categorized into quartiles based on AIP and TyG-BMI levels. In particular, participants with sarcopenia were predominantly Mexican American, with an education level of Some college or AA degree, occasional alcohol consumption, nonsmokers, and those without diabetes, hypertension, stroke and cancer. The participants with sarcopenia were older than the non-sarcopenic subjects and showed increased levels of red blood cell count, white blood cell count, serum uric acid, total cholesterol, and LDL-C, while having lower levels of serum total calcium and PIR. However, serum sodium, serum potassium levels, gender, and smoking status exhibited no significant differences between sarcopenia and non-sarcopenia participants. There was a gradual increase in the proportion of individuals with sarcopenia in both AIP and TyG-BMI quartile groupings.
Table 1.
Baseline characteristics of participants.
| Characteristic | Sarcopenia | P-value | |
|---|---|---|---|
| Yes, N=428 | No, N=4,407 | ||
| Total Calcium, mg/dL, M ± SD | 9.26 ± 0.35 | 9.32 ± 0.34 | <0.001 |
| Potassium, mmol/L, M ± SD | 4.01 ± 0.35 | 3.99 ± 0.32 | 0.420 |
| Sodium, mmol/L, M ± SD | 139.42 ± 2.46 | 139.26 ± 2.14 | 0.177 |
| Uric Acid, mg/dL, M ± SD | 5.59 ± 1.49 | 5.33 ± 1.38 | <0.001 |
| Red Blood Cell, million cells/uL, M ± SD | 4.85 ± 0.52 | 4.77 ± 0.48 | 0.002 |
| Age, years, M ± SD | 45 ± 11 | 39 ± 11 | <0.001 |
| PIR, M ± SD | 2.11 ± 1.52 | 2.50 ± 1.60 | <0.001 |
| White Blood Cell, 1000 cells/uL, M ± SD | 7.61 ± 2.25 | 6.76 ± 1.98 | <0.001 |
| Total Cholesterol, mg/dL, M ± SD | 199 ± 41 | 189 ± 40 | <0.001 |
| LDL-C, mg/dL, M ± SD | 121 ± 34 | 113 ± 34 | <0.001 |
| Alcohol Consumption, n (%) | <0.001 | ||
| Never | 110 (25.7%) | 641 (14.5%) | |
| Sometimes | 293 (68.5%) | 3,340 (75.8%) | |
| Often | 25 (5.8%) | 426 (9.7%) | |
| Smoking Status, n (%) | 0.918 | ||
| Yes | 171 (40.0%) | 1,772 (40.2%) | |
| No | 257 (60.0%) | 2,635 (59.8%) | |
| Hypertension, n (%) | <0.001 | ||
| Yes | 147 (34.3%) | 1,013 (23.0%) | |
| No | 281 (65.7%) | 3,394 (77.0%) | |
| Gender, n (%) | 0.870 | ||
| Male | 213 (49.8%) | 2,175 (49.4%) | |
| Female | 215 (50.2%) | 2,232 (50.6%) | |
| Race, n (%) | <0.001 | ||
| Mexican American | 142 (33.2%) | 583 (13.2%) | |
| Other Hispanic | 69 (16.1%) | 448 (10.2%) | |
| Non-Hispanic White | 122 (28.5%) | 1,587 (36.0%) | |
| Non-Hispanic Black | 23 (5.4%) | 944 (21.4%) | |
| Non-Hispanic Asian | 61 (14.3%) | 661 (15.0%) | |
| Other Race | 11 (2.6%) | 184 (4.2%) | |
| Education Level, n (%) | <0.001 | ||
| Less than 9th grade | 82 (19.2%) | 231 (5.2%) | |
| 9-11th grade | 69 (16.1%) | 533 (12.1%) | |
| High school graduate/GED or equivalent | 99 (23.1%) | 936 (21.2%) | |
| Some college or AA degree | 111 (25.9%) | 1,418 (32.2%) | |
| College graduate or above | 67 (15.7%) | 1,289 (29.2%) | |
| Diabetes, n (%) | <0.001 | ||
| Yes | 65 (15.2%) | 296 (6.7%) | |
| No | 363 (84.8%) | 4,111 (93.3%) | |
| Stroke, n (%) | 0.014 | ||
| Yes | 12 (2.8%) | 58 (1.3%) | |
| No | 416 (97.2%) | 4,349 (98.7%) | |
| Cancer, n (%) | 0.232 | ||
| Yes | 20 (4.7%) | 156 (3.5%) | |
| No | 408 (95.3%) | 4,251 (96.5%) | |
| AIP, n (%) | <0.001 | ||
| Q1 [-1.25,-0.33) | 46 (10.7%) | 1,123 (25.5%) | |
| Q2 [-0.33,-0.1) | 65 (15.2%) | 1,174 (26.6%) | |
| Q3 [-0.1,0.14) | 149 (34.8%) | 1,067 (24.2%) | |
| Q4 [0.14,1.65] | 168 (39.3%) | 1,043 (23.7%) | |
| TyG-BMI, n (%) | <0.001 | ||
| Q1 [115,196) | 25 (5.8%) | 1,184 (26.9%) | |
| Q2 [196,236) | 58 (13.6%) | 1,150 (26.1%) | |
| Q3 [236,283) | 107 (25.0%) | 1,102 (25.0%) | |
| Q4 [283,571] | 238 (55.6%) | 971 (22.0%) | |
PIR, ratio of family income to poverty; LDL-C, low-density lipoproteins cholesterol; AIP, atherogenic index of plasma; TyG-BMI, triglyceride glucose-body mass index; M, mean values; SD, standard deviation.
3.2. Multivariate logistic regression analysis of AIP and TyG-BMI levels with sarcopenia
Table 2 illustrates the correlation between AIP and TyG-BMI levels and sarcopenia. Individuals were stratified into four quartile groups according to AIP and TyG-BMI levels. Within the AIP quartile groups, the risk of sarcopenia was positively correlated with AIP levels, according to Model 1. Compared to the first quartile, the risk of sarcopenia increased by 241% and 293% in the third and fourth quartiles, respectively (OR=3.41, 95% CI: 2.42–4.79; OR=3.93, 95% CI: 2.81–5.51). After adjusting for gender, age, education level, ethnicity, and PIR, the risk of sarcopenia was positively correlated with AIP levels in Model 2. Compared to the first quartile, the risk of sarcopenia increased by 147% and 148% in the third and fourth quartiles, respectively (OR=2.47, 95% CI: 1.74–3.53, OR=2.48, 95% CI: 1.73–3.54). In Model 3, after controlling for gender, age, education level, ethnicity, PIR, total calcium, potassium, sodium, uric acid, red blood cell count, white blood cell count, total cholesterol, LDL-C, diabetes, hypertension, stroke, cancer, alcohol consumption, and smoking status, the risk of sarcopenia was positively correlated with AIP levels. Compared to the first quartile, the risk of sarcopenia increased by 83% and 58% in the third and fourth quartiles, respectively (OR=1.83, 95% CI: 1.26–2.68, OR=1.58, 95% CI: 1.07–2.34). In the TyG-BMI quartile groups, the risk of sarcopenia was positively correlated with TyG-BMI levels according to Model 1. Compared to the first quartile, the risk of sarcopenia rose by139%, 360% and 1061% in the second, third, and fourth quartiles, respectively (OR=2.39, 95% CI: 1.48–3.84; OR=4.60, 95% CI: 2.95–7.16; OR= 11.61, 95% CI: 7.62–17.68). In model 2, the risk of sarcopenia was positively correlated with TyG-BMI levels. Compared to the first quartile, the risk of sarcopenia increased by92%, 244%, and 934% in the second, third, and fourth quartiles, respectively (OR=1.92, 95% CI: 1.18–3.13; OR=3.44, 95% CI: 2.17–5.44; OR=10.34, 95% CI: 6.63–16.14). In model 3, the risk of sarcopenia was positively correlated with TyG-BMI levels. Compared to the first quartile, the risk of sarcopenia was increased by 82%, 204%, and 783% in the second, third, and fourth quartiles, respectively (OR=1.82, 95% CI: 1.11–2.99; OR=3.04, 95% CI: 1.89–4.89; OR= 8.83, 95% CI: 5.46–14.26).
Table 2.
Association between AIP and TyG-BMI with sarcopenia.
| OR (95% CI), P-value | |||
|---|---|---|---|
| Model 1 | Model 2 | Model 3 | |
| AIP | |||
| Q1 [-1.25,-0.33) | 1.00(reference) | 1.00(reference) | 1.00(reference) |
| Q2 [-0.33,-0.1) | 1.35 (0.92-1.99, p=0.126) | 1.14 (0.77-1.69, p=0.523) | 0.97 (0.64-1.45, p=0.867) |
| Q3 [-0.1,0.14) | 3.41 (2.42-4.79, p<0.001) | 2.47 (1.74-3.53, p<0.001) | 1.83 (1.26-2.68, p=0.002) |
| Q4 [0.14,1.65] | 3.93 (2.81-5.51, p<0.001) | 2.48 (1.73-3.54, p<0.001) | 1.58 (1.07-2.34, p=0.023) |
| TyG-BMI | |||
| Q1 [115,196) | 1.00(reference) | 1.00(reference) | 1.00(reference) |
| Q2 [196,236) | 2.39 (1.48-3.84, p<0.001) | 1.92 (1.18-3.13, p=0.009) | 1.82 (1.11-2.99, p=0.017) |
| Q3 [236,283) | 4.60 (2.95-7.16, p<0.001) | 3.44 (2.17-5.44, p<0.001) | 3.04 (1.89-4.89, p<0.001) |
| Q4 [283,571] | 11.61 (7.62-17.68, p<0.001) | 10.34 (6.63-16.14, p<0.001) | 8.83 (5.46-14.26, p<0.001) |
Model 1: crude model.
Model 2: adjusted for age, gender, race, education level and ratio of family income to poverty.
Model 3: adjusted for age, gender, race, education level, ratio of family income to poverty, total calcium, potassium, sodium, uric acid, red blood cell count, white blood cell count, total cholesterol, LDL-C, alcohol consumption, smoking status, hypertension, diabetes, cancer and stroke.
OR, odds ratio; CI, confidence interval.
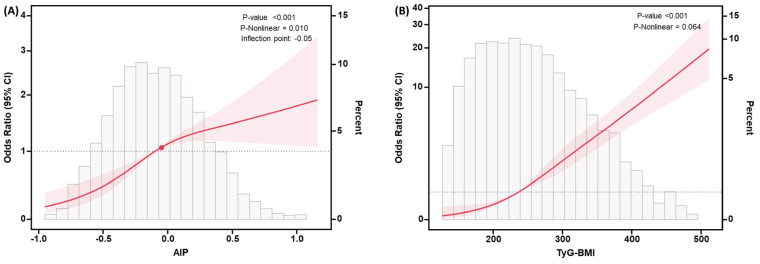
3.3. RCS analysis investigating the relationship between AIP and TyG-BMI levels and sarcopenia
We modeled and visualized the association between AIP and TyG-BMI levels and sarcopenia using RCS analysis in Figure 2 . After adjusting for the covariates including gender, age, ethnicity, education level, and PIR, a non-linear positive connection was found between AIP and sarcopenia (P-value<0.001, P-Nonlinear=0.010), and the OR curve was steeper when AIP<-0.05 than when AIP>-0.05, where the range of AIP was from -1.25 to 1.65. Conversely, after adjusting for the same covariates, a linear positive connection was discovered between TyG-BMI and sarcopenia (P-value<0.001, P-Nonlinear=0.064), where the range of TyG-BMI was from 114.52 to 571.42.
Figure 2.
RCS curves for the association between AIP (A), TyG-BMI (B) levels and the risk of developing sarcopenia. The model was adjusted for covariates including gender, age, ethnicity, education level, and PIR. The pink-shaded regions indicated the 95% confidence intervals and turning point was illustrated using a red dot. Abbreviations: OR, odds ratio; CI, confidence interval.
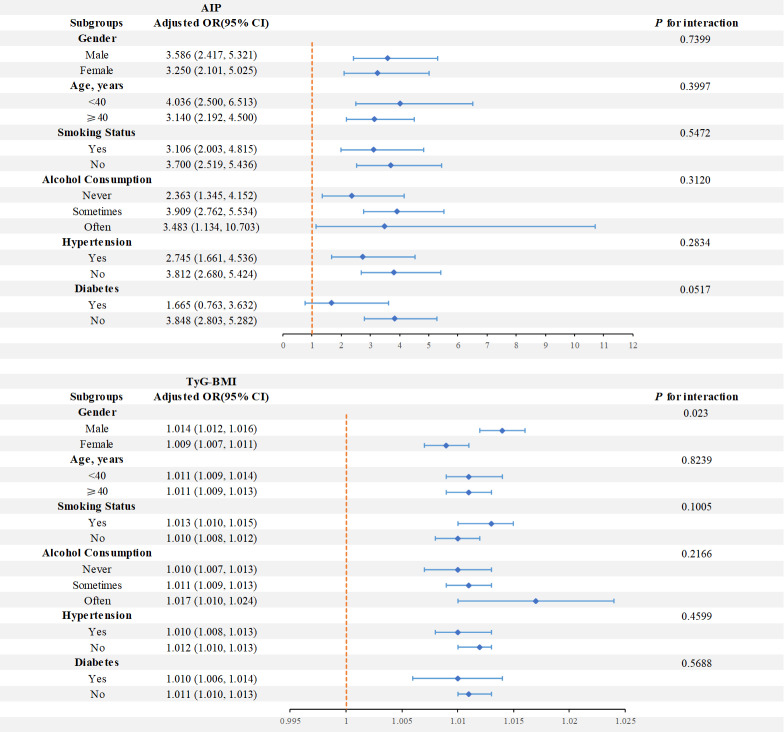
3.4. Subgroup analysis investigating the association between AIP and TyG-BMI levels and sarcopenia
We conducted subgroup analyses stratified by gender, age, smoking status, alcohol consumption, diabetes, and hypertension to delve deeper into the correlation between AIP and TyG-BMI levels and sarcopenia, and performed interaction tests ( Figure 3 ). Concerning the correlation between AIP and sarcopenia, the findings indicated that the link between AIP and sarcopenia was not significantly affected by the variables of gender, age, alcohol consumption, smoking status, diabetes, and hypertension (P for interaction>0.05). Concerning the correlation between TyG-BMI and sarcopenia, the findings indicated that gender had a significant interaction effect on the interaction between TyG-BMI and sarcopenia (P for interaction = 0.023). The TyG-BMI range for female participants was from 114.52 to 543.15, and for male participants, it was from 115.4 to 571.42. However, this connection was not significantly impacted by age, alcohol consumption, smoking status, hypertension, or diabetes (P for interaction > 0.05).
Figure 3.
Forest plots of stratified analyses of AIP and TyG-BMI with sarcopenia. Age, gender, alcohol consumption, smoking status, and a history of diabetes and hypertension were all adjusted except the stratification variable itself.
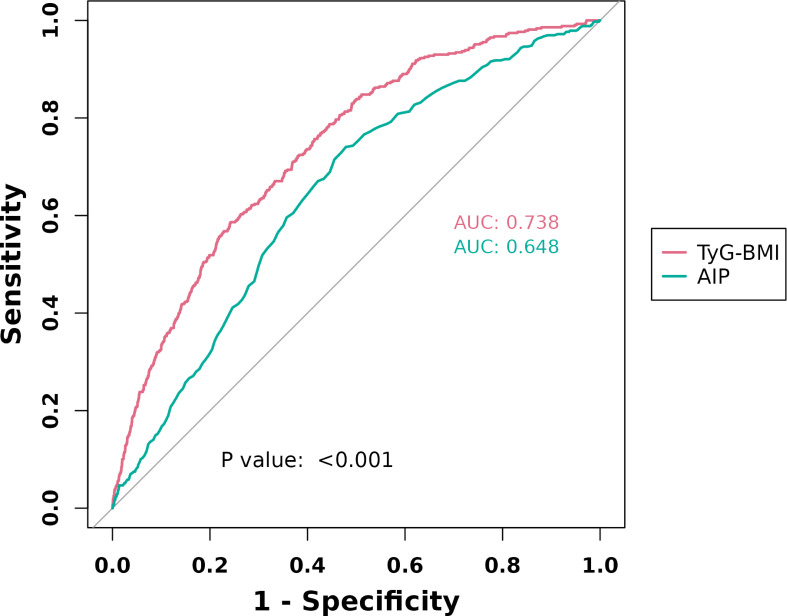
3.5. ROC curves of AIP and TyG-BMI levels in diagnosing sarcopenia
The ROC curve ( Figure 4 ) revealed that TyG-BMI exhibited superior diagnostic validity for sarcopenia (AUC: 0.738, 95% CI: 0.714 - 0.761) compared to AIP, which demonstrated lower diagnostic validity (AUC: 0.648, 95% CI: 0.622 - 0.673). The cutoff value of AIP was -0.1050, and that of TyG-BMI was 277.5750. When AIP was -0.1050, the sensitivity for diagnosing sarcopenia was 0.741, and the specificity was 0.521. When TyG-BMI was 277.5750, the sensitivity for diagnosing sarcopenia was 0.5864, and the specificity was 0.7579.
Figure 4.
ROC curves of AIP and TyG-BMI levels in diagnosing sarcopenia.
4. Discussion
This cross-sectional study utilized data from the 2011–2018 NHANES surveys, concentrating on individuals aged 20 to 59 years, to examine the relationship between TyG-BMI and AIP and sarcopenia. After controlling for pertinent confounding factors, we observed that increased levels of AIP and TyG-BMI showed significant associations with a heightened risk of sarcopenia in our analysis of 4,835 subjects. We detected a nonlinear positive link between AIP and sarcopenia after adjusting for covariates, alongside a linear positive relationship between TyG-BMI and sarcopenia. Furthermore, we observed that the link between AIP levels and heightened sarcopenia risk did not significantly differ across subgroups based on gender, age, hypertension, diabetes, smoking status, or alcohol consumption. However, the link between increased TyG-BMI levels and elevated sarcopenia risk was notably more prominent within the male subgroup. Furthermore, compared to AIP, TyG-BMI shown better diagnostic validity for sarcopenia.
To our knowledge, this study represents the first attempt to explore the correlation between AIP and TyG-BMI and sarcopenia, as well as to compare their diagnostic efficacy for sarcopenia. Numerous pathophysiological mechanisms, such as hormone fluctuations, denervation, and mitochondrial dysfunction, are thought to be involved with sarcopenia (25). Additionally, inflammation is a major factor in sarcopenia. Increased levels of inflammatory cytokines associated with aging and disease processes can exacerbate age-related anorexia and cause a reduction in food intake, which in turn can lead to muscle loss (26). Sarcopenia may ensue from this cascade, and sarcopenia may have negative health effects like functional decline, falls, weakness, and mortality from the loss of lean body mass (25, 27). Although sarcopenia is usually linked to advanced aging, it is now known to start earlier than 60, with a decrease in muscular strength starting at roughly 40 (2, 27). Apart from the elderly population, individuals who are underweight, women, and those with other chronic conditions are also at a higher risk of developing sarcopenia (28). Scholars that have delved into biomarkers associated with sarcopenia have become more numerous in recent years. Many studies have underscored the robust relationship between sarcopenia and various biomarkers, such as the serum creatinine/cystatin C ratio (29), γ-glutamyltransferase (30), HOMA-IR index (31), etc., elucidating the intricate mechanisms underlying the condition. Prior research has demonstrated a heightened risk of sarcopenia in individuals with diabetes and its complications, along with an association between sarcopenia and heart disease (32). Our study reaffirmed these connections between the musculoskeletal, endocrine, and cardiovascular systems using two biomarkers, AIP and TyG-BMI.
AIP was first described by Dobiásová et al. in 2000 (33). It is regarded as a promising cardiovascular disease(CVD) prediction biomarker (34). Although there have been no studies specifically addressing the correlation between AIP and sarcopenia, Liu X et al. (35) have shown that there is a unidirectional causal relationship between sarcopenia and cardiovascular disease, and the loss of muscle mass and strength has a significant causal role in promoting the occurrence and development of CVD. Gao K et al. (36) also found that among Chinese individuals in their middle and later years, sarcopenia and potential sarcopenia were linked to an increased risk of cardiovascular disease. Our investigation turned up a substantial positive connection between AIP and sarcopenia in the American community. Previous investigations have looked into the correlation between AIP and various diseases using a range of epidemiological techniques. Kim, S.H. et al. (37) revealed an established correlation between AIP and the risk of CVD after adjusting for traditional risk factors, suggesting that AIP may be utilized to identify patients at high risk for cardiovascular events. AIP outperformed other lipid components in predicting diabetes risk, according to a meta-analysis of 15 case-control studies (38). Additionally, several researches suggest that AIP may serve as a potential marker of inflammation and impaired lipid metabolism (39, 40).
TyG-BMI serves as an indicator for evaluating insulin resistance, with Er LK et al. (41) demonstrating its superiority over other insulin resistance scores, including HOMA-IR, for identifying insulin resistance. Additionally, Lim J et al. (42) reported that TyG-BMI outperformed TyG and triglyceride glucose-waist circumference (TyG-WC) in predicting insulin resistance. Sarcopenia and TyG-BMI levels were found to be significantly positively correlated by our investigation. While the association between TyG-BMI levels and sarcopenia has not been specifically studied, previous research in various populations has linked TyG levels with sarcopenia. A study conducted in a Korean population (43) revealed that individuals with skeletal muscle mass relative to the lowest quartile of body weight exhibited a twofold increased risk of type 2 diabetes development compared to those in the highest quartile. Chen Y. et al. (44) found a negative correlation between the TyG index and the incidence of sarcopenia among elderly Chinese individuals. However, this association lost significance after adjustment for body mass index. In a non-diabetic cohort receiving maintenance hemodialysis, Chen R et al. (45) identified a significant correlation between the risk of sarcopenia and the TyG index. Lower skeletal muscle mass was revealed to be independently correlated with insulin resistance in older adults by Lee SW et al. (46). These findings underscore a plausible association between diabetes and sarcopenia.
To date, no study has delineated the mechanistic underpinnings of the association between AIP and TyG-BMI and sarcopenia. We posit that inflammation could serve as a pivotal mechanism in this relationship. One notable risk factor for diabetes and cardiovascular disease is chronic inflammation (47). Atherosclerosis initiates with endothelial damage, leading to the accumulation and oxidation of cholesterol-containing LDL particles in the arterial wall, triggering an unresolvable inflammatory response (48), which itself generates, activates, and sustains antigens, perpetuating the inflammatory response (47). On the other hand, chronic, low-grade systemic inflammation correlates with type 2 diabetes, exerting detrimental effects on glucose and muscle homeostasis (49–51). Elevated levels of inflammatory markers such C-reactive protein (CRP), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α) are commonly observed in patients with type 2 diabetes (52, 53). Furthermore, inflammatory conditions impair vascular reactivity and insulin delivery, fostering insulin resistance (54). Additionally, inflammation indirectly diminishes the concentrations of growth hormone and insulin-like growth factor-1 (IGF-1), thereby adversely impacting skeletal muscle (55, 56) and fostering sarcopenia development. TNF-α impedes muscle protein synthesis through modulation of the PI3K/Akt/mTOR signaling pathway and fosters muscle atrophy via upregulating the expression of various muscle growth inhibitory factors, including atrogin1, nuclear factor κB (NF-κB), and myostatin (57, 58). Moreover, sarcopenia is regarded as an age-related condition, and increasing inflammatory markers and associated variables are frequently observed with aging (59). Age-related increases in circulation levels of inflammatory markers, such as TNF-α, CRP, interleukin-1β (IL-1β), and IL-6, are directly linked to a decline in muscle mass and strength (60–62). Therefore, we supposed that inflammation is one of the important mechanisms as a mediator of AIP and TyG-BMI prediction of sarcopenia.
This study offers several advantages. Firstly, it serves as a significant reference for further delineating and predicting the emergence of sarcopenia within the American population. Secondly, by using a nationally representative sample and a standardized experimental testing technique, along with data from NHANES, the study’s errors were significantly reduced. This approach bolstered the reliability of the experimental outcomes and guaranteed the study’s representativeness. Nonetheless, the study bears limitations. Primarily, due to its cross-sectional nature, establishing a causal link between elevated levels of AIP and TyG-BMI and heightened sarcopenia risk was unattainable. Furthermore, only individuals aged 8–59 underwent dual-energy X-rays for sarcopenia confirmation, neglecting those aged 60 and above. These limitations suggest the necessity for cohort studies in the future to determine a causal association between the two indices and sarcopenia, as well as additional studies targeting sarcopenia in the elderly population.
In summary, our research showed a correlation between higher levels of AIP and TyG-BMI and heightened sarcopenia risk in US adults aged 20–59 years. Moreover, TyG-BMI has better diagnostic validity than AIP.
Acknowledgments
The authors would like to express their gratitude to the participants and researchers involved in the National Health and Nutrition Examination Survey (NHANES), as well as to NHANES for providing accessible and open-source data.
Funding Statement
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was financially supported by the Geriatric Health Research Project of Jiangsu Commission of Health (LR2022008 and LSD2022036), Jiangsu Province Senile Health Scientific Research Project (LKM2023050, LKM2023022, and LKZ2022012), Jiangsu Health Development Research Center (JSHD2022054).
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.cdc.gov/nchs/nhanes/index.htm.
Ethics statement
The studies involving humans were approved by the ethical review board of the National Center for Health Statistics. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
RP: Writing – original draft, Writing – review & editing. TW: Writing – original draft, Writing – review & editing. RT: Writing – review & editing. ZQ: Writing – review & editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Anker SD, Morley JE, von Haehling S. Welcome to the ICD-10 code for sarcopenia. J Cachexia Sarcopenia Muscle. (2016) 7:512–4. doi: 10.1002/jcsm.12147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cruz-Jentoft AJ, Sayer AA. Sarcopenia. Lancet. (2019) 393:2636–46. doi: 10.1016/S0140-6736(19)31138-9 [DOI] [PubMed] [Google Scholar]
- 3. Yuan S, Larsson SC. Epidemiology of sarcopenia: Prevalence, risk factors, and consequences. Metabolism. (2023) 144:155533. doi: 10.1016/j.metabol.2023.155533 [DOI] [PubMed] [Google Scholar]
- 4. Shachar SS, Williams GR, Muss HB, Nishijima TF. Prognostic value of sarcopenia in adults with solid tumours: A meta-analysis and systematic review. Eur J Cancer. (2016) 57:58–67. doi: 10.1016/j.ejca.2015.12.030 [DOI] [PubMed] [Google Scholar]
- 5. Shu X, Lin T, Wang H, Zhao Y, Jiang T, Peng X, et al. Diagnosis, prevalence, and mortality of sarcopenia in dialysis patients: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. (2022) 13:145–58. doi: 10.1002/jcsm.12890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tantai X, Liu Y, Yeo YH, Praktiknjo M, Mauro E, Hamaguchi Y, et al. Effect of sarcopenia on survival in patients with cirrhosis: A meta-analysis. J Hepatol. (2022) 76:588–99. doi: 10.1016/j.jhep.2021.11.006 [DOI] [PubMed] [Google Scholar]
- 7. Feng L, Gao Q, Hu K, Wu M, Wang Z, Chen F, et al. Prevalence and risk factors of sarcopenia in patients with diabetes: A meta-analysis. J Clin Endocrinol Metab. (2022) 107:1470–83. doi: 10.1210/clinem/dgab884 [DOI] [PubMed] [Google Scholar]
- 8. Larsson L, Degens H, Li M, Salviati L, Lee YI, Thompson W, et al. Sarcopenia: aging-related loss of muscle mass and function. Physiol Rev. (2019) 99:427–511. doi: 10.1152/physrev.00061.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dalle S, Rossmeislova L, Koppo K. The role of inflammation in age-related sarcopenia. Front Physiol. (2017) 8:1045. doi: 10.3389/fphys.2017.01045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tisdale MJ. Catabolic mediators of cancer cachexia. Curr Opin Support Palliat Care. (2008) 2:256–61. doi: 10.1097/SPC.0b013e328319d7fa [DOI] [PubMed] [Google Scholar]
- 11. Mittal A, Bhatnagar S, Kumar A, Lach-Trifilieff E, Wauters S, Li H, et al. The TWEAK-Fn14 system is a critical regulator of denervation-induced skeletal muscle atrophy in mice. J Cell Biol. (2010) 188:833–49. doi: 10.1083/jcb.200909117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sadeghi M, Heshmat-Ghahdarijani K, Talaei M, Safaei A, Sarrafzadegan N, Roohafza H. The predictive value of atherogenic index of plasma in the prediction of cardiovascular events; a fifteen-year cohort study. Adv Med Sci. (2021) 66:418–23. doi: 10.1016/j.advms.2021.09.003 [DOI] [PubMed] [Google Scholar]
- 13. Peng H, Zhang J, Huang X, Xu M, Huang J, Wu Y, et al. Development and validation of an online dynamic nomogram based on the atherogenic index of plasma to screen nonalcoholic fatty liver disease. Lipids Health Dis. (2023) 22:44. doi: 10.1186/s12944-023-01808-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yi Q, Ren Z, Bai G, Zhu S, Li S, Li C, et al. The longitudinal effect of the atherogenic index of plasma on type 2 diabetes in middle-aged and older Chinese. Acta Diabetol. (2022) 59:269–79. doi: 10.1007/s00592-021-01801-y [DOI] [PubMed] [Google Scholar]
- 15. Li YW, Kao TW, Chang PK, Chen WL, Wu LW. Atherogenic index of plasma as predictors for metabolic syndrome, hypertension and diabetes mellitus in Taiwan citizens: a 9-year longitudinal study. Sci Rep. (2021) 11:9900. doi: 10.1038/s41598-021-89307-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yang S, Shi X, Liu W, Wang Z, Li R, Xu X, et al. Association between triglyceride glucose-body mass index and heart failure in subjects with diabetes mellitus or prediabetes mellitus: a cross-sectional study. Front Endocrinol (Lausanne). (2023) 14:1294909. doi: 10.3389/fendo.2023.1294909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Huang YC, Huang JC, Lin CI, Chien HH, Lin YY, Wang CL, et al. Comparison of innovative and traditional cardiometabolic indices in estimating atherosclerotic cardiovascular disease risk in adults. Diagnostics (Basel). (2021) 11(4):603. doi: 10.3390/diagnostics11040603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ahn SH, Lee JH, Lee JW. Inverse association between triglyceride glucose index and muscle mass in Korean adults: 2008-2011 KNHANES. Lipids Health Dis. (2020) 19:243. doi: 10.1186/s12944-020-01414-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kim B, Kim G, Lee Y, Taniguchi K, Isobe T, Oh S. Triglyceride-glucose index as a potential indicator of sarcopenic obesity in older people. Nutrients. (2023) 15(3):555. doi: 10.3390/nu15030555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Diao H, Yan F, He Q, Li M, Zheng Q, Zhu Q, et al. Association between dietary inflammatory index and sarcopenia: A meta-analysis. Nutrients. (2023) 15(1):219. doi: 10.3390/nu15010219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Oliveira SR, Kallaur AP, Lopes J, Colado Simão AN, Reiche EM, de Almeida ERD, et al. Insulin resistance, atherogenicity, and iron metabolism in multiple sclerosis with and without depression: Associations with inflammatory and oxidative stress biomarkers and uric acid. Psychiatry Res. (2017) 250:113–20. doi: 10.1016/j.psychres.2016.12.039 [DOI] [PubMed] [Google Scholar]
- 22. Niroumand S, Khajedaluee M, Khadem-Rezaiyan M, Abrishami M, Juya M, Khodaee G, et al. Atherogenic Index of Plasma (AIP): A marker of cardiovascular disease. Med J Islam Repub Iran. (2015) 29:240. [PMC free article] [PubMed] [Google Scholar]
- 23. Huang X, He J, Wu G, Peng Z, Yang B, Ye L. TyG-BMI and hypertension in Normoglycemia subjects in Japan: A cross-sectional study. Diabetes Vasc Dis Res. (2023) 20:14791641231173617. doi: 10.1177/14791641231173617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Batsis JA, Mackenzie TA, Jones JD, Lopez-Jimenez F, Bartels SJ. Sarcopenia, sarcopenic obesity and inflammation: Results from the 1999-2004 National Health and Nutrition Examination Survey. Clin Nutr. (2016) 35:1472–83. doi: 10.1016/j.clnu.2016.03.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lang T, Streeper T, Cawthon P, Baldwin K, Taaffe DR, Harris TB. Sarcopenia: etiology, clinical consequences, intervention, and assessment. Osteoporos Int. (2010) 21:543–59. doi: 10.1007/s00198-009-1059-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Michaud M, Balardy L, Moulis G, Gaudin C, Peyrot C, Vellas B, et al. Proinflammatory cytokines, aging, and age-related diseases. J Am Med Dir Assoc. (2013) 14:877–82. doi: 10.1016/j.jamda.2013.05.009 [DOI] [PubMed] [Google Scholar]
- 27. Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. (2019) 48:16–31. doi: 10.1093/ageing/afy169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Petermann-Rocha F, Chen M, Gray SR, Ho FK, Pell JP, Celis-Morales C. Factors associated with sarcopenia: A cross-sectional analysis using UK Biobank. Maturitas. (2020) 133:60–7. doi: 10.1016/j.maturitas.2020.01.004 [DOI] [PubMed] [Google Scholar]
- 29. Kim SW, Jung HW, Kim CH, Kim KI, Chin HJ, Lee H. A new equation to estimate muscle mass from creatinine and cystatin C. PloS One. (2016) 11:e0148495. doi: 10.1371/journal.pone.0148495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Petermann-Rocha F, Gray SR, Pell JP, Celis-Morales C, Ho FK. Biomarkers profile of people with sarcopenia: A cross-sectional analysis from UK biobank. J Am Med Dir Assoc. (2020) 21:2017.e1–2017.e9. doi: 10.1016/j.jamda.2020.05.005 [DOI] [PubMed] [Google Scholar]
- 31. Lee JH, Kim S, Kim MK, Yun BH, Cho S, Choi YS, et al. Relationships between 25(OH)D concentration, sarcopenia and HOMA-IR in postmenopausal Korean women. Climacteric. (2018) 21:40–6. doi: 10.1080/13697137.2017.1395410 [DOI] [PubMed] [Google Scholar]
- 32. Gao Q, Hu K, Yan C, Zhao B, Mei F, Chen F, et al. Associated factors of sarcopenia in community-dwelling older adults: A systematic review and meta-analysis. Nutrients. (2021) 15(1):219. doi: 10.3390/nu13124291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dobiásová M, Frohlich J. The new atherogenic plasma index reflects the triglyceride and HDL-cholesterol ratio, the lipoprotein particle size and the cholesterol esterification rate: changes during lipanor therapy. Vnitr Lek. (2000) 46:152–6. [PubMed] [Google Scholar]
- 34. Wu J, Zhou Q, Wei Z, Wei J, Cui M. Atherogenic index of plasma and coronary artery disease in the adult population: A meta-analysis. Front Cardiovasc Med. (2021) 8:817441. doi: 10.3389/fcvm.2021.817441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Liu X, Wang Y, Wang Z, Li L, Yang H, Liu J, et al. Association between sarcopenia-related traits and cardiovascular diseases: a bi-directional Mendelian randomization study. Front Endocrinol (Lausanne). (2023) 14:1237971. doi: 10.3389/fendo.2023.1237971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gao K, Cao LF, Ma WZ, Gao YJ, Luo MS, Zhu J, et al. Association between sarcopenia and cardiovascular disease among middle-aged and older adults: Findings from the China health and retirement longitudinal study. EClinicalMedicine. (2022) 44:101264. doi: 10.1016/j.eclinm.2021.101264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kim SH, Cho YK, Kim YJ, Jung CH, Lee WJ, Park JY, et al. Association of the atherogenic index of plasma with cardiovascular risk beyond the traditional risk factors: a nationwide population-based cohort study. Cardiovasc Diabetol. (2022) 21:81. doi: 10.1186/s12933-022-01522-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhu XW, Deng FY, Lei SF. Meta-analysis of Atherogenic Index of Plasma and other lipid parameters in relation to risk of type 2 diabetes mellitus. Prim Care Diabetes. (2015) 9:60–7. doi: 10.1016/j.pcd.2014.03.007 [DOI] [PubMed] [Google Scholar]
- 39. Mazidi M, Katsiki N, Mikhailidis DP, Banach M. Association of ideal cardiovascular health metrics with serum uric acid, inflammation and atherogenic index of plasma: A population-based survey. Atherosclerosis. (2019) 284:44–9. doi: 10.1016/j.atherosclerosis.2018.09.016 [DOI] [PubMed] [Google Scholar]
- 40. Zhu X, Yu L, Zhou H, Ma Q, Zhou X, Lei T, et al. Atherogenic index of plasma is a novel and better biomarker associated with obesity: a population-based cross-sectional study in China. Lipids Health Dis. (2018) 17:37. doi: 10.1186/s12944-018-0686-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Er LK, Wu S, Chou HH, Hsu LA, Teng MS, Sun YC, et al. Triglyceride glucose-body mass index is a simple and clinically useful surrogate marker for insulin resistance in nondiabetic individuals. PloS One. (2016) 11:e0149731. doi: 10.1371/journal.pone.0149731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lim J, Kim J, Koo SH, Kwon GC. Comparison of triglyceride glucose index, and related parameters to predict insulin resistance in Korean adults: An analysis of the 2007-2010 Korean National Health and Nutrition Examination Survey. PloS One. (2019) 14:e0212963. doi: 10.1371/journal.pone.0212963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hong S, Chang Y, Jung HS, Yun KE, Shin H, Ryu S. Relative muscle mass and the risk of incident type 2 diabetes: A cohort study. PloS One. (2017) 12:e0188650. doi: 10.1371/journal.pone.0188650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chen Y, Liu C, Hu M. Association between Triglyceride-glucose index and sarcopenia in China: A nationally representative cohort study. Exp Gerontol. (2024) 190:112419. doi: 10.1016/j.exger.2024.112419 [DOI] [PubMed] [Google Scholar]
- 45. Chen R, Zhang L, Zhang M, Wang Y, Liu D, Li Z, et al. The triglyceride-glucose index as a novel marker associated with sarcopenia in non-diabetic patients on maintenance hemodialysis. Ren Fail. (2022) 44:1615–21. doi: 10.1080/0886022X.2022.2128373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lee SW, Youm Y, Lee WJ, Choi W, Chu SH, Park YR, et al. Appendicular skeletal muscle mass and insulin resistance in an elderly korean population: the korean social life, health and aging project-health examination cohort. Diabetes Metab J. (2015) 39:37–45. doi: 10.4093/dmj.2015.39.1.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ferrucci L, Fabbri E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol. (2018) 15:505–22. doi: 10.1038/s41569-018-0064-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. (2011) 473:317–25. doi: 10.1038/nature10146 [DOI] [PubMed] [Google Scholar]
- 49. de Rekeneire N, Peila R, Ding J, Colbert LH, Visser M, Shorr RI, et al. Diabetes, hyperglycemia, and inflammation in older individuals: the health, aging and body composition study. Diabetes Care. (2006) 29:1902–8. doi: 10.2337/dc05-2327 [DOI] [PubMed] [Google Scholar]
- 50. Vozarova B, Weyer C, Lindsay RS, Pratley RE, Bogardus C, Tataranni PA. High white blood cell count is associated with a worsening of insulin sensitivity and predicts the development of type 2 diabetes. Diabetes. (2002) 51:455–61. doi: 10.2337/diabetes.51.2.455 [DOI] [PubMed] [Google Scholar]
- 51. Haddad F, Zaldivar F, Cooper DM, Adams GR. IL-6-induced skeletal muscle atrophy. J Appl Physiol. (1985) 98:911–7. doi: 10.1152/japplphysiol.01026.2004 [DOI] [PubMed] [Google Scholar]
- 52. Goyal R, Faizy AF, Siddiqui SS, Singhai M. Evaluation of TNF-α and IL-6 levels in obese and non-obese diabetics: pre- and postinsulin effects. N Am J Med Sci. (2012) 4:180–4. doi: 10.4103/1947-2714.94944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. King DE, Mainous AG, 3rd, Buchanan TA, Pearson WS. C-reactive protein and glycemic control in adults with diabetes. Diabetes Care. (2003) 26:1535–9. doi: 10.2337/diacare.26.5.1535 [DOI] [PubMed] [Google Scholar]
- 54. Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. (1999) 340:448–54. doi: 10.1056/NEJM199902113400607 [DOI] [PubMed] [Google Scholar]
- 55. Boutin RD, Yao L, Canter RJ, Lenchik L. Sarcopenia: current concepts and imaging implications. AJR Am J Roentgenol. (2015) 205:W255–66. doi: 10.2214/AJR.15.14635 [DOI] [PubMed] [Google Scholar]
- 56. Roubenoff R, Hughes VA. Sarcopenia: current concepts. J Gerontol A Biol Sci Med Sci. (2000) 55:M716–24. doi: 10.1093/gerona/55.12.M716 [DOI] [PubMed] [Google Scholar]
- 57. Sakuma K, Aoi W, Yamaguchi A. Molecular mechanism of sarcopenia and cachexia: recent research advances. Pflugers Arch. (2017) 469:573–91. doi: 10.1007/s00424-016-1933-3 [DOI] [PubMed] [Google Scholar]
- 58. Huang KC, Chiang YF, Huang TC, Chen HY, Lin PH, Ali M, et al. Capsaicin alleviates cisplatin-induced muscle loss and atrophy in vitro and in vivo . J Cachexia Sarcopenia Muscle. (2023) 14:182–97. doi: 10.1002/jcsm.13120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Jensen GL. Inflammation: roles in aging and sarcopenia. JPEN J Parenter Enteral Nutr. (2008) 32:656–9. doi: 10.1177/0148607108324585 [DOI] [PubMed] [Google Scholar]
- 60. Kamper RS, Alcazar J, Andersen LL, Haddock B, Jørgensen NR, Hovind P, et al. Associations between inflammatory markers, body composition, and physical function: the Copenhagen Sarcopenia Study. J Cachexia Sarcopenia Muscle. (2021) 12:1641–52. doi: 10.1002/jcsm.12832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Pan L, Xie W, Fu X, Lu W, Jin H, Lai J, et al. Inflammation and sarcopenia: A focus on circulating inflammatory cytokines. Exp Gerontol. (2021) 154:111544. doi: 10.1016/j.exger.2021.111544 [DOI] [PubMed] [Google Scholar]
- 62. Schaap LA, Pluijm SM, Deeg DJ, Harris TB, Kritchevsky SB, Newman AB, et al. Higher inflammatory marker levels in older persons: associations with 5-year change in muscle mass and muscle strength. J Gerontol A Biol Sci Med Sci. (2009) 64:1183–9. doi: 10.1093/gerona/glp097 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.cdc.gov/nchs/nhanes/index.htm.