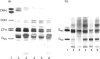Abstract
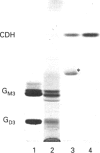
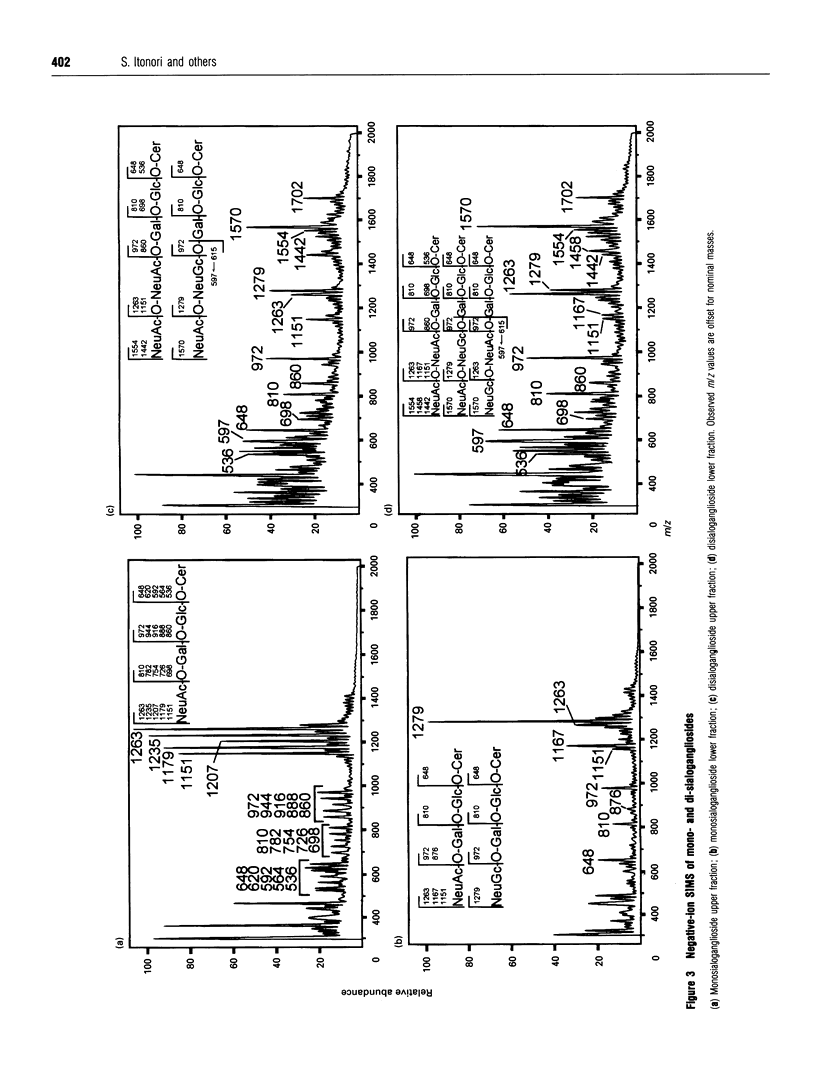
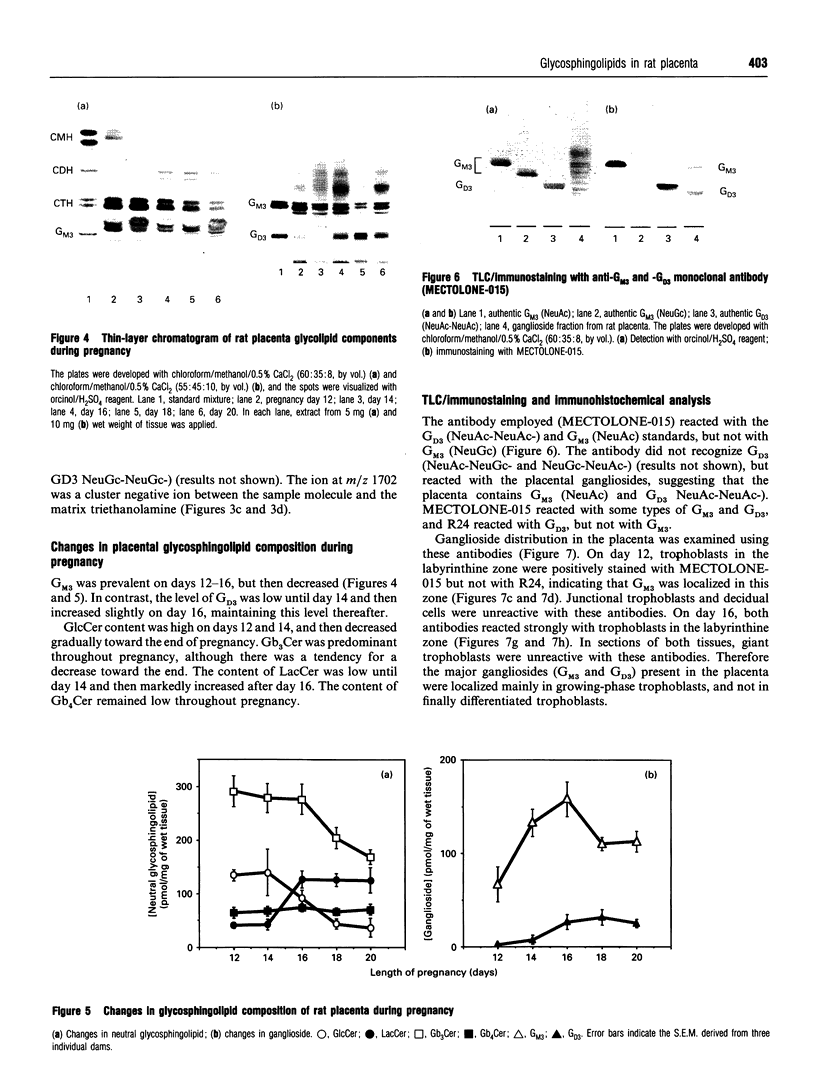
The composition of glycolipids and their changes in the placenta were investigated in the normal pregnant rat. Total lipid fractions extracted from the placenta between days 12 and 20 of pregnancy (day 0 = oestrus) were subjected to glycolipid analysis using DEAE-Sephadex chromatography, silica-gel HPLC, silica-gel TLC, TLC/immunostaining, matrix-assisted secondary-ion mass spectrometry in the negative-ion mode and 1H NMR. Glycolipids identified in the rat placenta were: gangliosides GM3 (NeuAcLacCer and NeuGcLacCer) and GD3 (NeuAcNeuAcLacCer, NeuAcNeuGcLacCer and NeuGcNeuAcLacCer), and neutral glycolipids ceramide monosaccharide (CMH) (GlcCer), ceramide disaccharide (CDH) (LacCer), ceramide trisaccharide (CTH) (Gb3Cer) and ceramide tetrasaccharide (CQH) (Gb4Cer). The content of neutral glycolipids was higher than that of gangliosides throughout pregnancy. Of the neutral glycolipids, CMH and CTH predominated and the level of CDH was low at mid-pregnancy. During late pregnancy, CMH and CTH decreased and CDH increased markedly. CQH remained at a low level throughout pregnancy. Of the gangliosides, GM3 was predominant on days 12-16 and then decreased, whereas GD3, which was low on day 12, increased slightly on day 16 and maintained the same level thereafter. Immunohistochemical studies indicated that these changes in the expression of major gangliosides from GM3 to GD3 occurred in labyrinthine trophoblasts. Thus expression of these glycolipids appears to change markedly during pregnancy.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bremer E. G., Hakomori S., Bowen-Pope D. F., Raines E., Ross R. Ganglioside-mediated modulation of cell growth, growth factor binding, and receptor phosphorylation. J Biol Chem. 1984 Jun 10;259(11):6818–6825. [PubMed] [Google Scholar]
- Bremer E. G., Hakomori S. GM3 ganglioside induces hamster fibroblast growth inhibition in chemically-defined medium: ganglioside may regulate growth factor receptor function. Biochem Biophys Res Commun. 1982 Jun 15;106(3):711–718. doi: 10.1016/0006-291x(82)91769-7. [DOI] [PubMed] [Google Scholar]
- Cheresh D. A., Pierschbacher M. D., Herzig M. A., Mujoo K. Disialogangliosides GD2 and GD3 are involved in the attachment of human melanoma and neuroblastoma cells to extracellular matrix proteins. J Cell Biol. 1986 Mar;102(3):688–696. doi: 10.1083/jcb.102.3.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J., Glasser S. R. Histological and fine structural observations on the placenta of the rat. Acta Anat (Basel) 1968;69(4):542–608. doi: 10.1159/000143100. [DOI] [PubMed] [Google Scholar]
- Furuyama N., Shiota K., Takahashi M. Two distinct placental lactogen-like substances in serum during mid-pregnancy in the rat. Endocrinol Jpn. 1991 Oct;38(5):533–540. doi: 10.1507/endocrj1954.38.533. [DOI] [PubMed] [Google Scholar]
- Hanai N., Dohi T., Nores G. A., Hakomori S. A novel ganglioside, de-N-acetyl-GM3 (II3NeuNH2LacCer), acting as a strong promoter for epidermal growth factor receptor kinase and as a stimulator for cell growth. J Biol Chem. 1988 May 5;263(13):6296–6301. [PubMed] [Google Scholar]
- Hannun Y. A., Bell R. M. Functions of sphingolipids and sphingolipid breakdown products in cellular regulation. Science. 1989 Jan 27;243(4890):500–507. doi: 10.1126/science.2643164. [DOI] [PubMed] [Google Scholar]
- Hansson G. C., Wazniowska K., Rock J. A., Ness P. M., Kickler T. S., Shirey R. S., Niebyl J. R., Zopf D. The glycosphingolipid composition of the placenta of a blood group P fetus delivered by a blood group Pk1 woman and analysis of the anti-globoside antibodies found in maternal serum. Arch Biochem Biophys. 1988 Jan;260(1):168–176. doi: 10.1016/0003-9861(88)90438-9. [DOI] [PubMed] [Google Scholar]
- Hidari K., Itonori S., Sanai Y., Ohashi M., Kasama T., Nagai Y. Isolation and characterization of a monosialosylgangliopentaosyl ceramide from Xenopus laevis oocyte. J Biochem. 1991 Sep;110(3):412–416. doi: 10.1093/oxfordjournals.jbchem.a123595. [DOI] [PubMed] [Google Scholar]
- Hirosawa M., Miura R., Min K. S., Hattori N., Shiota K., Ogawa T. A cDNA encoding a new member of the rat placental lactogen family, PL-I mosaic (PL-Im). Endocr J. 1994 Aug;41(4):387–397. doi: 10.1507/endocrj.41.387. [DOI] [PubMed] [Google Scholar]
- Houghton A. N., Mintzer D., Cordon-Cardo C., Welt S., Fliegel B., Vadhan S., Carswell E., Melamed M. R., Oettgen H. F., Old L. J. Mouse monoclonal IgG3 antibody detecting GD3 ganglioside: a phase I trial in patients with malignant melanoma. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1242–1246. doi: 10.1073/pnas.82.4.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itonori S., Hidari K., Sanai Y., Taniguchi M., Nagai Y. Involvement of the acyl chain of ceramide in carbohydrate recognition by an anti-glycolipid monoclonal antibody: the case of an anti-melanoma antibody, M2590, to GM3-ganglioside. Glycoconj J. 1989;6(4):551–560. doi: 10.1007/BF01053777. [DOI] [PubMed] [Google Scholar]
- Kawano T., Kozutsumi Y., Takematsu H., Kawasaki T., Suzuki A. Regulation of biosynthesis of N-glycolylneuraminic acid-containing glycoconjugates: characterization of factors required for NADH-dependent cytidine 5'monophosphate-N-acetylneuraminic acid hydroxylation. Glycoconj J. 1993 Feb;10(1):109–115. doi: 10.1007/BF00731194. [DOI] [PubMed] [Google Scholar]
- Kotani M., Kawashima I., Ozawa H., Terashima T., Tai T. Differential distribution of major gangliosides in rat central nervous system detected by specific monoclonal antibodies. Glycobiology. 1993 Apr;3(2):137–146. doi: 10.1093/glycob/3.2.137. [DOI] [PubMed] [Google Scholar]
- Lampio A., Airaksinen A., Maaheimo H. UDP-galactose:lactosylceramide alpha-galactosyltransferase activity in human placenta. Glycoconj J. 1993 Apr;10(2):165–169. doi: 10.1007/BF00737713. [DOI] [PubMed] [Google Scholar]
- Levery S. B., Nudelman E. D., Salyan M. E., Hakomori S. Novel tri-and tetrasialosylpoly-N-acetyllactosaminyl gangliosides of human placenta: structure determination of pentadeca- and eicosaglycosylceramides by methylation analysis, fast atom bombardment mass spectrometry, and 1H NMR spectroscopy. Biochemistry. 1989 Sep 19;28(19):7772–7781. doi: 10.1021/bi00445a037. [DOI] [PubMed] [Google Scholar]
- Mitchell M. D., Trautman M. S., Dudley D. J. Cytokine networking in the placenta. Placenta. 1993 May-Jun;14(3):249–275. doi: 10.1016/s0143-4004(05)80426-6. [DOI] [PubMed] [Google Scholar]
- Ozawa H., Kawashima I., Tai T. Generation of murine monoclonal antibodies specific for N-glycolylneuraminic acid-containing gangliosides. Arch Biochem Biophys. 1992 May 1;294(2):427–433. doi: 10.1016/0003-9861(92)90707-4. [DOI] [PubMed] [Google Scholar]
- Pukel C. S., Lloyd K. O., Travassos L. R., Dippold W. G., Oettgen H. F., Old L. J. GD3, a prominent ganglioside of human melanoma. Detection and characterisation by mouse monoclonal antibody. J Exp Med. 1982 Apr 1;155(4):1133–1147. doi: 10.1084/jem.155.4.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg A., Sauer A., Noble E. P., Gross H. J., Chang R., Brossmer R. Developmental patterns of ganglioside sialosylation coincident with neuritogenesis in cultured embryonic chick brain neurons. J Biol Chem. 1992 May 25;267(15):10607–10612. [PubMed] [Google Scholar]
- Rueda R., Tabsh K., Ladisch S. Detection of complex gangliosides in human amniotic fluid. FEBS Lett. 1993 Aug 9;328(1-2):13–16. doi: 10.1016/0014-5793(93)80955-t. [DOI] [PubMed] [Google Scholar]
- SVENNERHOLM L. CHROMATOGRAPHIC SEPARATION OF HUMAN BRAIN GANGLIOSIDES. J Neurochem. 1963 Sep;10:613–623. doi: 10.1111/j.1471-4159.1963.tb08933.x. [DOI] [PubMed] [Google Scholar]
- Saito T., Hakomori S. I. Quantitative isolation of total glycosphingolipids from animal cells. J Lipid Res. 1971 Mar;12(2):257–259. [PubMed] [Google Scholar]
- Sariola H., Aufderheide E., Bernhard H., Henke-Fahle S., Dippold W., Ekblom P. Antibodies to cell surface ganglioside GD3 perturb inductive epithelial-mesenchymal interactions. Cell. 1988 Jul 15;54(2):235–245. doi: 10.1016/0092-8674(88)90556-9. [DOI] [PubMed] [Google Scholar]
- Shaw L., Schneckenburger P., Carlsen J., Christiansen K., Schauer R. Mouse liver cytidine-5'-monophosphate-N-acetylneuraminic acid hydroxylase. Catalytic function and regulation. Eur J Biochem. 1992 May 15;206(1):269–277. doi: 10.1111/j.1432-1033.1992.tb16925.x. [DOI] [PubMed] [Google Scholar]
- Shiota K., Furuyama N., Takahashi M. Placental lactogen secretion during prolonged-pregnancy in the rat: the ovary plays a pivotal role in the control of placental function. Endocrinol Jpn. 1991 Oct;38(5):541–549. doi: 10.1507/endocrj1954.38.541. [DOI] [PubMed] [Google Scholar]
- Soares M. J., Faria T. N., Roby K. F., Deb S. Pregnancy and the prolactin family of hormones: coordination of anterior pituitary, uterine, and placental expression. Endocr Rev. 1991 Nov;12(4):402–423. doi: 10.1210/edrv-12-4-402. [DOI] [PubMed] [Google Scholar]
- Song W. X., Vacca M. F., Welti R., Rintoul D. A. Effects of gangliosides GM3 and De-N-acetyl GM3 on epidermal growth factor receptor kinase activity and cell growth. J Biol Chem. 1991 Jun 5;266(16):10174–10181. [PubMed] [Google Scholar]
- Svejcar J., Ehrlich-Rogozinski S., Riedel D., Müthing J., Sharon N. Developmental changes in neutral glycosphingolipids of mouse placenta. Glycoconj J. 1993 Jun;10(3):247–250. doi: 10.1007/BF00702207. [DOI] [PubMed] [Google Scholar]
- Svennerholm L. Gangliosides and other glycolipids of human placenta. Acta Chem Scand. 1965;19(6):1506–1507. doi: 10.3891/acta.chem.scand.19-1506. [DOI] [PubMed] [Google Scholar]
- Tai T., Kawashima I., Furukawa K., Lloyd K. O. Monoclonal antibody R24 distinguishes between different N-acetyl- and N-glycolylneuraminic acid derivatives of ganglioside GD3. Arch Biochem Biophys. 1988 Jan;260(1):51–55. doi: 10.1016/0003-9861(88)90423-7. [DOI] [PubMed] [Google Scholar]
- Taki T., Matsuo K., Yamamoto K., Matsubara T., Hayashi A., Abe T., Matsumoto M. Human placenta gangliosides. Lipids. 1988 Mar;23(3):192–198. doi: 10.1007/BF02535457. [DOI] [PubMed] [Google Scholar]
- Yu R. K., Macala L. J., Taki T., Weinfield H. M., Yu F. S. Developmental changes in ganglioside composition and synthesis in embryonic rat brain. J Neurochem. 1988 Jun;50(6):1825–1829. doi: 10.1111/j.1471-4159.1988.tb02484.x. [DOI] [PubMed] [Google Scholar]
- Zheng M., Fang H., Tsuruoka T., Tsuji T., Sasaki T., Hakomori S. Regulatory role of GM3 ganglioside in alpha 5 beta 1 integrin receptor for fibronectin-mediated adhesion of FUA169 cells. J Biol Chem. 1993 Jan 25;268(3):2217–2222. [PubMed] [Google Scholar]
- von dem Borne A. E., Bos M. J., Joustra-Maas N., Tromp J. F., van't Veer M. B., van Wijngaarden-du Bois R., Tetteroo P. A. A murine monoclonal IgM antibody specific for blood group P antigen (globoside) Br J Haematol. 1986 May;63(1):35–46. doi: 10.1111/j.1365-2141.1986.tb07492.x. [DOI] [PubMed] [Google Scholar]