Abstract
Objectives
The pathophysiology of multiple sclerosis (MS) involves inflammatory neurodegeneration in the brainstem, cerebellum, and retina. The clinical relevance of oculomotor involvement in MS, however, remains uncertain.
Methods
In this cross-sectional study, we evaluated heterophoria as a (sub)clinical tool in 54 MS patients and 55 age-matched healthy controls (HCs). We quantified heterophoria in prism diopters for distance and near range with orthoptic examination. Our primary outcome was high degrees of horizontal heterophoria (HDHH) defined as measurements beyond ±2 standard deviations from the mean prism diopter of heterophoria of our HCs.
Results
More than one-third (37%, n=20/54) of MS patients but only 11% (n=6/55) of HCs were classified as HDHH [distance, MS=9% (n=5/54) versus HC=6% (n=3/55); near, MS=19% (n=10/54) versus HC=5% (n=3/55)]. Our MS patients presented more combined vertical and horizontal deviations at near range [MS 19% (n=10/54) versus for HC 7% (n=4/55)]. We observed the combination of HDHH both at distance and at near testing in 9% (n=5/54) of MS patients but not at all in HCs (n=0/55).
Discussion
Despite the high prevalence of heterophoria, HDHH may be an additional (sub)clinical tool of subclinical involvement in MS. Thus, orthoptic examination may be an additional tool to improve MS diagnostic procedures.
Keywords: multiple sclerosis, heterophoria, clinical assessment, screening, orthoptic assessment
Introduction
The pathophysiology of multiple sclerosis (MS) involves inflammatory neurodegeneration in the brainstem and cerebellum. In-depth neuro-ophthalmological examinations are increasingly relevant for characterizing MS patients (1, 2). While retinal changes in MS have been extensively characterized and associated with disease progression (3, 4), the precise profile and extent of oculomotor involvement in MS compared to healthy control (HCs) remains less certain. Subtle inflammation and/or degeneration of the central nervous system may lead to heterophoria (5) or to manifest heterotropia (6) in MS patients. Considering that heterophoria and/or subclinical binocular vision anomalies in the general population may be considered common (7–9), we hypothesized that heterophoria is also frequently found in MS patients potentially in a more pronounced form. Brainstem/cerebellar pathology is known to be associated with a poor MS prognosis (10) and (subclinical) changes of binocular vision (11, 12). Measurement of heterophoria may become a valuable tool for characterizing MS patients.
Patients and methods
Patients and HCs were prospectively recruited at the Department of Neurology, Heinrich Heine University, Düsseldorf, Germany between 2018 and 2021. Inclusion criteria were >18 years of age and an MS diagnosis according to the McDonald criteria 2017. A complete and signed declaration of consent was required for participation. Patients with an acute relapse involving vision or brainstem functions and those with manifest oculomotor symptoms or heterotropia were excluded. However, due to the monocentric study design and heterogeneous follow-up times of the patients, we were not able to exclude patients with a history of prior relapses involving the brainstem or optic nerves.
All participants underwent orthoptic examinations following a defined protocol performed by a trained optometrist. Assessments included the alternating cover and Maddox rod test in primary position of the eyes, and full/low (100%/2.5%) habitually corrected contrast visual acuity testing using SLOAN charts of the Early Treatment Diabetic Retinopathy Study (ETDRS, Precision Vision, Inc.) at a distance of 2 m. A unilateral and alternating cover test was performed at 6 m (distance) and 40 cm (near) to separate heterotropia from heterophoria. Horizontal (esophoria/exophoria) and/or vertical (hypophoria/hyperphoria) heterophoria was measured. The Maddox rod and corresponding tangent scale (Maddox cross) were used to quantify the horizontal and vertical deviation in prism diopters for each eye using a prism bar at distance (6 m) and near (40 cm) range. Maddox rod test–retest reliability was assessed with the same structured procedure in an independent cohort of 15 MS patients ( Supplementary Figure S1 ) prior to formal study recruitment.
A high degree of horizontal heterophoria (HDHH) was defined as a heterophoria exceeding the mean value ±2 standard deviations of the prism diopter angle in HCs. ( Supplementary Table S1 ).
Statistical evaluations
Statistical analyses were performed using IBM SPSS Statistics (version 20). The intraclass correlation coefficient was calculated using a two-way mixed model and absolute agreement type to evaluate the test–retest reliability of our assessments ( Supplementary Figure S1 ).
Group comparisons between patients with MS and HC (independent variable) for continuous data [prism diopter (dependent variable)] were performed using Generalized Estimating Equation (GEE) models [model: constant term, analysis-type III (Wald)] with an exchangeable correlation matrix including all eyes, correcting for within subject inter-eye correlations, age, and sex for strength of deviation (horizontal, vertical) at distance and near range ( Supplementary Table S2 ). Probability values (p, two-tailed) <0.05 were considered significant.
To assess and quantify the strength of association between determined heterophorias (i.e., the prism diopter angle measured using Maddox rod test and alternating cover test, distance and near range), HDHH and history of double vision (DV) and group (MS vs. HCs), odds ratio (OR), and the associated 95% confidence interval (95%CI) were calculated. 95%CIs not including 1 were considered statistically significant.
Results
To investigate reproducibility, we repeated Maddox rod and alternating cover test assessments on consecutive days and at morning and evening in 15 MS patients. Test–retest reliability was excellent with intraclass correlation coefficients >0.85 for all testing conditions ( Supplementary Figure S1 ).
We included a cohort of 54 MS patients with a predominantly relapsing disease course (67% of patients) and 55 age-matched HCs ( Table 1 and Supplementary Figure S2 ). The mean age of MS patients was 41.9 ± 11.8 years. The mean disease duration was 11.9 ± 9.3 years, with a mean EDSS of 3.4 ± 2.2. The alternating cover test revealed that a higher proportion of MS patients had exophoria both at distance [MS n=13/54 (24%) versus HCs n=0/50 (0%), OR=1.32 (95%CI: 1.13–1.53)] and in near range [MS n=24/54 (44%) versus HCs n=14/50 (28%), OR=2.06 (95%CI: 0.91–4.67), Figures 1A, B ] compared to HCs. Upon Maddox rod testing, a more complex picture emerged with higher proportions of heterophoria and combined deviations in both MS patients and HCs [e.g., exophoria near; MS n=27/54 (50%) versus HCs n=27/55 (49%), Figures 1C, D ]. To scrutinize if unidirectional or combined heterophoria forms have a greater potential for distinguishing MS patients and HCs, we characterized heterophoria patterns ( Supplementary Figures S3A, B ). Here, our MS patients presented a trend to more combined horizontal/vertical deviations than HCs at near [MS n=10/54 (19%) versus HCs n=4/55 (7%), OR=2.90 (95%CI: 0.85–9.89)] and at distance [MS n=19/51 (37%) versus HCs n=16/54 (30%), OR=1.41 (95%CI: 0.63–3.18)]. Considered individually, the presence of horizontal and vertical deviations was similar between both groups ( Supplementary Table S2 ). The degree of horizontal phorias was more broadly distributed in MS patients compared to HCs, while the distribution of the degree of vertical phorias was similar between groups at both distances ( Supplementary Figures S3A, B ). The mean prism diopter value of vertical phorias did not deviate from zero prism diopters [(mean prism diopters ± SD) distance: MS 0 ± 0.7 prism diopters versus HCs 0 ± 0.5 prism diopters, p=0.689; near: MS 0 ± 0.7 prism diopters versus HCs 0 ± 0.5 prism diopters, p=0.881, Supplementary Figure S4 ]. Therefore, we focused on horizontal heterophoria analyses. The prevalence of horizontal heterophoria was similar between groups [ Supplementary Table S2 , e.g., esophoria distance: MS n=17/51 (33%) versus HCs n=25/54 (46%), OR=0.58 (95%CI: 0.26–1.28)]. The mean prism diopter value for the horizontal axis was shifted towards esophoria at distance and towards exophoria at near examination, in both HCs and MS cohort. The mean prism diopter value for exophoria was significantly more pronounced at near examination in MS patients [(mean prism diopters ± SD) distance: MS +0.8 ± 3.7 prism diopters versus HCs +1.4 ± 2.2 prism diopters, p=0.299, GEE; near: MS −4.5 ± 6.3 prism diopters versus HCs −2.3 ± 3.9 prism diopters, p=0.014, GEE]. We observed marked horizontal heterophoria in MS patients: 37% of MS patients (n=20/54) classified as HDHH, as compared to 11% of HCs [n=6/55; distance: OR=1.85 (95%CI: 0.42–8.17); near: OR=3.94 (95%CI: 1.02–15.22), Supplementary Figure S5 , Supplementary Table S2 ]. We observed the combination of HDHH both at distance and at near testing in n=5/54 (9%) MS patients but not at all in HCs, OR=1.1 (95%CI: 1.01–1.2). This was particularly prominent for exophoric deviations at near range: we found a significantly larger proportion of HDHH in our MS cohort, associated with higher angles of deviation. In order to take into account the influence of other clinical parameters such as previous optic neuritis (ON) or history of previous double vision (DV)/diplopia, these parameters ( Table 1 ) were also determined. According to records and history, 27 (50%) of our 54 MS patients had previous ON [MS-HDHH n=10/20 (50%), MS-non-HDHH n=17/34 (50%)]. However, the proportion of patients with a history of DV at any time point after MS diagnosis was significantly larger in the HDHH group with a moderate association [MS-HDHH n=16/20 (80%), MS-non-HDHH n=14/34 (41%), OR=5.71 (95%CI: 1.57–20.78)].
Table 1.
Demographic, clinical, and visual characteristics of the multiple sclerosis (MS) patients and healthy control (HC) participants.
| Characteristics | MS patients | HC participants | ||||
|---|---|---|---|---|---|---|
| TOTAL n = 54 |
HDHH n = 20 (37%) |
Non-HDHH n = 34 (63%) |
Total n = 55 |
HDHH n = 6 (11%) |
Non-HDHH n = 49 (89%) |
|
| Demographic | ||||||
| Age in years; mean (SD) | 41.9 (± 11.8) | 39.8 (± 13.4) | 43.2 (± 10.9) | 41.5 (± 13.2) | 49.3 (± 8.7) | 40.6 (± 13.4) |
| Female, n (%) | 39 (72) | 12 (60) | 27 (79) | 35 (64) | 2 (33) | 33 (67) |
| Visual | ||||||
|
HCVA; LogMAR
mean (SD) |
0.07 (± 0.32) | 0.09 (± 0.23) | 0.05 (± 0.36) | -0.04 (± 0.1) | -0.01 (± 0.1) | -0.04 (± 0.1) |
|
LCVA (2.5%); Letter
mean (SD) |
25 (± 14) | 21 (± 14) | 27 (± 13) | 40 (± 8) | 38 (± 9) | 40 (± 8) |
| Clinical | ||||||
| RRMS, n (%) | 36 (67) | 11 (55) | 25 (73) | N/A | N/A | N/A |
| SPMS, n (%) | 13 (24) | 7 (35) | 6 (18) | N/A | N/A | N/A |
| PPMS, n (%) | 5 (9) | 2 (10) | 3 (9) | N/A | N/A | N/A |
| Age at symptom onset, year; mean (SD) | 29.5 (± 9.2) | 26.1 (± 8.6) | 32.1 (± 9.4) | N/A | N/A | N/A |
|
Age at diagnosis,
year; mean (SD) |
32.5 (± 10.6) | 28.6 (± 11.3) | 34.8 (± 9.7) | N/A | N/A | N/A |
| Disease duration in year; mean (SD) | 11.9 (± 9.3) | 12.5 (± 9.2) | 11.4 (± 9.4) | N/A | N/A | N/A |
| EDSS at orthoptic assessment, mean (SD) | 3.4 (± 2.2) | 4.2 (± 2.3) | 3.0 (± 2.1) | N/A | N/A | N/A |
| History of ON pat., n (%) | 27 (50) | 10 (50) | 17 (50) | N/A | N/A | N/A |
| History of diplopia, n (%) | 30 (55.6) | 16 (80) | 14 (41) | N/A | N/A | N/A |
| Therapy | ||||||
| None, n (%) | 17 (32) | 6 (30) | 11 (33) | N/A | N/A | N/A |
| Platform a , n (%) | 12 (23) | 3 (15) | 9 (27) | N/A | N/A | N/A |
| High efficacy b , n (%) | 24 (45) | 11 (55) | 13 (40) | N/A | N/A | N/A |
EDSS, expanded disability status scale; ON, optic neuritis; SD, standard deviation; H/LCVA, high/low contrast visual acuity; RR/SP/PPMS, relapsing–remitting/secondary progressive/primary progressive multiple sclerosis, HDHH, high degree of horizontal heterophoria (outside the defined limits for horizontal heterophoria angles); non-HDHH, the part of the cohort inside of the defined limits for horizontal heterophoria.
Platform therapies were defined as Glatirameracetat, Dimethylfumarat, Teriflunomid, Interferon-ß-1b s.c., and Peginterferon.
High-efficacy therapies were defined as Fingolimod, Ocrelizumab, Natalizumab, Alemtuzumab, Rituximab, Mitoxantron, Cladribin, and Azathioprin.
Figure 1.
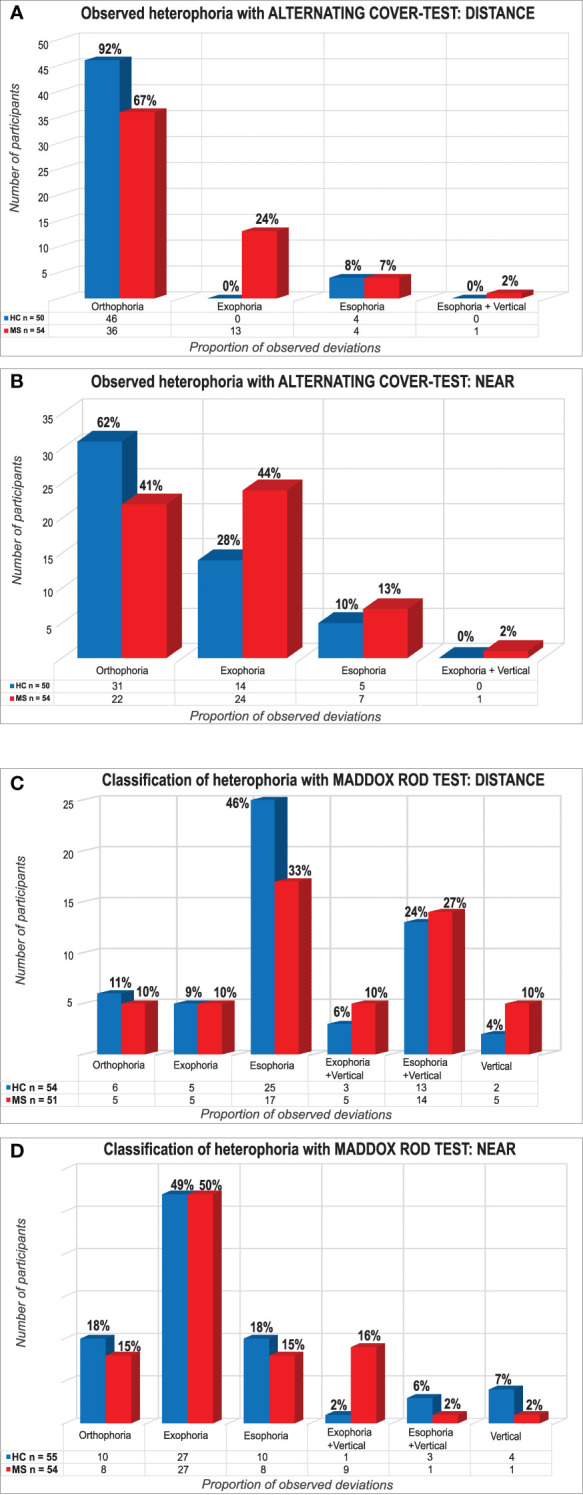
Proportion of multiple sclerosis (MS) patients and healthy controls (HCs) with and without heterophoria. Plots show results of the alternating cover test for distance (A) and near (B) range and the Maddox rod method for distance (C) and near (D) range, grouped by orthophoria, exophoria, and esophoria (“+ vertical” indicates the presence of an additional vertical deviation). Lack of Measurement data for five HCs (A, B). One HC and three MS patients were excluded because of suppression of fixation light distance (C).
Discussion
Characterizing oculomotor MS manifestations has been of special interest since the very first description of the disease by Charcot and the MS-related visual symptoms described by Uhthoff more than a century ago (13), as abnormalities of the afferent visual system and eye movements are common at any stage and course of the disease (14, 15). While these are accepted as integral parts of the clinical picture (1, 16), systematic characterizations of subclinical changes in binocular vision in MS based on orthoptic examination are lacking (17). In our cohort, we observed a higher prevalence of higher degrees of heterophoria in MS patients versus HCs. Specifically, HDHH was found in 37% of MS patients, more than three times the rate observed in HCs (11%). These orthoptic abnormalities may reflect subtle brainstem and cerebellar involvement in disease. These deviations may direct clinicians to investigate infratentorial demyelination, a known prognostic factor (10). To summarize, MS is a disease of the central nervous system, and inflammatory infiltrates involving the brain stem have commonly been identified by MRI imaging and histopathology (18, 19). While these often result in overt clinical manifestations such as oculomotor symptoms like double vision, we suggest that the high rates of HHO observed in our study are likely to be a subclinical manifestation of such pathology involving the oculomotor system in the brainstem. Further studies including high-resolution brain stem MRI imaging are warranted to corroborate this assumption.
Strengths of our study include the use of two independent orthoptic examination techniques, the exclusion of heterotropia, a good test–retest reliability, and the comparison with HCs. Limitations include the rather small sample size and the cross-sectional study design, which precludes prognostic or diagnostic conclusions from our observations. The examiner of the orthoptic measurements was not masked and was therefore aware of the subject’s diagnosis. In addition, no (cycloplegic) refraction or assessment of possible accommodation disorders and the influence of reduced visual acuity compared to the HCs was performed. Likewise, an effect of possible fatigue in the MS cohort cannot be completely ruled out. We cannot exclude that a history of ON may have influenced our findings. However, the similar rates of previous ON in the HDHH and the non-HDHH groups argue against a major influence of a history of ON.
At the same time, the higher rates of a history of a previous DV in the HDHH group may suggest that the HDHH may result from previous lesions involving the oculomotor system, which have since resolved. Thus, the presence of a higher grade heterophoria might be promising as a (sub)clinical tool for MS lesions that involve the oculomotor system. The relevance of our results should be confirmed in larger, longitudinal studies.
Funding Statement
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Data availability statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Heinrich-Heine-University Düsseldorf, registry number 5758R. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
JG: Writing – review & editing, Writing – original draft, Methodology, Investigation, Conceptualization. MW: Writing – review & editing, Writing – original draft, Visualization, Methodology, Formal analysis. TG: Writing – review & editing, Methodology, Investigation. CB: Writing – review & editing, Methodology, Formal analysis. MG: Writing – review & editing, Methodology. HL: Writing – review & editing, Methodology, Investigation. SK: Writing – review & editing, Methodology, Investigation, Conceptualization. WL: Writing – review & editing. SM: Writing – review & editing, Supervision, Conceptualization. OA: Writing – review & editing, Project administration. PA: Writing – review & editing, Writing – original draft, Supervision, Resources, Project administration, Methodology, Investigation, Formal analysis, Conceptualization.
Conflict of interest
JG has received within the past 3 years a Research Fellowship from the German Research Foundation DFG, project number 438899010. SM received honoraria for lecturing and travel expenses for attending meetings from Almirall, Amicus Therapeutics Germany, Bayer Health Care, Biogen, Celgene, Diamed, Genzyme, MedDay Pharmaceuticals, Merck Serono, Novartis, Novo Nordisk, ONO Pharma, Roche, Sanofi-Aventis, Chugai Pharma, QuintilesIMS, and Teva. His research funded by the German Ministry for Education and Research BMBF, German Research Foundation DFG, Else Kröner Fresenius Foundation, German Academic Exchange Service, Hertie Foundation, Interdisciplinary Center for Clinical Studies IZKF Muenster, German Foundation Neurology, and by Almirall, Amicus Therapeutics Germany, Biogen, Diamed, Fresenius Medical Care, Genzyme, Merck Serono, Novartis, ONO Pharma, Roche, and Teva. OA received, with approval of the Rector of Heinrich-Heine University, grants from the German Research Foundation DFG, the German Ministry for Education and Research BMBF as part of the German Competence Network Multiple Sclerosis KKNMS; for NEMOS NationNMO-PAT FKZ 01GI1602B, the Eugène Devic European Network EU-FP7, honoraria and travel/accommodation/meeting expenses from Alexion, Almirall, Biogen, Horizon, Janssen Cilag, Merck Serono, Novartis, Roche, and Sanofi-Genzyme. OA is founding member and co-coordinator of the German Neuromyelitis optica-Study Group NEMOS, www.nemos-net.de and member of the European Reference Network for Rare Eye Diseases ERN-EYE, co-funded by the Health Program of the European Union under the Framework Partnership Agreement No 739534 “ERN-EYE.” PA received, with approval of the Rector of Heinrich-Heine University and the CEO of University of Düsseldorf Hospital, personal fees, research grants, and/or non-financial support from Allergan, Biogen, Bristol Myers Squibb, Celgene, German Research Foundation DFG, EFRE-NRW, Janssen Cilag, Ipsen, Merck Serono, Merz Pharmaceuticals, Novartis, and Roche, and personal fees and non-financial support from Bayer Healthcare, Teva, and Sanofi-Aventis/Genzyme, outside the submitted work.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1431394/full#supplementary-material
References
- 1. Roberg BL, Bruce JM, Feaster HT, O’Bryan SR, Westervelt HJ, Glusman M, et al. Speedy eye movements in multiple sclerosis: association with performance on visual and nonvisual cognitive tests. J Clin Exp Neuropsychol. (2015) 37:1–15. doi: 10.1080/13803395.2014.983465 [DOI] [PubMed] [Google Scholar]
- 2. Moroso A, Ruet A, Deloire M, Lamargue-Hamel D, Cubizolle S, Charré-Morin J, et al. Cerebellar assessment in early multiple sclerosis. Cerebellum. (2017) 16:607–11. doi: 10.1007/s12311-016-0831-8 [DOI] [PubMed] [Google Scholar]
- 3. Albrecht P, Ringelstein M, Müller AK, Keser N, Dietlein T, Lappas A, et al. Degeneration of retinal layers in multiple sclerosis subtypes quantified by optical coherence tomography. Mult Scler. (2012) 18:1422–9. doi: 10.1177/1352458512439237 [DOI] [PubMed] [Google Scholar]
- 4. Martinez-Lapiscina EH, Arnow S, Wilson JA, et al. Retinal thickness measured with optical coherence tomography and risk of disability worsening in multiple sclerosis: a cohort study. Lancet Neurol. (2016) 15:574–84. doi: 10.1016/S1474-4422(16)00068-5 [DOI] [PubMed] [Google Scholar]
- 5. Gil-Casas A, Piñero-Llorens DP and Molina-Martin A. Binocular vision in patients with multiple sclerosis. Clin Optom (Auckl). (2021) 13:39–49. doi: 10.2147/OPTO.S286862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Reche Sainz JA, Espinet Badia R, Puig Ganau T. Divergence insufficiency and demyelinating disorder. Eur J Ophthalmol. (2002) 12:238–40. doi: 10.1177/112067210201200312 [DOI] [PubMed] [Google Scholar]
- 7. Dowley D. The orthophorization of heterophoria. Ophthal Physiol Opt. (1987) 7:169–74. doi: 10.1111/j.1475-1313.1987.tb01016.x [DOI] [PubMed] [Google Scholar]
- 8. Stidwill D. Epidemiology of strabismus. Ophthal Physiol Opt. (1997) 17:536–9. doi: 10.1111/j.1475-1313.1997.tb00094.x [DOI] [PubMed] [Google Scholar]
- 9. Larsson E, Holmström G and Rydberg A. Ophthalmological findings in 10-year-old full-term children–a population-based study. Acta Ophthalmol. (2015) 93:192–8. doi: 10.1111/aos.12476 [DOI] [PubMed] [Google Scholar]
- 10. Minneboo A, Barkhof F, Polman CH, Uitdehaag BMJ, Knol DL, Castelijns JA, et al. Infratentorial lesions predict long-term disability in patients with initial findings suggestive of multiple sclerosis. Arch Neurol. (2004) 61:217–21. doi: 10.1001/archneur.61.2.217 [DOI] [PubMed] [Google Scholar]
- 11. Teufel J, Strupp M, Linn J, Kalla R, Feil K. Conjugate Eye Deviation in Unilateral Lateral Medullary Infarction. J Clin Neurol (Seoul Korea). (2019) 15:228–34. doi: 10.3988/jcn.2019.15.2.228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Arnold AC. Ophthalmic manifestations of multiple sclerosis. Semin Ophthalmol. (1988) 3:229–43. doi: 10.3109/08820538809063825 [DOI] [Google Scholar]
- 13. Uhthoff W. Untersuchungen über die bei der multiplen Herdsklerose vorkommenden Augenstörungen. Archiv f Psychiatr. (1890) 21:55–116. doi: 10.1007/BF02162972 [DOI] [Google Scholar]
- 14. McDonald WI, Barnes D. The ocular manifestations of multiple sclerosis. 1. Abnormalities of the afferent visual system. J neurol neurosurg Psychiatry. (1992) 55:747–52. doi: 10.1136/jnnp.55.9.747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Barnes D, McDonald WI. The ocular manifestations of multiple sclerosis. 2. Abnormalities of eye movements. J neurol neurosurg Psychiatry. (1992) 55:863–8. doi: 10.1136/jnnp.55.10.863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Graves J, Balcer LJ. Eye disorders in patients with multiple sclerosis: natural history and management. Clin Ophthalmol (Auckland N.Z.). (2010) 4:1409–22. doi: 10.2147/OPTH.S6383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Casillas Casillas E, Rosenfield M. Comparison of subjective heterophoria testing with a phoropter and trial frame. Optom Vis Sci. (2006) 83:237–41. doi: 10.1097/01.opx.0000214316.50270.24 [DOI] [PubMed] [Google Scholar]
- 18. International Multiple Sclerosis Genetics Consortium and MultipleMS Consortium . Locus for severity implicates CNS resilience in progression of multiple sclerosis. Nature. (2023) 619:323–31. doi: 10.1038/s41586-023-06250-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Habek M. Evaluation of brainstem involvement in multiple sclerosis. Expert Rev Neurother. (2013) 13:299–311. doi: 10.1586/ern.13.18 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding author.


