Abstract
The Ideal Plant Architecture 1 (IPA1) transcription factor promotes rice yield and immunity through phosphorylation at its amino acid residue Ser163 as a switch. Although phosphorylated IPA1 mimic, IPA1(S163D), directly targets the promoter of immune response gene WRKY45, it cannot activate its expression. Here, we identified a co-activator of IPA1(S163D), a RING-finger E3 ligase IPA1 interactor 7 (IPI7), which fine-tunes the transcriptional activity of IPA1 to timely promote plant immunity and simultaneously maintain growth for yield. IPI7 interacts with IPA1 and promotes K29-polyubiquitination of IPA1 in vitro and in vivo. However, the stability of IPA1 protein is not affected by IPI7-mediated ubiquitination. The IPI7-promoted K29-polyubiquitination of IPA1 is induced by Magnaporthe oryzae infection and required for phosphorylated IPA1 to transactivate WRKY45 expression for immune response but not for plain IPA1 to transactivate DENSE AND ERECT PANICLES 1 (DEP1) expression for panicle development. IPI7 knockout impairs IPA1-mediated immunity but not yield. Our study reveals that plants utilize non-proteolytic K29-ubiquitination as a response to pathogen infection to fine-tune IPA1 transactivation activity for promoting immunity.
Subject terms: Plant immunity, Post-translational modifications, Plant molecular biology
In this paper, the E3 ubiquitin ligase IPI7 is found to fine-tune the transactivation activity of IPA1 through non-hydrolyzed K29-ubiquitination chain, thereby timely regulating rice disease resistance during Magnaporthe oryzae infection.
Introduction
To protect themselves from pathogen attacks, plants have developed a sophisticated immune system that includes pathogen-associated molecular pattern (PAMP)-triggered immunity (PTI) and effector-triggered immunity (ETI)1,2. Plants can recognize different kinds of pathogens and rapidly activate immune signaling pathways within individual cells to trigger a series of events, such as activation of mitogen activated protein kinase (MAPK) cascades, induction of pathogenesis-related (PR) genes, production of reactive oxygen species (ROS), and deposition of callose and lignin3.
Post-translational modifications (PTMs) are ubiquitous regulatory mechanisms critical for cell signaling transduction, regulating the molecular switches and crosstalk between linked pathways spatially and temporally. Ubiquitination is one of the major PTMs that regulates a wide range of cellular processes, including hormone signaling, cell cycle, plant senescence, flowering, plant architecture generation and the immune response4–10. Ubiquitination of a protein substrate requires a sequential activation of three enzymes: ubiquitin (Ub)-activating enzyme (E1), Ub-conjugating enzyme (E2), and Ub ligase (E3). Activated Ub is transferred from E1 to E2. Then, E2 forms a complex with E3 which transfers Ub to the substrate. The E1-E2-E3 enzymatic cascade is a repetitive process, resulting in the formation of mono-ubiquitin or a poly-Ub chain11. Ubiquitinated proteins undergo quite different fates depending on the number and site of the attached Ub molecules12. In plants, mono Ub in a cell can serve as a signal for endosomal punctual and histone structural change as well as protein stability13–15. The K48-linked poly-Ub chain is the first identified poly-Ub chain, helping the substrate to enter 26S proteasome for degradation16. Many non‐K48‐linked types of ubiquitination serve in non‐proteolytic roles, such as K63-linked ubiquitination, that often function in regulation of protein stability, internalization and sorting5,8,17. Although a plethora of novel data have emerged regarding the molecular functions of mono-, K48-, and K63-ubiquitin chain, rarely studies reported on other ubiquitin chains, especially in the plant kingdom.
During the ubiquitination process, E3 ligases play a critical role in determining substrate specificity for target proteins. Based on their conserved domains, E3 ligases can be divided into various types, such as Really Interesting New Gene (RING)/U-box, Homology to E6-AP C Terminus (HECT), Anaphase Promoting Complex (APC), SKP1-CUL1-F-box (SCF), CUL3-BTB, and CUL4-DDB1-DWD18. These different types of E3 ligases have been identified to be involved in plant immune responses, including effector recognition, receptor complexes initiation, and immune signal transduction19. Many effectors, such as AvrPtoB, XopK and XopAE, require E3 ligase activity for full virulence of pathogens and for interaction with hosts20–25. Direct ubiquitination of pattern recognition receptors by E3 ligases can attenuate plant innate immunity. For example, flg22 induces two E3 ligases, PUB12 and PUB13, to associate with and polyubiquitinate FLS2 leading to flagellin-induced FLS2 degradation, and pub12 and pub13 mutants display elevated immune responses to flagellin treatment26. MYB30-interacting E3 ligase 1 (MIEL1) ubiquitinates transcription factor MYB30, leading to MYB30 degradation by proteasome and repression of the immune response27,28. When microbe-associated molecular patterns (MAMPs) are recognized, E3 ubiquitin ligase RING-H2 FINGER A3A (RHA3A) monoubiquitinates receptor-like cytoplasmic kinase BOTRYTIS-INDUCED KINASE 1 (BIK1), contributing to the release of BIK1 from the FLS2-BAK1 complex and activation of immune signaling15.
Yield penalties usually accompany immune response activation29,30. Accordingly, plants have developed precise strategies to fine-tune immune responses for maintaining a low level of basal immunity under normal conditions but a rapid response upon pathogen infection. To minimize the fitness cost, plants employ different mechanisms to regulate the expression levels and stability of resistance-related proteins to an optimal level, including epigenetic regulation, miRNA-mediated regulation and proteasomal degradation. Moreover, creating pathogen-inducible transcriptional or translational control is also an effective way to enhance disease resistance without fitness cost31–34.
Transcriptional reprogramming in response to a pathogen challenge is regulated by a broad variety of transcription factor families. Controlling the stability and transcriptional activity levels of transcription factors is necessary for plant immune response. Ideal Plant Architecture 1 (IPA1) was identified as an important transcription factor contributing to activation of plant immunity to Magnaporthe oryzae and the development of an ideal plant architecture that generates stronger panicles and higher yield35–37. Previous studies found that several candidates were involved in the IPA1 signaling pathway that modulates plant architecture38 and established that the expression level and stability of IPA1 was essential for its function6,39,40.
Although IPA1 phosphorylation that serves to quickly adjust IPA1 function was found to be a critical PTM induced by M. oryzae infection37, we found that phosphorylation of IPA1 alone cannot directly activate the expression of immune response gene WRKY45. Therefore, there should be other co-activators serving to regulate IPA1 function in response to M. oryzae infection. In this study, we identified IPA1 interactor 7 (IPI7) as a coactivator for IPA1-mediated resistance to M. oryzae. IPI7, a RING-finger-containing E3 ligase, interacted with IPA1 and promoted the K29-polyubiquitination of IPA1 in vitro and in vivo. Although IPI7-mediated K29-polyubiquitination of IPA1 was induced by M. oryzae infection, the stability of IPA1 protein was not affected by IPI7. In vivo results indicated that IPI7-mediated K29-polyubiquitination was required for phosphorylated IPA1 to transactivate WRKY45 expression for immune response activation, but not for plain IPA1 to transactivate DEP1 expression for panicle development. Genetic evidence indicates that IPI7-knock out compromised IPA1-mediated resistance to M. oryzae but not panicle development. Our results reveal that ubiquitination mediated by IPI7 orchestrates with phosphorylation to control the transactivation activity of IPA1 for promoting plant immunity.
Results
Identification of IPA1-interacting protein IPI7
IPA1 protein becomes phosphorylated at its amino acid residue Ser163 upon M. oryzae infection, which serves to quickly adjust IPA1’s function in activating the immune response. IPA1(S163D), which mimics IPA1 phosphorylated at Ser163, preferentially binds to the WRKY45 promoter to activate downstream immune responses37. However, we found that IPA1(S163D) failed to activate WRKY45 expression in tobacco leaves (Supplementary Fig. 1), indicating the requirement of other factors for IPA1 action on WRKY45 expression.
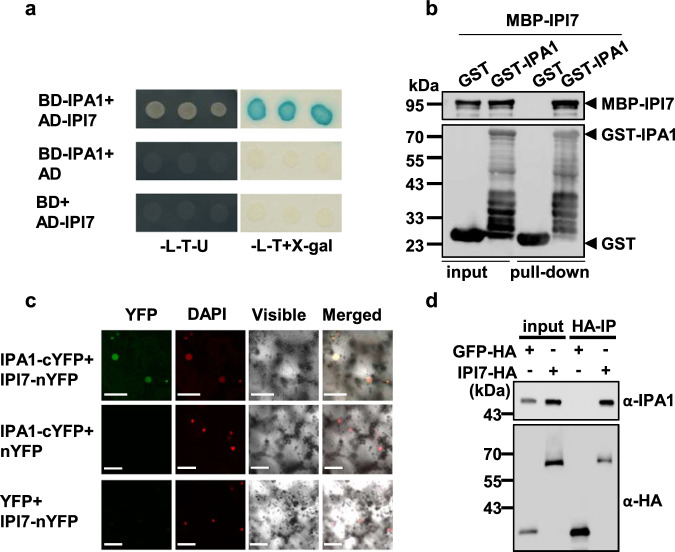
To search for factors affecting IPA1 activity, we performed a yeast two-hybrid assay to screen for IPA1-interacting (IPI) proteins and tested their potential ability to coactivate WRKY45 expression with IPA1(S163D) in tobacco leaf infiltration assays. We identified IPI7 that is capable of working with IPA1(S163D), but not with non-phosphorylated mutant IPA1, IPA1(S163A), to activate WRKY45 expression (Supplementary Fig. 2). The interaction between IPI7 and IPA1 was then validated by in vitro and in vivo experiments including yeast-two hybrid (Fig. 1a), GST-pull down (Fig. 1b), bi-molecular fluorescence complementation (BiFC) (Fig. 1c) and co-immunoprecipitation (Co-IP) (Fig. 1d) assays.
Fig. 1. IPI7 interacts with IPA1.
a Interaction between IPI7 and IPA1 in the yeast two-hybrid assay. IPA1 protein was fused with the GAL4 binding domain to generate BD-IPA1, and IPI7 with the GAL4 activation domain to form AD-IPI7. Blue clones in an X-Gal assay and clones grown on the SD-L-T-U medium indicate protein interaction in yeast cells. b GST pull-down assay for interaction between IPI7 and IPA1. MBP-IPI7, but not MBP, was pulled down with GST-IPA1 immobilized on glutathione-agarose beads. The immunoblot was probed separately with MBP and GST antibodies. Similar results were obtained from three independent biological experiments. c BiFC assay for interaction between IPI7 and IPA1 in tobacco leaves. IPA1 was fused with cYFP (C terminus of YFP) and IPI7 with nYFP (N terminus of YFP). Yellow fluorescence indicates interaction between IPI7 and IPA1 in the nucleus. DAPI was used as the nuclear marker. Bars = 10 μm. Similar results are obtained from three independent biological experiments. d IPI7 interacts with IPA1 in vivo. Total proteins from the protoplasts expressing IPI7-HA or GFP-HA were IP’d with a HA antibody. Proteins before (input) and after IP were detected with an antibody against IPA1 or HA. Similar results are obtained from two independent biological experiments.
IPA1 is ubiquitinated by IPI7
Sequence analysis revealed that IPI7 encodes a RING-finger-containing E3 ligase previously reported as APIP641. We examined the E3 ligase activity of IPI7 and determined whether IPA1 is a substrate of IPI7. In in vitro ubiquitination assays, intact MBP-IPI7 showed E3 ligase activity and promoted polyubiquitination of His-TF-IPA1 in the presence of E1, E2 and ubiquitin proteins (Fig. 2a and Supplementary Fig. 3). However, MBP-IPI7(H58Y), carrying a mutation on histidine 58 in the RING finger domain, lost its E3 ligase activity and failed to promote the ubiquitination of IPA1 (Fig. 2a).
Fig. 2. IPI7 ubiquitinates IPA1.
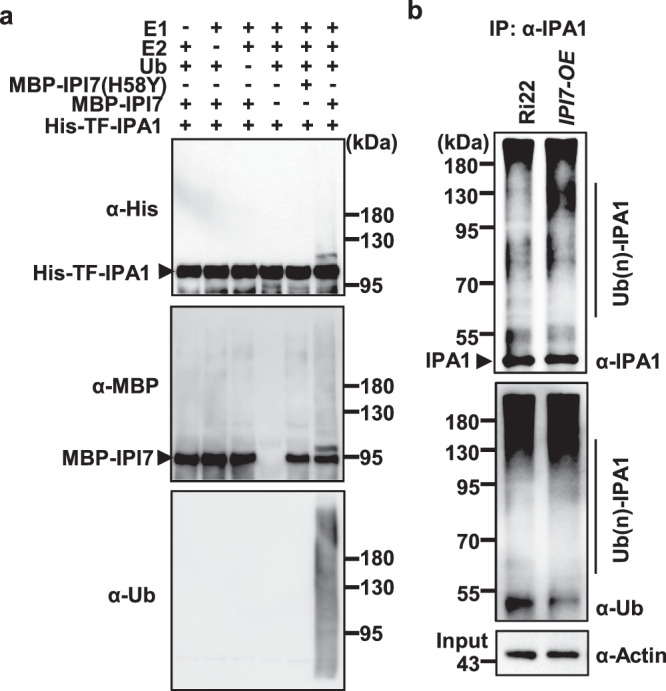
a In vitro ubiquitination of His-IPA1 by MBP-IPI7. MBP-IPI7(H58Y) and MBP were used as negative controls. Immunoblotting was performed with antibodies against His, MBP or Ub. The presence (+) or absence (−) of components in the reaction mixture was indicated. Similar results were obtained from three independent biological experiments. b IPI7 promotes ubiquitination of IPA1 in vivo. The assay was performed with rice variety Ri22 and Ri22-derived transgenic plants overexpressing IPI7 driven by a rice ubiquitin promoter (IPI7-OE). Immunoprecipitation was performed with an IPA1 antibody and immunoblotting was performed with an antibody against Ub or IPA1. Similar results were obtained from three independent biological experiments.
To confirm in vivo ubiquitination of IPA1 by IPI7, we co-expressed IPA1-HA and IPI7-MYC in tobacco leaves via Agrobacterium tumefaciens infiltration then immunoprecipitated IPA1 with an HA antibody, and found that stronger smear bands of ubiquitinated IPA1 were detected for IPA1-HA in the presence of IPI7-MYC (Supplementary Fig. 4). We then generated IPI7-overexpressing (IPI7-OE) transgenic plants in rice variety Ri22 (Supplementary Fig. 5a), which carries the ipa1-1D allele that contains a point mutation in the miR156 target site resulting in a higher level of IPA1 protein35. We detected a higher IPA1 ubiquitination level in the IPI7-OE plants than in Ri22 plants (Fig. 2b). These results indicate that IPI7 targets IPA1 for polyubiquitination.
The stability of IPA1 protein is not affected by IPI7
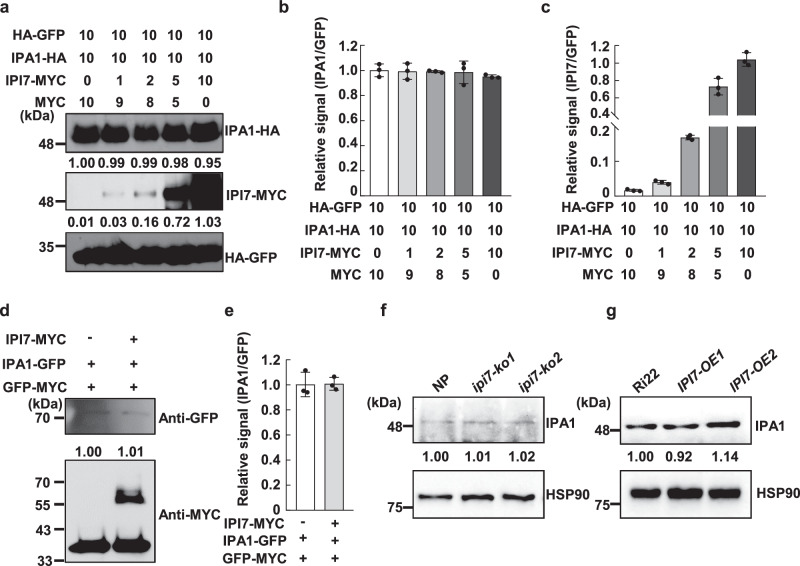
To test whether IPI7 affects IPA1 protein stability, we firstly co-expressed IPA1-HA and IPI7-MYC in tobacco leaves (Fig. 3a–c) or rice protoplast cells (Fig. 3d, e). Similar results indicated that the IPA1 protein level was stable with increased levels of IPI7-MYC protein. Then, we employed the CRISPR/Cas9 technology using two independent guide RNAs that target two different regions in the IPI7 coding sequence to generate IPI7-knockout (ipi7-ko) transgenic plants in the Nipponbare (NP) rice genetic background and confirmed the mutations (Supplementary Fig. 6). We found no significant differences in IPA1 protein levels between ipi7-ko and wild-type NP plants (Fig. 3f and Supplementary Fig. 7a, b). Similar results were obtained in IPI7 RNA interference (RNAi) plants where the IPA1 RNA level and protein level remained stable when IPI7 expression was reduced by RNAi (IPI7-Ri) (Supplementary Fig. 7c–f).
Fig. 3. The stability of IPA1 protein is not affected by IPI7.
a Stability of IPA1 protein with increasing levels of IPI7. Numbers indicate relative concentrations of A. tumefaciens carrying constructs used for co-infiltration. HA-GFP is an internal control for protein expression. Quantitation of protein bands of IPA1/GFP (b) and IPI7/GFP (c) for (a). Each value represents mean ± SD (n = 3 independent assays). d Stability of IPA1 with or without added IPI7 in rice protoplasts. The indicated plasmids were used to transfect rice protoplasts. IPA1-GFP protein amounts were quantitated by densitometry and normalized to the GFP-MYC level. e Statistical analysis of protein bands in (d) was conducted with three independent assays. Data are mean ± SD. IPA1 abundance in Nipponbare (NP), ipi7-ko plants (f), Ri22, and IPI7-OE plants (g). Samples were collected from indicated plants. IPA1 protein was probed in immunoblots with an IPA1 antibody and quantified by densitometry normalized to Heat Shock Protein 90 (HSP90). Similar results were obtained from three independent biological experiments in the dataset.
Similarly, we found that IPA1 protein levels did not significantly alter in IPI7-OE transgenic plants compared to wild-type Ri22 (Fig. 3g and Supplementary Fig. 7g, h). These results suggest that the ubiquitination of IPA1 mediated by IPI7 does not lead to protein degradation, but may serve other purposes biochemically.
Ubiquitination mediated by IPI7 contributes to the transactivation of WRKY45 by IPA1(S163D)
To further validate that IPI7 is a co-activator in IPA1(S163D)-mediated transactivation of WRKY45, we firstly examined the interaction between IPA1(S163D) and IPI7 and found that the phosphorylation mimic of IPA1 on Ser163 did not change the interaction between IPA1 and IPI7 (Supplementary Fig. 8a). Next, we performed in vivo and in vitro ubiquitination assays to compare the polyubiquitination levels of IPA1, IPA1(S163A) and IPA(S163D) mediated by IPI7 and found that there were no significant differences (Supplementary Fig. 8b, c). As expected, the stability of IPA1(S163D) protein was also not affected by IPI7 (Supplementary Fig. 8d).
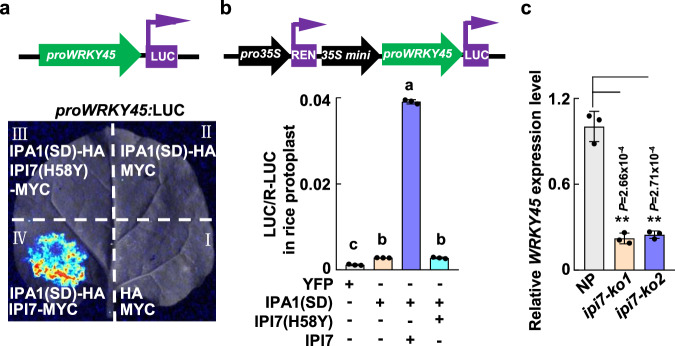
Then, we tested whether IPI7 could help IPA1(S163D) to transactivate the WRKY45 promoter fused to a luciferase (LUC) reporter in tobacco leaves. As shown in Fig. 4a, we detected significant light signals derived from proWRKY45:LUC only when co-expressed with both IPA1(S163D)-HA and IPI7-MYC, but not with IPA1(S163A) and IPI7-MYC (Fig. 4a and Supplementary Fig. 9). In rice protoplasts, we also found that IPI7 enhanced the effect of IPA1(S163D) to synergistically activate proWRKY45:LUC expression by nearly thirty-fold (Fig. 4b). However, IPI7(H58Y) lost nearly all its activity to enhance the transactivation activity of IPA1(S163D) on the WRKY45 promoter in tobacco leaves and rice protoplasts (Fig. 4a, b), suggesting that the E3 ligase activity of IPI7 is essential for its function in promoting the transactivation activity of IPA1(S163D). In agreement, ipi7-ko1 and ipi7-ko2 plants expressed significantly lower WRKY45 RNA levels than NP plants (Fig. 4c). These results indicate that ubiquitination of IPA1(S163D) mediated by IPI7 is required for IPA1(S163D) to activate WRKY45 expression.
Fig. 4. Ubiquitination of IPA1 mediated by IPI7 is essential for IPA1 to activate WRKY45 expression.
a, b Effects of IPA1(S163D) and IPI7 on transactivation of WRKY45 promoter in tobacco leaves or rice protoplasts. a A. tumefaciens carrying plasmids were infiltrated into tobacco leaves. b Plasmids were used to transfect rice protoplasts. The luciferase (LUC) reporter is driven by the WRKY45 promoter (proWRKY45:LUC); Renilla LUC was used as the internal reference. D-luciferin was applied as the LUC substrate. Each value represents mean ± SD (n = 3 independent biological samples). Different letters indicate significant differences determined by the Tukey–Kramer test, p < 0.05 (one-way ANOVA was conducted, followed by a two-sided honestly significant difference (HSD) test for multiple comparisons). p values are shown in the Source Data file. c WRKY45 expression levels in NP and ipi7-ko plants were determined by RT-qPCR (n = 3 independent biological samples). ** indicates p < 0.01 (Two-tailed t-test for statistical analysis). Source data are provided as a Source Data file.
Our previous study indicated that phosphorylation of IPA1 at amino acid Ser163 alters the DNA binding specificity of IPA1, leading to a switch in target genes for IPA1 and IPA1(S163D)37. Thus, we tested the effects of IPI7 on the target-selection and transactivation specificity of IPA1 and found that addition of IPI7 has no effects on the activity of IPA1 to transactivate the DEP1 promoter (Supplementary Fig. 10), which was identified as one of the key targets for IPA1, but not for IPA1(S163D)37,38. These results suggest that IPI7 is essential for the transactivation activity of IPA1(S163D) but not IPA1.
K29-polyubiquitination of IPA1 promoted by IPI7 is enhanced by M. oryzae infection
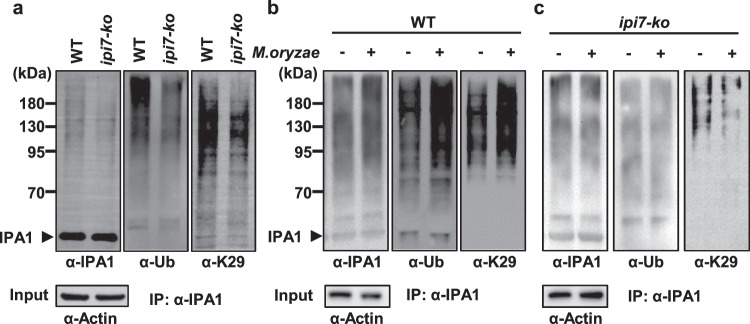
Different types of ubiquitination are suggested to lead to different the fates for substrates. To examine the ubiquitin-chain type covalently added on IPA1 in vivo that is promoted by IPI7, we used ubiquitin-chain-specific antibodies, including anti-K6, anti-K11, anti-K27, anti-K29, anti-K33, anti-K48, and anti-K63, to detect them in immunoblot assays. Anti-K6, anti-K29, and anti-K48 detected clear bands, but only anti-K29 detected a clear reduction in ipi7-ko plants for the K29-mediated ubiquitination of IPA1 (Fig. 5a and Supplementary Fig. 11a). These results suggest that IPI7 specifically promotes K29-polyubiquitination on IPA1 in vivo.
Fig. 5. IPI7-mediated K29-polyubiquitination of IPA1 is induced by M. oryzae.
a IPI7 is required for K29-polyubiquitination of IPA1. The assay was performed in wild-type plants and ipi7-ko plants. Immunoprecipitation was performed with an IPA1 antibody and immunoblotting was performed with an antibody against IPA1, Ub or K29-polyubiquitin chain (K29). Proteins before immunoprecipitation (input) were detected with an antibody against actin for normalization. Effects of M. oryzae infection on the ubiquitination of IPA1 in vivo in wild-type (b) and ipi7-ko (c) plants. Leaves were collected after inoculation with (+) or without (−) M. oryzae. Immunoprecipitation was performed with an IPA1 antibody and immunoblotting was performed with an antibody against IPA1, Ub or K29. Proteins before immunoprecipitation (input) were probed with an antibody against actin for normalization. Similar results were obtained from three independent biological experiments in the datasets.
M. oryzae infection induces IPA1 phosphorylation at Ser163, which is necessary for IPA1-mediated activation of WRKY45. Thus, we asked whether ubiquitination of IPA1 by IPI7 is also influenced by M. oryzae infection. We performed in vivo ubiquitination assays using wild-type rice leaves infected with M. oryzae and found that IPA1 ubiquitination was induced by M. oryzae infection (Fig. 5b left and middle panels). In particular, K29-ubiquitination of IPA1 started to accumulate at 3 h post-inoculation (hpi), peaked at 6 hpi, then subsided to near basal levels within 12 hpi (Supplementary Fig. 11b). Interestingly, this pattern largely coincides with IPA1 phosphorylation, which starts to accumulate at 3 hpi, peaks at 6 to 12 hpi, then subsides to near normal levels within 48 hpi37. We further found that this enhancement of ubiquitination was inhibited in ipi7-ko plants (Fig. 5c left and middle panels). These results indicate that IPI7 is required for the K29-polyubiquitination of IPA1 induced by M. oryzae infection.
Knockout of IPI7 blocks IPA1(S163D)-triggered immunity and rescues yield penalty
Examination of blast disease resistance by inoculation with M. oryzae showed that ipi7-ko1 and ipi7-ko2 plants developed significantly larger lesions and elevated fungal biomass (2-3 fold) than wild-type plants, indicating that ipi7-ko plants are compromised in resistance to M. oryzae (Supplementary Fig. 12a, b).
Because IPA1 was identified as a transcription factor contributing to panicle development35,36, we analyzed the panicle morphology of ipi7-ko plants. ipi7-ko1 and ipi7-ko2 plants showed no clear changes in panicle length, numbers of primary and secondary branches, or grains per panicle compared with wild-type plants (Supplementary Fig. 12c–g). Thus, our results suggest that IPI7 positively regulates plant immunity, but does not play a major role in panicle development.
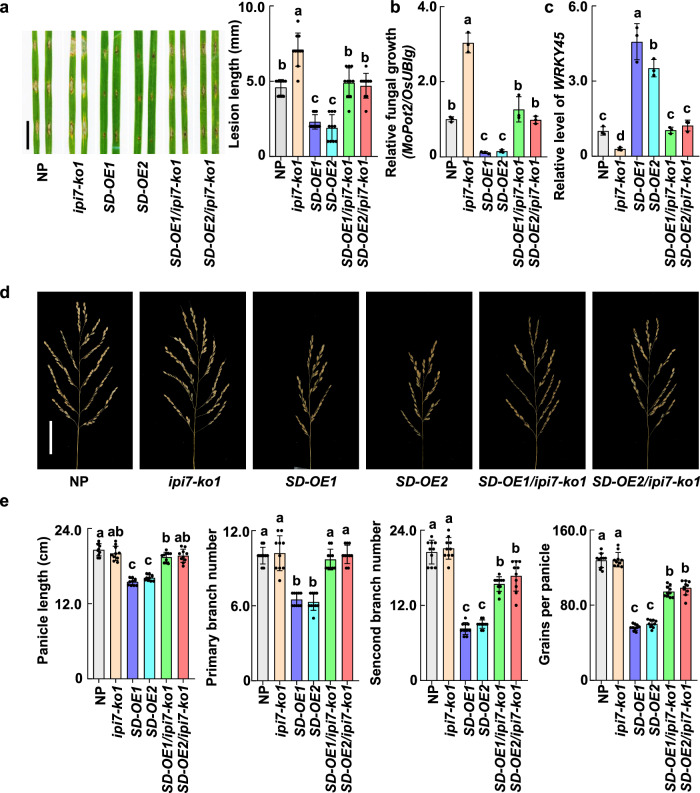
To validate the genetic role of IPI7 in orchestrating phosphorylation and ubiquitination in IPA1-mediated disease resistance and yield, we generated transgenic plants over-expressing IPA1(S163D) in IPI7 knockout genetic background (SD-OE/ipi7-ko) (Supplementary Fig. 13) and examined their disease resistance to M. oryzae. We found that SD-OE/ipi7-ko plants developed lesions twice as large as SD-OE plants and the same size as NP plants (Fig. 6a). Fungal biomass determined by DNA quantification confirmed that SD-OE/ipi7-ko leaves harbored significantly larger M. oryzae populations than SD-OE leaves, but similar amounts as NP leaves (Fig. 6b), indicating that ipi7-ko neutralizes the immunity-enhancing effect of SD-OE.
Fig. 6. IPI7 contributes to IPA1-mediated disease resistance with lower fitness cost.
a, b Effects of IPI7 knockout on IPA1(S163D)-mediated resistance to M. oryzae. Rice leaves were punch-inoculated with M. oryzae and incubated for a week. Lesion pictures are displayed, and 10 independent lesion lengths are measured (right panel) (a), M. oryzae populations determined based on fungal MoPot2 DNA content (b) are presented. Each value represents mean ± SD (n = 3 independent biological samples). c WRKY45 RNA levels in the leaves of NP, ipi7-ko, IPA1(S163D) over-expression (SD-OE) and SD-OE/ipi7-ko double transgenic plants were determined by RT-qPCR (n = 3 independent biological samples). d Main panicle morphology of NP, ipi7-ko1, SD-OE, and SD-OE/ipi7-ko plants. Scale bar = 5 cm. e Statistical analysis of main panicle length, primary and secondary branch numbers, and grains per main panicle of NP and different transgenic plants. Each value represents mean ± SD (n = 10 rice plants). Different letters indicate significant differences at p < 0.05 (one-way ANOVA was conducted, followed by two-sided HSD test for multiple comparisons). The corresponding p values can be found in the Source Data. Source data are provided as a Source Data file.
We also detected RNA levels of WRKY45, a validated target of IPA1(S163D). While WRKY45 RNA levels were highly induced in SD-OE plants, they were clearly reduced in SD-OE/ipi7-ko plants to the same level as in wild-type plants (Fig. 6c). These results provide further evidence that the impairment of IPI7 blocks IPA1(S163D)-triggered immunity by arresting WRKY45 activation. Furthermore, the detrimental effects of the hyper immune response triggered by IPA1(S163D) on rice yield traits, including panicle morphology, panicle length, primary and secondary branch numbers, and grains per panicle, were clearly alleviated in SD-OE/ipi7-ko plants (Fig. 6d, e), suggesting that ubiquitination orchestrates with phosphorylation for proper immune responses lowering fitness cost of IPA1 activation (Fig. 7).
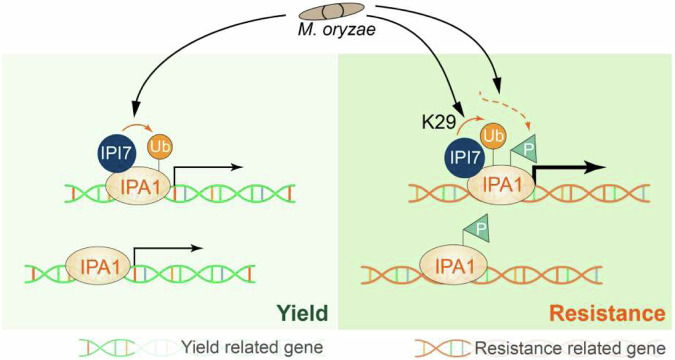
Fig. 7. A PTMs-monitoring model in which IPA1 promotes grain yield and disease resistance to M. oryzae.
Under normal conditions, IPA1 activates the promoters of various yield-related genes, promoting plant growth and yield. Upon M. oryzae challenge, IPA1 goes through four statuses: plain IPA1, ubiquitinated IPA1, phosphorylated IPA1, and ubiquitinated and phosphorylated IPA1. First, both plain IPA1 and ubiquitinated IPA1 promote expression of yield-related genes to increase grain yield. Second, phosphorylated IPA1 binds to promoters of the immune-related genes, including WRKY45 promoter, without transactivation activity. Third, ubiquitinated and phosphorylated IPA1, which represents “blast-activated” IPA1, binds to and transactivates immune-related genes to enhance host resistance to M. oryzae.
Discussion
Here, we discover that rice plants utilize non-proteolytic K29-ubiquitination that fine-tunes the transcriptional activator activity of IPA1 to promote immunity in plants (Fig. 7). Plain IPA1 prefers binding to yield-related gene promoters which contain the “GTAC” motif and activates their expression38. Upon M. oryzae infection, phosphorylation at S163 is a target switch device that helps IPA1 to alter binding to disease resistance-related gene promoters which contain the “TGGGCC” motif37; however, this binding itself is only required but not sufficient to activate plant immunity. Although the expression levels of IPI7 were not significantly changed by M. oryzae infection (Supplementary Fig. 14), IPI7-mediated K29-polyubiquitination of IPA1 is clearly induced by M. oryzae infection and serves as a booster to ensure that Ser163-phosphorylated IPA1 targets and transactivates immune-related genes such as WRKY45. These two post-translational modifications (ubiquitination and phosphorylation) of IPA1 protein play crucial roles in activating plant immunity. Thus, ubiquitination orchestrates with phosphorylation to form multiple regulatory layers that keep IPA1 in a balance among four different states: plain IPA1, phosphorylated IPA1, ubiquitinated IPA1, and ubiquitinated and phosphorylated IPA1. Only the ubiquitinated and phosphorylated IPA1 is the “pathogen-activated” IPA1, which binds to and transactivates immune-related genes like WRKY45 to enhance plant immunity against M. oryzae. Moreover, ubiquitinated but unphosphorylated IPA1 can also target growth-related genes for yield, which might compensate for the penalty caused by pathogen-activated IPA1. Therefore, these four states of IPA1 working together to build an effective system that timely enables IPA1 to promote both the immune response and yield in plants.
IPI7/APIP6 interacts with different proteins and targets them for ubiquitination. Previous studies indicated that IPI7/APIP6 targets Avr-Piz-t, OsELF3-2 and ROD1 for ubiquitination and promotes their degradation via the ubiquitin-proteasome system (UPS)32,41,42. Differently, our study found that ubiquitination of IPA1 mediated by IPI7 is not necessary for the degradation of IPA1 via the UPS (Fig. 3), but is critical for the transactivation of WRKY45 by IPA1 in response to pathogen attack (Figs. 4 and 6). Moreover, we found that the ubiquitination of IPA1 mediated by IPI7 did not affect its binding to downstream target genes (Supplementary Fig. 15). These results suggest that the molecular mechanism of ubiquitination of IPA1 promoted by IPI7 is different from previous studies and the substrates labeled with polyubiquitin chains by IPI7 have different fates.
Polyubiquitin chains are formed through the linkage of lysine residues other than K48 of the ubiquitin molecule, i.e., K6-, K11-, K27-, K29-, K33- and K63-linked ubiquitination43,44. Accordingly, the abundance, function, activity and subcellular distribution of substrates involved in different cellular and physiological processes can be regulated through different types of ubiquitin modifications. Of these various types of polyubiquitination, the K48-linked ubiquitination is the best characterized one which usually serves to proteolytically degrade substrates. By contrast, other types of ubiquitination are much less well understood in plants. We tested the ubiquitin chain types on IPA1 promoted by IPI7 using different ubiquitin-chain-specific antibodies and found that the IPI7-mediated enhancement of K29-polyubiquitination of IPA1 is crucial for the IPA1-mediated response to M. oryzae (Figs. 5, 6 and Supplementary Fig. 11). Our results suggest that the K29-polyubiquitin chain modification is involved in plant immune response by regulating IPA1(S163D) activity. However, the transcriptional activation activities of plain IPA1 and IPA1(S163A) were unaffected by IPI7-mediated ubiquitination (Supplementary Figs. 9b and 10), which might be due to the differences in the 3D structures of plain IPA1, phosphorylated IPA1, ubiquitinated IPA1, phosphorylated and ubiquitinated IPA1.
E3 ligases play a crucial role in governing substrate specificity, while the E2 ubiquitin–conjugating enzyme is often considered as a “carrier of ubiquitin” determining the topology of the polyubiquitin chain45. E3 selects the right E2 to generate the appropriate Ub signal on the target protein, thus controlling the fate of a given substrate46. Therefore, different E2s may be selected by IPI7 to work together in different signaling pathways.
Ubiquitination is also named “lysine ubiquitination” for many years because it occurs when a ubiquitin or ubiquitin-chain is covalently attached to a lysine (K) residue of the targeted protein. Sequence analysis reveals that there are seven K residues in IPA1 protein. However, we found that none of the seven single-K-site disruptions in IPA1 affected IPI7-mediated ubiquitination (Supplementary Fig. 16). Recently, it has been established that cysteine, serine and threonine residues also function as sites for ubiquitination, forming thioester, hydroxyester and peptide bonds, respectively, dependent on the free amino group of the N-terminus of target protein43. Therefore, the ability of IPA1 to be ubiquitinated by IPI7 on more than one type of amino acids creates many regulatory possibilities.
Plant immune responses often penalize growth and yield. Crop varieties with enhanced disease resistance and low fitness cost are highly desired by breeders. The utilization of a pathogen-inducible transcriptional and/or translational control is an effective way to restrict expression of resistance genes at the most appropriate level34,47. For example, a TBF1 cassette was used to regulate expression of a resistance gene to enhance broad-spectrum disease resistance without compromising plant fitness34. Epigenetic modification built an accurate expression pattern for PigmR and PigmS in nature, leading to activation of PigmR-mediated defense response in leaves while allowing grain production in panicles31. These findings have led to useful strategies to balance yield and immunity in breeding programs by regulating the expression levels of resistance genes. Our results yield new insights into how crops can achieve high yield and disease resistance simultaneously. Various disease resistance-related proteins require ubiquitination, phosphorylation or other PTMs to initiate their activation processes. The employment of PTMs to monitor the activity of immune-related proteins is an effective way to minimize fitness cost.
Methods
Plant materials and growth conditions
Rice (Oryza sativa) ssp japonica cultivated variety Nipponbare (NP), Ri22, ipi7-ko, and IPI7-OE plants were grown either in the greenhouse or experimental fields of the Sichuan Agricultural University (Chengdu, Sichuan, China).
The ipi7-ko plants were generated using CRISPR/Cas9 carried out by Biogle.cn. To minimize the potential off-target effects induced by CRISPR/Cas9, we performed two distinct transformations, using two sgRNAs targeting IPI7 at different locations: SG1 (TGCTGTATCTTCTGCACCTG) and SG2 (AGCTCATCCATGCCCATATG) (Supplementary Fig. 6). Individual sgRNA construct was created in the BGK03 vector containing Cas9, introduced into Agrobacterium tumefaciens strain EHA105, and transformed into NP. Six independent lines for each of SG1 and SG2 were obtained. To examine the CRISPR/Cas9-created lines, genomic DNA was extracted from transgenic plants and primer pairs flanking the designed target site were used for PCR amplification (Supplementary Data 1). Sequence alignment revealed that two independent mutants, ipi7-ko1 and ipi7-ko2, were obtained (Supplementary Fig. 6).
The Ubi-IPI7 and 35S:Flag-IPA1(S163D) plasmids were individually introduced into A. tumefaciens strain EHA105 and transformed into Ri22, NP or ipi7-ko plants as previously reported48. Independent lines with increased expression of IPI7 or IPA1 were obtained and used in further investigation.
Constructs
In brief, target fragments were generated by PCR amplification using primers listed in Supplementary Data 1. The linearized vector terminal 15–20 bp sequence was used as homologous sequence and added to the 5’ end of the gene-specific positive/reverse amplification primer sequences. The insert fragments with homologous sequence were ligated into desired vectors or cloned into desired vectors using ClonExpress® II One Step Cloning Kit (Vazyme, C112-01). The vectors used in the yeast two-hybrid assay were pDBLeu for bait and pPC86 for prey. pSPYCE and pSPYNE vectors were used for the BiFC assay49. The coding regions of targets were inserted into the pGEX-6P-1, pMAL-c2x or pColdTM-TF (TaKaRa, Cat# 3365) vector for protein expression in vitro. The promoters of DEP1 and WRKY45 were amplified from NP genomic DNA and ligated into the pCAMBIA1300-LUC vector to produce proDEP1:LUC and proWRKY45:LUC, respectively. Point mutation constructs were generated with the Quikchange site-directed mutagenesis kit (Stratagene, 200514). All of the primers used in constructs are listed in Supplementary Data 1.
Yeast Two-Hybrid assay
Yeast Two-Hybrid assay was performed as previously reported8. Plasmids were co-transferred into the MAV203 yeast strain, and transformed cells were grown on SD medium without Leu and Trp. The clones were then confirmed by growing on SD medium without Leu, Trp, and Ura for 2 days, or using X-gal assay.
Protein expression in vitro
GST-, MBP-, and His-fused proteins were individually expressed in Transetta(DE3) cells (TransGen Biotech, CD801). GST-fused proteins were purified using glutathione-conjugated Sepharose 4 Fast Flow (Sangon Biotech, 17-5132-01); the MBP-fused proteins were purified using Amylose Resin (Sangon Biotech, C500096-0005); the His-fused proteins were purified using Ni-Sepharose 6 Fast Flow (Sangon Biotech, C600033-002), according to manufacturer’s instructions.
Pull-down assay
Pull down assay was performed according to a previously described method50 with some modifications. Approximately 10 μg of GST or GST-IPA1 were incubated with 5 μL of prewashed glutathione agarose beads in 1 mL of pull-down buffer (20 mM Tris-HCl, PH 7.5, 1 mM β-mercaptoethanol, 3 mM EDTA, 1 mM DTT, 1% [v/v] Nonidet P-40, 1× Protease Inhibitor Cocktail, Beyotime) for 1 h at 4 °C with gentle shaking. The beads were harvested by centrifugation at 150 × g for 1 min, then mixed with 10 μg of MBP-IPI7 in 1 mL of pull-down buffer for 2 h at 4 °C with gentle shaking. Finally, the beads were harvested and washed three times with 1 mL of pull-down buffer and once more with 1 mL of 50 mM Tris-HCl, pH 7.5. The proteins pulled down were released from beads by boiling in SDS-PAGE sample loading buffer and analyzed by immunoblotting with a GST (Thermo Fisher Scientific MA4-004) or MBP antibody (NEW ENGLAND BioLabs, E8032S).
Transient expression, BiFC and transactivational activity assay
Transient expression assay was performed as previously reported51. Leaves of 4 weeks old N. benthamiana were infiltrated with A. tumefaciens carrying test constructs. In all cases, cultures were co-infiltrated with A. tumefaciens carrying a P19 suppressor in a gene silencing construct.
BiFC assay was performed in N. benthamiana leaves. N. benthamiana leaves were infiltrated with A. tumefaciens carrying constructs in the pSPYCE vector. Two days after infiltration, visible signals were examined under a confocal microscope (NiKon A1 i90, LSCM, Japan).
Transactivational activity assay was carried out in an Agrobacterium-mediated transient expression system. N. benthamiana leaves were infiltrated with A. tumefaciens carrying a proDEP1:LUC or proWRKY45:LUC reporter construct, together with A. tumefaciens carrying an IPA1(SD)-HA or IPA1-HA construct, and A. tumefaciens carrying an IPI7-MYC or IPI7(H58Y)-MYC construct. 60 h after infiltration, detached leaves were sprayed with 1 mM D-luciferin, potassium salt (InvitrogenTMAbcam, 115144-35-9) following manufacturer’s instructions. The Renilla LUC gene under control of the CaMV 35S promoter was co-transferred as an internal control. Luminescence signals were captured using a charge-coupled device (CCD) camera (BIO-RAD, ChemiDocTM Touch Imaging System). Then the luciferase activities were calculated using the Dual Luciferase Reporter Gene Assay Kit (Beyotime, RG027) according to manufacturer’s instructions. The ratio of LUC activities (firefly LUC/Renilla LUC) was calculated to normalize each assay.
In vitro ubiquitination assay
Ubiquitination assay was performed as previously described8 with some modifications: 1 μg GST-IPI7 or GST protein was incubated in a 20 μL reaction mixture containing 50 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 2 mM DTT, 5 mM ATP, 3 mM creatine phosphate (Solarbio, 922-32-7), 1 unit creatine kinase (Roche 10127566001), 100 ng E1 (Boston Biochem, E-305), 100 ng E2 (Boston Biochem, E2-607), and 4 μg ubiquitin (Boston Biochem, U-100). To confirm IPI7-mediated ubiquitination of IPA1, purified His-IPA1 protein (2 μg) was added to the reaction mixture and incubated at 30 °C for 1.5 h. The reaction was stopped by adding 5× SDS sample buffer and boiling at 100 °C for 10 min. The protein mixture was then separated in a 10% (w/v) SDS-PAGE.
In vivo ubiquitination assay
In vivo ubiquitination of IPA1 protein was performed as previously described8 with some modifications. Briefly, samples were ground into powder in liquid nitrogen and extracted in protein extraction buffer NB1 containing 50 mM Tris-MES (pH 8.0), 0.5 M sucrose, 1 mM MgCl2, 10 mM EDTA, 5 mM DTT (Solarbio, D8220), and 1× Protease Inhibitor Cocktail (APEXBIO, K1008). The crude extracts containing 500 μg protein were co-incubated with IPA1 polyclonal antibodies and 50 μM MG132 (Selleck.cn, S2619). After gentle shaking for 1 h, 30 μL Pierce™ Protein A/G Magnetic beads (Thermo Fisher Scientific, 88802) were added into the mixture and incubated for another 1 h with gentle shaking. The magnetic beads were washed with NB1 buffer for 3 times. After an equal volume of 2× SDS buffer was added and boiled at 100 °C for 10 min, the sample was run on a 10% (w/v) SDS-PAGE, immunoblotted, and probed with IPA1, ubiquitin (Beyotime, AF1705), and 7 different Ub chain-specific antibodies. Detailed information for the 7 Ub chain-specific antibodies can be found at the company website (https://abclonal.com.cn; https://www.bio-swamp.com) with the following catalog numbers: anti-K6 (Abclonal, A18106); anti-K11 (Bio-swamp, PAB46885); anti-K27 (Abclonal, A18202); anti-K29 (Abclonal, A18198); anti-K33 (Abclonal, A18199); anti-K48 (Abclonal, A3606); anti-K63 (Abclonal, A18164).
Ubiquitination combined with electrophoretic mobility shift assay
Firstly, an in vitro ubiquitination assay was performed as described before. Then, an EMSA assay was performed to detect the DNA binding activity of IPA1(S163D) protein in the in vitro ubiquitination assay reactions. Six μL products from the in vitro ubiquitination assay were incubated with single-biotin-labeled DNA probes of WRKY45 promoter for the EMSA assay37. Light Shift Chemiluminescent EMSA Kit (Beyotime, GS009) was used for detection in the EMSA assay. Detailed EMSA procedure followed manufacturer’s instructions. Photos were taken using a charge-coupled device (CCD) camera.
RNA extraction and reverse transcription quantitative PCR (RT-qPCR)
Total RNA was prepared with a TRIzol Kit (Thermo Fisher Scientific, 15596-018CN) according to user’s manual. cDNAs were generated using the PrimeScript RT Reagent Kit with gDNA Eraser (Perfect Real Time) (TaKaRa, Cat# RR047A). RT-qPCR was performed following the manufacturer’s instructions in the QuantiNova SYBR Green PCR Kit (QIAGEN, Cat# 208054). The primers used in RT-qPCR are listed in Supplementary Data 1. Ubiquitin (LOC_Os03g13170) was used as the internal control for normalization.
M. oryzae inoculation assay and protein extraction
For pathogen infection, a punch inoculation assay was carried out on rice leaf strips from 4-week-old plants as previously described52. Lesion size was measured after incubation for 5–7 days at 28 °C. Genomic DNA was isolated to measure the Pot2 gene of M. oryzae and calculated relative to a rice ubiquitin gene for normalization.
Leaves detached from 4-week-old plants were cut into 4-cm-long strips and incubated for 12 h in H2O to reduce residual wounding effects before further treatment. The leaf strips were treated with or without blast isolate ZHONG10-8-14 with a final concentration of 5 × 105 conidia/mL. Treated or non-treated samples were collected and ground into powder in liquid nitrogen and resuspended in NB1 buffer [50 mM 2-amino-2-(hydroxymethyl)−1,3-propanediol (TRIS)-MES pH 8.0, 0.5 M sucrose, 1 mM MgCl2, 10 mM EDTA, 1× Protease Inhibitor Cocktail (APExBio, K1008), 5 mM DTT (Solarbio, D8220)] on ice. Extracts were centrifuged at 13,000 × g at 4 °C for 10 min, and the supernatant was boiled at 100 °C for 10 min after adding 5× SDS buffer. The sample was run on a 10% (w/v) SDS-PAGE, immunoblotted, and probed with an IPA1 antibody. The protein level of actin was used as an internal control (Sangon Biotech, NO. D191048).
Western blots and analysis
Samples were boiled for 10 min and centrifuged at 11,000 × g for 2 min at room temperature. A total of 10–20 μL samples were loaded in an 8% or 10% SDS-PAGE gel. After electrophoresis, the proteins were transferred to a PVDF membrane using the TransBlot Turbo Transfer System (Bio-Rad, USA). The membranes were blocked with 4% non-fat milk in Tris-buffered saline with Tween 20 (TBST, containing 20 mM Tris, pH 7.6, 150 mM NaCl, 0.1% Tween 20) at room temperature for 2 h and then incubated overnight with a primary antibody at 4 °C. The primary antibodies were used in this study along with appropriate secondary antibody-HRP conjugates. The information of antibodies used is as indicated. Protein bands were visualized using an ECL chemiluminescence detection kit (Sangon, China, No. C500044), and they were imaged using a chemiluminescence imaging system (Bio-Rad, USA). The relative protein intensity was analyzed using ImageJ.
Statistics and reproducibility
Fluorescence images are collected using a Nikon A1 i90 LSCM confocal microscope. The ChemiDocTM Touch Imaging System from Bio-Rad is used for collecting protein images and Dul-Luciferase reporter images, while the ImageJ software is utilized for quantitative analysis of protein bands. The experimental data of the Dul-Luciferase reporter were collected using GLOMAXTM96. GraphPad 8.0 was used for all data analysis. The statistical analyses were conducted using SPSS 21.0. All values are presented as mean ± SD, and the number (n) of samples is indicated in the figure legend. Statistically significant differences between the control and experimental groups were determined using a one-way ANOVA with a two-sided honestly significant difference (HSD) multiple comparison test or t-test. Differences were considered statistically significant when the p value is <0.05. All experiments were repeated at least twice, and multiple biological replicates were used in each experiment. No data was excluded from the analyses. The investigators were not blinded to the allocation during experiments or outcome assessment.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Source data
Acknowledgements
We thank Mawsheng Chern for critical reading and editing of the manuscript. This work was supported by grants from the National Key Research and Development Program (2021YFA1300702); National Natural Science Foundation of China (31922066, 32072043, 32272116) to J.W., (31825022, 32121003) to X.C., (32072407) to X.Z., (32072041) to J.Y.; China Agriculture Research System (CARS-01-1) to J.L.; and the Sichuan Science and Technology Program (2024NSFTD0022) to J.W., (2022ZDZX0012) to S.L., (2022JDTD0023) to J.F.
Author contributions
J.W., J.L. and X.C. conceived and designed the experiments. J.W., H.S., J.Y. and Z.Z. performed most of the experiments and prepared the figures. H.Yi, L.X., X.Z., H.T. and Q.X. contributed to pathogen inoculation assays. Xi.C., X.L., W.L., Y.T., Q.H., L.S., Y.L. and L.W. collected the data. H.Yu, J.F., C.S., T.L., P.Q., W.W., S.L., J.L., X.C. and J.W. analyzed the data. J.W., J.L., X.C., H.Yu and M.H. wrote the manuscript.
Peer review
Peer review information
Nature Communications thanks the anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Data availability
All data are available in the main text and the Supplementary Information. Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Hui Shi, Junjie Yin, Zhangjie Zhao, Hong Yu.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-024-51962-x.
References
- 1.Jones, J. D. & Dangl, J. L. The plant immune system. Nature444, 323–329 (2006). 10.1038/nature05286 [DOI] [PubMed] [Google Scholar]
- 2.Veronese, P. et al. The membrane-anchored BOTRYTIS-INDUCED KINASE1 plays distinct roles in Arabidopsis resistance to necrotrophic and biotrophic pathogens. Plant Cell18, 257–273 (2006). 10.1105/tpc.105.035576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nürnberger, T., Brunner, F., Kemmerling, B. & Piater, L. Innate immunity in plants and animals: striking similarities and obvious differences. Immunol. Rev.198, 249–266 (2004). 10.1111/j.0105-2896.2004.0119.x [DOI] [PubMed] [Google Scholar]
- 4.Liu, L. J. et al. COP1-mediated ubiquitination of CONSTANS is implicated in cryptochrome regulation of flowering in Arabidopsis. Plant Cell20, 292–306 (2008). 10.1105/tpc.107.057281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martins, S. et al. Internalization and vacuolar targeting of the brassinosteroid hormone receptor BRI1 are regulated by ubiquitination. Nat. Commun.6, 6151 (2015). 10.1038/ncomms7151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miao, Y. & Zentgraf, U. A HECT E3 ubiquitin ligase negatively regulates Arabidopsis leaf senescence through degradation of the transcription factor WRKY53. Plant J.63, 179–188 (2010). 10.1111/j.1365-313X.2010.04233.x [DOI] [PubMed] [Google Scholar]
- 7.Mithoe, S. C. & Menke, F. L. Regulation of pattern recognition receptor signalling by phosphorylation and ubiquitination. Curr. Opin. Plant. Biol.45, 162–170 (2018). 10.1016/j.pbi.2018.07.008 [DOI] [PubMed] [Google Scholar]
- 8.Wang, J. et al. Tissue-specific ubiquitination by IPA1 INTERACTING PROTEIN1 modulates IPA1 protein levels to regulate plant architecture in rice. Plant Cell29, 697–707 (2017). 10.1105/tpc.16.00879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang, L. et al. Strigolactone signaling in arabidopsis regulates shoot development by targeting D53-like SMXL repressor proteins for ubiquitination and degradation. Plant Cell27, 3128–3142 (2015). 10.1105/tpc.15.00605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu, Y. et al. UBIQUITIN-SPECIFIC PROTEASE14 interacts with ULTRAVIOLET-B INSENSITIVE4 to regulate endoreduplication and cell and organ growth in arabidopsis. Plant Cell28, 1200–1214 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ciechanover, A. & Schwartz, A. L. The ubiquitin-proteasome pathway: the complexity and myriad functions of proteins death. Proc. Natl. Acad. Sci. USA95, 2727–2730 (1998). 10.1073/pnas.95.6.2727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sadowski, M., Suryadinata, R., Tan, A. R., Roesley, S. N. & Sarcevic, B. Protein monoubiquitination and polyubiquitination generate structural diversity to control distinct biological processes. IUBMB Life64, 136–142 (2012). 10.1002/iub.589 [DOI] [PubMed] [Google Scholar]
- 13.Braten, O. et al. Numerous proteins with unique characteristics are degraded by the 26S proteasome following monoubiquitination. Proc. Natl. Acad. Sci. USA113, E4639–E4647 (2016). 10.1073/pnas.1608644113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gu, X., Jiang, D., Wang, Y., Bachmair, A. & He, Y. Repression of the floral transition via histone H2B monoubiquitination. Plant J.57, 522–533 (2009). 10.1111/j.1365-313X.2008.03709.x [DOI] [PubMed] [Google Scholar]
- 15.Ma, X. et al. Ligand-induced monoubiquitination of BIK1 regulates plant immunity. Nature581, 199–203 (2020). 10.1038/s41586-020-2210-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pickart, C. M. & Fushman, D. Polyubiquitin chains: polymeric protein signals. Curr. Opin. Chem. Biol.8, 610–616 (2004). 10.1016/j.cbpa.2004.09.009 [DOI] [PubMed] [Google Scholar]
- 17.Leitner, J. et al. Lysine63-linked ubiquitylation of PIN2 auxin carrier protein governs hormonally controlled adaptation of Arabidopsis root growth. Proc. Natl. Acad. Sci. USA109, 8322–8327 (2012). 10.1073/pnas.1200824109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vierstra, R. D. The ubiquitin-26S proteasome system at the nexus of plant biology. Nat. Rev. Mol. Cell Biol.10, 385–397 (2009). 10.1038/nrm2688 [DOI] [PubMed] [Google Scholar]
- 19.Yin, J., Yi, H., Chen, X. & Wang, J. Post-translational modifications of proteins have versatile roles in regulating plant immune responses. Int. J. Mol. Sci.20, 2807 (2019). 10.3390/ijms20112807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen, H. et al. A bacterial type III effector targets the master regulator of salicylic acid signaling, NPR1, to subvert plant immunity. Cell Host Microbe22, 777–788.e777 (2017). 10.1016/j.chom.2017.10.019 [DOI] [PubMed] [Google Scholar]
- 21.Gimenez-Ibanez, S. et al. AvrPtoB targets the LysM receptor kinase CERK1 to promote bacterial virulence on plants. Curr. Biol.19, 423–429 (2009). 10.1016/j.cub.2009.01.054 [DOI] [PubMed] [Google Scholar]
- 22.Göhre, V. et al. Plant pattern-recognition receptor FLS2 is directed for degradation by the bacterial ubiquitin ligase AvrPtoB. Curr. Biol.18, 1824–1832 (2008). 10.1016/j.cub.2008.10.063 [DOI] [PubMed] [Google Scholar]
- 23.Popov, G., Majhi, B. B. & Sessa, G. Effector gene xopAE of Xanthomonas euvesicatoria 85-10 is part of an operon and encodes an E3 ubiquitin ligase. J. Bacteriol.200, e00104–e00118 (2018). 10.1128/JB.00104-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qin, J. et al. The Xanthomonas effector XopK harbours E3 ubiquitin-ligase activity that is required for virulence. New. Phytol.220, 219–231 (2018). 10.1111/nph.15287 [DOI] [PubMed] [Google Scholar]
- 25.Shan, L. et al. Bacterial effectors target the common signaling partner BAK1 to disrupt multiple MAMP receptor-signaling complexes and impede plant immunity. Cell Host Microbe4, 17–27 (2008). 10.1016/j.chom.2008.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu, D. et al. Direct ubiquitination of pattern recognition receptor FLS2 attenuates plant innate immunity. Science332, 1439–1442 (2011). 10.1126/science.1204903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marino, D. et al. Arabidopsis ubiquitin ligase MIEL1 mediates degradation of the transcription factor MYB30 weakening plant defence. Nat. Commun.4, 1476 (2013). 10.1038/ncomms2479 [DOI] [PubMed] [Google Scholar]
- 28.Marino, D. et al. Addendum: Arabidopsis ubiquitin ligase MIEL1 mediates degradation of the transcription factor MYB30 weakening plant defence. Nat. Commun.10, 1475 (2019). 10.1038/s41467-019-09341-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown, J. K. A cost of disease resistance: paradigm or peculiarity? Trends Genet.19, 667–671 (2003). 10.1016/j.tig.2003.10.008 [DOI] [PubMed] [Google Scholar]
- 30.Bergelson, J. & Purrington, C. B. Surveying patterns in the cost ofresistance in plants. Am. Nat.148, 536–558 (1996). 10.1086/285938 [DOI] [Google Scholar]
- 31.Deng, Y. et al. Epigenetic regulation of antagonistic receptors confers rice blast resistance with yield balance. Science355, 962–965 (2017). 10.1126/science.aai8898 [DOI] [PubMed] [Google Scholar]
- 32.Gao, M. et al. Ca(2+) sensor-mediated ROS scavenging suppresses rice immunity and is exploited by a fungal effector. Cell184, 5391–5404.e5317 (2021). 10.1016/j.cell.2021.09.009 [DOI] [PubMed] [Google Scholar]
- 33.Wang, H. et al. Suppression of rice miR168 improves yield, flowering time and immunity. Nat. Plants7, 129–136 (2021). 10.1038/s41477-021-00852-x [DOI] [PubMed] [Google Scholar]
- 34.Xu, G. et al. uORF-mediated translation allows engineered plant disease resistance without fitness costs. Nature545, 491–494 (2017). 10.1038/nature22372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiao, Y. et al. Regulation of OsSPL14 by OsmiR156 defines ideal plant architecture in rice. Nat. Genet.42, 541–544 (2010). 10.1038/ng.591 [DOI] [PubMed] [Google Scholar]
- 36.Miura, K. et al. OsSPL14 promotes panicle branching and higher grain productivity in rice. Nat. Genet.42, 545–549 (2010). 10.1038/ng.592 [DOI] [PubMed] [Google Scholar]
- 37.Wang, J. et al. A single transcription factor promotes both yield and immunity in rice. Science361, 1026–1028 (2018). 10.1126/science.aat7675 [DOI] [PubMed] [Google Scholar]
- 38.Lu, Z. et al. Genome-wide binding analysis of the transcription activator ideal plant architecture1 reveals a complex network regulating rice plant architecture. Plant Cell25, 3743–3759 (2013). 10.1105/tpc.113.113639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Song, X. et al. Targeting a gene regulatory element enhances rice grain yield by decoupling panicle number and size. Nat. Biotechnol.40, 1403–1411 (2022). 10.1038/s41587-022-01281-7 [DOI] [PubMed] [Google Scholar]
- 40.Zhang, L. et al. A natural tandem array alleviates epigenetic repression of IPA1 and leads to superior yielding rice. Nat. Commun.8, 14789 (2017). 10.1038/ncomms14789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park, C. H. et al. The Magnaporthe oryzae effector AvrPiz-t targets the RING E3 ubiquitin ligase APIP6 to suppress pathogen-associated molecular pattern-triggered immunity in rice. Plant Cell24, 4748–4762 (2012). 10.1105/tpc.112.105429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ning, Y. et al. OsELF3-2, an ortholog of Arabidopsis ELF3, interacts with the E3 ligase APIP6 and negatively regulates immunity against Magnaporthe oryzae in rice. Mol. Plant8, 1679–1682 (2015). 10.1016/j.molp.2015.08.004 [DOI] [PubMed] [Google Scholar]
- 43.McDowell, G. S. & Philpott, A. New insights into the role of ubiquitylation of proteins. Int. Rev. Cell. Mol. Biol.325, 35–88 (2016). 10.1016/bs.ircmb.2016.02.002 [DOI] [PubMed] [Google Scholar]
- 44.Yau, R. & Rape, M. The increasing complexity of the ubiquitin code. Nat. Cell Biol.18, 579–586 (2016). 10.1038/ncb3358 [DOI] [PubMed] [Google Scholar]
- 45.Nakamura, N. Ubiquitin system. Int. J. Mol. Sci.19, 1080 (2018). 10.3390/ijms19041080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cook, B. W., Lacoursiere, R. E. & Shaw, G. S. Recruitment of ubiquitin within an E2 chain elongation complex. Biophys. J.118, 1679–1689 (2020). 10.1016/j.bpj.2020.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu, M. et al. Inducible overexpression of ideal plant architecture1 improves both yield and disease resistance in rice. Nat. Plants5, 389–400 (2019). 10.1038/s41477-019-0383-2 [DOI] [PubMed] [Google Scholar]
- 48.Hiei, Y., Ohta, S., Komari, T. & Kumashiro, T. Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T-DNA. Plant J.6, 271–282 (1994). 10.1046/j.1365-313X.1994.6020271.x [DOI] [PubMed] [Google Scholar]
- 49.Waadt, R. et al. Multicolor bimolecular fluorescence complementation reveals simultaneous formation of alternative CBL/CIPK complexes in planta. Plant J.56, 505–516 (2008). 10.1111/j.1365-313X.2008.03612.x [DOI] [PubMed] [Google Scholar]
- 50.Zhou, J. et al. Differential phosphorylation of the transcription factor WRKY33 by the protein kinases CPK5/CPK6 and MPK3/MPK6 cooperatively regulates camalexin biosynthesis in arabidopsis. Plant Cell32, 2621–2638 (2020). 10.1105/tpc.19.00971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu, L. et al. An efficient system to detect protein ubiquitination by agroinfiltration in Nicotiana benthamiana. Plant J.61, 893–903 (2010). 10.1111/j.1365-313X.2009.04109.x [DOI] [PubMed] [Google Scholar]
- 52.Li, W. et al. A natural allele of a transcription factor in rice confers broad-spectrum blast resistance. Cell170, 114–126.e115 (2017). [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
All data are available in the main text and the Supplementary Information. Source data are provided with this paper.