Abstract
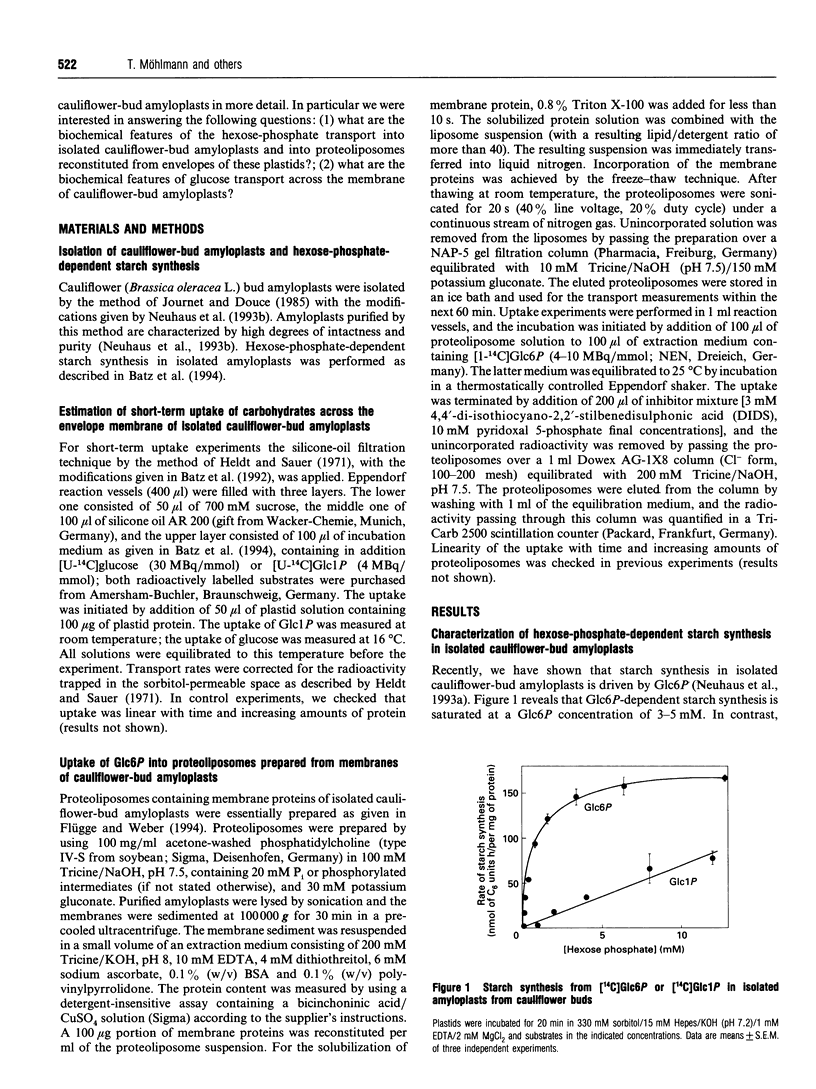
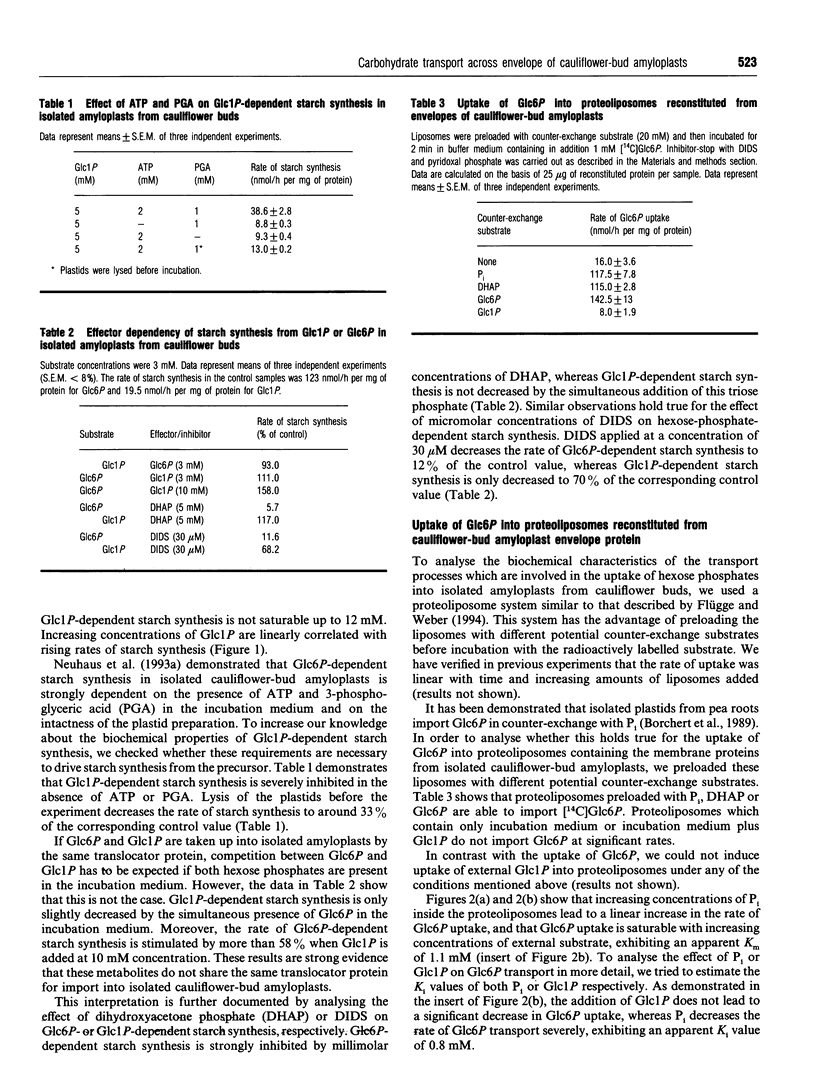
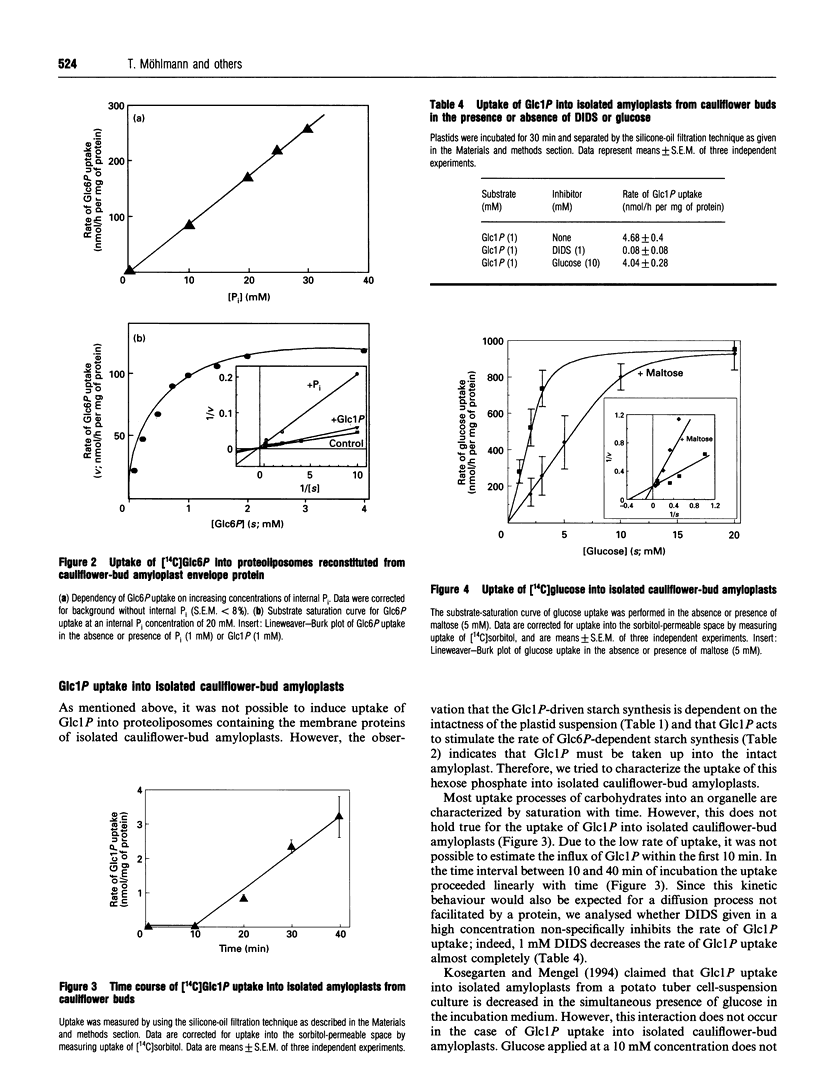
Using isolated amyloplasts from cauliflower buds, we have characterized the interaction and transport of various carbohydrates across the envelope membrane of a heterotrophic plastid. According to our results, glucose 6-phosphate (Glc6P) and glucose 1-phosphate (Glc1P) do not share the same transport protein for uptake into cauliflower-bud amyloplasts. Glc6P-dependent starch synthesis is strongly inhibited in the presence of dihydroxyacetone phosphate (DHAP) or 4,4'-di-isothiocyano-2,2'- stilbenedisulphonic acid (DIDS), whereas Glc1P-dependent starch synthesis is hardly affected by these compounds. Analysis of the Glc6P uptake into proteoliposomes reconstituted from the envelope proteins of cauliflower-bud amyloplasts indicate that Glc6P is taken up in a counter-exchange mode with Pi, DHAP or Glc6P, whereas Glc1P does not act as a counter-exchange substrate. Pi is a strong competitive inhibitor of Glc6P uptake (Ki 0.8 mM) into proteoliposomes, whereas Glc1P does not significantly inhibit Glc6P transport. Beside a hexose-phosphate translocator, these amyloplasts possess an envelope protein mediating the transport of glucose across the membrane. This translocator exhibits an apparent Km for glucose of 2.2 mM and is inhibited by low concentrations of phloretin, known to be a specific inhibitor of glucose-transport proteins. Maltose inhibits the uptake of glucose (Ki 2.3 mM), indicating that both carbohydrates share the same translocator.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Batz O., Maass U., Henrichs G., Scheibe R., Neuhaus H. E. Glucose- and ADPGlc-dependent starch synthesis in isolated cauliflower-bud amyloplasts. Analysis of the interaction of various potential precursors. Biochim Biophys Acta. 1994 Jul 6;1200(2):148–154. doi: 10.1016/0304-4165(94)90129-5. [DOI] [PubMed] [Google Scholar]
- Batz O., Scheibe R., Neuhaus H. E. Identification of the putative hexose-phosphate translocator of amyloplasts from cauliflower buds. Biochem J. 1993 Aug 15;294(Pt 1):15–17. doi: 10.1042/bj2940015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batz O., Scheibe R., Neuhaus H. E. Transport Processes and Corresponding Changes in Metabolite Levels in Relation to Starch Synthesis in Barley (Hordeum vulgare L.) Etioplasts. Plant Physiol. 1992 Sep;100(1):184–190. doi: 10.1104/pp.100.1.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borchert S., Harborth J., Schunemann D., Hoferichter P., Heldt H. W. Studies of the Enzymic Capacities and Transport Properties of Pea Root Plastids. Plant Physiol. 1993 Jan;101(1):303–312. doi: 10.1104/pp.101.1.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flügge U. I., Weber A. A rapid method for measuring organelle-specific substrate transport in homogenates from plant tissues. Planta. 1994;194(2):181–185. [PubMed] [Google Scholar]
- Heldt H. W., Sauer F. The inner membrane of the chloroplast envelope as the site of specific metabolite transport. Biochim Biophys Acta. 1971 Apr 6;234(1):83–91. doi: 10.1016/0005-2728(71)90133-2. [DOI] [PubMed] [Google Scholar]
- Herold A., Leegood R. C., McNeil P. H., Robinson S. P. Accumulation of Maltose during Photosynthesis in Protoplasts Isolated from Spinach Leaves Treated with Mannose. Plant Physiol. 1981 Jan;67(1):85–88. doi: 10.1104/pp.67.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Journet E. P., Douce R. Enzymic capacities of purified cauliflower bud plastids for lipid synthesis and carbohydrate metabolism. Plant Physiol. 1985 Oct;79(2):458–467. doi: 10.1104/pp.79.2.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeling P. L., Wood J. R., Tyson R. H., Bridges I. G. Starch Biosynthesis in Developing Wheat Grain : Evidence against the Direct Involvement of Triose Phosphates in the Metabolic Pathway. Plant Physiol. 1988 Jun;87(2):311–319. doi: 10.1104/pp.87.2.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhaus H. E., Henrichs G., Scheibe R. Characterization of Glucose-6-Phosphate Incorporation into Starch by Isolated Intact Cauliflower-Bud Plastids. Plant Physiol. 1993 Feb;101(2):573–578. doi: 10.1104/pp.101.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozueta-Romero J., Frehner M., Viale A. M., Akazawa T. Direct transport of ADPglucose by an adenylate translocator is linked to starch biosynthesis in amyloplasts. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5769–5773. doi: 10.1073/pnas.88.13.5769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer G., Heber U. Glucose transport into spinach chloroplasts. Plant Physiol. 1977 Aug;60(2):286–289. doi: 10.1104/pp.60.2.286. [DOI] [PMC free article] [PubMed] [Google Scholar]


