Abstract
Posttransplantation complications pose a major challenge to the long‐term survival and quality of life of organ transplant recipients. These complications encompass immune‐mediated complications, infectious complications, metabolic complications, and malignancies, with each type influenced by various risk factors and pathological mechanisms. The molecular mechanisms underlying posttransplantation complications involve a complex interplay of immunological, metabolic, and oncogenic processes, including innate and adaptive immune activation, immunosuppressant side effects, and viral reactivation. Here, we provide a comprehensive overview of the clinical features, risk factors, and molecular mechanisms of major posttransplantation complications. We systematically summarize the current understanding of the immunological basis of allograft rejection and graft‐versus‐host disease, the metabolic dysregulation associated with immunosuppressive agents, and the role of oncogenic viruses in posttransplantation malignancies. Furthermore, we discuss potential prevention and intervention strategies based on these mechanistic insights, highlighting the importance of optimizing immunosuppressive regimens, enhancing infection prophylaxis, and implementing targeted therapies. We also emphasize the need for future research to develop individualized complication control strategies under the guidance of precision medicine, ultimately improving the prognosis and quality of life of transplant recipients.
Keywords: infection, malignancy, organ transplantation, posttransplant complications, rejection, T cell
The most common posttransplant complications (PTC) include immune‐mediated complications (rejection and graft‐versus‐host disease), infections, metabolic complications (diabetes, hypertension, and dyslipidemia), and malignancies.
The mechanisms underlying PTC mainly involve allogeneic antigen‐activated innate immunity, cellular immunity, and humoral immunity responses, as well as the side effects and toxicity of immunosuppressants.
Treatment strategies for PTC include the use of immunosuppressants with fewer side effects, Treg cell therapy, infection screening and vaccination, mTOR inhibitors for cancer treatment, and CAR‐T cell therapy and Treg depletion therapy.

1. INTRODUCTION
Organ transplantation is the definitive therapeutic option for chronic and end‐stage organ failure, which can significantly prolong patient survival. 1 , 2 However, the improvement in long‐term survival of transplant recipients is limited, primarily attributed to the negative impact of posttransplant complications. 3 , 4 Although modern immunosuppressive regimens have been effective in reducing the incidence of acute rejection, immune‐mediated complications, such as chronic rejection and chronic graft insufficiency, remain a major challenge in organ transplantation. 5 Moreover, infectious complications pose another major threat to the lives of transplant recipients. Immunosuppressive therapy, while reducing the risk of rejection, simultaneously increases the incidence of infection. 6 Metabolic complications, such as posttransplant diabetes, dyslipidemia, and hypertension, also significantly impact the prognosis of recipients. 7 , 8 , 9 The increasing incidence of posttransplant malignancies, concomitant with the prolonged survival of transplant recipients, is of particular concern. 10 In view of these challenges, posttransplantation complications have become a major hurdle, limiting the further development of transplantation medicine and hindering the long‐term benefits for transplant recipients.
In‐depth investigation into the mechanisms underlying posttransplantation complications is of paramount importance for guiding clinical practice and improving transplantation outcomes. In recent years, advancements in molecular biology techniques have greatly facilitated the study of molecular mechanisms associated with posttransplantation complications. Regarding immune‐mediated complications, the roles of immune response pathways involving T cells and B cells, as well as cytokine and chemokine networks, in graft rejection and injury have been gradually elucidated. 5 , 11 , 12 Studies on immunosuppression have further elucidated the mechanisms by which immunosuppressive agents induce metabolic disorders and organ toxicity, as well as the immunological basis for their propensity to promote infection and malignancy. 1 , 13 , 14 These research advances have yielded novel insights and strategies for preventing and treating posttransplantation complications. However, our current understanding of posttransplantation complications remains limited and lacks depth, with many aspects requiring further investigation.
This review aims to provide a comprehensive summary of the clinical features and risk factors of posttransplantation complications, with a focus on the molecular mechanisms underlying their development, and to explore potential prevention and intervention strategies based on these findings. The paper is divided into three parts: (1) a summary of the epidemiological characteristics, clinical manifestations, and risk factors of major posttransplant complications, including immune‐mediated complications, infectious complications, metabolic complications, and malignant cancers; (2) an in‐depth examination of the molecular mechanisms underlying posttransplant complications, encompassing immune dysregulation, immunosuppressant side‐effects, and the molecular pathways of graft injury; and (3) a discussion of potential prevention and intervention strategies. Building upon the foundation laid by the first two parts, the third part summarizes the research progress in the prevention and treatment of posttransplantation complications and provides an outlook on future research directions.
2. MAJOR POSTTRANSPLANT COMPLICATIONS
Posttransplantation complications encompass a wide range of conditions that significantly impact the long‐term survival and quality of life of transplant recipients. This section provides a comprehensive overview of the major posttransplant complications, including immune‐mediated complications, infectious complications, metabolic and cardiovascular complications, malignancies, and other notable complications. By discussing the epidemiology, clinical manifestations, and risk factors associated with each complication category, this section lays the foundation for understanding the complex pathophysiology and management challenges in the posttransplantation setting.
2.1. Immune‐mediated complications
Posttransplant immune‐mediated complications, primarily consisting of rejection and graft‐versus‐host disease (GVHD), pose a significant challenge in the field of organ transplantation. Despite substantial progress in immunosuppressive therapy, rejection and GVHD continue to be critical factors impacting long‐term graft and patient survival.
Rejection can occur in nearly all types of allogeneic organ transplantation. Based on the onset timing and underlying mechanisms, rejection can be classified as hyperacute, acute, or chronic. 15 Hyperacute rejection typically manifests within minutes to hours posttransplantation and is primarily mediated by preexisting donor‐specific antibodies, such as those encountered in ABO blood group‐incompatible kidney transplantation. 16 Acute rejection most commonly develops days to weeks posttransplantation and is characterized by a cytotoxic response driven by donor‐specific lymphocytes, accompanied by humoral immune responses. Although the incidence of acute rejection following kidney and other organ transplantation has been markedly reduced with the use of immunosuppressive agents, the resulting graft damage and delayed graft function (DGF) continue to jeopardize recipient outcomes. 17 Chronic rejection, occurring months to years posttransplantation, is a progressive pathological process characterized by graft vasculopathy and interstitial fibrosis. In solid organ transplantation (SOT), such as heart and other organs, these pathological changes can manifest early and exhibit a high incidence in the late posttransplant period. For instance, within 1 year following kidney transplantation, over 81% of patients develop interstitial fibrosis and tubular atrophy lesions stemming from cellular arteritis, with these lesions progressing to severe damage in more than 50% of transplanted kidneys within 5 years. 18
GVHD is one of the most prevalent and severe complications following allogeneic hematopoietic stem cell transplantation (allo‐HSCT), frequently occurring in cases of incomplete donor–recipient (D–R) human leukocyte antigen (HLA) matching. Consequent to this histocompatibility disparity, immunocompetent cells within the graft mount an attack against recipient tissues, leading to dysregulation and dysfunction. 19 Based on the onset timing and clinical manifestations, GVHD can be classified into two forms: acute and chronic. Acute GVHD typically manifests within 100 days posttransplantation, primarily affecting the skin, liver, and gastrointestinal tract. In contrast, chronic GVHD usually develops beyond 100 days posttransplantation and can involve any organ system. 20 Research indicates that the incidence of acute GVHD ranges from 28 to 40% in moderate‐to‐severe cases, while chronic GVHD can affect up to 70% of patients. 21 , 22 As one of the primary factors influencing allo‐HSCT outcomes, GVHD is associated with a mortality rate of up to 25% in patients with chronic GVHD. 23 Despite the implementation of various preventive and therapeutic approaches, including immunosuppressive agents and cellular therapies, GVHD continues to pose a significant challenge in the context of allo‐HSCT.
2.2. Infectious complication
Posttransplant infections contribute significantly to morbidity and mortality among transplant recipients. Approximately 70% of kidney transplant recipients are estimated to develop an infection within 3 years posttransplantation. 24 Following cardiovascular disease, infections are the second most common cause of mortality in transplant recipients. In the late posttransplant period (4–10 years), infections are the primary cause of mortality, particularly among diabetic patients. 25 Immunosuppressive therapy, administered to prevent rejection, increases the risk of infections in transplant recipients, primarily including donor‐derived infections, nosocomial and community‐acquired infections, and reactivation of latent pathogens. 26 Frequently encountered bacterial infections comprise those caused by gram‐negative bacteria, such as Escherichia coli and Klebsiella pneumoniae, and gram‐positive bacteria, including staphylococci and enterococci. These infections can affect various organ systems and are strongly associated with the type of transplanted organ. For instance, urinary tract infections are linked to kidney transplantation, intra‐abdominal infections to liver transplantation, and pneumonia to lung transplantation. 27 Cytomegalovirus (CMV) is the most prevalent viral infection following transplantation, potentially leading to fever, leukopenia, and organ involvement. 28 Epstein–Barr virus (EBV), herpes simplex virus (HSV), varicella‐zoster virus (VZV), human herpesviruses (HHVs), and other human viruses are frequently encountered viruses in the posttransplantation period. In addition to the aforementioned viruses, human herpesvirus (HHV)‐6 and BK virus can also cause infections in transplant recipients. 29 In general, Pneumocystis jirovecii pneumonia and candidiasis are prevalent fungal infections following transplantation, whereas SOT recipients infected with invasive fungi, such as Aspergillus and Mucorales species, experience higher morbidity and mortality rates. 30 , 31 The incidence of posttransplant infections exhibits a distinct temporal pattern. In the first month posttransplantation, infections are commonly associated with surgical complications, nosocomial exposures, and donor‐derived pathogens, including multidrug‐resistant bacteria such as methicillin‐resistant Staphylococcus aureus, vancomycin‐resistant enterococci, carbapenem‐resistant Enterobacteriaceae, and Clostridioides difficile. Opportunistic infections are more prevalent between 1 and 6 months posttransplantation, coinciding with the period of more intensive immunosuppression. During this time, reactivation of latent pathogens, such as BK virus, hepatitis C virus (HCV), and Mycobacterium tuberculosis, may also occur. Pneumocystis jirovecii pneumonia, herpesvirus infections (including CMV, HSV, VZV, and EBV), and hepatitis B virus (HBV) infections are less common during this period due to the use of prophylactic medications. Beyond 6 months posttransplantation, the majority of patients are on lower levels of immunosuppression, and infections are primarily community acquired. Moreover, there remains a risk of recurrent infections and delayed‐onset viral infections, particularly CMV. 32 , 33 Consequently, pretransplantation screening of donors and recipients, immunization, optimal antimicrobial and antiviral prophylaxis, and prudent use of antibiotics can mitigate the impact of infections. Furthermore, screening donors for bacterial infections, such as urinary tract infections and bacteremia, is essential.
2.3. Metabolic and cardiovascular complications
Posttransplant diabetes mellitus (PTDM) is a prevalent endocrine metabolic disorder following SOT in adults, affecting 10% to 40% of recipients. New‐onset diabetes after transplantation (NODAT), defined as diabetes that develops posttransplantation in patients without a prior history of diabetes, is a common and severe complication following transplantation of the kidney, liver, heart, and various other organs. 34 NODAT‐related complications develop more rapidly compared with those in the general population with type 2 diabetes. Moreover, NODAT is associated with poorer outcomes, including an elevated risk of major cardiovascular events, graft failure, and mortality. 35 The primary pathogenic mechanism underlying NODAT is believed to be β‐cell dysfunction. Other pathophysiologic processes involved in NODAT development include impaired insulin sensitivity and uncontrolled glucagon release. 36 , 37 , 38 , 39 Notable risk factors for NODAT include advanced age and obesity. 40 , 41 Importantly, the use of posttransplant immunosuppressive agents, such as glucocorticoids, calcineurin inhibitors (CNIs), and mammalian target of rapamycin (mTOR) inhibitors, is strongly associated with the development of NODAT. 42 , 43
Dyslipidemia and hypertension are prevalent metabolic complications among various solid organ transplant recipients. Dyslipidemia, encompassing both hyperlipidemia and hypercholesterolemia, exhibits a high incidence following renal, hepatic, and cardiac transplantation. 44 , 45 , 46 In addition to nonmodifiable risk factors, such as advanced age and genetic predisposition, the use of immunosuppressive agents, including corticosteroids, CNIs, and mTOR inhibitors, are significant potential contributors to posttransplant dyslipidemia. 47 Hypertension is one of the most frequent cardiovascular complications following renal, cardiac, and pulmonary SOT. 48 , 49 , 50 The presence of common risk factors for hypertension in recipients, such as alcohol abuse, smoking, and obesity, along with transplant renal artery stenosis, allograft rejection, and immunosuppression, are contributing factors to posttransplant hypertension. 51 , 52 , 53 Notably, considering the substantial cardiovascular damage caused by dyslipidemia and hypertension, the management of blood lipids and blood pressure is crucial, particularly in heart transplant recipients. 54 , 55
Cardiovascular disease is the primary cause of mortality in early SOT recipients. 56 The risk of cardiovascular complications, such as coronary artery disease, heart failure, arrhythmias, and pulmonary hypertension, is substantially higher in the transplant population compared with the general population. 51 Moreover, factors such as smoking and obesity are also prevalent risk factors for posttransplant cardiovascular disease. 57 Notably, the aforementioned conditions, including diabetes, hyperlipidemia, hypertension, and the closely associated use of immunosuppressive agents, are all regarded as risk factors for the development of posttransplant cardiovascular disease. 58
2.4. Malignant cancer
Posttransplantation malignancies are among the most common postoperative complications in SOT and HSCT recipients, with an overall prevalence ranging from 4 to 18%. 1 High‐risk types of de novo posttransplant malignancies include skin cancer, genitourinary cancers, and posttransplant lymphoproliferative disease (PTLD). 59 In addition to traditional risk factors, such as smoking, sun exposure, and family history of cancer, the transplant population has unique risk factors for malignancy, including immunosuppression, oncogenic viruses, and donor‐derived transmission. 60 Epidemiologic data demonstrate that the incidence of de novo malignancies within 10 years posttransplantation is two to three times higher in transplant recipients compared with the general population, with the incidence of nonmelanoma skin cancers being up to 13 times higher than in the nontransplant population. 61 In general, the prognosis of de novo malignancies following transplantation is poor, with the 5‐year survival rate being substantially lower compared with patients with similar malignancies in the general population. At present, malignancies have become a leading cause of long‐term mortality in transplant recipients, necessitating close follow‐up, and surveillance.
In addition to novo neoplastic malignancies, virus‐associated cancers constitute a significant category of posttransplantation malignancies. Frequent viral infection‐associated posttransplantation malignancies include EBV‐associated PTLD, HHV‐8‐associated Kaposi's sarcoma (KS), and human papillomavirus‐associated skin cancer, anogenital cancer, and head and neck cancer. 62 , 63 , 64 Among these, PTLD, one of the most common posttransplantation malignancies, is associated with EBV infection in approximately 55−65% of cases. 65 , 66 Upon entering the human body, EBV typically first infects the oropharyngeal epithelial cells. As the viral infection subsides, the small amount of EBV present in B cells enters a latent period, evading the host immune system. Following reactivation of the virus due to various factors, the lifespan of the virus‐infected B lymphocytes is prolonged, with an increased potential for mutations. 65 , 67 , 68 Patients with early‐onset PTLD are typically EBV‐positive and have a remarkably high mortality rate. 69 , 70 Moreover, HHV‐8‐associated virologic markers are positive in nearly all transplant recipients with KS. 64 KS in the immunosuppressed state is typically more aggressive and fatal compared with immunocompetent recipients. 71 Notably, all malignancies with a substantially increased risk compared with the general population were associated with viruses, underscoring the crucial role of oncogenic viruses in posttransplant cancers. 60
2.5. Other complications
DGF is one of the most common early complications following kidney transplantation, with an incidence of approximately 25−30%. 72 Although there are various definitions of DGF, the most widely accepted one is the failure of the transplanted kidney to function at the expected level within 7 days posttransplantation, often necessitating dialysis. 73 The occurrence of DGF is closely associated with donor factors (e.g., advanced age, deceased donor transplantation), recipient factors (e.g., immune hyperreactivity, recurrence of kidney disease), and perioperative factors (e.g., ischemia–reperfusion injury, immunosuppressant toxicity). 74 Notably, both acute and chronic rejection are important causes of DGF exacerbation, which significantly reduces posttransplant survival. 75 Substantial clinical evidence demonstrates a strong association between DGF and subsequent chronic allograft dysfunction. 76 , 77
Transplant recipients frequently experience a range of comorbid gastrointestinal and neurologic complications. Gastrointestinal symptoms, including nausea, vomiting, and diarrhea, may be associated with factors such as immunosuppressant medications (e.g., mycophenolate mofetil [MMF]) and infections (e.g., CMV enteritis). 78 , 79 Moreover, transplant recipients have an increased risk of developing severe gastrointestinal complications, such as diverticulitis and gastrointestinal perforation. 80 , 81 Targeted modification of immunosuppressive regimens, prevention and treatment of related infections, and symptomatic supportive care are essential for managing posttransplant gastrointestinal complications. 82 Neurologic complications, such as headache, tremor, and sensory abnormalities, may be associated with immunosuppressant neurotoxicity (e.g., tacrolimus), infections (e.g., cryptococcal meningitis), and metabolic disturbances (e.g., hypomagnesemia). 83 , 84
3. MOLECULAR MECHANISMS OF POSTTRANSPLANTATION COMPLICATIONS
The molecular mechanisms underlying posttransplantation complications involve a complex interplay of immunological, metabolic, and oncogenic processes. This section delves into the intricate molecular pathways and cellular interactions that contribute to the development of immune‐mediated complications, the effects of immune oversuppression, and the pathogenesis of posttransplant malignancies. Additionally, it addresses posttransplant metabolic and cardiovascular complications. By providing a comprehensive analysis of the key molecular players and signaling cascades involved in each complication category, this section offers valuable insights into potential therapeutic targets and strategies for mitigating posttransplantation morbidity and mortality.
3.1. Posttransplant complications due to overimmunosuppression
SOT and allo‐HSCT are crucial clinical treatments for numerous end‐stage diseases. However, the posttransplantation period is frequently characterized by severe immune complications, primarily manifested as allograft rejection and GVHD. Although the contexts of occurrence and clinical manifestations of these two complications differ, the underlying immune activation and effector mechanisms share substantial similarities. This section will systematically summarize and discuss the mechanisms underlying these two types of complications (Figure 1).
FIGURE 1.
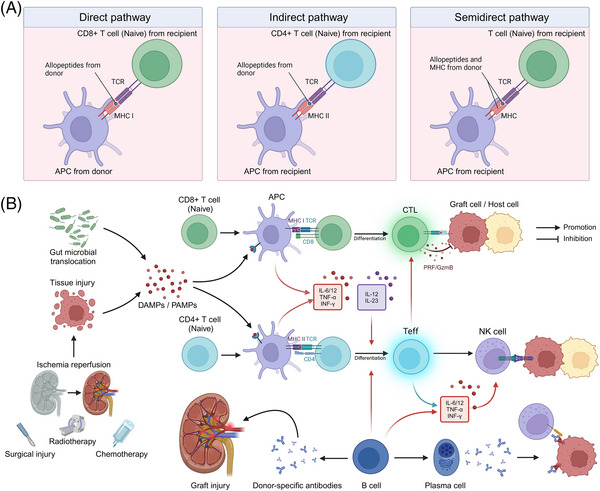
Mechanisms of complications associated with immune overactivation following transplantation. (A) Three pathways of allorecognition. In the direct pathway, donor antigen‐presenting cells (APCs) interact directly with recipient T cells. In the indirect pathway, recipient APCs present processed donor alloantigen peptides to recipient T cells, resembling a typical immune response. In the semi‐direct pathway, recipient APCs acquire donor HLA molecules that directly present peptides to recipient T cells. (B) Mechanisms of immune activation associated with transplantation. Tissue damage and the release of damage‐associated molecular patterns (DAMPs), such as ATP, uric acid, IL‐33, and HMGB‐1, can result from ischemia–reperfusion injury and surgical trauma during allogeneic solid organ transplantation, as well as preoperative chemotherapy and radiotherapy for hematopoietic stem cell transplantation. Intestinal barrier damage can also lead to alterations in gut microbiota and the release of pathogen‐associated molecular patterns (PAMPs). These DAMPs and PAMPs serve as danger signals that interact with the recipient's innate immune cell surface and intracellular pattern recognition receptors (PRRs), initiating and sustaining innate immune responses. Upon APC stimulation, naïve CD8+ T cells differentiate into cytotoxic T cells, which can destroy donor organs or host cells through the release of granules and perforin. Following APC stimulation, naïve CD4+ T cells differentiate into effector T (Teff) cells. Teff cells enhance the cytotoxic activity of natural killer (NK) cells and cytotoxic T lymphocytes (CTLs) by secreting proinflammatory cytokines, including IL‐6, IL‐12, and TNF‐α. B cells can function as specific antigen‐presenting cells and modulate CD4+ T cell activation via indirect allorecognition pathways. Furthermore, activated B cells can differentiate into plasma cells that produce antibodies, potentially leading to antibody‐mediated rejection (ABMR) through mechanisms such as antibody‐dependent cell‐mediated cytotoxicity (ADCC) and direct cytotoxic effects. Substantial quantities of donor‐specific antibodies produced by recipient B cells can bind to the vascular endothelium of the graft, rapidly inducing inflammatory infiltration and vascular damage. This figure was created using tools provided by Biorender.com (accessed July 2, 2024).
3.1.1. Innate immune activation
Innate immunity plays a crucial role in the immune response to transplantation. In the context of SOT, ischemia–reperfusion injury, surgical trauma, and other factors result in extensive cell death within the transplanted organ, leading to the release of damage‐associated molecular patterns (DAMPs), including high mobility group protein B1 (HMGB1), heat shock proteins, ATP, and extracellular DNA. 85 Likewise, extensive tissue damage caused by pretransplant conditioning regimens for allo‐HSCT, such as radiotherapy and total body irradiation, can result in a substantial release of DAMPs, including ATP, uric acid, interleukin (IL)−33, and HMGB‐1. 86 Moreover, damage to the intestinal barrier can lead to alterations in the gut microbiome, resulting in the translocation of gut microbes and the release of pathogen‐associated molecular patterns (PAMPs). 87 These DAMPs and PAMPs serve as danger signals that can bind to pattern recognition receptors (PRRs) on the surface and within the cytoplasm of the recipient's innate immune cells, such as Toll‐like receptors and NOD‐like receptors, initiating and sustaining the innate immune response. 88 Activation of PRRs triggers the activation of downstream inflammatory signaling pathways, including nuclear factor‐κB (NF‐κB) and other related pathways, and promotes the secretion of proinflammatory cytokines, such as IL‐1, IL‐6, and tumor necrosis factor‐alpha (TNF‐α). 89 , 90 In the context of graft rejection, these proinflammatory responses can lead to graft damage, resulting in acute or chronic rejection. Furthermore, activation of the complement system (e.g., C3a and C5a) can directly activate T cells and antigen‐presenting cells (APCs) within the graft. 91 In the context of GVHD, innate immune activation promotes the production of stimulatory cytokines, such as IL‐12 and IL‐23, which facilitate the differentiation of donor T cells into various effector CD4+ T cell (Teff) lineages. Notably, in addition to their antigen‐presenting functions, neutrophils, as part of the innate immune system, are believed to directly damage gastrointestinal tissues through the release of reactive oxygen species. 92
3.1.2. Allogeneic antigen recognition
In the context of allogeneic rejection, the graft expresses donor‐derived alloantigens, primarily major histocompatibility complex (MHC) molecules and minor histocompatibility antigens. 93 , 94 The recipient's immune system recognizes these alloantigens through direct and indirect pathways, which subsequently initiate an alloimmune response. 95 In the direct pathway, immature dendritic cells (DCs), which are prevalent in the donor organ, are stimulated by inflammatory signals to mature and migrate from the graft to the paracortex of the recipient's lymph nodes. These mature DCs exhibit high expression of MHC class I molecules and elevated levels of MHC costimulatory molecules, which can stimulate graft‐specific CD8+ T‐cell activation within the lymph nodes, thereby inducing an acute allogeneic rejection response. As donor APCs become depleted, the role of recipient APCs in the rejection response gradually becomes predominant. Graft antigens are recognized and processed by recipient APCs and subsequently bind to recipient MHC class II molecules. The antigenic peptide–MHC class II complex is then presented to CD4+ T cells, thereby inducing a slower and less intense immune response through this indirect pathway. 96 Notably, a semi‐direct pathway also contributes to allogeneic rejection. In this pathway, recipient APCs directly acquire intact donor MHC molecules through cell‐to‐cell interactions and present them to recipient T cells. This pathway is characterized by recipient APCs presenting both donor and recipient MHC molecules, thereby activating T cells through both direct and indirect pathways, and plays a crucial role in both acute and chronic rejection. 97 , 98
During the development of acute GVHD, donor T cells, mediated by L‐selectin, CCR7, and other molecules, migrate to lymphoid organs or host tissues, where they proliferate and differentiate extensively upon stimulation by host antigens presented by APCs via MHC class I or class II molecules. 99 Furthermore, costimulatory pathways, including CD28, CD278, and TNFR superfamily receptors (e.g., CD40L and OX40), are also essential for T cell activation. 100 , 101 , 102 , 103 Various cytokines, such as interferon‐gamma (IFN‐γ), TNF‐α, and others, play crucial roles in T cell activation. Among these cytokines, IFN‐γ promotes antigen presentation by upregulating the expression of adhesion molecules, chemokines, and HLA molecules. Moreover, inflammatory chemokines, such as C‐X‐C motif chemokine ligand (CXCL9), CXCL10, and CXCL11, can recruit activated Teff to infiltrate GVHD target organs. 104
3.1.3. T‐ and B‐cell‐mediated adaptive immune responses
T cells activated by allogeneic recognition or innate immune activation play a pivotal role in mediating the allogeneic immune response and inducing graft or host injury. The damaging effects of T cells on the graft or host primarily involve direct lysis of graft cells by cytotoxic T cells (CD8+ T cells) and the secretion of cytokines by helper T cells (CD4+ T cells) to promote inflammatory responses. Upon APC presentation and stimulation, which relies on T cell receptor (TCR) recognition of alloantigens, naïve CD8+ T cells are activated and rapidly proliferate and differentiate into effector T cells and memory T cells. 105 Influenced by a specific cytokine microenvironment, the initial CD8+ T cells differentiate into various CD8+ T cell lineages, including IFN‐γ‐producing Tc1, IL‐4‐producing Tc2, IL‐9‐producing Tc9, IL‐17‐producing Tc17, and IL‐22‐producing Tc22 cell subpopulations. 106 , 107 Among these subpopulations, typical cytotoxic T cells (CTLs), such as Tc1, Tc2, and Tc22, can mediate rejection by expressing granzymes, perforin, and other cytotoxic molecules. 108 , 109 , 110 In GVHD, CTLs are the primary effector cells that also mediate host cell lysis through the Fas/FasL pathway and cytotoxic molecules (e.g., perforin and granzyme B). 111 In the indirect pathway of rejection and GVHD, MHC class II complexes presented by APCs in the draining lymph nodes stimulate naïve CD4+ T cells by binding to the TCR, inducing their proliferation and differentiation into various subpopulations of CD4+ helper T (Th) cells. The Th subpopulations exhibit varying levels of surface marker expression and produce different cytokines, which play a broad and complex role in the allogeneic rejection response and GVHD. Among these subpopulations, the subpopulation that recognizes and removes antigens while promoting the immune response is referred to as Teff. 112 Common Teff include Th1 cells (which primarily secrete IFN‐γ, IL‐2, and TNF‐α, and mediate cellular immune responses), Th2 cells (which mainly secrete IL‐4, IL‐5, and IL‐13, promote B‐cell differentiation, maturation, and antibody production, and mediate humoral immune responses), Th17 cells (which primarily secrete IL‐17 and IL‐22), and T follicular helper cells (which produce IL‐21 and promote humoral immunity in germinal centers). 113 , 114 , 115
In immune rejection, memory T cells in transplant recipients can be derived from alloreactive memory T cells generated by previous exposure to alloantigens (e.g., blood transfusion, pregnancy, or a previous transplant) or from the differentiation of CD8+ T cells activated by direct or indirect pathways after transplantation. 116 Notably, viral or other microbial infections can also induce the generation of alloreactive memory T cells through antigen‐mimetic mechanisms, enabling these cells to cross‐recognize allogeneic antigens and participate in transplant rejection. 117 Compared with naïve T cells, these memory T cells have a lower activation threshold, a more rapid initiation effect, and enhanced proliferation, differentiation, and cytokine secretion. 118 , 119 These properties enable memory T cells to play a more prominent role in transplant rejection.
B cells also play a crucial role in allogeneic rejection immunization in transplantation. On the one hand, B cells can function as specific APCs and influence the activation of CD4+ T cells through the indirect recognition pathway. 120 , 121 For instance, heart transplantation in a mouse model with defective indirect presentation of alloantigens by B cells resulted in significantly prolonged survival of heart grafts, highlighting the importance of B cell presentation in mediating graft rejection. 122 Moreover, B cells play a vital role in the proliferation and subsequent differentiation of alloreactive T cells into memory T cells, thereby accelerating subsequent rejection. 123 In this process, a wide array of cytokines (e.g., IL‐6 and IFN‐γ) secreted by B cells play a crucial stimulatory role. 124 On the other hand, activated B cells can differentiate into plasma cells that produce antibodies, potentially leading to antibody‐mediated rejection. These antibodies can damage the graft through various mechanisms, such as complement‐dependent cytotoxicity, antibody‐dependent cell‐mediated cytotoxicity (ADCC), and direct cytotoxic effects. In hyperacute rejection, preexisting donor‐specific antibodies in the recipient's blood bind to the endothelium of the graft vasculature and activate the complement system. This initiates a cascade of pathological processes, including neutrophil infiltration, vascular injury, hemorrhage, fibrin deposition, and platelet aggregation, ultimately leading to irreversible graft damage within a short period. 125 , 126 In ADCC, the Fab segment of the alloantibody binds to the antigen on the graft cell surface, while its Fc segment cross‐links with the Fc receptor on the surface of the recipient's effector cells (e.g., natural killer [NK] cells and macrophages), activating them and inducing the release of cytotoxic substances, ultimately damaging the graft. 127 , 128 Furthermore, alloantibodies can directly induce apoptosis or necrosis by binding to specific antigens on the graft cell surface. 129
3.1.4. Disorders of immune regulation
Regulatory T cells (Tregs) are a subpopulation of T cells that employ various inhibitory mechanisms to regulate the activity of other immune cells and modulate immune system homeostasis. 130 Research has demonstrated that one of the primary functions of Tregs is to suppress the activation, proliferation, and cytokine production of effector T cells. 131 , 132 By inducing and maintaining transplantation tolerance, Tregs play a crucial role in preventing or attenuating immune rejection and GVHD. Consequently, when the number or function of Tregs is reduced or suppressed, it may disrupt immune homeostasis, leading to rejection and GVHD. Numerous observational studies in various transplant types, such as kidney and liver, have suggested a correlation between the relative reduction of Tregs and short‐term rejection after transplantation. 133 , 134 Likewise, several studies have observed an insufficient upregulation of Tregs in target organ biopsies of GVHD patients, such as the intestines and skin. 135 , 136 At present, numerous preclinical animal experiments and clinical trials have demonstrated that Tregs play a pivotal role in preventing severe GVHD and rejection. 137 , 138 , 139
3.2. Posttransplant complications due to under‐immunosuppression
Immunosuppressive therapies play a crucial role in transplantation antirejection therapy; however, they can also cause severe adverse reactions through various pathways. A comprehensive understanding of the mechanisms of action of immunosuppressive therapies is essential for optimizing immunosuppressive regimens and minimizing the risk of complications. This section will explore the molecular mechanisms underlying the heightened susceptibility to infections and increased risk of tumorigenesis associated with immunosuppressive therapies (Table 1 and Figure 2).
TABLE 1.
Summary of mechanisms for side effects of immunosuppressive therapy.
| Complications | Major mechanism | Details | References |
|---|---|---|---|
| PTDM | Immunosuppressive therapy‐related metabolic disorders | Increased insulin resistance occurs in peripheral tissues | 172, 175 |
| Direct damage occurs to pancreatic β‐cells | 173, 174 | ||
| Dyslipidemia | Key enzymes are upregulated in the cholesterol biosynthesis process | 177 | |
| LDL receptor binding to LDL is interfered with, and lipoprotein lipase activity is reduced | 178 | ||
| Hypertension | Vasodilators are decreased, and vasoconstrictors are increased | 180, 181 | |
| Aldosterone levels are mediated, and sympathetic nerve activation leads to sodium retention | 182, 183, 184 | ||
| Infection and malignancy | Immunosuppressive therapy‐induced impaired immune surveillance | Proliferation and activation are inhibited in CD8+ T cells and CD4+ T cells | 140, 141 |
| Th17 cell differentiation is inhibited | 142, 143 | ||
| Adhesion and penetration capabilities are reduced in CD8+ T cells | 144, 145, 146 | ||
| Proinflammatory cytokine release is reduced | 147, 148 | ||
| Cellular communication is suppressed, impacting T cell immune effects | 150 | ||
| Immunosuppressive therapy‐induced enhancement of Tregs immunosuppressive function | Immunosuppressants affect Foxp3 expression level | 153, 154 | |
| Co‐inhibitory receptors, such as TIGIT, PD‐1, CTLA‐4, and so on, are overexpressed by Tregs | 155, 156, 157, 158 | ||
| Immunosuppressive cytokine secretion by Tregs occurs, inhibiting Teff cells and consuming IL‐2 | 159 | ||
| Cytotoxic substance and immunosuppressive metabolite production is increased in Tregs | 160, 161 |
Abbreviations: CTLA‐4, cytotoxic T lymphocyte‐associated antigen‐4; IL‐2, interleukin‐2; LDL, low‐density lipoprotein; PD‐1, programmed cell death protein‐1; PTDM, posttransplantation diabetes mellitus; TIGIT, T cell immunoreceptor with immunoglobulin and ITIM domain.
FIGURE 2.
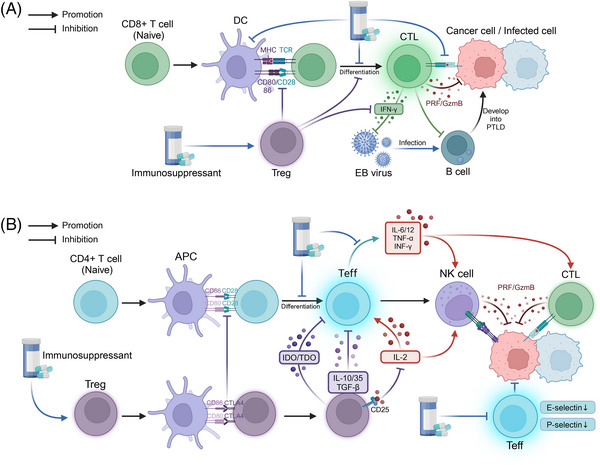
Mechanisms of complications associated with immunosuppression following transplantation. (A) Under normal circumstances, antigen‐presenting cells (APCs) stimulate the differentiation of naïve CD8+ T cells into cytotoxic T cells, which can eliminate infected or malignant cells through the release of granules and perforin. However, the excessive use of immunosuppressive agents can disrupt this process. Immunosuppressants can inhibit the activation, differentiation, and cytotoxic function of naïve CD8+ T cells. Moreover, immunosuppressants can activate regulatory T cells (Tregs), which may hinder the development of naïve CD8+ T cells and the production of interferon‐γ (IFN‐γ) by cytotoxic T cells. Furthermore, reduced IFN‐γ secretion may elevate the risk of Epstein–Barr virus (EBV) infection in B cells, consequently promoting the development of EBV‐associated posttransplant lymphoproliferative disorder (PTLD). Additionally, the suppression of cytotoxic T cell function against EBV further increases the risk of posttransplant malignancies. (B) When the body's antitumor immune system is functioning optimally, APCs stimulate the differentiation of naïve CD4+ T cells into effector T (Teff) cells. Teff cells enhance the anti‐infective and antitumor effects of natural killer (NK) cells and cytotoxic T lymphocytes (CTLs) by secreting proinflammatory cytokines, including IL‐6, IL‐12, and TNF‐α. However, the overuse of immunosuppressive agents can disrupt this process by inhibiting the differentiation of naïve CD4+ T cells and impairing the ability of Teff cells to secrete proinflammatory cytokines. Moreover, excessive immunosuppression promotes the competitive binding of cytotoxic T‐lymphocyte‐associated protein 4 (CTLA‐4) on Teff cells to CD80/86 on APCs, thereby inhibiting the activation of naïve CD4+ T cells. Furthermore, Tregs suppress Teff cell function and subsequent antitumor effects by secreting immunomodulatory metabolites (e.g., indoleamine 2,3‐dioxygenase [IDO] and tryptophan 2,3‐dioxygenase [TDO]) and anti‐inflammatory cytokines (e.g., IL‐10 and IL‐35), as well as by inhibiting IL‐2 production. This figure was created using tools provided by Biorender.com (accessed July 2, 2024). GzmB, granzyme B; IDO, indoleamine 2,3‐dioxygenase; PRF, perforin; TDO, tryptophan 2,3‐dioxygenase.
3.2.1. Impaired immune surveillance
Transplant recipients require long‐term use of immunosuppressive drugs to prevent rejection; however, this also leads to impaired immune surveillance function and an increased risk of infection and malignancy. Immunosuppressants inhibit the function of various types of immune cells, including T lymphocytes, through multiple mechanisms, thereby weakening the body's defense against pathogenic microorganisms and cancer cells.
Dysfunction of T cells, including CD8+ T cells and CD4+ T cells, is a crucial component of impaired transplant‐associated immune surveillance. The use of immunosuppressive agents can impair the proliferation and activation of CD8+ T cells and Teff, thereby diminishing their immune effects. Common immunosuppressive agents include CNIs, inosine monophosphate dehydrogenase inhibitors, and dihydroorotate dehydrogenase inhibitors, among others. For instance, even at very low doses, everolimus significantly inhibits the proliferation of CD8+ T cells. 140 Cyclosporine has been demonstrated to inhibit the maturation and differentiation of thymocytes by suppressing protein kinase C activation and reducing CD4/CD8 expression levels. 141 For Teff, the inhibition of Tim‐1 ligand, which is used to inhibit chronic rejection of cardiac grafts, is believed to be closely associated with the inhibition of Th17 cell differentiation and development. 142 Likewise, 15‐deoxyspergualin effectively inhibits the growth of naïve CD4+ T cells following early activation and reduces their polarization towards Th1 effector cells. 143 Furthermore, immunosuppressive agents also impact lymphocyte‐mediated immunity. In the presence of IL‐2, cyclosporine inhibits the reactivation of quiescent, antigen‐dependent cytotoxic T cells and directly suppresses their effector phase. 144 Likewise, when ex vivo expanded cytotoxic somatic cells were exposed to cyclosporine for one week, a significant decrease in their ability to lyse target cells was observed. 145 Moreover, MMF inhibited the adhesion and penetration of CD8+ T cells to target effector cells, which may be related to the downregulation of specific endothelial membrane molecules and the loss of protein localization in lymphocyte protrusions. 146 Similarly, the reduced release of proinflammatory cytokines due to immunosuppression decreases the immune function of Teff, including IFN‐γ, TNF‐α, IL‐12, and IL‐6. 147 , 148 Additionally, immunosuppressive agents may also inhibit the direct cytotoxic effects of Teff cells, such as adhesion and infiltration. 146 Notably, the inhibition of cells that communicate with T cells may affect their immune effects. 149 For instance, kinsenoside, a potential immunosuppressive drug for autoimmune hepatitis, can inhibit CD8+ T cell activity by reducing crosstalk between the metabolism‐associated PI3K–AKT pathway and the inflammation‐associated JAK2–STAT3 pathway in DCs. 149
For transplant patients, the use of immunosuppressants such as mTOR inhibitors and prednisolone is the main cause of elevated levels of Tregs, which can inhibit the function of effector T cells, such as CD8+ T cells and Th cells, through multiple molecular pathways. 150 , 151 Forkhead box P3 (Foxp3), a transcription factor, is highly associated with Tregs and is responsible for regulating their development and function. 130 , 131 , 132 , 133 , 134 , 135 , 136 , 137 , 138 , 139 , 140 , 141 , 142 , 143 , 144 , 145 , 146 , 147 , 148 , 149 , 150 , 151 , 152 Common posttransplantation immunosuppressants, such as cyclosporine A and rapamycin (RAPA), can affect the expression level of Foxp3, thereby modulating the function of Tregs. For instance, Qu et al. 153 demonstrated that RAPA significantly increased the ratio of Foxp3+ Treg cells to CD4+ T cells in the spleen and thymus of mice. Battaglia et al.’s 154 study showed that RAPA selectively expanded naturally occurring Foxp3+ Tregs in mice in vitro, and these Tregs could inhibit the proliferation of homologous T cells. Moreover, in the presence of immunosuppressive agents, Tregs can exert regulatory immune effects by overexpressing coinhibitory receptors, such as T‐cell immunoglobulin and ITIM domain (TIGIT), programmed cell death protein 1 (PD‐1), and CTL‐associated protein 4 (CTLA‐4). 155 , 156 For instance, Zeng et al. 157 demonstrated that the immunosuppressive function of Tregs was more potent after the combination of tacrolimus and MMF compared with tacrolimus alone, and the immunosuppressive effect of Tregs was attenuated by the use of PD‐1 or TIGIT antibodies. In vitro cellular experiments by Chen et al. 158 showed that the treatment of Tregs with RAPA can upregulate the expression of PD‐1‐related mRNA in mice. In the context of transplantation‐associated immunosuppression, the expanded Tregs can inhibit the proliferation and effects of effector T cells through the secretion of immunosuppressive cytokines, such as IL‐10, transforming growth factor‐β, and IL‐35. Moreover, the high expression of CD25 (IL‐2 receptor α chain) on Tregs enables them to deplete IL‐2 from the surrounding environment via their high‐affinity IL‐2 receptor, thereby limiting the binding of other effector T cells to IL‐2 and the subsequent activating effect. 159 Furthermore, Tregs can produce cytotoxic substances that act on effector T cells, such as granzymes and perforin, which directly inhibit their number and function. 160 Tregs‐associated immunosuppressive metabolites, such as indoleamine 2,3‐dioxygenase, and tryptophan 2,3‐dioxygenase, also inhibit the function and proliferation of effector T cells. 161
In conclusion, immunosuppressive agents impair cellular immunity in transplant recipients through multiple mechanisms, leading to compromised immune surveillance. First, the functions of key effector cells, such as CD8+ T cells and effector CD4+ T cells, which are crucial for anti‐infection and anticancer responses, are attenuated. Second, the immunosuppressive effects of Tregs are relatively enhanced. This immunosuppression‐associated impairment of immune surveillance is a significant contributor to the increased risk of infection and malignancy in transplant recipients. An in‐depth investigation of the molecular mechanisms underlying immunosuppression and impaired immune surveillance in transplant recipients can facilitate the optimization of immunosuppressive regimens. The goal is to prevent rejection while preserving the body's defense mechanisms to the greatest extent possible, ultimately improving transplantation outcomes.
3.2.2. Correlation between infection and carcinogenesis
The close association of CD8+ T cells with EBV infection‐associated PTLD has garnered attention, considering the crucial role of viral infection in posttransplantation tumorigenesis and progression. During EBV‐associated PTLD, the activation of EBV‐specific CD8+ T cells acts as a potent cancer suppressor, exerting cytotoxic effects early in the infection and maintaining EBV suppression over time. 162 However, although EBV stimulation is usually accompanied by an increase in CD8+ T cell numbers and hyperfunction, CTLs can be infected by EBV during the killing of EBV‐infected target cells in the presence of extensive, uncontrolled EBV replication, which may impair the cytotoxic function of CTLs. 163 , 164 For instance, Tanaka et al. 165 reported a case of a patient with severe aplastic anemia who had a persistently high peripheral blood EBV‐associated DNA load after allo‐HSCT; the patient was characterized by oligoclonal TCR Vβ profiles in peripheral lymphocytes after treatment with rituximab, suggesting that CTLs may be affected by EBV infection. 165 Moreover, in immunocompetent hosts, the EBV genome is immortalized and forms episomes in latently infected B cells. 166 When transplantation‐associated immunosuppression leads to a decline in the function of T cells, such as CTLs, the concomitant dysfunctional activation of antiviral functions, such as a decrease in IFN‐γ production, may be accompanied by uncontrolled proliferation of B cells, thereby promoting the development of B‐cell‐derived PTLD. 166 , 167
3.3. Metabolic and cardiovascular complication
Metabolic and cardiovascular complications are significant concerns in the posttransplant period, substantially impacting patient outcomes and quality of life. These complications arise from a complex interplay of factors, including the effects of immunosuppressive medications, infections, and the underlying health conditions of transplant recipients. Understanding the mechanisms behind these complications is crucial for developing effective prevention and treatment strategies. This section will explore two major contributors to posttransplant metabolic and cardiovascular complications: drug‐induced metabolic disorders and toxicity, and the critical role of infections.
3.3.1. Drug‐induced metabolic disorders and toxicity
Immunosuppressive drugs play a crucial role in the prevention and treatment of GVHD; however, they can also cause metabolic disorders and toxic reactions through various mechanisms. Among the numerous immunosuppressive agents, a growing body of research has demonstrated that CNIs and mTOR inhibitors carry the potential risk of causing PTDM. 168 , 169 , 170 , 171 Numerous animal experiments and clinical studies suggest that the core pathogenesis of immunosuppressant‐associated PTDM involves immunosuppressants causing abnormal glucose tolerance through direct damage to pancreatic β‐cells and increased insulin resistance in peripheral tissues. For instance, a clinical study by Duijnhoven et al. 172 demonstrated a significant decrease in insulin sensitivity and secretion in renal transplant patients receiving tacrolimus. Conversely, a study by Boots et al. 173 found that steroid withdrawal reduced insulin resistance levels in renal transplant patients, and that reduced tacrolimus levels increased pancreatic β‐cell secretion. Furthermore, mouse experiments by Shivaswamy et al. 174 demonstrated the damaging effects of tacrolimus treatment on pancreatic β‐cells and the inhibitory effects of sirolimus on insulin signaling. Likewise, Larsen et al. 175 observed tacrolimus‐ and sirolimus‐induced insulin resistance in a rat model. Notably, immunosuppressant‐induced insulin resistance and hyperglycemia can exacerbate other complications. For instance, corticosteroid‐associated insulin resistance and resulting hyperinsulinemia increase the risk of posttransplant dyslipidemia by enhancing hepatic uptake of free fatty acids and cholesterol synthesis. 176
In addition to the aforementioned insulin resistance, the mechanisms of immunosuppressant‐induced posttransplant dyslipidemia include abnormal cholesterol and lipoprotein metabolism. 177 , 178 First, corticosteroids and cyclosporine can increase the activity of key enzymes in cholesterol biosynthesis, such as 3‐hydroxy‐3‐methylglutaryl coenzyme A (HMG‐CoA) reductase, thereby enhancing the synthesis of very low‐density lipoprotein (VLDL) cholesterol. 177 Second, cyclosporine can also impair the body's ability to remove low‐density lipoprotein (LDL) and VLDL by interfering with LDL receptor binding to LDL and decreasing lipoprotein lipase activity. 179 Regarding posttransplant hypertension, immunosuppression, particularly CNI‐induced vasoconstriction and sodium retention, plays a crucial role. First, CNIs such as cyclosporine can lead to a reduction in vasodilators (e.g., nitric oxide) and an elevation in vasoconstrictors (e.g., endothelin and angiotensin II). 180 , 181 Second, CNIs may cause sodium retention by mediating aldosterone levels and sympathetic activation, which are thought to be strongly associated with hypertension. 182 , 183 , 184 Moreover, the organ toxicity of immunosuppressants should not be disregarded. CNI‐related nephrotoxicity is believed to be closely associated with posttransplant chronic kidney disease, and the resulting endothelial dysfunction, alterations in vascular tone, and vascular calcification are all risk factors for posttransplant hypertension. 185 , 186 , 187 It is noteworthy that the aforementioned metabolic complications, such as PTDM, posttransplant dyslipidemia, and hypertension, can act as risk factors that indirectly elevate the risk of posttransplant cardiovascular diseases. 51
3.3.2. The critical role of infection
In addition to the adverse effects of immunosuppressants, infection represents a significant factor contributing to metabolic disorders and cardiovascular diseases following transplantation. Viral infections, particularly CMV and HCV, play a crucial role in the development of metabolic complications. Currently, several retrospective cohort studies have identified CMV infection as a risk factor for PTDM. 188 , 189 Furthermore, numerous clinical studies have demonstrated that prior infection with hepatitis viruses, especially HCV, is associated with the incidence of PTDM. 189 , 190 , 191 Notably, the study by Roccaro et al. 192 confirmed an independent correlation between HCV eradication and a reduction in PTDM incidence following liver transplantation. Viral infection leading to pancreatic β‐cell damage is one of the mechanisms underlying PTDM. Substantial evidence supports the deleterious effects of CMV and HCV infection on pancreatic β‐cells. 193 , 194 Specifically, viral infection directly induces cell death, and the subsequent proinflammatory cytokine‐mediated immune response serves as the primary mechanism. 193 , 194 , 195 Additionally, chronic infection results in decreased pancreatic β‐cell secretory function, which is a significant contributor to diabetes development. 196 Viral infections such as CMV and HCV can also reduce insulin sensitivity in transplant recipients through various pathways. Insulin receptor substrate (IRS) is a key molecule in downstream insulin signaling. Both CMV and HCV core protein (HCVCP) can promote insulin resistance by downregulating IRS levels. 197 , 198 Specifically, CMV protein can cause sustained activation of mTOR complex 1 (mTORC1), which in turn leads to IRS degradation through phosphorylation. 198 Regarding HCV, HCVCP can lead to the phosphorylation of Serine 312 of IRS‐1, thereby causing IRS‐1 degradation. 199 Moreover, HCV infection can inhibit IRS‐1 function by activating the mTOR/S6K1 pathway and disrupt glucose metabolism by downregulating the glucose transporter GLUT4 and upregulating the gluconeogenic enzyme PCK2, thereby inducing insulin resistance. 200 Furthermore, HCVCP can promote insulin resistance by inducing endoplasmic reticulum stress in the liver and hepatocytes. 201 Regarding hyperlipidemia, mounting evidence suggests that HCV infection plays a crucial role. For instance, HCV core protein downregulates microsomal triglyceride transfer protein, an enzyme that mediates lipid translocation to the endoplasmic reticulum membrane, thereby reducing the assembly of VLDL. 202
The development of posttransplant hypertension is influenced by CMV infection in transplant recipients. 203 Cheng et al. 204 proposed that CMV infection promotes inflammatory responses and activates the renin–angiotensin system, leading to elevated arterial blood pressure. Furthermore, CMV infection can induce endothelial dysfunction by reducing responses to bradykinin and glyceryl trinitrate. CMV can also directly infect endothelial cells, trigger inflammation, and promote thrombosis by enhancing the expression of von Willebrand factor, intercellular adhesion molecule‐1, and vascular cell adhesion molecule‐1, thereby further compromising endothelial cell function. 205 , 206 , 207 , 208 The primary types of infections that increase the postoperative cardiovascular risk in transplant recipients include HCV and CMV. The study by Maggi et al. 209 demonstrated that liver transplant patients with previous or current HCV infection have a higher risk of cardiovascular disease. HCV infection can induce chronic inflammation, which is believed to be closely associated with endothelial dysfunction. 210 , 211 Moreover, direct infection of cardiovascular tissues by HCV may also play a significant role. For instance, numerous studies have isolated HCV RNA from carotid plaque tissue and blood–brain barrier endothelial cells of infected individuals. 212 , 213 Additionally, the aforementioned HCV‐related dyslipidemia is an important factor in cardiovascular diseases such as atherosclerosis. 214 Conversely, CMV infection has been extensively documented as a significant risk factor for cardiovascular disease. 215 , 216 Vascular endothelial damage and dysfunction caused by CMV infection are considered the foundation for various cardiovascular diseases, including atherosclerosis and acute myocardial infarction. 217 Furthermore, CMV infection may cause impaired arterial reactivity, tachycardia, and hypotension by inducing arterial smooth muscle dysfunction, which is thought to be related to sympathetic nerve activation. 218 It is noteworthy that the exceptionally high risk of cardiovascular disease caused by simultaneous infection with multiple viruses warrants special attention. For example, Aguilera et al. 219 conducted a retrospective analysis of liver transplant patients hospitalized for HCV cirrhosis and found that CMV reactivation was associated with an increased risk of cardiovascular events.
4. THERAPEUTIC INTERVENTIONS AND MANAGEMENT STRATEGIES
The prevention and management of posttransplantation complications require a multifaceted approach that integrates insights from the clinical features and molecular mechanisms discussed in the previous sections. This section explores various therapeutic interventions and strategies aimed at reducing the incidence and severity of posttransplant complications. By discussing the current state of immunosuppressive regimens, infection prophylaxis, metabolic management, and targeted therapies for malignancies, this section highlights the importance of personalized, evidence‐based approaches to optimize posttransplantation outcomes. Furthermore, this section identifies key areas for future research and emphasizes the need for ongoing efforts to develop novel therapeutic modalities and biomarkers for early detection and intervention.
4.1. Immunosuppressive therapies and novel cellular therapies
To prevent rejection or GVHD associated with transplantation and to induce tolerance, immunosuppressive therapies are now widely used in posttransplantation patients. The three‐signal model of T‐cell activation and proliferation provides a basis for understanding the molecular mechanisms of immunosuppression. 220 In this model, signal 1 is the presentation of foreign antigen by APCs to T cells, activation of their TCR, and signaling through the transduction apparatus of the CD3 complex. Signal 2 is an antigen‐nonspecific costimulatory signal that results from the interaction of multiple ligand molecules on the surface of the APC with multiple receptors on the surface of the T cell, such as CD28 and CD154. Both signal 1 and signal 2 activate signal transduction pathways, including the calcium–calmodulin‐dependent phosphatase pathway, the mitogen‐activated protein kinase pathway, and the NF‐κB pathway. Signal 3 refers to the stimulation of the cell cycle by increased production of IL‐2 through the IL‐2 receptor. This process requires the mTOR enzyme to translate mRNA translation and cell proliferation. 221 , 222
Immunosuppressive therapy is primarily divided into induction therapy and maintenance therapy, based on the different treatment objectives. 223 Induction therapy primarily aims to rapidly and potently suppress the body's immune response in the short term before and after transplantation surgery to prevent the occurrence of acute rejection. It mainly includes various antibodies. These antibodies have different mechanisms of action, depending on their target. For example, belatacept blocks the interaction of APCs with CD28 molecules on T lymphocytes, thereby blocking signal 2. 224 IL‐2 receptor antagonists, such as basiliximab, inhibit lymphocyte activation and replication, thereby blocking signal 3. 225 Moreover, polyclonal antibodies, such as antithymocyte globulin, can recognize multiple markers and exhibit a broad immunosuppressive profile. 224 Maintenance therapy aims to control chronic graft rejection and maintain stable graft survival in the long term. Commonly used drugs include CNIs, antimetabolites, mTOR inhibitors, and glucocorticoids. CNIs, including cyclosporine A and tacrolimus, primarily exert their immunosuppressive effects by inhibiting calcineurin. 226 mTOR inhibitors, such as sirolimus and everolimus, exert their immunosuppressive effects by inhibiting the proliferation and differentiation of T cells and B cells, as well as antibody production. 227 , 228 Antimetabolites, such as azathioprine and MMF, inhibit lymphocyte proliferation by inhibiting nucleic acid synthesis. 222 , 224 Glucocorticoids, such as methylprednisolone and prednisone, exert immunosuppressive and anti‐inflammatory effects. 229 It is crucial to note that these immunosuppressive regimens are based on appropriate combinations of drugs with different mechanisms of action and need to be tailored to the individual patient. Currently, a commonly used regimen is based on triple drug therapy containing CNIs, corticosteroids, and antimetabolites. 223
Considering the immunocompromise and toxicity inevitably associated with immunosuppressants, cellular therapy, particularly Treg‐based therapy, is of great importance in reducing the use of immunosuppressive therapy in patients. Currently, several clinical trials have demonstrated that Treg cell therapy has shown promising results in renal, hepatic, and other SOT patients. 230 , 231 , 232 , 233 , 234 For example, the results of the study by Sawitzki et al. 232 (The ONE Study) showed that it is feasible and safe for living kidney transplant recipients to receive Treg cell therapy with a relatively lower risk of infection. Additionally, a study by Todo et al. 234 demonstrated that living liver transplant recipients who received Treg therapy to induce immune tolerance had 7 out of 10 patients maintaining immunosuppression‐free status for more than 6 years. The safety and efficacy of this therapy need to be further confirmed by additional studies.
4.2. Prevention and treatment of infections
The prevention of common infections in transplant recipients requires a multifaceted and comprehensive approach. On the one hand, comprehensive pretransplant screening of donors and recipients for infectious diseases is crucial. According to the recommendations in the American Society of Transplantation Clinical Practice Guidelines, transplant recipients should be screened preoperatively for various pathogens, including human immunodeficiency virus (HIV), tuberculosis, CMV, influenza, and pneumococcus. Moreover, in patients with active or recent peritonitis, it is recommended to delay transplantation to minimize the risk of infection. 235 On the other hand, the guidelines also recommend immunization of transplant recipients well before transplantation, including pneumococcal, measles, mumps, rubella, diphtheria, tetanus, and pertussis vaccines. 235 Furthermore, the resumption of vaccinations should be delayed after transplantation until immunosuppression is minimized and the vaccine's efficacy is serologically confirmed. 236 Additionally, the importance of preoperative prophylactic medication against the risk of infection should be emphasized. For instance, in transplant recipients at high risk of CMV infection, preoperative oral ganciclovir is beneficial for graft survival. 237 For donors, screening of donor organs for HBV, HCV, HIV, tuberculosis, schistosomiasis, and other infections should be emphasized to prevent the transmission of latent infections from the donor through infected organs. 238 , 239
When posttransplantation infection occurs, the timely selection of the appropriate treatment regimen and continuous monitoring are crucial. On the one hand, appropriately reducing the use of immunosuppressants is beneficial for the recovery of the body's ability to fight infection and improves therapeutic efficacy. For instance, in patients with EBV‐associated PTLD, reducing the use of immunosuppressants can lead to remission in 23−86% of patients. 240 On the other hand, it is crucial to select the appropriate highly effective drugs for treatment based on the infecting pathogen. For viral infections, ganciclovir/valganciclovir for CMV, rituximab for EBV‐associated PTLD, and tenofovir or entecavir for HBV have shown promising results. 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 , 110 , 111 , 112 , 113 , 114 , 115 , 116 , 117 , 118 , 119 , 120 , 121 , 122 , 123 , 124 , 125 , 126 , 127 , 128 , 129 , 130 , 131 , 132 , 133 , 134 , 135 , 136 , 137 , 138 , 139 , 140 , 141 , 142 , 143 , 144 , 145 , 146 , 147 , 148 , 149 , 150 , 151 , 152 , 153 , 154 , 155 , 156 , 157 , 158 , 159 , 160 , 161 , 162 , 163 , 164 , 165 , 166 , 167 , 168 , 169 , 170 , 171 , 172 , 173 , 174 , 175 , 176 , 177 , 178 , 179 , 180 , 181 , 182 , 183 , 184 , 185 , 186 , 187 , 188 , 189 , 190 , 191 , 192 , 193 , 194 , 195 , 196 , 197 , 198 , 199 , 200 , 201 , 202 , 203 , 204 , 205 , 206 , 207 , 208 , 209 , 210 , 211 , 212 , 213 , 214 , 215 , 216 , 217 , 218 , 219 , 220 , 221 , 222 , 223 , 224 , 225 , 226 , 227 , 228 , 229 , 230 , 231 , 232 , 233 , 234 , 235 , 236 , 237 , 238 , 239 , 240 , 241 ‐ 242 For HIV infection or bacterial infections, the appropriate antibiotics should be chosen to prevent or control the infection. Furthermore, active tuberculosis requires a 6‐month treatment regimen with isoniazid, rifampicin, pyrazinamide, and ethambutol, using all four drugs for the first 2 months and isoniazid and rifampicin for the remaining 4 months. 243
4.3. Metabolic and cardiovascular risk management
The treatment of patients with PTDM should be chosen according to different stages. Intravenous insulin infusion is usually used in the early postoperative stage, followed by a transition to subcutaneous insulin injections. For long‐term glycemic management of PTDM, long‐term insulin use is also crucial. 7 Additionally, common posttransplant oral hypoglycemic agents include metformin, sulfonylureas, and repaglinide. 244 , 245 , 246 Among these, metformin has many advantages as a potential first‐line agent for posttransplant PTDM. 247 Shivaswamy et al. 248 demonstrated that in rats, metformin tended to reduce tacrolimus/sirolimus treatment‐induced endocrine cell apoptosis and ameliorate hyperglycemia induced by these immunosuppressive agents. Moreover, several studies have not observed any impairment or significant side effects of metformin on long‐term graft survival, suggesting its relative safety. 249 , 250 For patients with posttransplant dyslipidemia, statins are effective in lowering LDL levels and are recommended as the first‐line treatment and drug of choice for posttransplant dyslipidemia. 178 , 179 , 180 , 181 , 182 , 183 , 184 , 185 , 186 , 187 , 188 , 189 , 190 , 191 , 192 , 193 , 194 , 195 , 196 , 197 , 198 , 199 , 200 , 201 , 202 , 203 , 204 , 205 , 206 , 207 , 208 , 209 , 210 , 211 , 212 , 213 , 214 , 215 , 216 , 217 , 218 , 219 , 220 , 221 , 222 , 223 , 224 , 225 , 226 , 227 , 228 , 229 , 230 , 231 , 232 , 233 , 234 , 235 , 236 , 237 , 238 , 239 , 240 , 241 , 242 , 243 , 244 , 245 , 246 , 247 , 248 , 249 , 250 , 251 It is worth noting that statins are also believed to improve endothelial function, thereby benefiting cardiovascular health. 252 Furthermore, drugs such as fibrates, niacin, and HMG‐CoA reductase inhibitors have shown promising therapeutic effects on posttransplant dyslipidemia due to their ability to LDL cholesterol levels. 178 , 179 , 180 , 181 , 182 , 183 , 184 , 185 , 186 , 187 , 188 , 189 , 190 , 191 , 192 , 193 , 194 , 195 , 196 , 197 , 198 , 199 , 200 , 201 , 202 , 203 , 204 , 205 , 206 , 207 , 208 , 209 , 210 , 211 , 212 , 213 , 214 , 215 , 216 , 217 , 218 , 219 , 220 , 221 , 222 , 223 , 224 , 225 , 226 , 227 , 228 , 229 , 230 , 231 , 232 , 233 , 234 , 235 , 236 , 237 , 238 , 239 , 240 , 241 , 242 , 243 , 244 , 245 , 246 , 247 , 248 , 249 , 250 , 251 , 252 , 253 Calcium channel blockers, which antagonize the vasoconstrictive effects of CNIs with fewer side effects, are considered first‐line agents in the management of posttransplant hypertension. 182 , 183 , 184 , 185 , 186 , 187 , 188 , 189 , 190 , 191 , 192 , 193 , 194 , 195 , 196 , 197 , 198 , 199 , 200 , 201 , 202 , 203 , 204 , 205 , 206 , 207 , 208 , 209 , 210 , 211 , 212 , 213 , 214 , 215 , 216 , 217 , 218 , 219 , 220 , 221 , 222 , 223 , 224 , 225 , 226 , 227 , 228 , 229 , 230 , 231 , 232 , 233 , 234 , 235 , 236 , 237 , 238 , 239 , 240 , 241 , 242 , 243 , 244 , 245 , 246 , 247 , 248 , 249 , 250 , 251 , 252 , 253 , 254 Moreover, thiazide diuretics have demonstrated antihypertensive efficacy and safety in posttransplant hypertension. 255 For posttransplant cardiovascular disease, it is crucial to manage the aforementioned metabolic complications. 56 Notably, for metabolic complications and cardiovascular disease, lifestyle modifications such as smoking cessation and exercise are essential for prevention and improving prognosis. 256 , 257
For many transplant‐related metabolic and cardiovascular diseases, it is crucial to minimize the potential risk of complications associated with immunosuppressive therapy. In fact, several immunosuppressive agents have a relatively lower risk of complications and are considered more favorable options. A 12‐month, multicenter, prospective, randomized controlled trial by Wissing et al. 258 found that therapeutic switching from tacrolimus to cyclosporine A significantly improved glucose metabolism in renal transplant recipients postoperatively and had the potential to reverse PTDM in the first year after transplantation. A randomized controlled trial by Kim et al. 259 demonstrated that everolimus exhibited lower insulin resistance compared with low‐dose tacrolimus. Moreover, a study by Wen et al. 260 demonstrated that the costimulation blocker belatacept, alone or in combination with tacrolimus, had a lower risk of PTDM compared with tacrolimus alone. However, the effect of tacrolimus on posttransplant hyperlipidemia was less pronounced than that of cyclosporine, and replacing cyclosporine with tacrolimus led to a 25% reduction in LDL cholesterol. 261 Moreover, numerous studies have shown that the use of belatacept, either without or with reduced CNI exposure, can lower posttransplant lipid levels. 262 , 263 In patients with posttransplant hypertension, tapering, discontinuing, or avoiding corticosteroids and CNIs in favor of belatacept or mTOR inhibitors can be effective in controlling blood pressure. 262 , 263 , 264 ‐ 265 Considering the significance of each metabolic complication in the development of cardiovascular disease, the impact of these immunosuppressive regimen changes on cardiovascular disease risk has also garnered considerable research attention. For instance, Vincenti et al. 266 found a lower risk of serious cardiac events compared with cyclosporine by evaluating the long‐term safety profile of belatacept over a 5‐year period. Table 2 summarized the therapeutic interventions and management strategies in posttransplant complications.
TABLE 2.
Summary of therapeutic interventions and management strategies in posttransplant complications.
| Complications | Interventions | Details | References |
|---|---|---|---|
| Immune rejection and GVHD | Immunosuppressants | Calcineurin inhibitors, such as cyclosporine A and tacrolimus, inhibit calcineurin activity | 226 |
| mTOR inhibitors, such as sirolimus and everolimus, inhibit the proliferation and differentiation of T and B cells | 227, 228 | ||
| Antimetabolites, such as azathioprine and mycophenolate, inhibit nucleic acid synthesis | 229, 230 | ||
| Glucocorticoids, such as methylprednisolone and prednisone, have immunosuppressive and anti‐inflammatory effects | 231 | ||
| Novel cellular therapy | Treg cell therapy | 230, 231, 232, 233, 234 | |
| Infection | Pathogen screening | HIV, tuberculosis, cytomegalovirus, and other pathogens | 235, 238, 239 |
| Vaccination | Pneumococcal, measles, mumps, and rubella vaccines | 236 | |
| Antiviral drugs | Ganciclovir and so on | 33, 241, 242 | |
| Tuberculosis treatment scheme | Isoniazid, rifampicin, and so on | 243 | |
| Metabolic and cardiovascular risk | Drug therapy | Insulin and oral hypoglycemics such as metformin are used to manage diabetes | 244, 248, 249, 250 |
| Statins are used to lower LDL cholesterol and improve cardiovascular health | 251, 252 | ||
| Bile acid sequestrants, niacin, and HMG‐CoA reductase inhibitors are used to reduce LDL cholesterol | 254 | ||
| Calcium channel blockers are used to antagonize the vasoconstriction caused by CNIs | 255 | ||
| Diuretics such as thiazides are used to treat posttransplant hypertension | 257 | ||
| Conversion of immunosuppressive therapy | Converting from tacrolimus to cyclosporine, everolimus, or belatacept can reduce the risk of diabetes | 258, 259, 260 | |
| Using belatacept and other agents can relatively reduce the risk of dyslipidemia | 262, 263 | ||
| Using belatacept and mTOR inhibitors can help control blood pressure | 264, 265 |
Abbreviations: CNIs, calcineurin Inhibitors; GVHD, graft‐versus‐host disease; HIV, human immunodeficiency virus; HMG‐CoA, 3‐hydroxy‐3‐methylglutaryl‐coenzyme A; LDL, low‐density lipoprotein; mTOR, mammalian target of rapamycin.
4.4. Diagnosis and treatment of posttransplantation cancers
The high incidence and poor prognosis of posttransplant cancers underscore the importance of screening and early detection. Individualized cancer screening protocols for transplant recipients can help identify and intervene in cancers as early as possible, thereby improving prognosis. Considering that immune disorders are critical for posttransplantation tumorigenesis, the potential value of ILs as diagnostic markers for posttransplantation cancers has garnered considerable attention. Pontrelli et al. 267 identified differentially expressed genes in patients with and without posttransplantation malignancies. The results showed that these differentially expressed genes were closely associated with the cancer pathway and that the IL‐27‐related genes were the most downregulated. 267 Zhang et al. 268 demonstrated that, compared with primary cutaneous squamous cell carcinoma (SCC), the number of IL‐22‐secreting CD8+ T cells in skin SCC tissues after skin transplantation was higher, and the expression of IL‐22 receptor in tissues was more diffuse. Furthermore, they found that increased IL‐22 and IL‐22R expression accelerated tumor growth in transplant patients. 268 Moreover, T‐lymphocyte‐associated cell surface markers demonstrated promising predictive value. CD200, a membrane protein with immunosuppressive function expressed in hematopoietic tumors, suppresses the body's antitumor immunity by binding to the receptor CD200R, inducing Treg expression, and inhibiting tumor‐specific T‐cell function. 269 , 270 , 271 The study by Vaughan et al. 272 suggests the potential value of CD200 in posttransplantation cancers. Their study showed that 23.7% (nine out of 38) of PTLD patients exhibited CD200 positivity and that tumor cells exhibited membrane and cytoplasmic CD200 positivity. Furthermore, they found that PTLD patients with CD200 positivity had higher levels of Tregs compared with those with CD200 negativity. 272 CD57 is a marker expressed on highly differentiated T cells, and an increased frequency of CD57‐positive T cells has been associated with various cancers. The reduced proliferative capacity of these cells suggests that the organism is in a state of chronic immune activation and senescence. 273 , 274 Bottomley et al. 275 demonstrated that in kidney transplant recipients with cutaneous SCC, participants who highly expressed CD57 were more likely to develop SCC during follow‐up and tended to develop and experience recurrence earlier. They concluded that the percentage of CD8+ T cells expressing CD57 is a robust immune predictor of SCC occurrence and recurrence in long‐term, high‐risk kidney transplant recipients. 275 Courivaud et al. 276 found that CMV‐exposed renal transplant recipients were found to have a higher cumulative incidence of tumors compared with the unexposed population and a relatively shorter mean time to tumor onset. Notably, their results also demonstrated that CD57‐depleted T cells were significantly expanded in CMV‐exposed patients. 276 Collectively, these studies suggest the potential significance of CD57 in predicting posttransplantation cancers. Moreover, the efficacy of EBV DNA for the diagnosis of PTLD has been widely recognized as a risk factor for the development of PTLD. 277 A study by Rosselet et al. 278 demonstrated that in seven out of 10 EBV‐positive PTLD patients, serum EBV DNA levels could be monitored in five of them. In contrast, EBV DNA was not detected in any of the 38 control subjects. Furthermore, a study by Suresh et al. 279 demonstrated that in pediatric renal transplant recipients, an acute elevation of urinary CXCL10/creatinine ratio was associated with the development of transplant‐associated Burkitt's lymphoma, suggesting its potential value as a diagnostic biomarker.
Targeting the mechanisms of posttransplantation cancer development and progression is crucial for prevention and treatment. For example, sirolimus, which can reduce the activating effect of IL‐2 on T cells by inhibiting the mTOR receptor, has been used for immunosuppression in transplantation medicine, particularly after renal transplantation. 280 , 281 Notably, despite their immunosuppressive effects, some mTOR inhibitors can also suppress posttransplant tumorigenesis under certain circumstances. 282 A randomized controlled trial by Knoll et al. 283 demonstrated that a combination regimen was associated with a reduced risk of malignant tumors and nonmelanoma skin cancers in transplant recipients, compared with an immunosuppressive regimen without sirolimus. A meta‐analysis by Yanik et al. 284 revealed that sirolimus use in renal transplant recipients was associated with a lower risk of renal carcinogenesis. Considering the central role of the PI3K/Akt/mTOR pathway in the growth, proliferation, and other functions of cancer cells, this may be a potential mechanism by which mTOR inhibitors suppress tumorigenesis after transplantation. 285 Given the dual role of mTOR inhibitors in suppressing posttransplantation rejection and reducing the risk of posttransplantation tumorigenesis, they are essential for the prevention and treatment of specific types of posttransplantation cancers. Moreover, targeted therapies aimed at inhibiting Tregs in the management of posttransplantation tumors have garnered significant interest. Numerous studies have investigated the potential efficacy of ipilimumab (anti‐CTLA‐4), basiliximab (anti‐CD25), and denileukin diftitox in depleting intratumoral Tregs. 286 , 287 , 288 , 289 Similarly, targeting the chemokine receptor CCR8 to inhibit Tregs has gained traction in recent years, as it is believed to facilitate dose‐dependent, potent, and long‐lasting antitumor immune responses. 290 Furthermore, targeted therapies directed against the transcription factor Foxp3, such as TCR mimetic antibodies that selectively bind to Foxp3‐expressing Tregs, have demonstrated considerable promise in Treg depletion. 291 At present, these Treg depletion strategies have exhibited favorable antitumor effects in various malignancies, including gastric, head and neck, and lung cancers. 287 , 288 , 289 , 290 , 291 , 292 , 293 In the context of treating posttransplantation tumor recurrence following hematologic transplantation, several Treg depletion approaches have yielded promising outcomes. 288 , 289 , 290 , 291 , 292 , 293 , 294 For patients with recurrent multiple myeloma undergoing autologous stem cell transplantation (ASCT), two strategies—the elimination of Tregs in ASCT grafts using anti‐CD25 microbeads and the in vivo depletion of Tregs using basiliximab—significantly reduced the frequency of Tregs post‐ASCT. 288 Among recipients of allo‐HSCT, the inhibition of Tregs using antitumor necrosis factor receptor type 2 (TNFR2) therapy was demonstrated to elicit an allogeneic response and consequently induce substantial antitumor effects. 294 Moreover, for patients with recurrent hematologic malignancies following allo‐HSCT, ipilimumab has been shown to restore the body's antitumor responsiveness by enhancing the graft‐versus‐tumor effect. 295 In summary, while additional studies and clinical validation are still required, the potential utility of Treg depletion strategies in managing posttransplantation tumors presents a promising therapeutic approach. The therapeutic potential of allogeneic cell therapy (ACT), including allogeneic T‐cell therapy and antiviral ACT, has garnered significant attention. In SOT, such as kidney and heart transplantation, numerous case reports have indicated that chimeric antigen receptor T‐cell (CAR‐T) therapy has demonstrated therapeutic efficacy in PTLD. 296 , 297 , 298 Moreover, evidence suggests that CAR‐T therapy may also be effective in treating PTLD and drug‐resistant or refractory PTLD after allo‐HSCT. 299 , 300 Furthermore, a systematic evaluation study by Liu et al. 301 demonstrated that, in addition to post‐HSCT PTLD, virus‐specific CTLs targeting EBV (EBV‐CTLs) exhibited safe therapeutic efficacy. Donor‐derived CMV‐specific CTLs (CMV‐CTLs) reduce the severity of CMV infection after transplantation, which may increase the risk of tumorigenesis in renal transplant recipients. 302 , 303 Moreover, Blyth et al. 304 observed that donor‐derived CMV‐CTLs reduced the need for CMV‐directed drug therapy in allogeneic stem cell transplant recipients. Figure 3 summarized the diagnostic and therapeutic strategies for posttransplant cancers. Additionally, Table 3 compiles recent preclinical and clinical studies on the treatment of posttransplant complications from the past five years.
FIGURE 3.
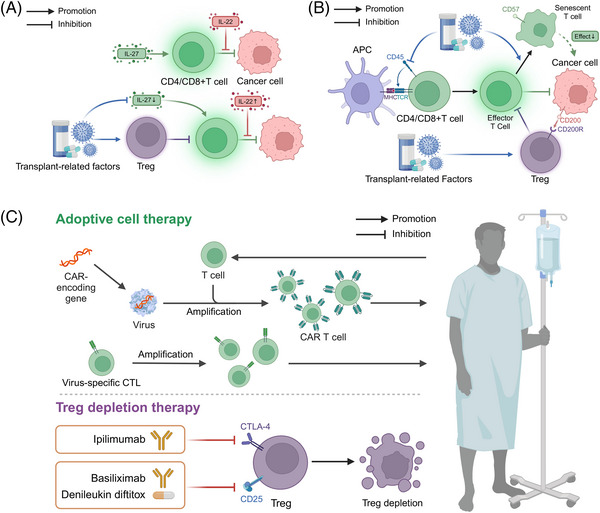
Diagnostic biomarkers and therapeutic strategies for posttransplant cancers. (A) Interleukins. IL‐27 can promote the anticancer response of T cells and has an inhibitory effect on Treg activity. IL‐22 released by T cells is considered to be associated with the occurrence and development of posttransplant cancers originating from epithelial tissues. Both of these cytokines have potential value as diagnostic biomarkers. (B) Cell surface biomarkers. CD45 on T cells can promote the activation of naïve T cells by APCs, but transplant‐related factors may inhibit CD45, affecting T‐cell function. CD200, expressed on the cell surface in some hematopoietic system cancers, can activate Tregs, thereby inhibiting the anticancer effects of effector T cells. CD57 is expressed on late‐stage T cells, which lack proliferative capacity, resulting in weakened anticancer effects. These markers have the potential to serve as diagnostic biomarkers. (C) Adoptive T‐cell therapy and Treg depletion therapy hold significant value for treating posttransplant cancers. ACT includes CAR‐T cell therapy and antiviral ACT. CAR‐T cell therapy involves the genetic modification of patient T cells by introducing CAR genes, allowing them to recognize cancer antigens with high specificity. The modified CAR‐T cells are expanded in vitro and then reinfused into the patient's body to enhance the immune system's attack on cancers. Antiviral ACT involves the in vitro expansion of posttransplant cancer‐specific T cells, which are then reinfused into the patient's body to rebuild virus‐specific immunity, thereby inhibiting cancer development. Treg depletion therapy primarily utilizes drugs targeting CTLA‐4, such as ipilimumab, drugs targeting CD25, such as basiliximab, and denileukin diftitox, which can inhibit the procancer effects of Tregs, thereby enhancing the anticancer effects of the immune system. This figure was created using tools provided by Biorender.com (accessed July 2, 2024).
TABLE 3.
Current summary of preclinical animal or cell‐based experiments and clinical research on the treatment of posttransplantation complications.
| Complications | Therapeutic strategy | Study types | Functions of discovered strategy | References |
|---|---|---|---|---|
| GVHD | MSCs | In vitro, in vivo | Enhance thymic regeneration, modulating oxidative stress, reducing PD‐1 expression on T cells, and polarizing macrophages to an anti‐inflammatory state. | 305, 306, 307 |
| Donor expanded Tregs | In vitro, in vivo | Enhances NK cell recovery and function, providing early immunity against tumors and pathogens post‐allo‐HSCT | 308 | |
| CB‐SCs | In vitro, in vivo | Modulate allogeneic CD4+ T cell activation and proliferation using soluble factors and extracellular vesicles | 309 | |
| JAK inhibitors | In vivo | Inhibit T cell activation, modulating Th cell differentiation, and disrupting the IFN‐g/CXCR3/CXCL10 axis | 310 | |
| Rejection | MSCs | In vitro, in vivo | Downregulate TBK1/IRF3 phosphorylation and reduce IFN‐γ production (in combination with tacrolimus), and promote macrophage polarization to the M2 phenotype via CXCR4 regulation | 311, 312 |
| IL‐10–BM‐MSCs | In vivo | Upregulate lncRNA 003946 in infiltrating CD68+ macrophages, thereby enhancing immunoinhibitory effects and reducing antigen presentation | 313 | |
| miRNAs | In vivo | Induce M2 polarization of macrophages through the PI3K/Akt (miR‐27a‐5p) and Myd88/TRAF6 pathways (miR‐505‐5p), thereby enhancing immunoinhibitory effects and reducing inflammation | 314, 315 | |
| MICs | In clinical research | Increase tolerogenic Bregs that produce IL‐10, leading to specific donor tolerance without adverse immune reactions | 316 | |
| IL‐35‐ASCs | In clinical research | Utilize IL‐35‐ASC‐derived exosomes to upregulate Tregs and enhance immunosuppressive properties, thereby prolonging graft survival | 317 | |
| Interleukin‐10 | In vitro, in vivo | Downregulate miR‐155 and activates SOCS5, promoting macrophage M2 polarization and relieving chronic rejection after heart transplantation | 318 | |
| Interleukin‐37 | In vitro, in vivo | Reduce Th1 and Th17 cells, increase Tregs, decrease TNF‐α and IFN‐γ levels, increase IL‐10 levels, and inhibit p‐mTOR expression in CD4+ cells | 319 | |
| Malignancy | CAR‐T | In clinical research | CAR‐T therapy shows promising efficacy and safety in treating relapsed/refractory SOT‐related PTLD, with 22 patients included in the study, achieving a 64% overall response rate and approximately one‐third of patients achieving sustained remission | 300 |
| Virus‐specific CTLs | In vitro | Immunosuppressive drug‐resistant HBV‐specific TCR‐redirected T cells can effectively lyse circulating HBV‐HCC cells in whole blood, even in the presence of immunosuppressants, potentially preventing posttransplant tumor recurrence | 320 | |
| PTDM | SGLT2 inhibitors | In clinical research | Improve glycemic control, reduce body weight, blood pressure, proteinuria, and serum uric acid levels, while increasing serum magnesium and hemoglobin levels in PTDM | 321, 322 |
| DPP4 inhibitors | In clinical research | Improve glycemic control and reduce body weight in PTDM patients by enhancing glucose excretion through the kidneys | 323, 324 | |
| FXR agonists | In vitro, in vivo | Inhibit gluconeogenesis and promoting glucose uptake through FXR activation in a PGC1α/FOXO1‐dependent manner | 325 | |
| GLP1R agonists | In clinical research | Improve glycemic control and reduce insulin requirements in PTDM patients without affecting immunosuppressant levels or kidney function | 326, 327 | |
| Sitagliptin | In clinical research | May help prevent the development of PTDM by improving glucose tolerance when administered early posttransplant | 328 | |
| Dyslipidemia | PCSK9 inhibitors | In clinical research | Effectively and safely reduce lipid levels in heart transplant patients with hyperlipidemia unresponsive to statins, without adverse effects on liver and kidney parameters | 329, 330 |
| Cardiovascular diseases | RASBs | In clinical research | Potentially reduce stroke events in kidney transplant recipients by mitigating cardiovascular risks | 331 |
| SGLT2 inhibitors | In clinical research | Reduce the risk of major adverse cardiovascular events, particularly myocardial infarction and cardiovascular mortality, in kidney transplant recipients with diabetes | 332 | |
| PCSK9 inhibitors | In vivo, in clinical research | Reduce vascular stenosis, inhibit inflammatory responses, and suppress vascular smooth muscle cell migration and proliferation through mechanisms involving the downregulation of NLRP3 inflammasome activation | 333, 334 |
Abbreviations: allo‐HSCT, allogeneic hematopoietic stem cell transplantation; ASCs, adipose‐derived mesenchymal stem cells; Bregs, regulatory B cells; CAR‐T, chimeric antigen receptor T‐cell; CB‐SCs, cord blood derived‐multipotent stem cells; CTLs, cytotoxic T cells; CXCL, C‐X‐C motif chemokine ligand; CXCR, C‐X‐C motif chemokine receptor; DPP4, dipeptidyl peptidase 4; FXR, farnesoid X receptor; GLP1R, glucagon‐like peptide 1 receptor; GVHD, graft‐versus‐host disease; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; IFN‐γ, interferon‐gamma; IL, interleukin; JAK, Janus kinase; MICs, modified immune cells; MSCs, mesenchymal stromal cells; mTOR, mammalian target of rapamycin; NLRP3, NOD‐, LRR‐, and pyrin domain‐containing protein 3; PCSK9, proprotein convertase subtilisin/kexin‐9; PD‐1, programmed cell death protein‐1; PTLD, posttransplant lymphoproliferative disorders; RASBs, renin–angiotensin system blockers; SGLT2, sodium–glucose cotransporter 2; SOT, solid organ transplantation; TCR, T cell receptor; TNF‐α, tumor necrosis factor‐alpha; Tregs, regulatory T cells.
4.5. Pharmacogenetics and immunogenetics in personalized immunosuppression and risk stratification
Pharmacogenetics and immunogenetics have emerged as critical areas of investigation in personalized medicine, particularly in the realm of immunosuppression management and risk stratification for transplant recipients. Currently, one of the most extensively studied pharmacogenetic markers is the cytochrome P450 3A5 (CYP3A5) polymorphism, which significantly influences the metabolism of tacrolimus, a widely used immunosuppressant in transplantation medicine. Individuals possessing a CYP3A5 expression genotype require a longer duration to achieve target tacrolimus plasma concentrations compared with nonexpressers. 335 Given the pivotal role of immunosuppressants in posttransplantation complications, numerous studies are presently exploring the impact of CYP3A5 expression on the risk of such complications. Research conducted by Cheng et al. 336 demonstrated that among renal transplant recipients receiving tacrolimus, patients with the CYP3A5*1*1 genotype necessitated higher drug doses to attain target plasma concentrations, potentially resulting in insufficient immunosuppression and consequently elevating the risk of acute rejection. Multiple studies have corroborated this increased rejection risk associated with the CYP3A5*1 allele. 337 , 338 Furthermore, among allo‐HSCT recipients receiving tacrolimus, those expressing CYP3A5 exhibited a higher incidence of GVHD. 339 These findings suggest that pretransplant administration of immunosuppressants based on genotypes of genes such as CYP3A5 may facilitate the achievement of desired therapeutic concentrations, thereby optimizing transplant outcomes and mitigating adverse effects associated with immune overactivation. Regarding immunogenetic markers, HLA typing remains a crucial factor in organ transplantation. Matching donor and recipient HLA alleles can significantly reduce the risk of graft rejection and improve long‐term outcomes. 340 The degree of HLA compatibility between donor and recipient is inversely correlated with the risk of rejection. 341 Consequently, adjusting the dosage of recipient immunosuppressants according to the degree of HLA matching may be advantageous in preventing immunosuppression‐related complications, such as infections. It is noteworthy that a substantial body of research has demonstrated the critical importance of matching ABO blood group antigens and MHC class I polypeptide‐related sequence A (MICA) between donor and recipient in controlling rejection. 342 , 343 Moreover, variations in genes encoding cytokines and their receptors have been associated with distinct immune responses, which may inform risk stratification and guide tailored immunosuppressive strategies. 344 For instance, the study by Dukat‐Mazurek et al. 345 confirmed a potential correlation between acute GVHD following HSCT and IL‐6 genotype C/C homozygosity. Additionally, they identified a significant protective effect of the IL‐10 gene GCC/ATA haplotype on the development and severity of GVHD. 345
5. FUTURE OUTLOOK AND SUMMARY
In summary, immune system dysfunction plays a crucial role in the development of posttransplantation complications. Despite extensive research efforts to elucidate the underlying mechanisms, several key aspects require further investigation. These include a more detailed understanding of the role of specific immune cell subsets in the pathogenesis of posttransplantation complications, the development of more accurate diagnostic methods for early detection and monitoring, and the identification of novel, effective prevention and treatment strategies. Here, we highlight four key points that warrant further exploration (Figure 4).
FIGURE 4.
Future research directions regarding posttransplant complications. This figure was created using tools provided by Biorender.com (accessed July 24, 2024).
5.1. The role of more immune cells in posttransplant complications
In the previous section, we discussed the pivotal roles of CD4+ and CD8+ T cells in posttransplant complications. However, the involvement of additional T cell subsets in the development of posttransplant complications remains unclear. Indeed, accumulating evidence suggests that other T cell subsets can be equally influential in the development of posttransplant complications. 346 , 347 Natural killer T (NKT) cells are a distinct class of innate T lymphocytes that recognize lipid antigens presented by the MHC class I molecule CD1d. During liver transplantation‐associated infections with pathogens such as HBV or HCV, different NKT cell subtypes can eliminate infected cells and pathogens through direct cytotoxic effects and by promoting the activation of CTLs. 348 , 349 In antitumor immunity, NKT cells can sense tumor‐mediated changes in lipid metabolism by recognizing endogenous lipids and modulate anticancer immunity by secreting a series of cytokines. 346 Indeed, numerous studies have observed an association between transplantation‐related factors and NKT cells. 350 , 351 Moiseev et al. 350 demonstrated that higher levels of NKT cells in peripheral blood stem cell transplant donors were associated with impaired immune recovery in recipients treated with posttransplant cyclophosphamide. Furthermore, a study by Hodge et al. 351 revealed that immunosuppressants, such as cyclosporine and prednisolone, inhibited the production of proinflammatory cytokines, including IFN‐γ and TNF‐α, by NKT cells. In addition to NKT cells, the potential role of gamma delta (γδ) T cells in posttransplantation complications is also of interest. In many cases, γδ T cells can directly recognize antigens through their γδ TCR without the involvement of APCs. 352 In transplantation‐associated complications, γδ T cells can play multiple roles. On the one hand, certain subsets of γδ T cells may suppress the immune function of transplant recipients. 353 In hematopoietic stem cell transplantation, IL‐17A‐producing γδ T cells can effectively enhance immunosuppression. 354 In liver transplant recipients, γδ T cells have also been reported to be associated with hepatic allograft tolerance. Therefore, these γδ T cell‐associated immune impairments may increase the risk of posttransplant infections and tumorigenesis. On the other hand, in some cases, γδ T cells can control the reactivation of viruses, such as EBV and CMV, which may enhance their ability to achieve posttransplant tumor suppression. 355 Currently, substantial evidence supports that transplantation‐related factors, such as the use of immunosuppressants and CMV infection, have a significant effect on γδ T cells. 347 , 348 , 349 , 350 , 351 , 352 , 353 , 354 , 355 , 358 Therefore, the impact of γδ T cells on posttransplantation cancers cannot be overlooked. However, there is still a paucity of studies to elucidate the precise role of T cell subsets, such as NKT and γδ T cells, in posttransplant infections or tumors. Therefore, more refined phenotyping criteria for T cell subsets need to be further investigated.
In addition, the crosstalk between other immune cells and T cells in the onset of impaired immune surveillance after transplantation warrants further investigation. In addition to NK cells and γδ T cells, other innate lymphoid cell (ILC) subpopulations, such as group 2 ILCs (ILC2s) and group 3 ILCs (ILC3s), play an equally important role in posttransplantation immunity. 357 ILC2s can promote antigen‐specific CD4+ T cell responses through the expression of MHC class II molecules and enhance NK cell cytotoxicity and T cell responses by secreting IL‐13. 358 , 359 On the other hand, ILC3s promote CD4+ and CD8+ T cell infiltration in tumors by producing CXCL10. 360 Furthermore, tumor‐associated macrophages (TAMs) also influence the antitumor immune function of T cells. Under specific circumstances, TAMs may inhibit CD8+ T cell recruitment by producing IL‐15/IL‐15 receptor alpha complexes while promoting Tregs by producing C‐C motif chemokine ligand 22. 361 , 362 Currently, the impact of these cells on posttransplantation infections or tumorigenesis needs to be further elucidated. Furthermore, it is necessary to thoroughly investigate whether other cells can also influence T cells, leading to impaired immune surveillance after transplantation.
5.2. Emerging clinical diagnostic technologies
The potential application of additional bioinformatics techniques in the diagnosis of posttransplantation complications requires further investigation. Given the central role of T cells in transplantation‐associated immunity, conventional techniques capable of identifying T cell‐associated biomarkers, such as single‐cell RNA sequencing (scRNA‐seq), spatial transcriptomics, and mass cytometry by time‐of‐flight, have been extensively employed in the diagnosis of posttransplantation complications, such as cancers. 363 , 364 , 365 Recently, novel techniques have been employed to evaluate T cell function, such as TCR sequencing (TCR‐seq) and peptide–MHC multimer technology. 366 , 367 , 368 These techniques have been applied to monitor posttransplant complications, such as the risk of immune rejection and T cell‐mediated antitumor immune responses. However, their potential application in the diagnosis of other posttransplant complications requires urgent investigation. 369 , 370 , 371 Importantly, diagnosing posttransplantation complications from a multiomics perspective is a promising research avenue. The development of multiomics‐based biomarkers for posttransplant rejection is currently a highly active area of research. 372 For instance, Lin and colleagues 187 developed a novel bioinformatics approach for identifying microRNA markers closely associated with kidney transplant rejection by integrating multiomics data to construct a molecular interactome network. Moreover, genomics, transcriptomics, and proteomics related to T cells have been extensively employed in detecting posttransplantation cancers. 373 , 374 , 375 , 376 Furthermore, other omics approaches have gained increasing attention, particularly metabolomics, which has potential diagnostic value for posttransplantation complications due to its ability to monitor T cell function. 377 For example, measuring the levels of mitochondrial and lipid metabolism in Tregs can reflect anti‐infective or antitumor immunity. 378 Additionally, given the role of various viruses and bacteria in posttransplant infections and tumors, the value of microbiome analysis in this context has also garnered attention. For instance, a study by Tellez et al. 379 demonstrated the reliability of microbial assay arrays in monitoring EBV infection in lymphoma. Although numerous studies suggest the potential value of multiomics approaches in diagnosing posttransplantation cancers, their application requires further investigation.
Interestingly, certain clinical diagnostic techniques focusing on T cells have potential applications in diagnosing posttransplant complications. Imaging is one such technique with potential applications in this context. 10 For example, a study by Anthony et al. 380 demonstrated that by injecting D‐luciferin into mice after bone marrow transplantation and using bioluminescence imaging, researchers could track specific T cell subpopulations and hypothesize about their contribution to GVHD at different time points. Notably, this noninvasive technique for monitoring T cell activity may also show promise in posttransplant tumor surveillance. 380 Similarly, pathological analyses targeting T cells also have potential applications in diagnosing posttransplantation tumors. In patients with posttransplantation tumors, Datta et al. 1 adequately assessed CD8+ T cell abundance in the tumor microenvironment by performing digital image analysis of tumor and adjacent tissue sections, finding that reduced abundance correlated with impaired cancer immune surveillance. Furthermore, Mohri et al. 381 used microscopy to observe the presence of numerous large atypical cells in the background of dense T cell aggregates, using this pathological feature as evidence to support the diagnosis of classical Hodgkin lymphoma‐type posttransplant lymphoproliferative disorder following renal transplantation. In the future, it will be essential to investigate additional clinical diagnostic techniques focusing on T cells and explore their potential applications in diagnosing posttransplantation tumors.
5.3. Advancing personalized management with cutting‐edge technologies
Multiomics technologies have demonstrated exceptional performance in the accurate and comprehensive prediction of posttransplant complications, which provide crucial guidance for improving transplant patient outcomes. In the field of genomics, Ma et al. 382 developed a 355‐gene transplant incidence panel encompassing cardiovascular disease, immunodeficiency, malignancy, and thrombosis. Their evaluation of this genetic panel, using exome sequencing data from 1590 kidney transplant recipients, revealed that approximately 9% of patients harbored variants predisposing them to these complications. This finding underscores the potential value of genetic testing in informing clinical decision‐making, such as implementing more intensive monitoring, adjusting immunosuppressive regimens, or initiating specialist referrals. 382 Proteomics has garnered significant attention in posttransplantation complication research. Hu et al. 383 conducted antibody array analyses on urine samples from kidney transplant recipients, identifying significantly elevated levels of 11 cytokines/chemokines in patients experiencing acute rejection compared with healthy controls. Their study further demonstrated that a combination of markers, including 10 kDa induced protein, IFN‐γ‐induced monokine and macrophage inflammatory protein‐1Delta, could differentiate between acute and chronic injury. 383 Additionally, the application of other multiomics approaches, such as transcriptomics and metabolomics, in posttransplantation complication studies has gained increasing traction. 384 , 385 Systems biology adopts a holistic perspective, integrating multiomics data to generate and validate novel hypotheses regarding organ function and failure. This approach necessitates addressing data variability, noise, and scale, while combining experimental big data with computational models to reconstruct and elucidate biological processes. 386 Sui et al.’s 387 research exemplifies the potential of systems biology in posttransplantation complications. By integrating proteomics and genomics technologies, they explored the expression levels of transcription factors, microRNAs, and long noncoding RNAs in biopsy samples from kidney transplant patients with acute rejection and healthy controls, identifying characteristic markers through association analysis. 387 Future research should focus on optimizing systems biology methods and integrating more multiomics data to develop accurate predictive models. These advancements aim to enable early detection and personalized treatment of posttransplantation complications, ultimately improving long‐term patient outcomes. Machine learning (ML) further enhances personalized management by leveraging large‐scale clinical and molecular data to identify patterns and predict outcomes with high accuracy. ML has been widely applied in D–R matching prediction prior to transplantation. 388 , 389 For instance, Briceño et al. 390 employed artificial neural networks for D–R matching in liver transplantation, demonstrating superior performance in predicting graft survival and probability of graft loss compared with validated graft survival scores. 391 ML‐based analyses hold promise for achieving precise D–R matching, potentially preventing posttransplantation complications. ML has also shown efficacy in predicting posttransplantation patient prognosis and exhibits significant potential in forecasting the risk of complications such as rejection, GVHD, and malignancy. 392 , 393 , 394 , 395 , 396 , 397 In many instances, ML demonstrates superior predictive capabilities compared with traditional methods. For example, Zare et al. 398 reported that ML models outperformed conventional logistic regression in predicting acute rejection in liver transplant patients. Notably, ML techniques offer substantial advantages in processing unstructured data, such as medical imaging. 399 In conclusion, advanced research methodologies including multiomics, systems biology, and ML are transforming the landscape of transplant medicine. These approaches are paving the way for more effective and personalized therapeutic interventions. The integrated application of these technologies is expected to significantly enhance the prevention, diagnosis, and treatment of posttransplantation complications, ultimately improving patients’ long‐term quality of life.
5.4. Balance of GVHD and posttransplantation cancers
Finally, the question of how to combat posttransplant tumors without causing GVHD is also an urgent one. Although the immunosuppressive effects of mTOR inhibitors are thought to counteract their anticancer effects, a substantial body of research suggests that mTOR inhibitors have a facilitating effect on the anticancer function of CD8+ T cells. 400 , 401 A randomized controlled trial by Knoll et al. 283 demonstrated that, compared with an immunosuppressive regimen without sirolimus, the combination regimen was associated with a reduced risk of malignant neoplasms and nonmelanoma skin cancers in transplant recipients. A meta‐analysis by Yanik et al. 284 revealed that sirolimus use in renal transplant recipients was associated with a lower risk of kidney cancer. This paradox arises from the fact that mTOR inhibitors are immunomodulators with a complex mechanism of action in adaptive immunity, capable of both promoting and suppressing immune responses. 400 Therefore, the effect of mTOR inhibitors on CD8+ T cells may depend on the relative predominance of their immunostimulatory and immunosuppressive effects. Given the dual effects of mTOR inhibitors in suppressing posttransplantation rejection and reducing the risk of posttransplantation tumorigenesis, their potential application in preventing and treating specific types of posttransplantation tumors warrants further investigation. Furthermore, γδ T cells may offer a promising strategy for resolving this issue. Numerous studies have demonstrated that γδ T cells, which lack allogeneic heterozygosity, can be safely used in hematopoietic stem cell transplantation and can mediate natural antitumor responses. 402 , 403 Consequently, γδ T cells have potential applications for simultaneously preventing GvHD and posttransplantation tumors in specific types of transplantation. Numerous studies support this concept. For instance, in allo‐HSCT recipients, γδ T cells are not believed to cause GvHD and may promote graft‐versus‐leukemia effects. 404 Furthermore, Wu et al. 405 , 406 observed that the proportion of CD27+Vδ1 Tregs, a subpopulation of γδ Tregs, in grafts transplanted with peripheral blood stem cells was negatively correlated with acute GVHD. They also found that γδ Tregs exhibited a cytotoxic effect on tumor cells following granulocyte colony‐stimulating factor treatment. 405 , 406 However, the specific mechanism of action and the potential role of γδ T cells in a broader range of transplantation settings require further investigation. Moreover, in addition to CAR‐T therapy, TCR engineering, a relay cell therapy, has also shown potential for suppressing tumors after transplantation. Currently, T cells recognizing EBV antigens through natural TCRs have been successfully used to treat EBV‐associated PTLD. 407 Furthermore, modified TCR‐genetically engineered virus‐specific T cells targeting CMV and EBV were found to possess both antiviral activity and transgenic TCR‐mediated tumor‐targeting activity. 408 Therefore, TCR engineering may also be a readily available cell therapy capable of specifically targeting tumors, and its further applications warrant additional investigation.
6. CONCLUSION
Posttransplant complications continue to pose significant challenges, limiting the long‐term survival and quality of life of transplant recipients. In this comprehensive review, we summarize the clinical features, risk factors, and molecular mechanisms underlying major posttransplant complications, including immune‐mediated, infectious, metabolic, and malignant complications.
The development of posttransplantation complications is driven by a complex interplay between immune activation, metabolic dysregulation, and immunosuppression. Innate and adaptive immune responses triggered by allogeneic antigens play a pivotal role in mediating graft rejection and GVHD. Metabolic complications, such as PTDM, dyslipidemia, and hypertension, are closely linked to the use of immunosuppressive agents and contribute substantially to cardiovascular morbidity in transplant recipients. Furthermore, posttransplant immunosuppression impairs the body's anti‐infective and antitumor immune responses, thereby increasing the patient's susceptibility to infections and malignancies. The reactivation of latent oncogenic viruses under immunosuppression is a key driver of posttransplant malignancy, underscoring the intricate relationship between immunosuppression and tumorigenesis. Drawing upon these mechanistic insights, we discuss potential prevention and intervention strategies for posttransplant complications. Optimizing immunosuppressive regimens to achieve a delicate balance between preventing rejection and minimizing adverse effects is of paramount importance. The development of novel immunosuppressive agents with higher specificity and lower toxicity holds promise for mitigating metabolic and cardiovascular complications. Moreover, enhanced infection prevention through vaccination, thorough D–R screening, and judicious use of antimicrobials can significantly reduce the burden of infectious complications. In the context of posttransplant malignancies, the implementation of antiviral medications, selection of appropriate immunosuppressive agents, and treatment with cellular therapies may improve the management of virus‐associated malignancies.
Despite significant advances in elucidating the mechanisms underlying posttransplant complications, several aspects remain to be fully understood. Future research should focus on elucidating the intricate interplay between immune, metabolic, and oncogenic pathways underlying these complications at the molecular level. Integrating multiomics approaches with systems biology will provide a more comprehensive understanding of the pathogenesis and facilitate the identification of novel therapeutic targets. Moreover, the development of personalized risk stratification and prevention strategies based on individual genetic and immunological profiles holds great promise for improving transplantation outcomes. A more profound understanding of the molecular mechanisms underlying these complications is crucial for developing effective prevention and treatment strategies. By integrating mechanistic insights with precision medicine approaches, we can optimize immunosuppression regimens, enhance complication monitoring, and implement targeted interventions to improve long‐term survival and quality of life for transplant recipients. Continuous research efforts in this field will usher in a new era of personalized transplant medicine, ultimately benefiting patients worldwide.
AUTHOR CONTRIBUTIONS
Yongguang Liu, Ming Zhao, and Peng Luo provided direction and guidance throughout the preparation of this manuscript. Xiaoyou Liu, Junyi Shen, and Hongyan Yan wrote and edited the manuscript. Yongguang Liu, Ming Zhao, and Peng Luo reviewed and made significant revisions to the manuscript. Xiaoyou Liu, Junyi Shen, and Hongyan Yan revised and edited the manuscript. Jianmin Hu, Guorong Liao, Ding Liu, Song Zhou, Jie Zhang, Jun Liao, Zefeng Guo, Yuzhu Li, Siqiang Yang, Shichao Li, Hua Chen, Ying Guo, Min Li, Lipei Fan, and Liuyang Li collected and prepared the related papers. All authors read and approved the final manuscript.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no conflict of interests.
ETHICS STATEMENT
Not applicable.
ACKNOWLEDGMENTS
This work was supported by the Basic Research Program Project of Guangzhou (Grant No. 2060206), the Basic and Applied Basic Research Foundation of Guangdong Province (Grant No. 2022A1515012304), and the National Natural Science Foundation of China (Grant No. 82170764).
Liu X, Shen J, Yan H, et al. Posttransplant complications: molecular mechanisms and therapeutic interventions. MedComm. 2024;5:e669. 10.1002/mco2.669
Contributor Information
Peng Luo, Email: luopeng@smu.edu.cn.
Ming Zhao, Email: zhaoming02@hotmail.com.
Yongguang Liu, Email: liuyg168@smu.edu.cn.
DATA AVAILABILITY STATEMENT
Not applicable.
REFERENCES
- 1. Datta RR, Schran S, Persa OD, et al. Post‐transplant malignancies show reduced T‐cell abundance and tertiary lymphoid structures as correlates of impaired cancer immunosurveillance. Clin Cancer Res. 2022;28(8):1712‐1723. [DOI] [PubMed] [Google Scholar]
- 2. Grinyó JM. Why is organ transplantation clinically important? Cold Spring Harb Perspect Med. 2013;3(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Conrad SA, Chhabra A, Vay D. Long‐term follow‐up and complications after cardiac transplantation. J La State Med Soc. 1993;145(5):217‐220. 223–5. [PubMed] [Google Scholar]
- 4. Sen A, Callisen H, Libricz S, Patel B. Complications of solid organ transplantation: cardiovascular, neurologic, renal, and gastrointestinal. Crit Care Clin. 2019;35(1):169‐186. [DOI] [PubMed] [Google Scholar]
- 5. Li Q, Lan P. Activation of immune signals during organ transplantation. Signal Transduct Target Ther. 2023;8(1):110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fishman JA. Infection in organ transplantation. Am J Transplant. 2017;17(4):856‐879. [DOI] [PubMed] [Google Scholar]
- 7. Shivaswamy V, Boerner B, Larsen J. Post‐transplant diabetes mellitus: causes, treatment, and impact on outcomes. Endocr Rev. 2016;37(1):37‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Massy ZA, Kasiske BL. Post‐transplant hyperlipidemia: mechanisms and management. J Am Soc Nephrol. 1996;7(7):971‐977. [DOI] [PubMed] [Google Scholar]
- 9. Tantisattamo E, Molnar MZ, Ho BT, et al. Approach and management of hypertension after kidney transplantation. Front Med (Lausanne). 2020;7:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Katabathina VS, Menias CO, Tammisetti VS, et al. Malignancy after solid organ transplantation: comprehensive imaging review. Radiographics. 2016;36(5):1390‐1407. [DOI] [PubMed] [Google Scholar]
- 11. Short S, Lewik G, Issa F. An immune atlas of T cells in transplant rejection: pathways and therapeutic opportunities. Transplantation. 2023;107(11):2341‐2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chong AS. Mechanisms of organ transplant injury mediated by B cells and antibodies: implications for antibody‐mediated rejection. Am J Transplant. 2020;20(Suppl 4):23‐32. Suppl 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Elalouf A. Infections after organ transplantation and immune response. Transpl Immunol. 2023;77:101798. [DOI] [PubMed] [Google Scholar]
- 14. Roberts MB, Fishman JA. Immunosuppressive agents and infectious risk in transplantation: managing the “net state of immunosuppression”. Clin Infect Dis. 2021;73(7):e1302‐e1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Justiz Vaillant AA, Mohseni M. Chronic Transplantation Rejection. StatPearls Publishing; 2024. Copyright ©. 2024. StatPearls Publishing LLC. [PubMed] [Google Scholar]
- 16. Yang JJ, Baek CH, Kim H, et al. Hyperacute rejection in ABO‐incompatible kidney transplantation: significance of isoagglutinin subclass. Transpl Immunol. 2021;69:101484. [DOI] [PubMed] [Google Scholar]
- 17. Moes DJ, Press RR, Ackaert O, et al. Exploring genetic and non‐genetic risk factors for delayed graft function, acute and subclinical rejection in renal transplant recipients. Br J Clin Pharmacol. 2016;82(1):227‐237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Solez K, Colvin RB, Racusen LC, et al. Banff ‘05 Meeting Report: differential diagnosis of chronic allograft injury and elimination of chronic allograft nephropathy ('CAN’). Am J Transplant. 2007;7(3):518‐526. [DOI] [PubMed] [Google Scholar]
- 19.Justiz Vaillant AA, Modi P, Mohammadi O. Graft‐Versus‐Host Disease. StatPearls Publishing. Copyright © 2024, StatPearls Publishing LLC. 2024. [PubMed] [Google Scholar]
- 20. Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft‐versus‐host disease: i. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11(12):945‐956. [DOI] [PubMed] [Google Scholar]
- 21. Greinix HT, Eikema DJ, Koster L, et al. Improved outcome of patients with graft‐versus‐host disease after allogeneic hematopoietic cell transplantation for hematologic malignancies over time: an EBMT mega‐file study. Haematologica. 2022;107(5):1054‐1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zecca M, Prete A, Rondelli R, et al. Chronic graft‐versus‐host disease in children: incidence, risk factors, and impact on outcome. Blood. 2002;100(4):1192‐1200. [DOI] [PubMed] [Google Scholar]
- 23. Grube M, Holler E, Weber D, Holler B, Herr W, Wolff D. Risk factors and outcome of chronic graft‐versus‐host disease after allogeneic stem cell transplantation‐results from a single‐center observational study. Biol Blood Marrow Transplant. 2016;22(10):1781‐1791. [DOI] [PubMed] [Google Scholar]
- 24. Dharnidharka VR, Agodoa LY, Abbott KC. Risk factors for hospitalization for bacterial or viral infection in renal transplant recipients–an analysis of USRDS data. Am J Transplant. 2007;7(3):653‐661. [DOI] [PubMed] [Google Scholar]
- 25. Weinrauch LA, D'Elia JA, Weir MR, et al. Infection and malignancy outweigh cardiovascular mortality in kidney transplant recipients: post hoc analysis of the FAVORIT trial. Am J Med. 2018;131(2):165‐172. [DOI] [PubMed] [Google Scholar]
- 26. Nambiar P, Silibovsky R, Belden KA. Infection in Kidney Transplantation. Contemporary Kidney Transplantation. Springer International Publishing; 2018:307‐327. eds. [Google Scholar]
- 27. Kritikos A, Manuel O. Bloodstream infections after solid‐organ transplantation. Virulence. 2016;7(3):329‐340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kotton CN. CMV: prevention, diagnosis and therapy. Am J Transplant. 2013;13(3):24‐40. Suppl. quiz 40. [DOI] [PubMed] [Google Scholar]
- 29. Miller S. Monitoring for viral infections in transplant patients. Clin Microbiol Newslett. 2016;38(16):129‐134. [Google Scholar]
- 30. Roux A, Canet E, Valade S, et al. Pneumocystis jirovecii pneumonia in patients with or without AIDS, France. Emerg Infect Dis. 2014;20(9):1490‐1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pappas PG, Alexander BD, Andes DR, et al. Invasive fungal infections among organ transplant recipients: results of the Transplant‐Associated Infection Surveillance Network (TRANSNET). Clin Infect Dis. 2010;50(8):1101‐1111. [DOI] [PubMed] [Google Scholar]
- 32. Fishman JA. Infection in solid‐organ transplant recipients. N Engl J Med. 2007;357(25):2601‐2614. [DOI] [PubMed] [Google Scholar]
- 33. Karuthu S, Blumberg EA. Common infections in kidney transplant recipients. Clin J Am Soc Nephrol. 2012;7(12):2058‐2070. [DOI] [PubMed] [Google Scholar]
- 34. Jenssen T, Hartmann A. Post‐transplant diabetes mellitus in patients with solid organ transplants. Nat Rev Endocrinol. 2019;15(3):172‐188. [DOI] [PubMed] [Google Scholar]
- 35. Hjelmesaeth J, Hartmann A, Leivestad T, et al. The impact of early‐diagnosed new‐onset post‐transplantation diabetes mellitus on survival and major cardiac events. Kidney Int. 2006;69(3):588‐595. [DOI] [PubMed] [Google Scholar]
- 36. Jørgensen MB, Hornum M, van Hall G, et al. The impact of kidney transplantation on insulin sensitivity. Transpl Int. 2017;30(3):295‐304. [DOI] [PubMed] [Google Scholar]
- 37. Nam JH, Mun JI, Kim SI, et al. beta‐Cell dysfunction rather than insulin resistance is the main contributing factor for the development of postrenal transplantation diabetes mellitus. Transplantation. 2001;71(10):1417‐1423. [DOI] [PubMed] [Google Scholar]
- 38. Zelle DM, Corpeleijn E, Deinum J, et al. Pancreatic β‐cell dysfunction and risk of new‐onset diabetes after kidney transplantation. Diabetes Care. 2013;36(7):1926‐1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Halden TA, Egeland EJ, Åsberg A, et al. GLP‐1 restores altered insulin and glucagon secretion in posttransplantation diabetes. Diabetes Care. 2016;39(4):617‐624. [DOI] [PubMed] [Google Scholar]
- 40. Shah T, Kasravi A, Huang E, et al. Risk factors for development of new‐onset diabetes mellitus after kidney transplantation. Transplantation. 2006;82(12):1673‐1676. [DOI] [PubMed] [Google Scholar]
- 41. Kasiske BL, Snyder JJ, Gilbertson D, Matas AJ. Diabetes mellitus after kidney transplantation in the United States. Am J Transplant. 2003;3(2):178‐185. [DOI] [PubMed] [Google Scholar]
- 42. Azadi S, Azarpira N, Roozbeh J, et al. Genetic polymorphisms of glucocorticoid receptor and their association with new‐onset diabetes mellitus in kidney transplant recipients. Gene. 2023;856:147138. [DOI] [PubMed] [Google Scholar]
- 43. Johnston O, Rose CL, Webster AC, Gill JS. Sirolimus is associated with new‐onset diabetes in kidney transplant recipients. J Am Soc Nephrol. 2008;19(7):1411‐1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gonyea JE, Anderson CF. Weight change and serum lipoproteins in recipients of renal allografts. Mayo Clin Proc. 1992;67(7):653‐657. [DOI] [PubMed] [Google Scholar]
- 45. Becker DM, Markakis M, Sension M, et al. Prevalence of hyperlipidemia in heart transplant recipients. Transplantation. 1987;44(2):323‐325. [DOI] [PubMed] [Google Scholar]
- 46. Dopazo C, Bilbao I, Castells LL, et al. Analysis of adult 20‐year survivors after liver transplantation. Hepatol Int. 2015;9(3):461‐470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Warden BA, Duell PB. Management of dyslipidemia in adult solid organ transplant recipients. J Clin Lipidol. 2019;13(2):231‐245. [DOI] [PubMed] [Google Scholar]
- 48. Bucharles SGE, Wallbach KKS, Moraes TP. Pecoits‐Filho R. Hypertension in patients on dialysis: diagnosis, mechanisms, and management. J Bras Nefrol. 2019;41(3):400‐411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Przybylowski P, Malyszko J, Malyszko JS, Kobus G, Sadowski J, Mysliwiec M. Blood pressure control in orthotopic heart transplant and kidney allograft recipients is far from satisfactory. Transplant Proc. 2010;42(10):4263‐4266. [DOI] [PubMed] [Google Scholar]
- 50. Savioli G, Surbone S, Giovi I, et al. Early development of metabolic syndrome in patients subjected to lung transplantation. Clin Transplant. 2013;27(3):E237‐243. [DOI] [PubMed] [Google Scholar]
- 51. Birdwell KA, Park M. Post‐transplant cardiovascular disease. Clin J Am Soc Nephrol. 2021;16(12):1878‐1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Laskow DA, Curtis JJ. Post‐transplant hypertension. Am J Hypertens. 1990;3(9):721‐725. [DOI] [PubMed] [Google Scholar]
- 53. Miller LW. Cardiovascular toxicities of immunosuppressive agents. Am J Transplant. 2002;2(9):807‐818. [DOI] [PubMed] [Google Scholar]
- 54. Poornima I, Power J, Doyle M. Significance of lipid management in cardiac transplant population. J Clin Lipidol. 2019;13(3):e42‐e43. [Google Scholar]
- 55. Campbell PT, Krim SR. Hypertension in cardiac transplant recipients: tackling a new face of an old foe. Curr Opin Cardiol. 2020;35(4):368‐375. [DOI] [PubMed] [Google Scholar]
- 56. Gillis KA, Patel RK, Jardine AG. Cardiovascular complications after transplantation: treatment options in solid organ recipients. Transplant Rev (Orlando). 2014;28(2):47‐55. [DOI] [PubMed] [Google Scholar]
- 57. Valantine H. Cardiac allograft vasculopathy after heart transplantation: risk factors and management. J Heart Lung Transplant. 2004;23:S187‐193. Suppl. [DOI] [PubMed] [Google Scholar]
- 58. Fellström B. Risk factors for and management of post‐transplantation cardiovascular disease. BioDrugs. 2001;15(4):261‐278. [DOI] [PubMed] [Google Scholar]
- 59. Krishnan A, Wong G, Teixeira‐Pinto A, Lim WH. Incidence and outcomes of early cancers after kidney transplantation. Transpl Int. 2022;35:10024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Rossi AP, Malignancy KleinCLPosttransplant. Posttransplant Malignancy. Surg Clin North Am. 2019;99(1):49‐64. [DOI] [PubMed] [Google Scholar]
- 61. Collett D, Mumford L, Banner NR, Neuberger J, Watson C. Comparison of the incidence of malignancy in recipients of different types of organ: a UK Registry audit. Am J Transplant. 2010;10(8):1889‐1896. [DOI] [PubMed] [Google Scholar]
- 62. Luskin MR, Heil DS, Tan KS, et al. The impact of EBV status on characteristics and outcomes of posttransplantation lymphoproliferative disorder. Am J Transplant. 2015;15(10):2665‐2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Verucchi G, Calza L, Trevisani F, et al. Human herpesvirus‐8‐related Kaposi's sarcoma after liver transplantation successfully treated with cidofovir and liposomal daunorubicin. Transpl Infect Dis. 2005;7(1):34‐37. [DOI] [PubMed] [Google Scholar]
- 64. Vajdic CM, van Leeuwen MT. Cancer incidence and risk factors after solid organ transplantation. Int J Cancer. 2009;125(8):1747‐1754. [DOI] [PubMed] [Google Scholar]
- 65. Holmes RD, Sokol RJ. Epstein‐Barr virus and post‐transplant lymphoproliferative disease. Pediatr Transplant. 2002;6(6):456‐464. [DOI] [PubMed] [Google Scholar]
- 66. Al‐Mansour Z, Nelson BP, Evens AM. Post‐transplant lymphoproliferative disease (PTLD): risk factors, diagnosis, and current treatment strategies. Curr Hematol Malig Rep. 2013;8(3):173‐183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Yao QY, Rowe M, Martin B, Young LS, Rickinson AB. The Epstein‐Barr virus carrier state: dominance of a single growth‐transforming isolate in the blood and in the oropharynx of healthy virus carriers. J Gen Virol. 1991;72(Pt 7):1579‐1590. [DOI] [PubMed] [Google Scholar]
- 68.Al Hamed R, Bazarbachi AH, Mohty M. Epstein‐Barr virus‐related post‐transplant lymphoproliferative disease (EBV‐PTLD) in the setting of allogeneic stem cell transplantation: a comprehensive review from pathogenesis to forthcoming treatment modalities. Bone Marrow Transplant. 2020;55(1):25‐39. [DOI] [PubMed] [Google Scholar]
- 69. Penn I. Cancers complicating organ transplantation. N Engl J Med. 1990;323(25):1767‐1769. [DOI] [PubMed] [Google Scholar]
- 70. Schober T, Framke T, Kreipe H, et al. Characteristics of early and late PTLD development in pediatric solid organ transplant recipients. Transplantation. 2013;95(1):240‐246. [DOI] [PubMed] [Google Scholar]
- 71. Chapman JR, Webster AC, Wong G. Cancer in the transplant recipient. Cold Spring Harb Perspect Med. 2013;3(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Mannon RB. Delayed graft function: the AKI of kidney transplantation. Nephron. 2018;140(2):94‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Siedlecki A, Irish W, Brennan DC. Delayed graft function in the kidney transplant. Am J Transplant. 2011;11(11):2279‐2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Ponticelli C, Reggiani F, Moroni G. Delayed graft function in kidney transplant: risk factors, consequences and prevention strategies. J Pers Med. 2022;12(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ojo AO, Wolfe RA, Held PJ, Port FK, Schmouder RL. Delayed graft function: risk factors and implications for renal allograft survival. Transplantation. 1997;63(7):968‐974. [DOI] [PubMed] [Google Scholar]
- 76. Domański L, Kłoda K, Kwiatkowska E, et al. Effect of delayed graft function, acute rejection and chronic allograft dysfunction on kidney allograft telomere length in patients after transplantation: a prospective cohort study. BMC Nephrol. 2015;16:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Cherukuri A, Mehta R, Sood P, Hariharan S. Early allograft inflammation and scarring associate with graft dysfunction and poor outcomes in renal transplant recipients with delayed graft function: a prospective single center cohort study. Transpl Int. 2018;31(12):1369‐1379. [DOI] [PubMed] [Google Scholar]
- 78. Gioco R, Corona D, Ekser B, et al. Gastrointestinal complications after kidney transplantation. World J Gastroenterol. 2020;26(38):5797‐5811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Dahman M, Krell R, Brayman K, et al. Simultaneous Clostridium difficile‐associated colitis and late‐onset intestinal cytomegalovirus disease in a renal transplant recipient. Ann Transplant. 2010;15(4):72‐76. [PubMed] [Google Scholar]
- 80. Rencuzogullari A, Ozuner G, Binboga S, Aytac E, Krishnamurthi V, Gorgun E. Colonic diverticulosis and diverticulitis in renal transplant recipients: management and long‐term outcomes. Am Surg. 2017;83(3):303‐307. [PubMed] [Google Scholar]
- 81. Lederman ED, Conti DJ, Lempert N, Singh TP, Lee EC. Complicated diverticulitis following renal transplantation. Dis Colon Rectum. 1998;41(5):613‐618. [DOI] [PubMed] [Google Scholar]
- 82. Calogero A, Gallo M, Sica A, et al. Gastroenterological complications in kidney transplant patients. Open Med (Wars). 2020;15(1):623‐634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Dhar R. Neurologic complications of transplantation. Handb Clin Neurol. 2017;141:545‐572. [DOI] [PubMed] [Google Scholar]
- 84. Tardieu L, Divard G, Lortholary O, et al. Cryptococcal meningitis in kidney transplant recipients: a two‐decade cohort study in France. Pathogens. 2022;11(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Tang Q, Dong C, Sun Q. Immune response associated with ischemia and reperfusion injury during organ transplantation. Inflamm Res. 2022;71(12):1463‐1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Çelik S, Kaynar L, Güven ZT, et al. The effect of danger‐associated molecular patterns on survival in acute graft versus host disease. Bone Marrow Transplant. 2024;59(2):189‐195. [DOI] [PubMed] [Google Scholar]
- 87. Hülsdünker J, Ottmüller KJ, Neeff HP, et al. Neutrophils provide cellular communication between ileum and mesenteric lymph nodes at graft‐versus‐host disease onset. Blood. 2018;131(16):1858‐1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Fukata M, Vamadevan AS, Abreu MT. Toll‐like receptors (TLRs) and Nod‐like receptors (NLRs) in inflammatory disorders. Semin Immunol. 2009;21(4):242‐253. [DOI] [PubMed] [Google Scholar]
- 89. Hayden MS, Ghosh S. NF‐κB in immunobiology. Cell Res. 2011;21(2):223‐244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Ghosh S, Karin M. Missing pieces in the NF‐kappaB puzzle. Cell. 2002;109:S81‐96. Suppl. [DOI] [PubMed] [Google Scholar]
- 91. West EE, Kolev M, Kemper C. Complement and the regulation of T cell responses. Annu Rev Immunol. 2018;36:309‐338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Schwab L, Goroncy L, Palaniyandi S, et al. Neutrophil granulocytes recruited upon translocation of intestinal bacteria enhance graft‐versus‐host disease via tissue damage. Nat Med. 2014;20(6):648‐654. [DOI] [PubMed] [Google Scholar]
- 93. Felix NJ, Donermeyer DL, Horvath S, et al. Alloreactive T cells respond specifically to multiple distinct peptide‐MHC complexes. Nat Immunol. 2007;8(4):388‐397. [DOI] [PubMed] [Google Scholar]
- 94. Abou‐Daya KI, Oberbarnscheidt MH. Innate allorecognition in transplantation. J Heart Lung Transplant. 2021;40(7):557‐561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. DeWolf S, Sykes M. Alloimmune T cells in transplantation. J Clin Invest. 2017;127(7):2473‐2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Ingulli E. Mechanism of cellular rejection in transplantation. Pediatr Nephrol. 2010;25(1):61‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Smyth LA, Lechler RI, Lombardi G. Continuous acquisition of MHC:peptide complexes by recipient cells contributes to the generation of anti‐graft CD8(+) T cell immunity. Am J Transplant. 2017;17(1):60‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Wood KJ, Zaitsu M, Goto R. Cell mediated rejection. Methods Mol Biol. 2013;1034:71‐83. [DOI] [PubMed] [Google Scholar]
- 99. Toubai T, Mathewson ND, Magenau J, Reddy P. Danger signals and graft‐versus‐host disease: current understanding and future perspectives. Front Immunol. 2016;7:539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Gardner D, Jeffery LE, Sansom DM. Understanding the CD28/CTLA‐4 (CD152) pathway and its implications for costimulatory blockade. Am J Transplant. 2014;14(9):1985‐1991. [DOI] [PubMed] [Google Scholar]
- 101. Solinas C, Gu‐Trantien C, Willard‐Gallo K. The rationale behind targeting the ICOS‐ICOS ligand costimulatory pathway in cancer immunotherapy. ESMO Open. 2020;5(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Grewal IS, Flavell RA. The role of CD40 ligand in costimulation and T‐cell activation. Immunol Rev. 1996;153:85‐106. [DOI] [PubMed] [Google Scholar]
- 103. Fu Y, Lin Q, Zhang Z, Zhang L. Therapeutic strategies for the costimulatory molecule OX40 in T‐cell‐mediated immunity. Acta Pharm Sin B. 2020;10(3):414‐433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Wysocki CA, Panoskaltsis‐Mortari A, Blazar BR, Serody JS. Leukocyte migration and graft‐versus‐host disease. Blood. 2005;105(11):4191‐4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Reina‐Campos M, Scharping NE, Goldrath AW. CD8(+) T cell metabolism in infection and cancer. Nat Rev Immunol. 2021;21(11):718‐738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. St Paul M, Ohashi PS. The roles of CD8(+) T cell subsets in antitumor immunity. Trends Cell Biol. 2020;30(9):695‐704. [DOI] [PubMed] [Google Scholar]
- 107. Delfs MW, Furukawa Y, Mitchell RN, Lichtman AH. CD8+ T cell subsets TC1 and TC2 cause different histopathologic forms of murine cardiac allograft rejection. Transplantation. 2001;71(5):606‐610. [DOI] [PubMed] [Google Scholar]
- 108. Mittrücker HW, Visekruna A, Huber M. Heterogeneity in the differentiation and function of CD8⁺ T cells. Arch Immunol Ther Exp (Warsz). 2014;62(6):449‐458. [DOI] [PubMed] [Google Scholar]
- 109. Kemp RA, Ronchese F. Tumor‐specific Tc1, but not Tc2, cells deliver protective antitumor immunity. J Immunol. 2001;167(11):6497‐6502. [DOI] [PubMed] [Google Scholar]
- 110. St Paul M, Saibil SD, Lien SC, et al. IL6 induces an IL22(+) CD8(+) T‐cell subset with potent antitumor function. Cancer Immunol Res. 2020;8(3):321‐333. [DOI] [PubMed] [Google Scholar]
- 111. Blazar BR, Taylor PA, Vallera DA. CD4+ and CD8+ T cells each can utilize a perforin‐dependent pathway to mediate lethal graft‐versus‐host disease in major histocompatibility complex‐disparate recipients. Transplantation. 1997;64(4):571‐576. [DOI] [PubMed] [Google Scholar]
- 112. Zemmour D, Kiner E, Benoist C. CD4(+) teff cell heterogeneity: the perspective from single‐cell transcriptomics. Curr Opin Immunol. 2020;63:61‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Helper ZhuJT. Cell differentiation, heterogeneity, and plasticity. Cold Spring Harb Perspect Biol. 2018;10(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Saravia J, Chapman NM, Chi H. Helper T cell differentiation. Cell Mol Immunol. 2019;16(7):634‐643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Zhou L, Chong MM, Littman DR. Plasticity of CD4+ T cell lineage differentiation. Immunity. 2009;30(5):646‐655. [DOI] [PubMed] [Google Scholar]
- 116. Benichou G, Gonzalez B, Marino J, Ayasoufi K, Valujskikh A. Role of memory T cells in allograft rejection and tolerance. Front Immunol. 2017;8:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Brehm MA, Daniels KA, Priyadharshini B, et al. Allografts stimulate cross‐reactive virus‐specific memory CD8 T cells with private specificity. Am J Transplant. 2010;10(8):1738‐1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Curtsinger JM, Lins DC, Mescher MF. CD8+ memory T cells (CD44high, Ly‐6C+) are more sensitive than naive cells to (CD44low, Ly‐6C‐) to TCR/CD8 signaling in response to antigen. J Immunol. 1998;160(7):3236‐3243. [PubMed] [Google Scholar]
- 119. Veiga‐Fernandes H, Walter U, Bourgeois C, McLean A, Rocha B. Response of naïve and memory CD8+ T cells to antigen stimulation in vivo. Nat Immunol. 2000;1(1):47‐53. [DOI] [PubMed] [Google Scholar]
- 120. Shiu KY, McLaughlin L, Rebollo‐Mesa I, et al. B‐lymphocytes support and regulate indirect T‐cell alloreactivity in individual patients with chronic antibody‐mediated rejection. Kidney Int. 2015;88(3):560‐568. [DOI] [PubMed] [Google Scholar]
- 121. Hong S, Zhang Z, Liu H, et al. B cells are the dominant antigen‐presenting cells that activate naive CD4(+) T cells upon immunization with a virus‐derived nanoparticle antigen. Immunity. 2018;49(4):695‐708. e4. [DOI] [PubMed] [Google Scholar]
- 122. Noorchashm H, Reed AJ, Rostami SY, et al. B cell‐mediated antigen presentation is required for the pathogenesis of acute cardiac allograft rejection. J Immunol. 2006;177(11):7715‐7722. [DOI] [PubMed] [Google Scholar]
- 123. Ng YH, Oberbarnscheidt MH, Chandramoorthy HC, Hoffman R, Chalasani G. B cells help alloreactive T cells differentiate into memory T cells. Am J Transplant. 2010;10(9):1970‐1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Dalloul A. B‐cell‐mediated strategies to fight chronic allograft rejection. Front Immunol. 2013;4:444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Feucht HE, Felber E, Gokel MJ, et al. Vascular deposition of complement‐split products in kidney allografts with cell‐mediated rejection. Clin Exp Immunol. 1991;86(3):464‐470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Baldwin WM, 3rd, Pruitt SK, Brauer RB, Daha MR, Sanfilippo F. Complement in organ transplantation. Contributions to inflammation, injury, and rejection. Transplantation. 1995;59(6):797‐808. [PubMed] [Google Scholar]
- 127. Seidel UJ, Schlegel P, Lang P. Natural killer cell mediated antibody‐dependent cellular cytotoxicity in tumor immunotherapy with therapeutic antibodies. Front Immunol. 2013;4:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Maeda A, Kogata S, Toyama C, et al. The innate cellular immune response in xenotransplantation. Front Immunol. 2022;13:858604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Wu GD, Jin YS, Salazar R, et al. Vascular endothelial cell apoptosis induced by anti‐donor non‐MHC antibodies: a possible injury pathway contributing to chronic allograft rejection. J Heart Lung Transplant. 2002;21(11):1174‐1187. [DOI] [PubMed] [Google Scholar]
- 130. Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299(5609):1057‐1061. [DOI] [PubMed] [Google Scholar]
- 131. Oparaugo NC, Ouyang K, Nguyen NPN, Nelson AM, Agak GW. Human regulatory T cells: understanding the role of Tregs in select autoimmune skin diseases and post‐transplant nonmelanoma skin cancers. Int J Mol Sci. 2023;24(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Asseman C, Mauze S, Leach MW, Coffman RL, Powrie F. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J Exp Med. 1999;190(7):995‐1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Bejugama K, Taduri G, Guditi S. Effect of regulatory T cells on short‐term graft outcome in kidney transplant recipients, a prospective observational, single‐center study. Transpl Immunol. 2022;73:101630. [DOI] [PubMed] [Google Scholar]
- 134. Han JW, Joo DJ, Kim JH, et al. Early reduction of regulatory T cells is associated with acute rejection in liver transplantation under tacrolimus‐based immunosuppression with basiliximab induction. Am J Transplant. 2020;20(8):2058‐2069. [DOI] [PubMed] [Google Scholar]
- 135. Rieger K, Loddenkemper C, Maul J, et al. Mucosal FOXP3+ regulatory T cells are numerically deficient in acute and chronic GvHD. Blood. 2006;107(4):1717‐1723. [DOI] [PubMed] [Google Scholar]
- 136. Fondi C, Nozzoli C, Benemei S, et al. Increase in FOXP3+ regulatory T cells in GVHD skin biopsies is associated with lower disease severity and treatment response. Biol Blood Marrow Transplant. 2009;15(8):938‐947. [DOI] [PubMed] [Google Scholar]
- 137. Hoffmann P, Ermann J, Edinger M, Fathman CG, Strober S. Donor‐type CD4(+)CD25(+) regulatory T cells suppress lethal acute graft‐versus‐host disease after allogeneic bone marrow transplantation. J Exp Med. 2002;196(3):389‐399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Taylor PA, Lees CJ, Blazar BR. The infusion of ex vivo activated and expanded CD4(+)CD25(+) immune regulatory cells inhibits graft‐versus‐host disease lethality. Blood. 2002;99(10):3493‐3499. [DOI] [PubMed] [Google Scholar]
- 139. Di Ianni M, Falzetti F, Carotti A, et al. Tregs prevent GVHD and promote immune reconstitution in HLA‐haploidentical transplantation. Blood. 2011;117(14):3921‐3928. [DOI] [PubMed] [Google Scholar]
- 140. Jin N, Malcherek G, Mani J, et al. Suppression of cytomegalovirus‐specific CD8(+)T cells by everolimus. Leuk Lymphoma. 2014;55(5):1144‐1150. [DOI] [PubMed] [Google Scholar]
- 141. Nakayama K, Nakauchi H. Cyclosporin A inhibits the decrease of CD4/CD8 expression induced by protein kinase C activation. Int Immunol. 1993;5(4):419‐426. [DOI] [PubMed] [Google Scholar]
- 142. Shi X, Zhang M, Liu F, et al. Tim‐1‐Fc suppresses chronic cardiac allograft rejection and vasculopathy by reducing IL‐17 production. Int J Clin Exp Pathol. 2014;7(2):509‐520. [PMC free article] [PubMed] [Google Scholar]
- 143. Holcombe H, Mellman I, Janeway CA, Jr. , Bottomly K, Dittel BN. The immunosuppressive agent 15‐deoxyspergualin functions by inhibiting cell cycle progression and cytokine production following naive T cell activation. J Immunol. 2002;169(9):4982‐4989. [DOI] [PubMed] [Google Scholar]
- 144. Havele C, Paetkau V. Cyclosporine blocks the activation of antigen‐dependent cytotoxic T lymphocytes directly by an IL‐2‐independent mechanism. J Immunol. 1988;140(10):3303‐3308. [PubMed] [Google Scholar]
- 145. Zhan X, Brown B, Slobod KS, Hurwitz JL. Inhibition of ex vivo‐expanded cytotoxic T‐lymphocyte function by high‐dose cyclosporine. Transplantation. 2003;76(4):739‐740. [DOI] [PubMed] [Google Scholar]
- 146. Blaheta RA, Leckel K, Wittig B, et al. Inhibition of endothelial receptor expression and of T‐cell ligand activity by mycophenolate mofetil. Transpl Immunol. 1998;6(4):251‐259. [DOI] [PubMed] [Google Scholar]
- 147. Lv QK, Liu JX, Li SN, et al. Mycophenolate mofetil modulates differentiation of Th1/Th2 and the secretion of cytokines in an active Crohn's disease mouse model. Int J Mol Sci. 2015;16(11):26654‐26666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Wang GN, Xiong Y, Ye J, Zhang LH, Ye XS. Synthetic N‐alkylated iminosugars as new potential immunosuppressive agents. ACS Med Chem Lett. 2011;2(9):682‐686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Xiang M, Liu T, Tan W, et al. Effects of kinsenoside, a potential immunosuppressive drug for autoimmune hepatitis, on dendritic cells/CD8(+) T cells communication in mice. Hepatology. 2016;64(6):2135‐2150. [DOI] [PubMed] [Google Scholar]
- 150. Tomita Y, Uehara S, Takiguchi S, Nakamura M. Effect of mammalian target of rapamycin inhibition on activated regulatory T‐cell expansion in kidney transplantation. Transplant Proc. 2023;55(4):792‐796. [DOI] [PubMed] [Google Scholar]
- 151. Janyst M, Kaleta B, Janyst K, Zagożdżon R, Kozlowska E, Lasek W. Comparative study of immunomodulatory agents to induce human T regulatory (Treg) cells: preferential Treg‐stimulatory effect of prednisolone and rapamycin. Arch Immunol Ther Exp (Warsz). 2020;68(4):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4(4):330‐336. [DOI] [PubMed] [Google Scholar]
- 153. Qu Y, Zhang B, Zhao L, et al. The effect of immunosuppressive drug rapamycin on regulatory CD4+CD25+Foxp3+T cells in mice. Transpl Immunol. 2007;17(3):153‐161. [DOI] [PubMed] [Google Scholar]
- 154. Battaglia M, Stabilini A, Roncarolo MG. Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells. Blood. 2005;105(12):4743‐4748. [DOI] [PubMed] [Google Scholar]
- 155. Anderson AC, Joller N, Kuchroo VK. Lag‐3, Tim‐3, and TIGIT: co‐inhibitory receptors with specialized functions in immune regulation. Immunity. 2016;44(5):989‐1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Stecher C, Battin C, Leitner J, et al. PD‐1 blockade promotes emerging checkpoint inhibitors in enhancing T cell responses to allogeneic dendritic cells. Front Immunol. 2017;8:572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Zeng Q, Yuan X, Cao J, et al. Mycophenolate mofetil enhances the effects of tacrolimus on the inhibitory function of regulatory T cells in patients after liver transplantation via PD‐1 and TIGIT receptors. Immunopharmacol Immunotoxicol. 2021;43(2):239‐246. [DOI] [PubMed] [Google Scholar]
- 158. Chen X, Li S, Long D, Shan J, Li Y. Rapamycin facilitates differentiation of regulatory T cells via enhancement of oxidative phosphorylation. Cell Immunol. 2021;365:104378. [DOI] [PubMed] [Google Scholar]
- 159. Setoguchi R, Hori S, Takahashi T, Sakaguchi S. Homeostatic maintenance of natural Foxp3(+) CD25(+) CD4(+) regulatory T cells by interleukin (IL)‐2 and induction of autoimmune disease by IL‐2 neutralization. J Exp Med. 2005;201(5):723‐735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Cao X, Cai SF, Fehniger TA, et al. Granzyme B and perforin are important for regulatory T cell‐mediated suppression of tumor clearance. Immunity. 2007;27(4):635‐646. [DOI] [PubMed] [Google Scholar]
- 161. Uyttenhove C, Pilotte L, Théate I, et al. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3‐dioxygenase. Nat Med. 2003;9(10):1269‐1274. [DOI] [PubMed] [Google Scholar]
- 162. Moosmann A, Bigalke I, Tischer J, et al. Effective and long‐term control of EBV PTLD after transfer of peptide‐selected T cells. Blood. 2010;115(14):2960‐2970. [DOI] [PubMed] [Google Scholar]
- 163. Volk V, Theobald SJ, Danisch S, et al. PD‐1 blockade aggravates Epstein‐Barr virus(+) post‐transplant lymphoproliferative disorder in humanized mice resulting in central nervous system involvement and CD4(+) T cell dysregulations. Front Oncol. 2020;10:614876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Brink AA, ten Berge RL, van den Brule AJ, Willemze R, Chott A, Meijer CJ. Epstein‐Barr virus is present in neoplastic cytotoxic T cells in extranodal, and predominantly in B cells in nodal T non‐Hodgkin lymphomas. J Pathol. 2000;191(4):400‐406. [DOI] [PubMed] [Google Scholar]
- 165. Tanaka T, Takizawa J, Miyakoshi S, et al. Manifestations of fulminant CD8 T‐cell post‐transplant lymphoproliferative disorder following the administration of rituximab for lymphadenopathy with a high level of Epstein‐Barr Virus (EBV) replication after allogeneic hematopoietic stem cell transplantation. Intern Med. 2014;53(18):2115‐2119. [DOI] [PubMed] [Google Scholar]
- 166. Jagadeesh D, Woda BA, Draper J, Evens AM. Post transplant lymphoproliferative disorders: risk, classification, and therapeutic recommendations. Curr Treat Options Oncol. 2012;13(1):122‐136. [DOI] [PubMed] [Google Scholar]
- 167. Macedo C, Donnenberg A, Popescu I, et al. EBV‐specific memory CD8+ T cell phenotype and function in stable solid organ transplant patients. Transpl Immunol. 2005;14(2):109‐116. [DOI] [PubMed] [Google Scholar]
- 168. Yousif E, Abdelwahab A. Post‐transplant diabetes mellitus in kidney transplant recipients in sudan: a comparison between tacrolimus and cyclosporine‐based immunosuppression. Cureus. 2022;14(2):e22285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Sato T, Inagaki A, Uchida K, et al. Diabetes mellitus after transplant: relationship to pretransplant glucose metabolism and tacrolimus or cyclosporine A‐based therapy. Transplantation. 2003;76(9):1320‐1326. [DOI] [PubMed] [Google Scholar]
- 170. Araki M, Flechner SM, Ismail HR, et al. Posttransplant diabetes mellitus in kidney transplant recipients receiving calcineurin or mTOR inhibitor drugs. Transplantation. 2006;81(3):335‐341. [DOI] [PubMed] [Google Scholar]
- 171. Lebranchu Y, Snanoudj R, Toupance O, et al. Five‐year results of a randomized trial comparing de novo sirolimus and cyclosporine in renal transplantation: the SPIESSER study. Am J Transplant. 2012;12(7):1801‐1810. [DOI] [PubMed] [Google Scholar]
- 172. Duijnhoven EMV, Boots JMM, Christiaans MHL, Wolffenbuttel BHR, Hooff JPV. Influence of tacrolimus on glucose metabolism before and after renal transplantation: a prospective study. J Am Soc Nephrol. 2001;12(3):583‐588. [DOI] [PubMed] [Google Scholar]
- 173. Boots JMM, van Duijnhoven EM, Christiaans MHL, Wolffenbuttel BHR, van Hooff JP. Glucose metabolism in renal transplant recipients on tacrolimus: the effect of steroid withdrawal and tacrolimus trough level reduction. J Am Soc Nephrol. 2002;13(1):221‐227. [DOI] [PubMed] [Google Scholar]
- 174. Shivaswamy V, Bennett RG, Clure CC, et al. Tacrolimus and sirolimus have distinct effects on insulin signaling in male and female rats. Transl Res. 2014;163(3):221‐231. [DOI] [PubMed] [Google Scholar]
- 175. Larsen JL, Bennett RG, Burkman T, et al. Tacrolimus and sirolimus cause insulin resistance in normal sprague dawley rats. Transplantation. 2006;82(4):466‐470. [DOI] [PubMed] [Google Scholar]
- 176. Pagano G, Bruno A, Cavallo‐Perin P, Cesco L, Imbimbo B. Glucose intolerance after short‐term administration of corticosteroids in healthy subjects. Prednisone, deflazacort, and betamethasone. Arch Intern Med. 1989;149(5):1098‐1101. [PubMed] [Google Scholar]
- 177. Kobashigawa JA, Kasiske BL. Hyperlipidemia in solid organ transplantation. Transplantation. 1997;63(3):331‐338. [DOI] [PubMed] [Google Scholar]
- 178. Agarwal A, Prasad GV. Post‐transplant dyslipidemia: mechanisms, diagnosis and management. World J Transplant. 2016;6(1):125‐134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. de Groen PC. Cyclosporine, low‐density lipoprotein, and cholesterol. Mayo Clin Proc. 1988;63(10):1012‐1021. [DOI] [PubMed] [Google Scholar]
- 180. Bloom IT, Bentley FR, Garrison RN. Acute cyclosporine‐induced renal vasoconstriction is mediated by endothelin‐1. Surgery. 1993;114(2). 480‐487. discussion 487–8. [PubMed] [Google Scholar]
- 181. Taler SJ, Textor SC, Canzanello VJ, Schwartz L. Cyclosporin‐induced hypertension: incidence, pathogenesis and management. Drug Saf. 1999;20(5):437‐449. [DOI] [PubMed] [Google Scholar]
- 182. Thomas B, Weir MR. The evaluation and therapeutic management of hypertension in the transplant patient. Curr Cardiol Rep. 2015;17(11):95. [DOI] [PubMed] [Google Scholar]
- 183. Curtis JJ, Luke RG, Jones P, Diethelm AG. Hypertension in cyclosporine‐treated renal transplant recipients is sodium dependent. Am J Med. 1988;85(2):134‐138. [DOI] [PubMed] [Google Scholar]
- 184. Moss NG, Powell SL, Falk RJ. Intravenous cyclosporine activates afferent and efferent renal nerves and causes sodium retention in innervated kidneys in rats. Proc Natl Acad Sci USA. 1985;82(23):8222‐8226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Lakkis JI, Weir MR. Treatment‐resistant hypertension in the transplant recipient. Semin Nephrol. 2014;34(5):560‐570. [DOI] [PubMed] [Google Scholar]
- 186. Bahous SA, Stephan A, Blacher J, Safar ME. Aortic stiffness, living donors, and renal transplantation. Hypertension. 2006;47(2):216‐221. [DOI] [PubMed] [Google Scholar]
- 187. Lin Y, Wang L, Ge W, et al. Multi‐omics network characterization reveals novel microRNA biomarkers and mechanisms for diagnosis and subtyping of kidney transplant rejection. J Transl Med. 2021;19(1):346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Cheng CY, Feng YT, Wang HY. Incidence and relative risk factors in posttransplant diabetes mellitus patients: a retrospective cohort study. Korean J Transplant. 2020;34(4):213‐237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Driscoll CJ, Cashion AK, Hathaway DK, et al. Posttransplant diabetes mellitus in liver transplant recipients. Prog Transplant. 2006;16(2):110‐116. [DOI] [PubMed] [Google Scholar]
- 190. Mohammad KG, Idrees MK, Ali T, Akhtar F. Posttransplant diabetes mellitus among live‐related kidney transplant recipients: Sindh Institute of Urology and Transplantation experience. Saudi J Kidney Dis Transpl. 2018;29(6):1320‐1325. [DOI] [PubMed] [Google Scholar]
- 191. Mazali FC, Lalli CA, Alves‐Filho G, Mazzali M. Posttransplant diabetes mellitus: incidence and risk factors. Transplant Proc. 2008;40(3):764‐766. [DOI] [PubMed] [Google Scholar]
- 192. Roccaro GA, Mitrani R, Hwang WT, Forde KA, Reddy KR. Sustained virological response is associated with a decreased risk of posttransplant diabetes mellitus in liver transplant recipients with hepatitis C‐related liver disease. Liver Transpl. 2018;24(12):1665‐1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Hjelmesaeth J, Müller F, Jenssen T, Rollag H, Sagedal S, Hartmann A. Is there a link between cytomegalovirus infection and new‐onset posttransplantation diabetes mellitus? Potential mechanisms of virus induced beta‐cell damage. Nephrol Dial Transplant. 2005;20(11):2311‐2315. [DOI] [PubMed] [Google Scholar]
- 194. Masini M, Campani D, Boggi U, et al. Hepatitis C virus infection and human pancreatic beta‐cell dysfunction. Diabetes Care. 2005;28(4):940‐941. [DOI] [PubMed] [Google Scholar]
- 195. Wang Q, Chen J, Wang Y, Han X, Chen X. Hepatitis C virus induced a novel apoptosis‐like death of pancreatic beta cells through a caspase 3‐dependent pathway. PLoS One. 2012;7(6):e38522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Chen J, Wang F, Zhou Y, et al. Chronic hepatitis C virus infection impairs insulin secretion by regulation of p38δ MAPK‐dependent exocytosis in pancreatic β‐cells. Clin Sci (Lond). 2020;134(5):529‐542. [DOI] [PubMed] [Google Scholar]
- 197. Kawaguchi T, Yoshida T, Harada M, et al. Hepatitis C virus down‐regulates insulin receptor substrates 1 and 2 through up‐regulation of suppressor of cytokine signaling 3. Am J Pathol. 2004;165(5):1499‐1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Domma AJ, Henderson LA, Goodrum FD, Moorman NJ, Kamil JP. Human cytomegalovirus attenuates AKT activity by destabilizing insulin receptor substrate proteins. J Virol. 2023;97(10):e0056323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Banerjee S, Saito K, Ait‐Goughoulte M, Meyer K, Ray RB, Ray R. Hepatitis C virus core protein upregulates serine phosphorylation of insulin receptor substrate‐1 and impairs the downstream akt/protein kinase B signaling pathway for insulin resistance. J Virol. 2008;82(6):2606‐2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Bose SK, Shrivastava S, Meyer K, Ray RB, Ray R. Hepatitis C virus activates the mTOR/S6K1 signaling pathway in inhibiting IRS‐1 function for insulin resistance. J Virol. 2012;86(11):6315‐6322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Jia B, Wang Y, Yu G, et al. Naringenin ameliorates insulin resistance by modulating endoplasmic reticulum stress in hepatitis C virus‐infected liver. Biomed Pharmacother. 2019;115:108848. [DOI] [PubMed] [Google Scholar]
- 202. Perlemuter G, Sabile A, Letteron P, et al. Hepatitis C virus core protein inhibits microsomal triglyceride transfer protein activity and very low density lipoprotein secretion: a model of viral‐related steatosis. Faseb J. 2002;16(2):185‐194. [DOI] [PubMed] [Google Scholar]
- 203. Hui J, Qu YY, Tang N, et al. Association of cytomegalovirus infection with hypertension risk: a meta‐analysis. Wien Klin Wochenschr. 2016;128(15‐16):586‐591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Cheng J, Ke Q, Jin Z, et al. Cytomegalovirus infection causes an increase of arterial blood pressure. PLoS Pathog. 2009;5(5):e1000427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Grahame‐Clarke C, Chan NN, Andrew D, et al. Human cytomegalovirus seropositivity is associated with impaired vascular function. Circulation. 2003;108(6):678‐683. [DOI] [PubMed] [Google Scholar]
- 206. Bentz GL, Yurochko AD. Human CMV infection of endothelial cells induces an angiogenic response through viral binding to EGF receptor and beta1 and beta3 integrins. Proc Natl Acad Sci USA. 2008;105(14):5531‐5536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Rahbar A, Söderberg‐Nauclér C. Human cytomegalovirus infection of endothelial cells triggers platelet adhesion and aggregation. J Virol. 2005;79(4):2211‐2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Gredmark‐Russ S, Dzabic M, Rahbar A, et al. Active cytomegalovirus infection in aortic smooth muscle cells from patients with abdominal aortic aneurysm. J Mol Med (Berl). 2009;87(4):347‐356. [DOI] [PubMed] [Google Scholar]
- 209. Maggi P, Calò F, Messina V, et al. Cardiovascular disease risk in liver transplant recipients transplanted due to chronic viral hepatitis. PLoS One. 2022;17(3):e0265178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Chew KW, Hua L, Bhattacharya D, et al. The effect of hepatitis C virologic clearance on cardiovascular disease biomarkers in human immunodeficiency virus/hepatitis C virus coinfection. Open Forum Infect Dis. 2014;1(3):ofu104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Castellon X, Bogdanova V. Chronic inflammatory diseases and endothelial dysfunction. Aging Dis. 2016;7(1):81‐89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Boddi M, Abbate R, Chellini B, et al. Hepatitis C virus RNA localization in human carotid plaques. J Clin Virol. 2010;47(1):72‐75. [DOI] [PubMed] [Google Scholar]
- 213. Fletcher NF, Wilson GK, Murray J, et al. Hepatitis C virus infects the endothelial cells of the blood‐brain barrier. Gastroenterology. 2012;142(3):634‐643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Massy ZA. Hyperlipidemia and cardiovascular disease after organ transplantation. Transplantation. 2001;72:S13‐15. Suppl. [DOI] [PubMed] [Google Scholar]
- 215. Wang H, Peng G, Bai J, et al. Cytomegalovirus infection and relative risk of cardiovascular disease (ischemic heart disease, stroke, and cardiovascular death): a meta‐analysis of prospective studies up to 2016. J Am Heart Assoc. 2017;6(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Yates TA, Griffith GJ, Morris TT. Human cytomegalovirus and risk of incident cardiovascular disease in UK Biobank. J Infect Dis. 2022;225(7):1301‐1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Rodríguez‐Goncer I, Fernández‐Ruiz M, Aguado JM. A critical review of the relationship between post‐transplant atherosclerotic events and cytomegalovirus exposure in kidney transplant recipients. Expert Rev Anti Infect Ther. 2020;18(2):113‐125. [DOI] [PubMed] [Google Scholar]
- 218. Eerdmans PH, Persoons MC, Debets SJ, et al. Impaired arterial reactivity following cytomegalovirus infection in the immunosuppressed rat. Br J Pharmacol. 1996;119(4):637‐646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Aguilera V, Di Maira T, Conde I, et al. Cytomegalovirus reactivation in liver transplant recipients due to hepatitis C cirrhosis is associated with higher cardiovascular risk—an observational, retrospective study. Transpl Int. 2018;31(6):649‐657. [DOI] [PubMed] [Google Scholar]
- 220. Halloran PF. Immunosuppressive drugs for kidney transplantation. N Engl J Med. 2004;351(26):2715‐2729. [DOI] [PubMed] [Google Scholar]
- 221. Tönshoff B. Immunosuppressants in organ transplantation. Handb Exp Pharmacol. 2020;261:441‐469. [DOI] [PubMed] [Google Scholar]
- 222. Parlakpinar H, Gunata M. Transplantation and immunosuppression: a review of novel transplant‐related immunosuppressant drugs. Immunopharmacol Immunotoxicol. 2021;43(6):651‐665. [DOI] [PubMed] [Google Scholar]
- 223. Szumilas K, Wilk A, Wiśniewski P, et al. Current status regarding immunosuppressive treatment in patients after renal transplantation. Int J Mol Sci. 2023;24(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Bauer AC, Franco RF, Manfro RC. Immunosuppression in kidney transplantation: state of the art and current protocols. Curr Pharm Des. 2020;26(28):3440‐3450. [DOI] [PubMed] [Google Scholar]
- 225. Wagner SJ, Brennan DC. Induction therapy in renal transplant recipients. Drugs. 2012;72:671‐683. [DOI] [PubMed] [Google Scholar]
- 226. Wu Q, Wang X, Nepovimova E, Wang Y, Yang H, Kuca K. Mechanism of cyclosporine A nephrotoxicity: oxidative stress, autophagy, and signalings. Food Chem Toxicol. 2018;118:889‐907. [DOI] [PubMed] [Google Scholar]
- 227. Kajiwara M, Masuda S. Role of mTOR inhibitors in kidney disease. Int J Mol Sci. 2016;17(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Saran U, Foti M, Dufour JF. Cellular and molecular effects of the mTOR inhibitor everolimus. Clin Sci (Lond). 2015;129(10):895‐914. [DOI] [PubMed] [Google Scholar]
- 229. Ayroldi E, Cannarile L, Migliorati G, Nocentini G, Delfino DV, Riccardi C. Mechanisms of the anti‐inflammatory effects of glucocorticoids: genomic and nongenomic interference with MAPK signaling pathways. Faseb J. 2012;26(12):4805‐4820. [DOI] [PubMed] [Google Scholar]
- 230. Chandran S, Tang Q, Sarwal M, et al. Polyclonal regulatory T cell therapy for control of inflammation in kidney transplants. Am J Transplant. 2017;17(11):2945‐2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Mathew JM, HV J, LeFever A, et al. A phase I clinical trial with ex vivo expanded recipient regulatory T cells in living donor kidney transplants. Sci Rep. 2018;8(1):7428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Sawitzki B, Harden PN, Reinke P, et al. Regulatory cell therapy in kidney transplantation (The ONE Study): a harmonised design and analysis of seven non‐randomised, single‐arm, phase 1/2A trials. Lancet. 2020;395(10237):1627‐1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Sánchez‐Fueyo A, Whitehouse G, Grageda N, et al. Applicability, safety, and biological activity of regulatory T cell therapy in liver transplantation. Am J Transplant. 2020;20(4):1125‐1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Todo S, Yamashita K, Goto R, et al. A pilot study of operational tolerance with a regulatory T‐cell‐based cell therapy in living donor liver transplantation. Hepatology. 2016;64(2):632‐643. [DOI] [PubMed] [Google Scholar]
- 235. Kasiske BL, Cangro CB, Hariharan S, et al. The evaluation of renal transplantation candidates: clinical practice guidelines. Am J Transplant. 2001;1(2):3‐95. Suppl. [PubMed] [Google Scholar]
- 236. Danzinger‐Isakov L, Kumar D. Guidelines for vaccination of solid organ transplant candidates and recipients. Am J Transplant. 2009:S258‐262. Suppl. [DOI] [PubMed] [Google Scholar]
- 237. Kliem V, Fricke L, Wollbrink T, Burg M, Radermacher J, Rohde F. Improvement in long‐term renal graft survival due to CMV prophylaxis with oral ganciclovir: results of a randomized clinical trial. Am J Transplant. 2008;8(5):975‐983. [DOI] [PubMed] [Google Scholar]
- 238. Jha V. Post‐transplant infections: an ounce of prevention. Indian J Nephrol. 2010;20(4):171‐178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Schaffner A. Pretransplant evaluation for infections in donors and recipients of solid organs. Clin Infect Dis. 2001;33(1):S9‐14. Suppl. [DOI] [PubMed] [Google Scholar]
- 240. Asberg A, Humar A, Jardine AG, et al. Long‐term outcomes of CMV disease treatment with valganciclovir versus IV ganciclovir in solid organ transplant recipients. Am J Transplant. 2009;9(5):1205‐1213. [DOI] [PubMed] [Google Scholar]
- 241. Allen U, Preiksaitis J. Epstein‐barr virus and posttransplant lymphoproliferative disorder in solid organ transplant recipients. Am J Transplant. 2009;9(4):S87‐96. Suppl. [DOI] [PubMed] [Google Scholar]
- 242. KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant. 2009;9 Suppl (3):S1‐155. [DOI] [PubMed] [Google Scholar]
- 243. Subramanian A, Dorman S. Mycobacterium tuberculosis in solid organ transplant recipients. Am J Transplant. 2009;9(4):S57‐62. Suppl. [DOI] [PubMed] [Google Scholar]
- 244. Kwon S, Kim YC, Kwon H, et al. Metformin use and long‐term clinical outcomes in kidney transplant recipients. Am J Kidney Dis. 2023;82(3):290‐299. e1. [DOI] [PubMed] [Google Scholar]
- 245. Haidinger M, Antlanger M, Kopecky C, Kovarik JJ, Säemann MD, Werzowa J. Post‐transplantation diabetes mellitus: evaluation of treatment strategies. Clin Transplant. 2015;29(5):415‐424. [DOI] [PubMed] [Google Scholar]
- 246. Türk T, Pietruck F, Dolff S, et al. Repaglinide in the management of new‐onset diabetes mellitus after renal transplantation. Am J Transplant. 2006;6(4):842‐846. [DOI] [PubMed] [Google Scholar]
- 247. Sharif A. Should metformin be our antiglycemic agent of choice post‐transplantation? Am J Transplant. 2011;11(7):1376‐1381. [DOI] [PubMed] [Google Scholar]
- 248. Shivaswamy V, Bennett RG, Clure CC, Larsen JL, Hamel FG. Metformin improves immunosuppressant induced hyperglycemia and exocrine apoptosis in rats. Transplantation. 2013;95(2):280‐284. [DOI] [PubMed] [Google Scholar]
- 249. Kurian B, Joshi R, Helmuth A. Effectiveness and long‐term safety of thiazolidinediones and metformin in renal transplant recipients. Endocr Pract. 2008;14(8):979‐984. [DOI] [PubMed] [Google Scholar]
- 250. Stephen J, Anderson‐Haag TL, Gustafson S, Snyder JJ, Kasiske BL, Israni AK. Metformin use in kidney transplant recipients in the United States: an observational study. Am J Nephrol. 2014;40(6):546‐553. [DOI] [PubMed] [Google Scholar]
- 251. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Atherosclerosis. 2019;290:140‐205. [DOI] [PubMed] [Google Scholar]
- 252. Blum A, Shamburek R. The pleiotropic effects of statins on endothelial function, vascular inflammation, immunomodulation and thrombogenesis. Atherosclerosis. 2009;203(2):325‐330. [DOI] [PubMed] [Google Scholar]
- 253. Riella LV, Gabardi S, Chandraker A. Dyslipidemia and its therapeutic challenges in renal transplantation. Am J Transplant. 2012;12(8):1975‐1982. [DOI] [PubMed] [Google Scholar]
- 254. Terker AS, Yang CL, McCormick JA, et al. Sympathetic stimulation of thiazide‐sensitive sodium chloride cotransport in the generation of salt‐sensitive hypertension. Hypertension. 2014;64(1):178‐184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Taber DJ, Srinivas TM, Pilch NA, et al. Are thiazide diuretics safe and effective antihypertensive therapy in kidney transplant recipients? Am J Nephrol. 2013;38(4):285‐291. [DOI] [PubMed] [Google Scholar]
- 256. Anis KH, Weinrauch LA, D'Elia JA. Effects of smoking on solid organ transplantation outcomes. Am J Med. 2019;132(4):413‐419. [DOI] [PubMed] [Google Scholar]
- 257. Tuomilehto J, Lindström J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344(18):1343‐1350. [DOI] [PubMed] [Google Scholar]
- 258. Wissing KM, Abramowicz D, Weekers L, et al. Prospective randomized study of conversion from tacrolimus to cyclosporine A to improve glucose metabolism in patients with posttransplant diabetes mellitus after renal transplantation. Am J Transplant. 2018;18(7):1726‐1734. [DOI] [PubMed] [Google Scholar]
- 259. Kim HD, Chang JY, Chung BH, et al. Effect of everolimus with low‐dose tacrolimus on development of new‐onset diabetes after transplantation and allograft function in kidney transplantation: a multicenter, open‐label. Ann Transplant. 2021;26:e927984. Randomized Trial. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Wen X, Casey MJ, Santos AH, Hartzema A, Womer KL. Comparison of utilization and clinical outcomes for belatacept‐ and tacrolimus‐based immunosuppression in renal transplant recipients. Am J Transplant. 2016;16(11):3202‐3211. [DOI] [PubMed] [Google Scholar]
- 261. White M, Haddad H, Leblanc MH, et al. Conversion from cyclosporine microemulsion to tacrolimus‐based immunoprophylaxis improves cholesterol profile in heart transplant recipients with treated but persistent dyslipidemia: the Canadian multicentre randomized trial of tacrolimus vs cyclosporine microemulsion. J Heart Lung Transplant. 2005;24(7):798‐809. [DOI] [PubMed] [Google Scholar]
- 262. Vincenti F, Charpentier B, Vanrenterghem Y, et al. A phase III study of belatacept‐based immunosuppression regimens versus cyclosporine in renal transplant recipients (BENEFIT study). Am J Transplant. 2010;10(3):535‐546. [DOI] [PubMed] [Google Scholar]
- 263. Vanrenterghem Y, Bresnahan B, Campistol J, et al. Belatacept‐based regimens are associated with improved cardiovascular and metabolic risk factors compared with cyclosporine in kidney transplant recipients (BENEFIT and BENEFIT‐EXT studies). Transplantation. 2011;91(9):976‐983. [DOI] [PubMed] [Google Scholar]
- 264. Jardine AG, Fellström B, Logan JO, et al. Cardiovascular risk and renal transplantation: post hoc analyses of the assessment of lescol in renal transplantation (ALERT) Study. Am J Kidney Dis. 2005;46(3):529‐536. [DOI] [PubMed] [Google Scholar]
- 265. Soveri I, Holdaas H, Jardine A, Gimpelewicz C, Staffler B, Fellström B. Renal transplant dysfunction–importance quantified in comparison with traditional risk factors for cardiovascular disease and mortality. Nephrol Dial Transplant. 2006;21(8):2282‐2289. [DOI] [PubMed] [Google Scholar]
- 266. Vincenti F, Blancho G, Durrbach A, et al. Five‐year safety and efficacy of belatacept in renal transplantation. J Am Soc Nephrol. 2010;21(9):1587‐1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Pontrelli P, Rascio F, Zaza G, et al. Interleukin‐27 is a potential marker for the onset of post‐transplant malignancies. Nephrol Dial Transplant. 2019;34(1):157‐166. [DOI] [PubMed] [Google Scholar]
- 268. Zhang S, Fujita H, Mitsui H, et al. Increased Tc22 and Treg/CD8 ratio contribute to aggressive growth of transplant associated squamous cell carcinoma. PLoS One. 2013;8(5):e62154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Coles SJ, Hills RK, Wang EC, et al. Increased CD200 expression in acute myeloid leukemia is linked with an increased frequency of FoxP3+ regulatory T cells. Leukemia. 2012;26(9):2146‐2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Olteanu H, Harrington AM, Hari P, Kroft SH. CD200 expression in plasma cell myeloma. Br J Haematol. 2011;153(3):408‐411. [DOI] [PubMed] [Google Scholar]
- 271. Gorczynski RM. CD200 and its receptors as targets for immunoregulation. Curr Opin Investig Drugs. 2005;6(5):483‐488. [PubMed] [Google Scholar]
- 272. Vaughan JW, Shi M, Horna P, Olteanu H. Increased CD200 expression in post‐transplant lymphoproliferative disorders correlates with an increased frequency of FoxP3(+) regulatory T cells. Ann Diagn Pathol. 2020;48:151585. [DOI] [PubMed] [Google Scholar]
- 273. Van den Hove LE, Vandenberghe P, Van Gool SW, et al. Peripheral blood lymphocyte subset shifts in patients with untreated hematological tumors: evidence for systemic activation of the T cell compartment. Leuk Res. 1998;22(2):175‐184. [DOI] [PubMed] [Google Scholar]
- 274. Tsukishiro T, Donnenberg AD, Whiteside TL. Rapid turnover of the CD8(+)CD28(‐) T‐cell subset of effector cells in the circulation of patients with head and neck cancer. Cancer Immunol Immunother. 2003;52(10):599‐607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275. Bottomley MJ, Harden PN, Wood KJ. CD8+ immunosenescence predicts post‐transplant cutaneous squamous cell carcinoma in high‐risk patients. J Am Soc Nephrol. 2016;27(5):1505‐1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. Courivaud C, Bamoulid J, Gaugler B, et al. Cytomegalovirus exposure, immune exhaustion and cancer occurrence in renal transplant recipients. Transpl Int. 2012;25(9):948‐955. [DOI] [PubMed] [Google Scholar]
- 277. Lau E, Moyers JT, Wang BC, et al. Analysis of post‐transplant lymphoproliferative disorder (PTLD) outcomes with Epstein‐Barr Virus (EBV) assessments—a single tertiary referral center experience and review of literature. Cancers (Basel). 2021;13(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278. Rosselet A, Vu DH, Meylan P, et al. Associations of serum EBV DNA and gammopathy with post‐transplant lymphoproliferative disease. Clin Transplant. 2009;23(1):74‐82. [DOI] [PubMed] [Google Scholar]
- 279. Suresh S, Dix D, Wang L, Blydt‐Hansen TD. High urinary CXCL10/Cr with onset of Burkitt lymphoma in a pediatric kidney transplant recipient. Pediatr Transplant. 2022;26(7):e14354. [DOI] [PubMed] [Google Scholar]
- 280. Mukherjee S, Mukherjee U. A comprehensive review of immunosuppression used for liver transplantation. J Transplant. 2009;2009:701464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281. Diekmann F, Andrés A. Oppenheimer F. mTOR inhibitor‐associated proteinuria in kidney transplant recipients. Transplant Rev (Orlando). 2012;26(1):27‐29. [DOI] [PubMed] [Google Scholar]
- 282. Campbell SB, Walker R, Tai SS, Jiang Q, Russ GR. Randomized controlled trial of sirolimus for renal transplant recipients at high risk for nonmelanoma skin cancer. Am J Transplant. 2012;12(5):1146‐1156. [DOI] [PubMed] [Google Scholar]
- 283. Knoll GA, Kokolo MB, Mallick R, et al. Effect of sirolimus on malignancy and survival after kidney transplantation: systematic review and meta‐analysis of individual patient data. Bmj. 2014;349:g6679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284. Yanik EL, Siddiqui K, Engels EA. Sirolimus effects on cancer incidence after kidney transplantation: a meta‐analysis. Cancer Med. 2015;4(9):1448‐1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285. Engelman JA. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat Rev Cancer. 2009;9(8):550‐562. [DOI] [PubMed] [Google Scholar]
- 286. Dees S, Ganesan R, Singh S, Grewal IS. Regulatory T cell targeting in cancer: emerging strategies in immunotherapy. Eur J Immunol. 2021;51(2):280‐291. [DOI] [PubMed] [Google Scholar]
- 287. Rosskopf S, Leitner J, Zlabinger GJ, Steinberger P. CTLA‐4 antibody ipilimumab negatively affects CD4(+) T‐cell responses in vitro. Cancer Immunol Immunother. 2019;68(8):1359‐1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288. Derman BA, Zha Y, Zimmerman TM, et al. Regulatory T‐cell depletion in the setting of autologous stem cell transplantation for multiple myeloma: pilot study. J Immunother Cancer. 2020;8(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289. Thibodeaux SR, Barnett BB, Pandeswara S, et al. IFNα augments clinical efficacy of regulatory T‐cell depletion with denileukin diftitox in ovarian cancer. Clin Cancer Res. 2021;27(13):3661‐3673. [DOI] [PubMed] [Google Scholar]
- 290. Campbell JR, McDonald BR, Mesko PB, et al. Fc‐optimized anti‐CCR8 antibody depletes regulatory T cells in human tumor models. Cancer Res. 2021;81(11):2983‐2994. [DOI] [PubMed] [Google Scholar]
- 291. Dao T, Mun SS, Scott AC, et al. Depleting T regulatory cells by targeting intracellular Foxp3 with a TCR mimic antibody. Oncoimmunology. 2019;8(7):1570778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292. Oweida AJ, Darragh L, Phan A, et al. STAT3 modulation of regulatory T cells in response to radiation therapy in head and neck cancer. J Natl Cancer Inst. 2019;111(12):1339‐1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. Son CH, Bae JH, Shin DY, et al. Combination effect of regulatory T‐cell depletion and ionizing radiation in mouse models of lung and colon cancer. Int J Radiat Oncol Biol Phys. 2015;92(2):390‐398. [DOI] [PubMed] [Google Scholar]
- 294. Moatti A, Debesset A, Pilon C, et al. TNFR2 blockade of regulatory T cells unleashes an antitumor immune response after hematopoietic stem‐cell transplantation. J Immunother Cancer. 2022;10(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295. Davids MS, Kim HT, Bachireddy P, et al. Ipilimumab for patients with relapse after allogeneic transplantation. N Engl J Med. 2016;375(2):143‐153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296. Dang BN, Ch'ng J, Russell M, Cheng JC, Moore TB, Alejos JC. Treatment of post‐transplant lymphoproliferative disorder (PTLD) in a heart transplant recipient with chimeric antigen receptor T‐cell therapy. Pediatr Transplant. 2021;25(5):e13861. [DOI] [PubMed] [Google Scholar]
- 297. Oren D, DeFilippis EM, Lotan D, et al. Successful CAR T cell therapy in a heart and kidney transplant recipient with refractory PTLD. JACC CardioOncol. 2022;4(5):713‐716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298. Hernani R, Sancho A, Amat P, et al. CAR‐T therapy in solid transplant recipients with post‐transplant lymphoproliferative disease: case report and literature review. Curr Res Transl Med. 2021;69(4):103304. [DOI] [PubMed] [Google Scholar]
- 299. Clerico M, Dogliotti I, Aroldi A, et al. Post‐transplant lymphoproliferative disease (PTLD) after allogeneic hematopoietic stem cell transplantation: biology and treatment options. J Clin Med. 2022;11(24). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300. McKenna M, Epperla N, Ghobadi A, et al. Real‐world evidence of the safety and survival with CD19 CAR‐T cell therapy for relapsed/refractory solid organ transplant‐related PTLD. Br J Haematol. 2023;202(2):248‐255. [DOI] [PubMed] [Google Scholar]
- 301. Liu JY, Zhang JM, Zhan HS, Sun LY, Wei L. EBV‐specific cytotoxic T lymphocytes for refractory EBV‐associated post‐transplant lymphoproliferative disorder in solid organ transplant recipients: a systematic review. Transpl Int. 2021;34(12):2483‐2493. [DOI] [PubMed] [Google Scholar]
- 302. Fabrizio VA, Rodriguez‐Sanchez MI, Mauguen A, et al. Adoptive therapy with CMV‐specific cytotoxic T lymphocytes depends on baseline CD4+ immunity to mediate durable responses. Blood Adv. 2021;5(2):496‐503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303. Ke P, Bao X, Zhou J, et al. Donor CMV‐specific cytotoxic T lymphocytes successfully treated drug‐resistant cytomegalovirus encephalitis after allogeneic hematopoietic stem cell transplantation. Hematology. 2020;25(1):43‐47. [DOI] [PubMed] [Google Scholar]
- 304. Blyth E, Clancy L, Simms R, et al. Donor‐derived CMV‐specific T cells reduce the requirement for CMV‐directed pharmacotherapy after allogeneic stem cell transplantation. Blood. 2013;121(18):3745‐3758. [DOI] [PubMed] [Google Scholar]
- 305. Zhang X, He J, Zhao K, et al. Mesenchymal stromal cells ameliorate chronic GVHD by boosting thymic regeneration in a CCR9‐dependent manner in mice. Blood Adv. 2023;7(18):5359‐5373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306. Zhuoya W, Nannan Z, Aiping Z, et al. Human placenta derived mesenchymal stromal cells alleviate GVHD by promoting the generation of GSH and GST in PD‐1(+)T cells. Cell Immunol. 2020;352:104083. [DOI] [PubMed] [Google Scholar]
- 307. Geiger S, Ozay EI, Geumann U, et al. Alpha‐1 antitrypsin‐expressing mesenchymal stromal cells confer a long‐term survival benefit in a mouse model of lethal GvHD. Mol Ther. 2019;27(8):1436‐1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308. Proics E, David M, Mojibian M, et al. Preclinical assessment of antigen‐specific chimeric antigen receptor regulatory T cells for use in solid organ transplantation. Gene Ther. 2023;30(3‐4):309‐322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309. Descalzi‐Montoya DB, Yang Z, Fanning S, et al. Cord blood‐derived multipotent stem cells ameliorate in vitro/in vivo alloreactive responses, and this effect is associated with exosomal microvesicles in vitro. Transplant Cell Ther. 2024;30(4):396.e1‐396.e14. [DOI] [PubMed] [Google Scholar]
- 310. Sun X, He Q, Yang J, et al. Preventive and therapeutic effects of a novel JAK inhibitor SHR0302 in acute graft‐versus‐host disease. Cell Transplant. 2021;30:9636897211033778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311. Chen Y, Yan G, Ma Y, et al. Combination of mesenchymal stem cells and FK506 prolongs heart allograft survival by inhibiting TBK1/IRF3‐regulated‐IFN‐γ production. Immunol Lett. 2021;238:21‐28. [DOI] [PubMed] [Google Scholar]
- 312. Tuo L, Song H, Jiang D, Bai X, Song G. Mesenchymal stem cells transfected with anti‐miRNA‐204‐3p inhibit acute rejection after heart transplantation by targeting C‐X‐C motif chemokine receptor 4 (CXCR4) in vitro. J Thorac Dis. 2021;13(8):5077‐5092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313. Lu X, Ru Y, Chu C, et al. Lentivirus‐mediated IL‐10‐expressing bone marrow mesenchymal stem cells promote corneal allograft survival via upregulating lncRNA 003946 in a rat model of corneal allograft rejection. Theranostics. 2020;10(18):8446‐8467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314. Wang J, Ma Y, Wang J. miR‐27a‐5p inhibits acute rejection of liver transplantation in rats by inducing M2 polarization of Kupffer cells through the PI3K/Akt pathway. Cytokine. 2023;165:156085. [DOI] [PubMed] [Google Scholar]
- 315. Chai H, Lei Z, Liu Y, et al. miR‐505‐5p alleviates acute rejection of liver transplantation by inhibiting Myd88 and inducing M2 polarizationof Kupffer cells. Acta Biochim Biophys Sin (Shanghai). 2022;54(8):1148‐1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316. Morath C, Schmitt A, Kleist C, et al. Phase I trial of donor‐derived modified immune cell infusion in kidney transplantation. J Clin Invest. 2020;130(5):2364‐2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317. Guo H, Li B, Li N, et al. Exosomes: potential executors of IL‐35 gene‐modified adipose‐derived mesenchymal stem cells in inhibiting acute rejection after heart transplantation. Scand J Immunol. 2022;96(2):e13171. [DOI] [PubMed] [Google Scholar]
- 318. Kong G, Chen Y, Liu Z, Wang Y, Li H, Guo C. Adenovirus‐IL‐10 relieves chronic rejection after mouse heart transplantation by inhibiting miR‐155 and activating SOCS5. Int J Med Sci. 2023;20(2):172‐185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319. Shao B, Zhang JY, Ren SH, et al. Recombinant human IL‐37 attenuates acute cardiac allograft rejection in mice. Cytokine. 2024;179:156598. [DOI] [PubMed] [Google Scholar]
- 320. Lin M, Bhakdi SC, Tan D, et al. Lytic efficiency of immunosuppressive drug‐resistant armoured T cells against circulating HBV‐related HCC in whole blood. Immunother Adv. 2023;3(1):ltad015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321. Halden TAS, Kvitne KE, Midtvedt K, et al. Efficacy and safety of empagliflozin in renal transplant recipients with posttransplant diabetes mellitus. Diabetes Care. 2019;42(6):1067‐1074. [DOI] [PubMed] [Google Scholar]
- 322. Sánchez Fructuoso AI, Bedia Raba A, Banegas Deras E, et al. Sodium‐glucose cotransporter‐2 inhibitor therapy in kidney transplant patients with type 2 or post‐transplant diabetes: an observational multicentre study. Clin Kidney J. 2023;16(6):1022‐1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323. Thiruvengadam S, Hutchison B, Lim W, et al. Intensive monitoring for post‐transplant diabetes mellitus and treatment with dipeptidyl peptidase‐4 inhibitor therapy. Diabetes Metab Syndr. 2019;13(3):1857‐1863. [DOI] [PubMed] [Google Scholar]
- 324. Bae J, Kim Y, Cho Y, et al. Efficacy and safety of gemigliptin in post‐transplant patients with type 2 diabetes mellitus. Transplant Proc. 2019;51(10):3444‐3448. [DOI] [PubMed] [Google Scholar]
- 325. Li L, Zhao H, Chen B, et al. FXR activation alleviates tacrolimus‐induced post‐transplant diabetes mellitus by regulating renal gluconeogenesis and glucose uptake. J Transl Med. 2019;17(1):418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326. Thangavelu T, Lyden E, Shivaswamy V. A retrospective study of glucagon‐like peptide 1 receptor agonists for the management of diabetes after transplantation. Diabetes Ther. 2020;11(4):987‐994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327. Singh P, Taufeeq M, Pesavento TE, Washburn K, Walsh D, Meng S. Comparison of the glucagon‐like‐peptide‐1 receptor agonists dulaglutide and liraglutide for the management of diabetes in solid organ transplant: a retrospective study. Diabetes Obes Metab. 2020;22(5):879‐884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Delos Santos RB, Hagopian JC, Chen L, et al. Sitagliptin versus placebo to reduce the incidence and severity of posttransplant diabetes mellitus after kidney transplantation—a single‐center, randomized, double‐blind controlled trial. Transplantation. 2023;107(5):1180‐1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329. Kuhl M, Binner C, Jozwiak J, et al. Treatment of hypercholesterolaemia with PCSK9 inhibitors in patients after cardiac transplantation. PLoS One. 2019;14(1):e0210373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330. Uyanik‐Uenal K, Stoegerer‐Lanzenberger M, Auersperg K, Aliabadi‐Zuckermann A, Laufer G, Zuckermann A. Treatment of therapy‐resistant hyperlipidaemia after heart transplant with PCSK9‐inhibitors. J Heart Lung Transplant. 2019;38(4):S213‐S214. Supplement. [Google Scholar]
- 331. Tsuchimoto A, Masutani K, Ueki K, et al. Effect of renin‐angiotensin system blockade on graft survival and cardiovascular disease in kidney transplant recipients: retrospective multicenter study in Japan. Clin Exp Nephrol. 2020;24(4):369‐378. [DOI] [PubMed] [Google Scholar]
- 332. Lim J‐H, Kwon S, Seo YJ, et al. Cardioprotective effect of SGLT2 inhibitor in diabetic kidney transplant recipients: a multicenter propensity score matched study. Kidney Int Rep. 2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333. Alotaibi T, Nagib AM, Denewar A, et al. Inhibition of proprotein convertase subtilisin/kexin‐9 after kidney transplant: single‐center experience among patients with high cardiovascular risk. Exp Clin Transplant. 2024;22(1):315‐322. Suppl. [DOI] [PubMed] [Google Scholar]
- 334. Zou Y, Chen Z, Zhang X, et al. Targeting PCSK9 ameliorates graft vascular disease in mice by inhibiting NLRP3 inflammasome activation in vascular smooth muscle cells. Front Immunol. 2022;13:894789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335. Birdwell KA, Decker B, Barbarino JM, et al. Clinical pharmacogenetics implementation consortium (CPIC) guidelines for CYP3A5 genotype and tacrolimus dosing. Clin Pharmacol Ther. 2015;98(1):19‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336. Cheng Y, Li H, Meng Y, et al. Effect of CYP3A5 polymorphism on the pharmacokinetics of tacrolimus and acute rejection in renal transplant recipients: experience at a single centre. Int J Clin Pract Suppl. 2015(183):16‐22. [DOI] [PubMed] [Google Scholar]
- 337. Asempa TE, Rebellato LM, Hudson S, Briley K, Maldonado AQ. Impact of CYP3A5 genomic variances on clinical outcomes among African American kidney transplant recipients. Clin Transplant. 2018;32(1). [DOI] [PubMed] [Google Scholar]
- 338. Uesugi M, Kikuchi M, Shinke H, et al. Impact of cytochrome P450 3A5 polymorphism in graft livers on the frequency of acute cellular rejection in living‐donor liver transplantation. Pharmacogenet Genomics. 2014;24(7):356‐366. [DOI] [PubMed] [Google Scholar]
- 339. Marco DN, Molina M, Guio AM, et al. Effects of CYP3A5 Genotype on tacrolimus pharmacokinetics and graft‐versus‐host disease incidence in allogeneic hematopoietic stem cell transplantation. Pharmaceuticals (Basel). 2024;17(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340. Edinur HA, Manaf SM. Che Mat NF. Genetic barriers in transplantation medicine. World J Transplant. 2016;6(3):532‐541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341. Lan X, Zhang MM, Pu CL, et al. Impact of human leukocyte antigen mismatching on outcomes of liver transplantation: a meta‐analysis. World J Gastroenterol. 2010;16(27):3457‐3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342. Booth GS, Gehrie EA, Bolan CD, Savani BN. Clinical guide to ABO‐incompatible allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2013;19(8):1152‐1158. [DOI] [PubMed] [Google Scholar]
- 343. Suárez‐Alvarez B, López‐Vázquez A, Gonzalez MZ, et al. The relationship of anti‐MICA antibodies and MICA expression with heart allograft rejection. Am J Transplant. 2007;7(7):1842‐1848. [DOI] [PubMed] [Google Scholar]
- 344. Vakil MK, Mansoori Y, Al‐Awsi GRL, et al. Individual genetic variability mainly of proinflammatory cytokines, cytokine receptors, and toll‐like receptors dictates pathophysiology of COVID‐19 disease. J Med Virol. 2022;94(9):4088‐4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345. Dukat‐Mazurek A, Bieniaszewska M, Hellmann A, Moszkowska G, Trzonkowski P. Association of cytokine gene polymorphisms with the complications of allogeneic haematopoietic stem cell transplantation. Hum Immunol. 2017;78(11‐12):672‐683. [DOI] [PubMed] [Google Scholar]
- 346. Nair S, Dhodapkar MV. Natural killer T cells in cancer immunotherapy. Front Immunol. 2017;8:1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347. Gu Y, Hu Y, Hu K, et al. Rapamycin together with TGF‐β1, IL‐2 and IL‐15 induces the generation of functional regulatory γδT cells from human peripheral blood mononuclear cells. J Immunol Methods. 2014;402(1‐2):82‐87. [DOI] [PubMed] [Google Scholar]
- 348. Kaneko Y, Harada M, Kawano T, et al. Augmentation of Valpha14 NKT cell‐mediated cytotoxicity by interleukin 4 in an autocrine mechanism resulting in the development of concanavalin A‐induced hepatitis. J Exp Med. 2000;191(1):105‐114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349. Ito H, Ando K, Ishikawa T, et al. Role of Valpha14+ NKT cells in the development of Hepatitis B virus‐specific CTL: activation of Valpha14+ NKT cells promotes the breakage of CTL tolerance. Int Immunol. 2008;20(7):869‐879. [DOI] [PubMed] [Google Scholar]
- 350. Moiseev IS, Babenko EV, Epifanovskaya OS, et al. High prevalence of CD3, NK, and NKT cells in the graft predicts adverse outcome after matched‐related and unrelated transplantations with post transplantation cyclophosphamide. Bone Marrow Transplant. 2020;55(3):544‐552. [DOI] [PubMed] [Google Scholar]
- 351. Hodge G, Hodge S, Holmes‐Liew CL, Reynolds PN, Holmes M. Histone deacetylase 2 is decreased in peripheral blood pro‐inflammatory CD8+ T and NKT‐like lymphocytes following lung transplant. Respirology. 2017;22(2):394‐400. [DOI] [PubMed] [Google Scholar]
- 352. Deseke M, Prinz I. Ligand recognition by the γδ TCR and discrimination between homeostasis and stress conditions. Cell Mol Immunol. 2020;17(9):914‐924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353. Xu L, Feng J, Xu X, et al. IL‐17‐producing γδT cells ameliorate intestinal acute graft‐versus‐host disease by recruitment of Gr‐1(+)CD11b(+) myeloid‐derived suppressor cells. Bone Marrow Transplant. 2021;56(10):2389‐2399. [DOI] [PubMed] [Google Scholar]
- 354. Malone F, Carper K, Reyes J, Li W. gammadeltaT cells are involved in liver transplant tolerance. Transplant Proc. 2009;41(1):233‐235. [DOI] [PubMed] [Google Scholar]
- 355. Kabelitz D, Serrano R, Kouakanou L, Peters C, Kalyan S. Cancer immunotherapy with γδ T cells: many paths ahead of us. Cell Mol Immunol. 2020;17(9):925‐939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356. Mirchandani AS, Besnard AG, Yip E, et al. Type 2 innate lymphoid cells drive CD4+ Th2 cell responses. J Immunol. 2014;192(5):2442‐2448. [DOI] [PubMed] [Google Scholar]
- 357. Chiossone L, Dumas PY, Vienne M, Vivier E. Natural killer cells and other innate lymphoid cells in cancer. Nat Rev Immunol. 2018;18(11):671‐688. [DOI] [PubMed] [Google Scholar]
- 358. Scheper W, van Dorp S, Kersting S, et al. γδT cells elicited by CMV reactivation after allo‐SCT cross‐recognize CMV and leukemia. Leukemia. 2013;27(6):1328‐1338. [DOI] [PubMed] [Google Scholar]
- 359. Robinette ML, Colonna M. Innate lymphoid cells and the MHC. Hla. 2016;87(1):5‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360. Bruchard M, Geindreau M, Perrichet A, et al. Recruitment and activation of type 3 innate lymphoid cells promote antitumor immune responses. Nat Immunol. 2022;23(2):262‐274. [DOI] [PubMed] [Google Scholar]
- 361. Pan Y, Yu Y, Wang X, Zhang T. Tumor‐associated macrophages in tumor immunity. Front Immunol. 2020;11:583084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362. Zhang W, Zhang Q, Yang N, et al. Crosstalk between IL‐15Rα(+) tumor‐associated macrophages and breast cancer cells reduces CD8(+) T cell recruitment. Cancer Commun (Lond). 2022;42(6):536‐557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363. Liang Y, Tan Y, Guan B, et al. Single‐cell atlases link macrophages and CD8(+) T‐cell subpopulations to disease progression and immunotherapy response in urothelial carcinoma. Theranostics. 2022;12(18):7745‐7759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364. Garnier AS, Planchais M, Riou J, et al. Pre‐transplant CD45RC expression on blood T cells differentiates patients with cancer and rejection after kidney transplantation. PLoS One. 2019;14(3):e0214321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365. Greenfield HM, Gharib MI, Turner AJ, et al. The impact of monitoring Epstein‐Barr virus PCR in paediatric bone marrow transplant patients: can it successfully predict outcome and guide intervention? Pediatr Blood Cancer. 2006;47(2):200‐205. [DOI] [PubMed] [Google Scholar]
- 366. Pai JA, Satpathy AT. High‐throughput and single‐cell T cell receptor sequencing technologies. Nat Methods. 2021;18(8):881‐892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367. Reguzova AY, Karpenko LI, Mechetina LV, Belyakov IM. Peptide‐MHC multimer‐based monitoring of CD8 T‐cells in HIV‐1 infection and AIDS vaccine development. Expert Rev Vaccines. 2015;14(1):69‐84. [DOI] [PubMed] [Google Scholar]
- 368. Zhang Z, Xiong D, Wang X, Liu H, Wang T. Mapping the functional landscape of T cell receptor repertoires by single‐T cell transcriptomics. Nat Methods. 2021;18(1):92‐99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369. Sigdel TK, Fields PA, Liberto J, et al. Perturbations of the T‐cell immune repertoire in kidney transplant rejection. Front Immunol. 2022;13:1012042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370. Pauken KE, Lagattuta KA, Lu BY, et al. TCR‐sequencing in cancer and autoimmunity: barcodes and beyond. Trends Immunol. 2022;43(3):180‐194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371. Bentzen AK, Marquard AM, Lyngaa R, et al. Large‐scale detection of antigen‐specific T cells using peptide‐MHC‐I multimers labeled with DNA barcodes. Nat Biotechnol. 2016;34(10):1037‐1045. [DOI] [PubMed] [Google Scholar]
- 372. Kohut TJ, Barandiaran JF, Keating BJ. Genomics and liver transplantation: genomic biomarkers for the diagnosis of acute cellular rejection. Liver Transpl. 2020;26(10):1337‐1350. [DOI] [PubMed] [Google Scholar]
- 373. Margolskee E, Jobanputra V, Jain P, et al. Genetic landscape of T‐ and NK‐cell post‐transplant lymphoproliferative disorders. Oncotarget. 2016;7(25):37636‐37648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374. Zhi Y, Li M, Lv G. Into the multi‐omics era: progress of T cells profiling in the context of solid organ transplantation. Front Immunol. 2023;14:1058296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375. Peters FS, Peeters AMA, van den Bosch TPP, et al. Disrupted regulation of serpinB9 in circulating T cells is associated with an increased risk for post‐transplant skin cancer. Clin Exp Immunol. 2019;197(3):341‐351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376. Nguyen TH, Bird NL, Grant EJ, et al. Maintenance of the EBV‐specific CD8(+) TCRαβ repertoire in immunosuppressed lung transplant recipients. Immunol Cell Biol. 2017;95(1):77‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377. Geiger R, Rieckmann JC, Wolf T, et al. L‐arginine modulates T cell metabolism and enhances survival and anti‐tumor activity. Cell. 2016;167(3):829‐842. e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378. Matias MI, Yong CS, Foroushani A, et al. Regulatory T cell differentiation is controlled by αKG‐induced alterations in mitochondrial metabolism and lipid homeostasis. Cell Rep. 2021;37(5):109911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379. Tellez J, Jaing C, Wang J, Green R, Chen M. Detection of Epstein‐Barr virus (EBV) in human lymphoma tissue by a novel microbial detection array. Biomark Res. 2014;2(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380. Anthony BA, Hadley GA. Induction of graft‐versus‐host disease and in vivo T cell monitoring using an MHC‐matched murine model. J Vis Exp. 2012(66):e3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381. Mohri T, Ikura Y, Hirakoso A, et al. Classical Hodgkin lymphoma type post‐transplant lymphoproliferative disorder in a kidney transplant recipient: a diagnostic pitfall. Int J Hematol. 2018;108(2):218‐227. [DOI] [PubMed] [Google Scholar]
- 382. Ma BM, Elefant N, Tedesco M, et al. Developing a genetic testing panel for evaluation of morbidities in kidney transplant recipients. Kidney Int. 2024;106(1):115‐125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383. Hu H, Kwun J, Aizenstein BD, Knechtle SJ. Noninvasive detection of acute and chronic injuries in human renal transplant by elevation of multiple cytokines/chemokines in urine. Transplantation. 2009;87(12):1814‐1820. [DOI] [PubMed] [Google Scholar]
- 384. Reikvam H, Bruserud Ø, Hatfield KJ. Pretransplant systemic metabolic profiles in allogeneic hematopoietic stem cell transplant recipients—identification of patient subsets with increased transplant‐related mortality. Transplant Cell Ther. 2023;29(6):375.e1‐375.e14. [DOI] [PubMed] [Google Scholar]
- 385. Hruba P, Klema J, Le AV, et al. Novel transcriptomic signatures associated with premature kidney allograft failure. EBioMedicine. 2023;96:104782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386. Bontha SV, Maluf DG, Mueller TF, Mas VR. Systems biology in kidney transplantation: the application of multi‐omics to a complex model. Am J Transplant. 2017;17(1):11‐21. [DOI] [PubMed] [Google Scholar]
- 387. Sui W, Lin H, Peng W, et al. Molecular dysfunctions in acute rejection after renal transplantation revealed by integrated analysis of transcription factor, microRNA and long noncoding RNA. Genomics. 2013;102(4):310‐322. [DOI] [PubMed] [Google Scholar]
- 388. Briceño J, Ayllón MD, Ciria R. Machine‐learning algorithms for predicting results in liver transplantation: the problem of donor‐recipient matching. Curr Opin Organ Transplant. 2020;25(4):406‐411. [DOI] [PubMed] [Google Scholar]
- 389. Guijo‐Rubio D, Briceño J, Gutiérrez PA, Ayllón MD, Ciria R, Hervás‐Martínez C. Statistical methods versus machine learning techniques for donor‐recipient matching in liver transplantation. PLoS One. 2021;16(5):e0252068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390. Briceño J, Cruz‐Ramírez M, Prieto M, et al. Use of artificial intelligence as an innovative donor‐recipient matching model for liver transplantation: results from a multicenter Spanish study. J Hepatol. 2014;61(5):1020‐1028. [DOI] [PubMed] [Google Scholar]
- 391. Ayllón MD, Ciria R, Cruz‐Ramírez M, et al. Validation of artificial neural networks as a methodology for donor‐recipient matching for liver transplantation. Liver Transpl. 2018;24(2):192‐203. [DOI] [PubMed] [Google Scholar]
- 392. Ayers B, Sandholm T, Gosev I, Prasad S, Kilic A. Using machine learning to improve survival prediction after heart transplantation. J Card Surg. 2021;36(11):4113‐4120. [DOI] [PubMed] [Google Scholar]
- 393. Miller R, Tumin D, Cooper J, Hayes DJr, Tobias JD. Prediction of mortality following pediatric heart transplant using machine learning algorithms. Pediatr Transplant. 2019;23(3):e13360. [DOI] [PubMed] [Google Scholar]
- 394. Tian D, Yan HJ, Huang H, et al. Machine learning‐based prognostic model for patients after lung transplantation. JAMA Netw Open. 2023;6(5):e2312022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395. Chandra G, Wang J, Siirtola P, Röning J. Leveraging machine learning for predicting acute graft‐versus‐host disease grades in allogeneic hematopoietic cell transplantation for T‐cell prolymphocytic leukaemia. BMC Med Res Methodol. 2024;24(1):112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396. Tanaka T, Voigt MD. Decision tree analysis to stratify risk of de novo non‐melanoma skin cancer following liver transplantation. J Cancer Res Clin Oncol. 2018;144(3):607‐615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397. Jo SJ, Park JB, Lee KW. Prediction of very early subclinical rejection with machine learning in kidney transplantation. Sci Rep. 2023;13(1):22387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398. Zare A, Zare MA, Zarei N, et al. A neural network approach to predict acute allograft rejection in liver transplant recipients using routine laboratory data. Research article. Hepat Mon. 2017;17(12):e55092. [Google Scholar]
- 399. Ram S, Verleden SE, Kumar M, et al. Computed tomography‐based machine learning for donor lung screening before transplantation. J Heart Lung Transplant. 2024;43(3):394‐402. [DOI] [PubMed] [Google Scholar]
- 400. El Hage A, Dormond O. Combining mTOR inhibitors and T cell‐based immunotherapies in cancer treatment. Cancers (Basel). 2021;13(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401. Havenith SH, Yong SL, van Donselaar‐van der Pant KA, van Lier RA, ten Berge IJ, Bemelman FJ. Everolimus‐treated renal transplant recipients have a more robust CMV‐specific CD8+ T‐cell response compared with cyclosporine‐ or mycophenolate‐treated patients. Transplantation. 2013;95(1):184‐191. [DOI] [PubMed] [Google Scholar]
- 402. Rozenbaum M, Meir A, Aharony Y, et al. Gamma‐Delta CAR‐T cells show CAR‐directed and independent activity against leukemia. Front Immunol. 2020;11:1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403. Sebestyen Z, Prinz I, Déchanet‐Merville J, Silva‐Santos B, Kuball J. Translating gammadelta (γδ) T cells and their receptors into cancer cell therapies. Nat Rev Drug Discov. 2020;19(3):169‐184. [DOI] [PubMed] [Google Scholar]
- 404. Godder KT, Henslee‐Downey PJ, Mehta J, et al. Long term disease‐free survival in acute leukemia patients recovering with increased gammadelta T cells after partially mismatched related donor bone marrow transplantation. Bone Marrow Transplant. 2007;39(12):751‐757. [DOI] [PubMed] [Google Scholar]
- 405. Xuan L, Wu X, Qiu D, et al. Regulatory γδ T cells induced by G‐CSF participate in acute graft‐versus‐host disease regulation in G‐CSF‐mobilized allogeneic peripheral blood stem cell transplantation. J Transl Med. 2018;16(1):144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406. Ye W, Kong X, Zhang W, Weng Z, Wu X. The roles of γδ T cells in hematopoietic stem cell transplantation. Cell Transplant. 2020;29:963689720966980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407. Gaballa MR, Ramos CA. Cellular immunotherapy in lymphoma: beyond CART cells. Curr Treat Options Oncol. 2020;21(3):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408. Bajwa G, Lanz I, Cardenas M, Brenner MK, Arber C. Transgenic CD8αβ co‐receptor rescues endogenous TCR function in TCR‐transgenic virus‐specific T cells. J Immunother Cancer. 2020;8(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


