Abstract
Ependymomas are rare nervous system tumors that can arise anywhere in the neuraxis. While having a high propensity for leptomeningeal dissemination, retrograde dissemination (from the spine to the CNS) remains infrequent. We describe the case of a 31-year-old female who presented with hydrocephalus secondary to an intracranial leptomeningeal metastasis of a giant spinal ependymoma with mixed (classic and myxopapillary) histopathologic features, successfully treated with surgical resection and radiotherapy of the entire neuraxis. This case highlights the importance of including ependymomas in the differential diagnosis for lesions in atypical extra-axial locations, of systematically obtaining imaging of the entire neuraxis when suspecting it, and of considering retrograde dissemination when both intracranial and spinal lesions are present.
Keywords: Spinal ependymoma, Myxopapillary ependymoma, CSF dissemination, Metastasis, Hydrocephalus
Introduction
Ependymomas are WHO grade 2 or 3 tumors that can arise anywhere in the neuraxis, and represent 3%-9% of all gliomas [1]. While bearing a relatively good prognosis, ependymomas are prone to a high recurrence rate, mostly due to their propensity for leptomeningeal dissemination. Retrograde intracranial dissemination, however, remains infrequent. We describe the case of a young adult female initially presenting with hydrocephalus secondary to an intracranial leptomeningeal metastasis of a giant, mixed-type (classic and myxopapillary) spinal ependymoma, initially misdiagnosed because of incomplete imaging of the rest of the neuraxis, and then successfully treated with surgical radiation and pan-neuraxis radiotherapy.
Case presentation
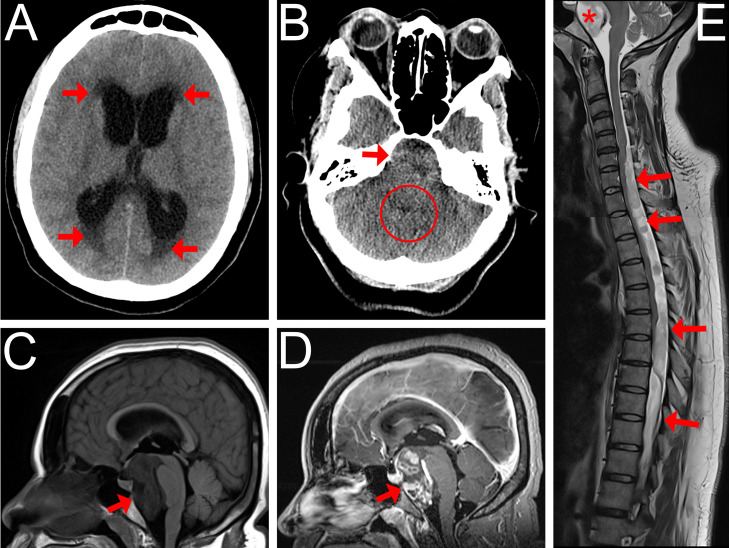
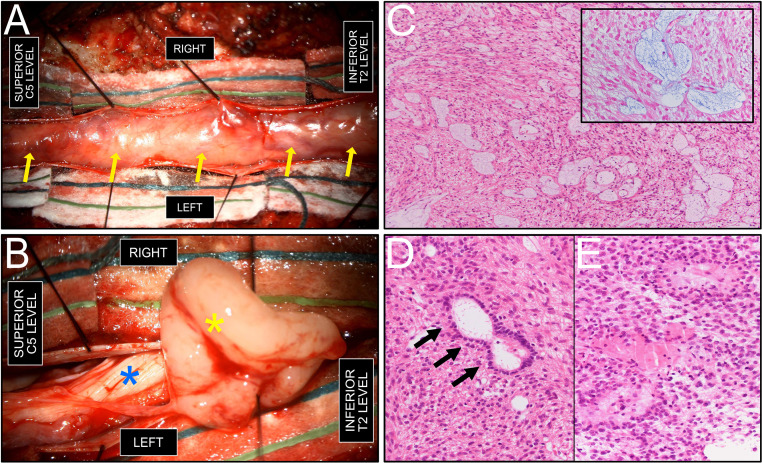
A 31-year-old female with a history of gestational diabetes and obesity, who was initially admitted for labor induction at 38 weeks of pregnancy, presented with new-onset severe headaches for 2 weeks. All her vitals and blood tests were normal. On postpartum day 2, her headaches worsened, and she began to experience diplopia. Noncontrast head CT showed significant triventricular hydrocephalus with signs of transependymal resorption (Fig. 1A), and an ill-defined mass centered in the prepontine cistern (Fig. 1B). A brain MRI confirmed the presence of a large, enhancing, extra-axial retrochiasmatic and retroclival mass with focal cystic areas (Fig. 1C and D). The initial presumed diagnosis was that of a craniopharyngioma, with atypical ependymoma and germ cell tumor listed as differential diagnoses. The patient underwent urgent left parietal ventriculoperitoneal shunt placement, with subsequent substantial improvement of her headache. The patient was adamant to obtain her discharge as soon as possible, and was finally discharged 4 days postdelivery in good clinical state with a close scheduled follow-up. On her follow-up appointment 1 month after discharge, after thorough questioning, she reported having a progressive bilateral lower limb weakness for a few months before delivery. Neurological exam revealed moderate paraparesis, paraparesthesis and hyperreflexia. A spine MRI showed a large intradural extramedullary lesion extending from C5 to L1, presenting similar signal characteristics as the intracranial lesion (Fig. 1E). An ependymoma with spinal metastasis became the preferred diagnosis, with germ cell tumor not excluded. The patient underwent a 2-stage gross resection of the spine lesion (Figs. 2A and B). Her postoperative course was complicated by bilateral pulmonary emboli which resolved after 6 months of anticoagulant therapy. The intracranial lesion, radiologically monitored until then upon tumor board recommendation and in accordance with patient's will to delay surgery after the cessation of breastfeeding, demonstrated interval progression, and partial resection using a transclival endonasal approach was performed. Gross total resection was not achievable because of its engulfment of the basilar artery (Fig. 1D). Histopathological analysis revealed a metastatic hybrid spinal ependymoma (classic + myxopapillary), WHO grade 2 (Figs. 2C-E). The patient then received radiotherapy of the entire neuraxis and remains periodically followed, with mild residual paraparesis and no progression or recurrence until now.
Fig. 1.
CT and MR findings of a mixed-type metastatic spinal ependymoma in a 31-year-old female patient. (A) Axial noncontrast head CT image showing severe obstructive hydrocephalus with signs of transependymal resorption (arrows). (B) Axial noncontrast head CT image at the level of posterior fossa, showing an ill-defined, heterogenous mass effect filling the prepontine cistern (arrow). Note the small caliber of the 4th ventricle (circle). (C,D) Pre- and postcontrast Sagittal Brain MR T1-weighted image showing a large T1-hypointense prepontine extra-axial mass (arrow) extending from the retro-chiasmatic region to the level of the inferior aspect of the clivus and causing a compression of the brainstem and optic chiasma. It displays an avid, irregular, patchy enhancement (arrow). The basilar artery was engulfed but remained patent. (E) Sagittal cervical and thoracic spine MR T2-weighted reconstructed image showing an extensive posterior intradural extramedullary mass, beginning at the C5 level and extending caudally to the upper lumbar spine level, which displays heterogenous, T2 hyperintense signal (arrows). Note the resulting spinal cord compression, as well as the similar T2 signal in the prepontine mass (asterisk).
Fig. 2.
Intraoperative and histopathologic findings. (A) intraoperative view, following C5-T2 laminectomy and dural sac opening, showing a tan-colored, well-demarcated encapsulated intradural tumor (arrows), without areas of macroscopic necrosis. (B) Intraoperative view, after partial dissection of the intradural extramedullary mass (yellow asterisk) from the plane of the spinal cord (blue asterisk). (C) Glial epithelial proliferation with focal perivascular myxoid microcysts, typical of myxopapillary ependymoma. Mitotic activity was low. (hematoxylin and eosin (H&E) stain, 100X original magnification). Inset: Alcian Blue staining highlighting the myxoid content in light blue within microcysts (400X magnification). A strong positivity for GFAP immunohistochemistry was also noted (not shown). (D) ‘True’ ependymal rosette (arrows), consisting of tumor cells arranged around an empty central lumen, characteristic of ‘classic’ spinal ependymoma (H&E stain, 200X magnification). (E) Glial proliferation with tumor cells arranged in perivascular pseudorosettes (H&E stain, 200X magnification).
Discussion
The diagnosis of ependymomas can be clinically challenging, given the numerous possible clinical manifestations depending on the location of the lesions. In our case, the only initial symptom reported by the patient was a new-onset, nonspecific headache in a peripartum context, without any clinical features orienting towards pre-eclampsia or other diseases, and which was only investigated by imaging after delivery. The differential diagnosis of ependymomas on imaging is location and age dependent [2]. In our case, the atypical retroclival location and the presence of spinal involvement raised suspicion for 2 additional diagnoses: germ cell tumor and papillary craniopharyngioma, which can both present with leptomeningeal seeding (although very rare for the latter, usually after resection [3]). While it can be difficult to identify which of the leptomeningeal lesions is the primary (and decide between drop or retrograde dissemination [4]), we assumed the spinal lesion to be the primary one, given its large size compared to the retroclival lesion, the onset of symptoms related to the spine component which seems to have begun before those related to the intracranial component, and the presence of histological features in both tumors suggesting myxopapillary contingent.
Leptomeningeal dissemination of spinal ependymoma generally stays confined to the spine level. Intracranial (retrograde) dissemination from spinal ependymomas remains very rare, with only a few reported cases in the literature. Most of them were either anaplastic and/or occurred postoperatively [[5], [6], [7]]. Only 3 prior cases reported preoperative intracranial dissemination from spinal ependymoma [4,[8], [9], [10]], from which our case differs in many ways: first, our patient was older than the reported (mostly pediatric) patients. Second, the 2 other cases causally attributed the intracranial dissemination to trauma or to spontaneous rupture secondary to hemorrhage, while none of this occurred in our case. Third, our case was of mixed-type on histopathologic assessment, while the reported ones were purely myxopapillary. Finally, none of them initially presented with obstructive hydrocephalus.
Gross total resection is the mainstay of treatment of ependymomas, regardless of location [1]. When total resection is not feasible, or when in presence of anaplastic ependymoma (WHO grade 3), postoperative radiotherapy is indicated, as in our case. Prognosis mainly depends on tumor grade, presence of leptomeningeal spread at initial diagnosis, and extent of resection. Posterior Fossa type A ependymoma, supratentorial ependymoma with ZFTA fusion, and spinal ependymoma with MYCN amplification, are molecular subtypes that are associated with poor prognosis. The 5-year survival rate for ependymoma (regardless of location and type) is 84%. Myxopapillary ependymoma is associated with an excellent 10-year survival rate of 92.4%, however with high local recurrence (up to 84%) and leptomeningeal spread (9.3%) [1].
Conclusion
Our unusual case highlights the importance of systematic imaging of the entire neuraxis when a diagnosis of ependymoma is considered, of the inclusion of ependymoma in the differential diagnosis of lesions in atypical extra-axial locations, and of considering retrograde leptomeningeal spread when both intracranial and spinal ependymomas are found.
Patient consent
Written informed consent for publication was obtained from the patient.
Author contributions
Each author has significantly contributed to the conception, drafting, and critical revision of this article. They all approve its final version and agree to be accountable for all parts.
Footnotes
Competing Interests: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments: None.
References
- 1.Ruda R, Bruno F, Pellerino A, Soffietti R. Ependymoma: evaluation and management updates. Curr Oncol Rep. 2022;24(8):985–993. doi: 10.1007/s11912-022-01260-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Osborn AG, Hedlund GL, Salzman KL. Osborn’s brain : imaging, pathology, and anatomy. Second edition ed. Elsevier; Philadelphia, PA: 2018. Ependymal tumors; pp. 559–568. [Google Scholar]
- 3.Du C, Feng CY, Yuan J, Yuan X. Ectopic recurrence of pediatric craniopharyngiomas after gross total resection: a report of two cases and a review of the literature. Childs Nerv Syst. 2016;32(8):1523–1529. doi: 10.1007/s00381-016-3050-1. [DOI] [PubMed] [Google Scholar]
- 4.Wang M, Wang H, Zhou Y, Zhan R, Wan S. Myxopapillary ependymoma in the third ventricle area and sacral canal: dropped or retrograde metastasis? Neurol Med Chir (Tokyo) 2013;53(4):237–241. doi: 10.2176/nmc.53.237. [DOI] [PubMed] [Google Scholar]
- 5.Guo Z, Wan J, Zhao B. Extensive craniospinal disseminated metastasis after the resection of intradural extramedullary ependymoma in the craniocervical junction: a case report and literature review. Int J Neurosci. 2021;131(9):919–926. doi: 10.1080/00207454.2020.1759585. [DOI] [PubMed] [Google Scholar]
- 6.Garg K, Sharma R, Dash C, Agrawal D, Sharma BS. Spinal intradural extramedullary ependymoma with intracranial metastasis and leptomeningeal spread: a case report and comprehensive review of literature. Neurol India. 2019;67(5):1352–1357. doi: 10.4103/0028-3886.271269. [DOI] [PubMed] [Google Scholar]
- 7.Mridha AR, Sharma MC, Sarkar C, Suri V, Rishi A, Garg A, et al. Myxopapillary ependymoma of lumbosacral region with metastasis to both cerebellopontine angles: report of a rare case. Childs Nerv Syst. 2007;23(10):1209–1213. doi: 10.1007/s00381-007-0423-5. [DOI] [PubMed] [Google Scholar]
- 8.Awaya T, Nishimura Y, Eguchi K, Nagashima Y, Ando R, Akahori S, et al. Preoperative intracranial dissemination of spinal myxopapillary ependymoma attributed to tumor hemorrhage. World Neurosurg. 2021;145:13–18. doi: 10.1016/j.wneu.2020.08.169. [DOI] [PubMed] [Google Scholar]
- 9.Khalatbari MR, Jalaeikhoo H, Hamidi M, Moharamzad Y. Craniospinal dissemination of filum myxopapillary ependymoma following spinal trauma: case report and literature review. Childs Nerv Syst. 2013;29(1):149–152. doi: 10.1007/s00381-012-1927-1. [DOI] [PubMed] [Google Scholar]
- 10.Woesler B, Moskopp D, Kuchelmeister K, Schul C, Wassmann H. Intracranial metastasis of a spinal myxopapillary ependymoma. A case report. Neurosurg Rev. 1998;21(1):62–65. doi: 10.1007/bf01111488. [DOI] [PubMed] [Google Scholar]