Abstract
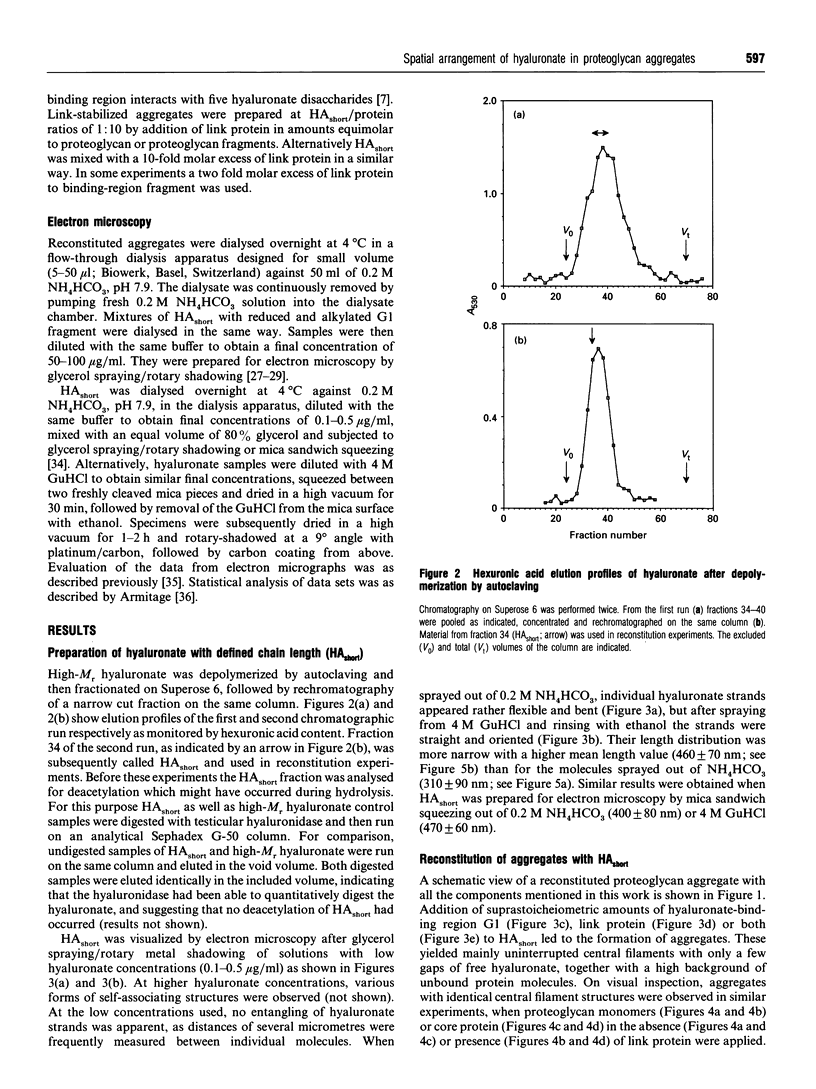
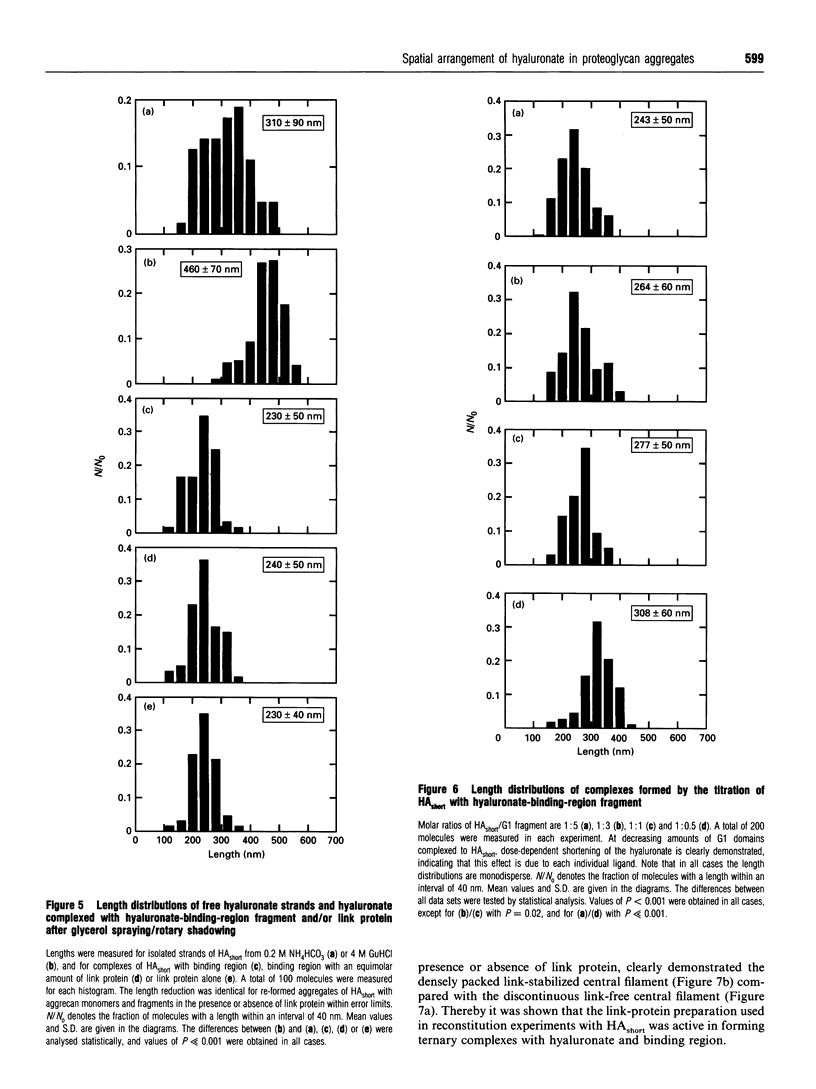
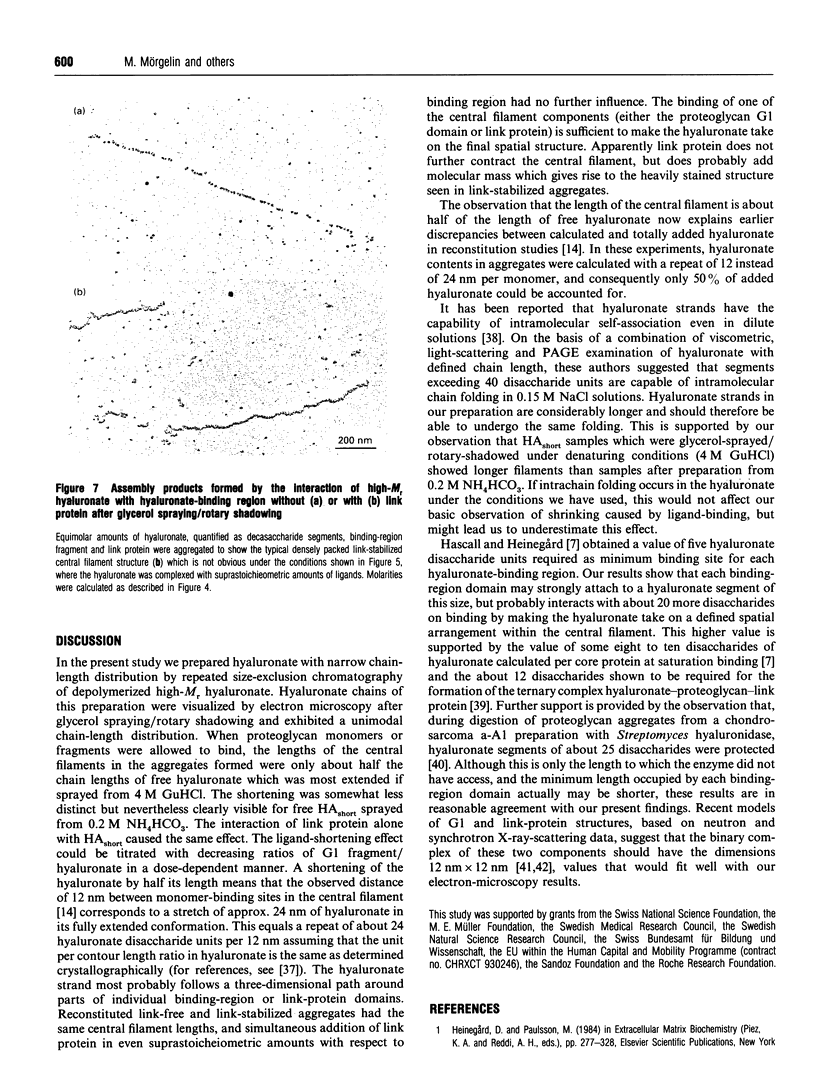
Aggregates of proteoglycans from the Swarm rat chondrosarcoma reassembled in vitro have been studied by rotary-shadowing electron microscopy, and shown to be similar to native structures that have never been dissociated [Mörgelin, Engel, Heinegård and Paulsson (1992) J. Biol. Chem. 267, 14275-14284]. A hyaluronate with defined chain length (HAshort) has now been prepared by autoclaving high-Mr hyaluronate and fractionation to a narrow size distribution by gel filtration. Proteoglycan monomers, core protein, hyaluronate-binding region and link protein were combined with HAshort. Free chains of HAshort and reconstituted complexes with proteoglycan, link protein and aggrecan fragments were examined by electron microscopy after rotary shadowing. Length measurements showed that the hyaluronate was condensed to about half of its original length on binding intact aggrecan monomers, any aggrecan fragment or link protein alone. This strongly implies that hyaluronate adopts a defined spatial arrangement within the central filament of the aggregate, probably different from its secondary structure in solution. No differences in length were observed between link-free and link-stabilized aggregates.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Bonnet F., Dunham D. G., Hardingham T. E. Structure and interactions of cartilage proteoglycan binding region and link protein. Biochem J. 1985 May 15;228(1):77–85. doi: 10.1042/bj2280077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doege K. J., Sasaki M., Kimura T., Yamada Y. Complete coding sequence and deduced primary structure of the human cartilage large aggregating proteoglycan, aggrecan. Human-specific repeats, and additional alternatively spliced forms. J Biol Chem. 1991 Jan 15;266(2):894–902. [PubMed] [Google Scholar]
- Doege K., Fernandez P., Hassell J. R., Sasaki M., Yamada Y. Partial cDNA sequence encoding a globular domain at the C terminus of the rat cartilage proteoglycan. J Biol Chem. 1986 Jun 25;261(18):8108–8111. [PubMed] [Google Scholar]
- Doege K., Sasaki M., Horigan E., Hassell J. R., Yamada Y. Complete primary structure of the rat cartilage proteoglycan core protein deduced from cDNA clones. J Biol Chem. 1987 Dec 25;262(36):17757–17767. [PubMed] [Google Scholar]
- Engel J., Furthmayr H. Electron microscopy and other physical methods for the characterization of extracellular matrix components: laminin, fibronectin, collagen IV, collagen VI, and proteoglycans. Methods Enzymol. 1987;145:3–78. doi: 10.1016/0076-6879(87)45003-9. [DOI] [PubMed] [Google Scholar]
- Faltz L. L., Caputo C. B., Kimura J. H., Schrode J., Hascall V. C. Structure of the complex between hyaluronic acid, the hyaluronic acid-binding region, and the link protein of proteoglycan aggregates from the swarm rat chondrosarcoma. J Biol Chem. 1979 Feb 25;254(4):1381–1387. [PubMed] [Google Scholar]
- Fosang A. J., Hardingham T. E. Isolation of the N-terminal globular protein domains from cartilage proteoglycans. Identification of G2 domain and its lack of interaction with hyaluronate and link protein. Biochem J. 1989 Aug 1;261(3):801–809. doi: 10.1042/bj2610801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler W. E., Erickson H. P. Trinodular structure of fibrinogen. Confirmation by both shadowing and negative stain electron microscopy. J Mol Biol. 1979 Oct 25;134(2):241–249. doi: 10.1016/0022-2836(79)90034-2. [DOI] [PubMed] [Google Scholar]
- Franzén A., Björnsson S., Heinegård D. Cartilage proteoglycan aggregate formation. Role of link protein. Biochem J. 1981 Sep 1;197(3):669–674. doi: 10.1042/bj1970669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham T. E., Muir H. The specific interaction of hyaluronic acid with cartillage proteoglycans. Biochim Biophys Acta. 1972 Sep 15;279(2):401–405. doi: 10.1016/0304-4165(72)90160-2. [DOI] [PubMed] [Google Scholar]
- Hardingham T. E. The role of link-protein in the structure of cartilage proteoglycan aggregates. Biochem J. 1979 Jan 1;177(1):237–247. doi: 10.1042/bj1770237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hascall V. C., Heinegård D. Aggregation of cartilage proteoglycans. II. Oligosaccharide competitors of the proteoglycan-hyaluronic acid interaction. J Biol Chem. 1974 Jul 10;249(13):4242–4249. [PubMed] [Google Scholar]
- Heinegård D., Axelsson I. Distribution of keratan sulfate in cartilage proteoglycans. J Biol Chem. 1977 Mar 25;252(6):1971–1979. [PubMed] [Google Scholar]
- Heinegård D., Hascall V. C. Aggregation of cartilage proteoglycans. 3. Characteristics of the proteins isolated from trypsin digests of aggregates. J Biol Chem. 1974 Jul 10;249(13):4250–4256. [PubMed] [Google Scholar]
- Heinegård D. Polydispersity of cartilage proteoglycans. Structural variations with size and buoyant density of the molecules. J Biol Chem. 1977 Mar 25;252(6):1980–1989. [PubMed] [Google Scholar]
- Heinegård D., Sommarin Y. Isolation and characterization of proteoglycans. Methods Enzymol. 1987;144:319–372. doi: 10.1016/0076-6879(87)44186-4. [DOI] [PubMed] [Google Scholar]
- Kimura J. H., Hardingham T. E., Hascall V. C., Solursh M. Biosynthesis of proteoglycans and their assembly into aggregates in cultures of chondrocytes from the Swarm rat chondrosarcoma. J Biol Chem. 1979 Apr 25;254(8):2600–2609. [PubMed] [Google Scholar]
- Mould A. P., Holmes D. F., Kadler K. E., Chapman J. A. Mica sandwich technique for preparing macromolecules for rotary shadowing. J Ultrastruct Res. 1985 Apr;91(1):66–76. doi: 10.1016/0889-1605(85)90077-1. [DOI] [PubMed] [Google Scholar]
- Mörgelin M., Engel J., Heinegård D., Paulsson M. Proteoglycans from the swarm rat chondrosarcoma. Structure of the aggregates extracted with associative and dissociative solvents as revealed by electron microscopy. J Biol Chem. 1992 Jul 15;267(20):14275–14284. [PubMed] [Google Scholar]
- Mörgelin M., Heinegård D., Engel J., Paulsson M. The cartilage proteoglycan aggregate: assembly through combined protein-carbohydrate and protein-protein interactions. Biophys Chem. 1994 May;50(1-2):113–128. doi: 10.1016/0301-4622(94)85024-0. [DOI] [PubMed] [Google Scholar]
- Mörgelin M., Paulsson M., Hardingham T. E., Heinegård D., Engel J. Cartilage proteoglycans. Assembly with hyaluronate and link protein as studied by electron microscopy. Biochem J. 1988 Jul 1;253(1):175–185. doi: 10.1042/bj2530175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neame P. J., Christner J. E., Baker J. R. Cartilage proteoglycan aggregates. The link protein and proteoglycan amino-terminal globular domains have similar structures. J Biol Chem. 1987 Dec 25;262(36):17768–17778. [PubMed] [Google Scholar]
- Oldberg A., Antonsson P., Heinegård D. The partial amino acid sequence of bovine cartilage proteoglycan, deduced from a cDNA clone, contains numerous Ser-Gly sequences arranged in homologous repeats. Biochem J. 1987 Apr 1;243(1):255–259. doi: 10.1042/bj2430255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsson M., Mörgelin M., Wiedemann H., Beardmore-Gray M., Dunham D., Hardingham T., Heinegård D., Timpl R., Engel J. Extended and globular protein domains in cartilage proteoglycans. Biochem J. 1987 Aug 1;245(3):763–772. doi: 10.1042/bj2450763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins S. J., Nealis A. S., Dunham D. G., Hardingham T. E., Muir I. H. Molecular modeling of the multidomain structures of the proteoglycan binding region and the link protein of cartilage by neutron and synchrotron X-ray scattering. Biochemistry. 1991 Nov 5;30(44):10708–10716. doi: 10.1021/bi00108a015. [DOI] [PubMed] [Google Scholar]
- Perkins S. J., Nealis A. S., Dunham D. G., Hardingham T. E., Muir I. H. Neutron and X-ray solution-scattering studies of the ternary complex between proteoglycan-binding region, link protein and hyaluronan. Biochem J. 1992 Jul 1;285(Pt 1):263–268. doi: 10.1042/bj2850263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg L., Hellmann W., Kleinschmidt A. K. Electron microscopic studies of proteoglycan aggregates from bovine articular cartilage. J Biol Chem. 1975 Mar 10;250(5):1877–1883. [PubMed] [Google Scholar]
- Rosenberg L., Hellmann W., Kleinschmidt A. K. Macromolecular models of proteinpolysaccharides from bovine nasal cartilage based on electron microscopic studies. J Biol Chem. 1970 Aug 25;245(16):4123–4130. [PubMed] [Google Scholar]
- Sai S., Tanaka T., Kosher R. A., Tanzer M. L. Cloning and sequence analysis of a partial cDNA for chicken cartilage proteoglycan core protein. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5081–5085. doi: 10.1073/pnas.83.14.5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shotton D. M., Burke B. E., Branton D. The molecular structure of human erythrocyte spectrin. Biophysical and electron microscopic studies. J Mol Biol. 1979 Jun 25;131(2):303–329. doi: 10.1016/0022-2836(79)90078-0. [DOI] [PubMed] [Google Scholar]
- Tengblad A. A comparative study of the binding of cartilage link protein and the hyaluronate-binding region of the cartilage proteoglycan to hyaluronate-substituted Sepharose gel. Biochem J. 1981 Nov 1;199(2):297–305. doi: 10.1042/bj1990297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner R. E., Lin P. Y., Cowman M. K. Self-association of hyaluronate segments in aqueous NaCl solution. Arch Biochem Biophys. 1988 Sep;265(2):484–495. doi: 10.1016/0003-9861(88)90153-1. [DOI] [PubMed] [Google Scholar]
- Tyler J. M., Branton D. Rotary shadowing of extended molecules dried from glycerol. J Ultrastruct Res. 1980 May;71(2):95–102. doi: 10.1016/s0022-5320(80)90098-2. [DOI] [PubMed] [Google Scholar]
- Wellauer P., Wyler T., Buddecke E. Electron microscopic and physico-chemical studies on bovine nasal cartilage proteoglycan. Hoppe Seylers Z Physiol Chem. 1972 Jul;353(7):1043–1052. doi: 10.1515/bchm2.1972.353.2.1043. [DOI] [PubMed] [Google Scholar]
- Wiedemann H., Paulsson M., Timpl R., Engel J., Heinegård D. Domain structure of cartilage proteoglycans revealed by rotary shadowing of intact and fragmented molecules. Biochem J. 1984 Nov 15;224(1):331–333. doi: 10.1042/bj2240331. [DOI] [PMC free article] [PubMed] [Google Scholar]





