Highlights
-
•
Lenvatinib plus pembrolizumab (LP) therapy may be effective for patients with uterine carcinosarcoma (UCS).
-
•
The overall response rate of LP in UCS was not worse than that in patients with other endometrial cancers.
-
•
More cases are required to investigate the efficacy of LP therapy in patients with UCS.
Keywords: Uterine carcinosarcoma, Lenvatinib, Pembrolizumab, Endometrial cancer
Abstract
Lenvatinib plus pembrolizumab (LP) therapy is currently used in patients with advanced or recurrent endometrial cancer. However, patients with uterine carcinosarcoma (UCS) were not included in the KEYNOTE-775, and the efficacy of LP therapy for patients with UCS in clinical practice remains unclear. We administered LP therapy to five patients with UCS. We aimed to report our clinical experience with LP therapy in these patients and investigate the genomic characteristics of those who responded to LP therapy.
We retrospectively reviewed patients with UCS (n = 5) who underwent LP therapy at our hospital from January 2019 to December 2023. Efficacy was assessed using the response rate according to the Response Evaluation Criteria in Solid Tumors version 1.1. Safety was evaluated in terms of adverse events.
The median age was 65 (55–78) years, and the mismatch repair status was proficient in all of the patients. One patient had stage II disease, and four had stage III. The median number of LP therapy courses was 8 (1–35). The overall response rate was 40%. None of the patients experienced adverse events that were grade 3 or higher. The median follow-up duration was 9 (1–26) months, median progression-free survival was 9.1 (0.16 to NA) months, and median overall survival was 10.2 (1.41 to NA) months.
LP therapy may be effective for patients with UCS. As this report was based on a limited number of patients, more cases are required to investigate the efficacy of LP therapy in patients with UCS.
1. Introduction
Uterine carcinosarcoma (UCS) is a rare tumor that accounts for less than 5 % of all uterine tumors and is responsible for 15 % of all deaths caused by uterine corpus malignancies (Cantrell et al., 2015, Bogani et al., 2023). Owing to its aggressive features, the rate of recurrence is high, and patients with UCS still have a poor prognosis.
First-line chemotherapy with a carboplatin/paclitaxel doublet regimen is recommended for patients with UCS (Powell et al., 2022). In contrast, immunotherapy has become the standard treatment after the failure of platinum-based chemotherapy in patients with endometrial cancer, and lenvatinib plus pembrolizumab (LP) therapy is now widely used in patients with advanced or recurrent endometrial cancer. However, clinical trials on immunotherapy for patients with endometrial cancer sometimes exclude patients with UCS based on their enrollment criteria (Eskander et al., 2023). UCS was also excluded from the KEYNOTE-775 (Makker et al., 2022). Thus, the efficacy of LP therapy in patients with UCS in clinical practice remains unclear.
We administered LP therapy to five patients with UCS. We aimed to report our clinical experience with LP therapy in patients with UCS and investigate the genomic characteristics of those who responded to LP therapy.
2. Materials and methods
This study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Ethics Committee of the National Cancer Center Hospital (NCCH) (2014-393). The patients were provided with the option to refuse to participate by providing opt-out consent.
Patients with UCS who were treated with LP therapy at the NCCH from April 2019 to December 2023 were included (n = 5).
We retrospectively reviewed their medical records, and efficacy was assessed by the response rate according to the revised Response Evaluation Criteria in Solid Tumors (RECIST) guidelines ver. 1.1. Safety was evaluated in terms of adverse events using the National Cancer Institute Common Terminology Criteria for Adverse Events v5.0 (https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_50).
Progression-free survival (PFS) and overall survival (OS) were estimated using the Kaplan-Meier method. Adverse events were evaluated to assess the safety of the therapy. Statistical analyses were performed using EZR version 1.54 (Saitama Medical Center, Jichi Medical University, Saitama, Japan).
3. Results
The patient characteristics are shown in Table 1. The median age was 65 (55–78) years, and the mismatch repair (MMR) status was proficient in all of the patients. One patient had stage II disease, and four had stage III disease. All of the patients underwent surgery as initial treatment. The number of prior regimens was one in one, two in three, and three in one patient. No patient received immunotherapy in a prior regimen.
Table 1.
Patients’ characteristics.
| Characteristics (n = 5) | n | (%) | |
|---|---|---|---|
| Age [range] | 65 | [55–78] | |
| PS (ECOG) | |||
| 0 | 4 | (80) | |
| 1 | 1 | (20) | |
| MMR status | |||
| Proficient | 5 | (100) | |
| Deficient | 0 | (0) | |
| FIGO Stage | |||
| I | 0 | (0) | |
| II | 1 | (20) | |
| III | 4 | (80) | |
| IV | 0 | (0) | |
| Prior chemotherapy regimens | |||
| 1 | 1 | (20) | |
| 2 | 3 | (60) | |
| ≥3 | 1 | (20) | |
PS, performance status; MMR, mismatch repair; FIGO, International Federation of Gynecology and Obstetrics.
The median number of cycles of LP therapy was 8 (1–35), and treatment was discontinued in four patients due to progressive disease (PD). Lenvatinib dose reduction was required in three patients, with reduced doses of 8, 10, and 14 mg. The reasons for dose reduction were hoarseness, urinary protein, and fatigue.
The overall response rate (ORR) was 40 %, with a partial response (PR) in two, stable disease (SD) in one, and PD in two patients (Table 2). The duration of response (DOR) was 23.3 months in patient 1 and 24.3 months in patient 2. LP therapy was discontinued because of the suspicion of a new lung lesion in patient 2; however, 21 months after the PD diagnosis, the lung lesions were reduced and disappeared on CT imaging which revealed to be asymptomatic interstitial lung disease. The patient continues to be followed without treatment, but her recurrent lesions did not worsen after the discontinuation of LP therapy.
Table 2.
Patients’ details.
| Patient No. | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Age | 65 | 78 | 58 | 68 | 55 |
| FIGO Stage | IIIC1 | IIIC2 | IIIC2 | II | IIIA |
| MMR status | pMMR | pMMR | pMMR | pMMR | pMMR |
| Number of prior regimens | 2 | 2 | 3 | 2 | 1 |
| Detail of prior regimens | TC/TC | TC/DXR | TC/IFM/DXR | TC/DXR | TC |
| Site of recurrence | Intra-abdominal | Extra-abdominal | Extra-abdominal | Extra-abdominal | Intra-abdominal |
| Initial dose of LEN (mg) | 20 | 20 | 20 | 20 | 20 |
| Cycles of LEN + PEM | 35 | 8 | 11 | 1 | 3 |
| Best response | PR | PR | SD | PD | PD |
| Discontinuation cause | − | Suspicious of PD | PD | PD | PD |
| DOR (months) | 23.3 | 24.3 | 7.3 | − | − |
| PFS (months) | − | − | 9.2 | 0.7 | 2.5 |
| OS (months) | 25.0, AWD | 27.1, AWD | 10.4, DOD | 1.4, DOD | 7.1, DOD |
FIGO, International Federation of Gynecology and Obstetrics; MMR, mismatch repair; pMMR, proficient MMR; TC, paclitaxel plus carboplatin; DXR, doxorubicin; IFM, ifosfamide: LEN, lenvatinib; PEM, pembrolizumab; SD, stable disease; PR, partial response; PD, progressive disease; DOR, duration of response; PFS, progression-free survival; OS, overall survival; AWD, alive with disease; DOD, dead of disease.
The two responders to LP therapy underwent a comprehensive genomic profiling test (FoundationOne CDx), and the results are shown in Table 3. Mutations in TP53 and NOTCH3 were identical in both patients (Table 3).
Table 3.
Results of the comprehensive genomic profiling; FoundationOne CDx.
| Patient No.1 | PPP2R1A | TP53 | BRCA2 | EPHB4 | GNAS | LTK | NOTCH3 | NOTCH3 | PBRM1 | CCNE1 | |||
| S256Y | Q136* | C2689Y | R902W | P97fs*8 | W707* | G1347R | G840E | truncation | Amplification | ||||
| Patient No.2 | PIK3CA | TP53 | CBL | CD22 | DAXX | ERCC4 | HSD3B1 | MTOR | NOTCH3 | RAD54L | SPEN | RB1 | MYCL |
| H1047Y | K132R | G838V | P788L | E451del | D271G | R186* | R1818C | P1166S | R542H | A2721T | Loss | Amplification | |
No patient had adverse events that were grade 3 or higher, and the most common adverse events were hypothyroidism in two, fatigue in two, proteinuria in two, and hand-foot syndrome in two patients.
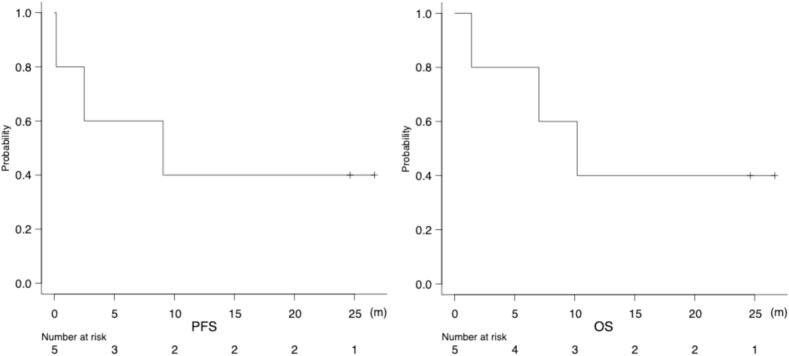
The median follow-up duration was 9 (1–26) months, the median PFS was 9.1 (0.16 to NA) months, and the median OS was 10.2 (1.41 to NA) months (Fig. 1).
Fig. 1.
Kaplan-Meier curves for progression-free survival (PFS) and overall survival (OS).
4. Discussion
Our results suggested that the ORR of LP therapy in patients with UCS was not worse than that in patients with other histological types of endometrial cancer, as seen in those included in the KEYNOTE-775.
Because carcinosarcoma was not included in the KEYNOTE-775 (Makker et al., 2022), the efficacy of LP therapy in patients with UCS can only be demonstrated in clinical settings. However, there are few reports regarding the clinical efficacy of LP therapy in patients with UCS owing to its rarity.
To the best of our knowledge, only three reports have shown the clinical efficacy of LP therapy in patients with UCS. One report had no partial or complete responses (0 of 7) in patients with UCS (Hunt et al., 2021). Another had response and clinical benefit rates of 25 % (3 out of 12) and 58.3 % (7 out of 12), respectively (How et al., 2021). One report included 9 (21 %) patients with UCS in the overall population, but did not describe ORR, PFS or OS specific to patients with UCS, only those in the overall population (Zammarrelli et al., 2023) (Table 4).
Table 4.
Summary of previous studies with lenvatinib plus pembrolizumab in patients with uterine carcinosarcoma.
| Study | Number of pts with UCS | Median follow-up (m) | ORR (%) | CBR (%) | Median PFS (%) | Median OS (%) |
|---|---|---|---|---|---|---|
| Hunt et al. 2021 | 7 | 2.8 | 0 | 28.5 | 2.6 | 2.8 |
| How et al., 2021/(overall) | 12/(70) | −/(7) | 25 | 58.3 | −/(4.6) | −/(8.6) |
| Zammarrelli et al., 2023/(overall) | 9/(43) | − | −/(32) | −/(73) | −/(6) | −/(18.3) |
| Our report | 5 | 9 | 40 | 60 | 9.1 | 10.2 |
pts, patients; UCS, uterine carcinosarcoma; m, months; ORR, overall response rate; CBR, clinical benefit rate; PFS, progression-free survival; OS, overall survival.
Compared with a previous report, our results showed a higher response rate of 40 % and a disease control rate of 60 %, which is similar to the results of the KEYNOTE-775 in which the majority of patients experienced tumor shrinkage regardless of histology (Makker et al., 2023).
The cause of the difference in the clinical efficacy of LP therapy is unclear but may be due to the small sample size and no standardized timing for disease assessment due to the retrospective study.
Although not investigated in the present study, PD-L1 expression may play a role in the efficacy of immune checkpoint inhibitors (ICIs). In one study that included 361 cases of carcinosarcoma, PD-L1 expression was observed in 25 % of the cases (Jones et al., 2017). Our study included two patients with a long-term treatment response, suggesting that PD-L1 expression may have contributed to the difference in the efficacy of ICI.
The Cancer Genome Atlas (TCGA) characterized endometrial carcinomas into four molecular subgroups: POLE ultramutated (POLE), microsatellite instability hypermutated, copy number low, and copy number high (CNH). These classifications are increasingly being applied clinically in endometrial cancer; however, there are few reports on the differences in the clinical response to LP therapy based on molecular classification (Chiba et al., 2024). Although this study had a limited number of cases, our findings suggest that the CNH group may have a shorter PFS and poorer prognosis even after LP therapy. The majority of UCS cases are classified as CNH, with a frequency of 74 % (How et al., 2021). Patients with UCS in the POLE group are shown to have a good prognosis, similar to that of patients with other histological types of endometrial cancer (Nakad Borrego et al., 2022, Travaglino et al., 2022). Another report concludes that POLE-mutated tumors are more immunogenic and that patients with pathogenic POLE mutations have clinical benefits for ICIs (Garmezy et al., 2022). Therefore, it is possible that these molecular classification groups differed in their response to LP therapy. For the two patients with PR, who had long-term responses, the TCGA subgroup was classified as CNH. This group included the majority of patients with UCS, which could not explain the difference in efficacy among the patients in our study. In addition, NOTCH3 mutations were found in both patients; however, it was not possible to determine whether these were associated with differences in response to LP therapy owing to the limited number of patients. Further investigation is needed to determine the differences in the efficacy of LP therapy between molecular classifications.
5. Conclusion
LP therapy may be effective for patients with UCS. As this report was based on a limited number of patients, more cases are required to investigate the efficacy of LP therapy in patients with UCS.
Consent statement
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of written consent is available for review by the Editor-in-Chief of this journal on request.
CRediT authorship contribution statement
Risako Ozawa: Writing – original draft, Data curation. Tadaaki Nishikawa: Writing – review & editing, Project administration, Conceptualization. Kasumi Yamamoto: Writing – review & editing. Tatsunori Shimoi: Writing – review & editing. Mitsuya Ishikawa: Writing – review & editing. Tomoyasu Kato: Writing – review & editing, Supervision. Kan Yonemori: Writing – review & editing, Supervision, Conceptualization.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: T. Nishikawa reports receiving Grants to his institution from Daiichi-Sankyo, and AstraZeneca. He also reports receiving honoraria for speakers' bureaus and manuscript writing from AstraZeneca, Chugai, Takeda, MSD, Eisai, Taiho, Roche Diagnostics and Sanofi.
K. Yonemori reports receiving grants or contracts from Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc.(Rahway, NJ, USA), Daiichi-Sankyo, AstraZeneca, Taiho, Pfizer, Novartis, Takeda, Chugai, Ono, Seattle Genetics, Eisai, Eli Lilly, Genmab, Boehringer Ingelheim, Kyowa Hakko Kirin, Nihon Kayaku, Sanofi and Haihe. He also reports receiving honoraria for lectures, presentations, and speakers' bureaus for Pfizer, Eisai, AstraZeneca, Eli lilly, Takeda, Chugai, MSD, FujiFilm Pharma, Bayer, Asteras, Boehringer Ingelheim, Daiichi Sankyo, PDR pharma, Sanofi.
References
- Cantrell L.A., Blank S.V., Duska L.R. Uterine carcinosarcoma: a review of the literature. Gynecol. Oncol. 2015;137:581–588. doi: 10.1016/j.ygyno.2015.03.041. [DOI] [PubMed] [Google Scholar]
- Bogani G., Ray-Coquard I., Concin N., Ngoi N.Y.L., Morice P., Caruso G., Enomoto T., Takehara K., Denys H., Lorusso D., Coleman R., Vaughan M.M., Takano M., Provencher D.M., Sagae S., Wimberger P., Póka R., Segev Y., Kim S.I., Kim J.W., Candido Dos Reis F.J., Ramirez P.T., Mariani A., Leitao M., Makker V., Abu-Rustum N.R., Vergote I., Zannoni G., Tan D., McCormack M., Paolini B., Bini M., Raspagliesi F., Benedetti Panici P., Di Donato V., Muzii L., Colombo N., Pignata S., Scambia G., Monk B.J. Endometrial carcinosarcoma. Int. J. Gynecol. Cancer. 2023;33:147–174. doi: 10.1136/ijgc-2022-004073. [DOI] [PubMed] [Google Scholar]
- Powell M.A., Filiaci V.L., Hensley M.L., Huang H.Q., Moore K.N., Tewari K.S., Copeland L.J., Secord A.A., Mutch D.G., Santin A., Warshal D.P., Spirtos N.M., DiSilvestro P.A., Ioffe O.B., Miller D.S. Randomized Phase III trial of paclitaxel and carboplatin versus paclitaxel and ifosfamide in patients with carcinosarcoma of the uterus or ovary: An NRG oncology trial. J. Clin. Oncol. 2022;40:968–977. doi: 10.1200/JCO.21.02050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskander R.N., Sill M.W., Beffa L., Moore R.G., Hope J.M., Musa F.B., Mannel R., Shahin M.S., Cantuaria G.H., Girda E., Mathews C., Kavecansky J., Leath C.A., Gien L.T., Hinchcliff E.M., Lele S.B., Landrum L.M., Backes F., O’Cearbhaill R.E., Al Baghdadi T., Hill E.K., Thaker P.H., John V.S., Welch S., Fader A.N., Powell M.A., Aghajanian C. Pembrolizumab plus chemotherapy in advanced endometrial cancer. N. Engl. J. Med. 2023;388:2159–2170. doi: 10.1056/NEJMoa2302312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makker V., Colombo N., Casado Herráez A., Santin A.D., Colomba E., Miller D.S., Fujiwara K., Pignata S., Baron-Hay S., Ray-Coquard I., Shapira-Frommer R., Ushijima K., Sakata J., Yonemori K., Kim Y.M., Guerra E.M., Sanli U.A., McCormack M.M., Smith A.D., Keefe S., Bird S., Dutta L., Orlowski R.J., Lorusso D., Study 309–KEYNOTE-775 Investigators Lenvatinib plus pembrolizumab for advanced endometrial cancer. N. Engl. J. Med. 2022;386:437–448. doi: 10.1056/NEJMoa2108330. [DOI] [PubMed] [Google Scholar]
- https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_50.
- Hunt J.T., Chambers L.M., Yao M., Joehlin-Price A., Debernardo R., Rose P.G. Lenvatinib plus pembrolizumab in patients with advanced or recurrent uterine carcinosarcoma. Gynecol. Oncol. Rep. 2021;37 doi: 10.1016/j.gore.2021.100840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- How J.A., Patel S., Fellman B., Lu K.H., Hwu P., Ramondetta L.M., Westin S.N., Fleming N.D., Soliman P.T., Jazaeri A.A. Toxicity and efficacy of the combination of pembrolizumab with recommended or reduced starting doses of lenvatinib for treatment of recurrent endometrial cancer. Gynecol. Oncol. 2021;162:24–31. doi: 10.1016/j.ygyno.2021.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zammarrelli W.A., 3rd., Ma W., Espino K., Gordhandas S., Yeoshoua E., Ehmann S., Zhou Q., Iasonos A., Abu-Rustum N.R., Aghajanian C., Green A.K., Rubinstein M.M., Makker V. Adverse events and oncologic outcomes with combination lenvatinib and pembrolizumab for the treatment of recurrent endometrial cancer. Gynecol. Oncol. 2023;178:27–35. doi: 10.1016/j.ygyno.2023.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makker V., Colombo N., Casado Herráez A., Monk B.J., Mackay H., Santin A.D., Miller D.S., Moore R.G., Baron-Hay S., Ray-Coquard I., Ushijima K., Yonemori K., Kim Y.M., Guerra Alia E.M., Sanli U.A., Bird S., Orlowski R., McKenzie J., Okpara C., Barresi G., Lorusso D. Lenvatinib plus pembrolizumab in previously treated advanced endometrial cancer: updated efficacy and safety from the randomized Phase III study 309/KEYNOTE-775. J. Clin. Oncol. 2023;41:2904–2910. doi: 10.1200/JCO.22.02152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones N.L., Xiu J., Chatterjee-Paer S., Buckley de Meritens A., Burke W.M., Tergas A.I., Wright J.D., Hou J.Y. Distinct molecular landscapes between endometrioid and nonendometrioid uterine carcinomas. Int. J. Cancer. 2017;140:1396–1404. doi: 10.1002/ijc.30537. [DOI] [PubMed] [Google Scholar]
- Chiba Y., Kagabu M., Osakabe M., Ito R., Sato S., Takatori E., Kaido Y., Nagasawa T., Shoji T., Yanagawa N., Baba T. A single-institution retrospective exploratory analysis on the effectiveness and safety of lenvatinib plus pembrolizumab for advanced endometrial cancer: insights from ProMisE molecular classification system. Jpn. J. Clin. Oncol. 2024;54:424–433. doi: 10.1093/jjco/hyad192. [DOI] [PubMed] [Google Scholar]
- Nakad Borrego S., Lengyel E., Kurnit K.C. Molecular characterizations of gynecologic carcinosarcomas: a focus on the immune microenvironment. Cancers (Basel). 2022;14:4465. doi: 10.3390/cancers14184465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travaglino A., Raffone A., Raimondo D., Arciuolo D., Angelico G., Valente M., Scaglione G., D’alessandris N., Casadio P., Inzani F., Mollo A., Santoro A., Seracchioli R., Franco Zannoni G. Prognostic value of the TCGA molecular classification in uterine carcinosarcoma. Int. J. Gynaecol. Obstet. 2022;158:13–20. doi: 10.1002/ijgo.13937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garmezy B., Gheeya J., Lin H.Y., Huang Y., Kim T., Jiang X., Thein K.Z., Pilié P.G., Zeineddine F., Wang W., Shaw K.R., Rodon J., Shen J.P., Yuan Y., Meric-Bernstam F., Chen K., Yap T.A. Clinical and molecular characterization of POLE mutations as predictive biomarkers of response to immune checkpoint inhibitors in advanced cancers. JCO Precis. Oncol. 2022;6 doi: 10.1200/PO.21.00267. [DOI] [PMC free article] [PubMed] [Google Scholar]