Highlights
-
•
We compared circulating levels of neutrophil extracellular traps between endometrial cancer (EC) patients and healthy women.
-
•
Patients with EC showed higher levels of histone-DNA complex, cell-free DNA, and neutrophil elastase than controls.
-
•
In EC patients, high cell-free dsDNA was a poor prognostic factor for both progression-free survival and overall survival.
Keywords: Endometrial cancer, Neutrophil extracellular trap, Neutrophil elastase, Prognostic marker
Abstract
Objective
Neutrophils produce neutrophil extracellular traps (NETs) by releasing nuclear contents into the extracellular environment. NETs are associated with systemic inflammation and cancer development and progression. We aimed to investigate whether NET markers are associated with the prognosis of endometrial cancer.
Methods
Circulating levels of three NET markers (histone-DNA complex, cell-free double-stranded DNA (dsDNA), and neutrophil elastase) were measured in 98 patients with endometrial cancer who underwent surgery as primary treatment between January 2015 and June 2018 and 45 healthy women. Area under the receiver operating characteristic curve (AUC) analyses were conducted to investigate the diagnostic and prognostic utility of the markers for endometrial cancer.
Results
Patients with endometrial cancer showed significantly higher levels of the three NET markers than those in healthy controls. In discriminating endometrial cancer patients from healthy controls, the three NET markers showed AUC values in the following order: cell-free dsDNA (0.832; 95 % CI, 0.760–0.889), histone-DNA complex (0.740; 95 % CI, 0.660–0.809), and neutrophil elastase (0.689; 95 % CI, 0.607–0.764), comparable to those of CA-125 (0.741; 95 % CI, 0.659–0.813). Multivariate analysis adjusting for FIGO stage, histology, and lymphovascular space invasion, and lymph node involvement revealed that cell-free dsDNA level (cutoff: 95.2 ng/mL) was an independent prognostic marker for poor progression-free (adjusted HR, 2.75; 95 % CI, 1.09—6.92; P = 0.032) and overall survival (adjusted HR, 11.51; 95 % CI, 2.06—64.22; P = 0.005) for patients with endometrial cancer.
Conclusion
High levels of circulating NET markers were observed in patients with endometrial cancer. Cell-free dsDNA levels may play a role as prognostic markers for endometrial cancer.
Introduction
Endometrial cancer is a global burden, with 417,367 new cases expected to occur annually worldwide [1]. Endometrial cancer is the fourth most common cancer in women [2]. In Korea, the incidence of endometrial cancer is continuously increasing in conjunction with a Western lifestyle and obese women [3]. In the era of precision cancer medicine, early diagnosis and accurate prognosis of endometrial cancer are the first steps.
Cancer development and progression are predisposed to inflammation [4]. The inflammatory process can stimulate neutrophils and create neutrophil extracellular traps (NETs), which are web-like filamentous structures containing mixtures of DNA-histone complexes, cell-free double-stranded DNA (dsDNA), and cytoplasmic enzymes, including neutrophil elastase, myeloperoxidase, and cathepsin G [5,6]. NETs promote cancer progression by activating dormant cancer cells, inducing immunosuppression, and angiogenesis [[7], [8], [9], [10]]. NETs also induce cancer metastasis by shielding cancer cells in the circulatory system and aiding their adherence to distant organs [11].
Considering that NET formation actively occurs under inflammatory conditions, endometrial cancer with a florid inflammatory microenvironment is likely to show high NET formation in both tumor tissue and circulation, which may affect cancer prognosis. Furthermore, obesity, a well-known risk factor for developing endometrial cancer, is associated with chronic inflammation and contributes to the secretion of inflammatory cytokines, such as IL-6, and adipocytokines, such as adiponectin, leptin, and resistin, from adipocytes [12]. Inflammatory cytokines and adipocytokines can cause hyperinsulinemia [13] and stimulate estrogen synthesis [14], both of which promote endometrial cell proliferation. Therefore, an increase in NET markers may be profound in endometrial cancer and may be associated with a poor prognosis. However, few studies have reported elevated levels of circulating NET markers in endometrial cancer [15].
To the best of our knowledge, there are no reports on the prognostic impact of circulating NET markers in endometrial cancer. Thus, we aimed to investigate the prognostic value of three circulating NET markers in endometrial cancer: histone-DNA complex, cell-free dsDNA, and neutrophil elastase.
Methods
Study population
This retrospective cohort study was approved by the Institutional Review Board of Seoul National University Hospital (SNUH; No. 2302-007-1400) and was conducted according to the principles of the Declaration of Helsinki and its later amendments. From institution's endometrial cancer cohort, we identified patients who met the following criteria: (i) aged ≥18 years; (ii) diagnosed with endometrial cancer between January 2015 and June 2018; (iii) underwent primary surgery at our institution; (iv) provided informed consent to donate blood samples for scientific purposes; and (v) had blood samples taken one day before surgery and stored at SNUH Human Biobank. However, we excluded patients who had malignancies other than endometrial cancer; had received chemotherapy, radiation, or hormone therapy before surgery; had severe comorbidities, such as uncontrolled diabetes mellitus, long-term corticosteroid use, or end-stage renal disease, were lost to follow-up during primary treatment; or had insufficient clinicopathologic data.
The healthy controls were women who met the following criteria: (i) aged ≥ 18 years; (ii) had no history of the disease being diagnosed; (iii) provided informed consent to donate blood samples for scientific purposes; and (iv) had blood samples taken at the time of routine health check-up and stored at SNUH Human Biobank.
In total, 98 patients with endometrial cancer (study group) and 45 healthy women (control group) were included.
Data collection
The baseline characteristics, including age, were recorded. For the study group, we collected the following clinicopathologic characteristics by reviewing medical records and pathological reports: histological subtype and grade, 2009 International Federation of Gynecology and Obstetrics (FIGO) stage, ESGO–ESP–ESTRO risk classification [16], and postoperative adjuvant treatment. After primary treatment, the patients underwent physical examination and serum CA-125 levels were measured every three to four months for the first two years, every six months for the next two years, and annually thereafter. Imaging studies were conducted according to the physician's preference or when symptoms or examination findings were suspicious for recurrence. In terms of survival outcomes, progression-free survival (PFS) and overall survival (OS) were defined as the time interval from the date of surgery to the date of disease progression confirmed by imaging studies, as per the Response Evaluation Criteria in Solid Tumors version 1.1 [17] and cancer-related death or the last follow-up date, respectively.
Measurement of the circulating markers
Circulating NET markers were measured as previously described [18]. Briefly, peripheral whole blood samples were collected into sodium citrate tubes (Becton Dickinson, San Jose, CA, USA). After centrifugation for 15 min at 1550 × g, the plasma was aliquoted, stored at -70 °C, and thawed before analysis. Histone-DNA complex levels were measured using a cell death detection ELISA kit (Roche Diagnostics, Basel, Switzerland). Cell-free dsDNA levels were measured using Quant-iT PicoGreen dsDNA reagent (Molecular Probes, Eugene, Oregon, USA) and a Fluoroskan Ascent microplate fluorometer (Thermo Fisher Scientific Inc., Waltham, Massachusetts, USA). Neutrophil elastase levels were measured using a human neutrophil elastase platinum ELISA kit (eBioscience, Vienna, Austria). The initial serum CA-125 levels were evaluated using an immunoradiometric assay kit (Institute of Isotopes, Budapest, Hungary).
Statistical analysis
Continuous variables were compared between the two groups using Student's T test or Mann–Whitney U test, and categorical variables were compared using chi-squared or Fisher's exact test. Receiver operating characteristic (ROC) curve analysis was performed and Youden index was used to establish the optimal cutoff values for each plasma biomarker and to evaluate its diagnostic performance in identifying endometrial cancer. For survival analysis, Kaplan–Meier method and log-rank test were used. In multivariate analysis, Cox proportional hazard regression analysis was conducted, and adjusted hazard ratios (HRs) and 95 % confidence intervals (CIs) were calculated. All statistical analyses were performed using IBM SPSS Statistics version 26.0 (IBM Corp., Armonk, NY, USA), GraphPad Prism version 9.3.0 (GraphPad Software, San Diego, CA, USA), and MedCalc Software version 20.027 (MedCalc Software, Ostend, Belgium). P < 0.05 was considered statistically significant.
Results
Elevation of circulating NET markers in endometrial cancer
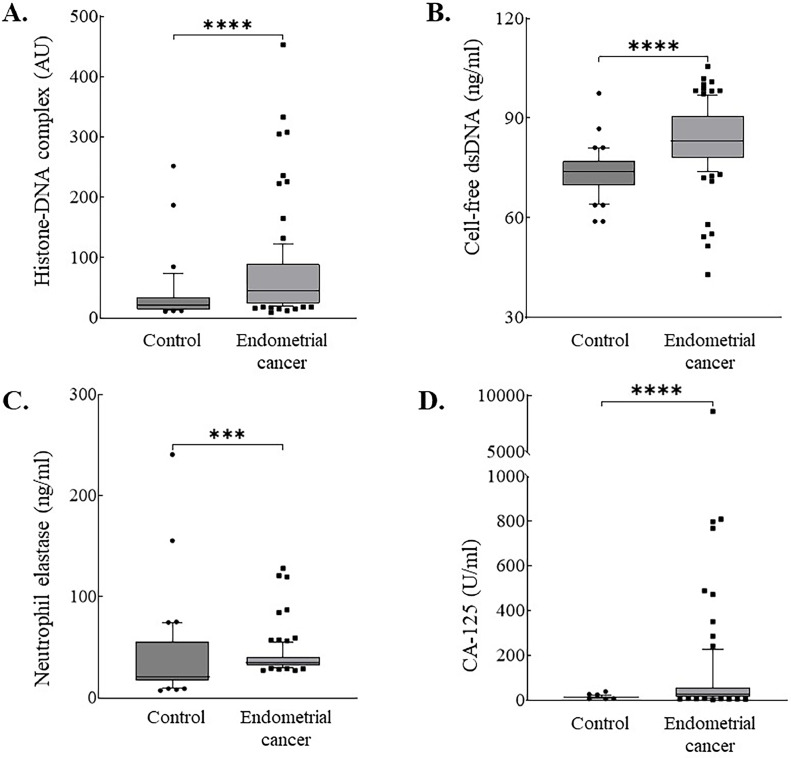
Patients with endometrial cancer were significantly older than the controls (median, 57.2 vs. 42.0 years; P < 0.001) (Table 1). Patients with endometrial cancer also showed significantly increased histone-DNA complex (median, 44.5 vs. 22.0 AU; P < 0.001), cell-free dsDNA (median, 83.0 vs. 74.0 ng/mL; P < 0.001), neutrophil elastase (median, 35.3 vs. 21.1 ng/mL; P < 0.001), and CA-125 levels (median, 24.5 vs. 13.7 IU/mL; P < 0.001), compared with healthy controls (Table 1 and Fig. 1).
Table 1.
Comparison of markers between patients with endometrial cancer and healthy controls.
| Variables | Patients (n = 98) | Controls (n = 45) | P value |
|---|---|---|---|
| Age (years) | 57.2 (49.8–63.6) | 42.0 (37.0–48.0) | <0.001 |
| Histone-DNA complex (AU) | 44.5 (24.0–89.0) | 22.0 (14.0–34.0) | <0.001 |
| Cell-free dsDNA (ng/ml) | 83.0 (78.1–90.5) | 74.0 (69.6–76.8) | <0.001 |
| Neutrophil elastase (ng/ml) | 35.3 (31.9–41.0) | 21.1 (17.3–54.7) | <0.001 |
| CA-125 (IU/ml) | 24.5 (14.3–57.0) | 13.7 (9.7–16.1)* | <0.001 |
| FIGO stage | |||
| I | 53 (54.0) | ||
| II | 11 (11.2) | ||
| III | 28 (28.6) | ||
| IV | 6 (6.2) | ||
| Histologic subtype | |||
| Endometrioid | 84 (85.7) | ||
| Serous | 13 (13.3) | ||
| Clear cell | 1 (1.0) | ||
| Histologic grade | |||
| 1 | 23 (23.5) | ||
| 2 | 41 (41.8) | ||
| 3 | 34 (34.7) | ||
| ESGO–ESP–ESTRO risk classification | |||
| Low | 19 (19.4) | ||
| Intermediate | 9 (9.2) | ||
| High-intermediate | 17 (17.3) | ||
| High | 49 (50.0) | ||
| Advanced/metastatic | 4 (4.1) | ||
| Lymphovascular space invasion | |||
| Yes | 41 (41.8) | ||
| No | 57 (58.2) | ||
| Lymph node metastasis | |||
| Yes | 24 (24.5) | ||
| No | 74 (75.5) | ||
| Adjuvant treatment | |||
| No | 27 (27.6) | ||
| Radiation only | 29 (29.6) | ||
| Chemotherapy only | 18 (18.4) | ||
| CCRT | 24 (24.5) | ||
Data are presented as medians (interquartile ranges) for continuous variables and numbers (percentages) for categorical variables.
Missing data: *8.
Abbreviations: AU, arbitrary unit; dsDNA, double-stranded DNA; FIGO, International Federation of Gynecology and Obstetrics; CCRT, concurrent chemoradiation therapy.
Fig. 1.
Box and whisker plots of (A) Histone-DNA complex; (B) Cell-free double-stranded DNA (dsDNA); (C) Neutrophil elastase; (D) CA-125 levels in healthy controls (A–C, n = 45; D, n = 37) and patients with endometrial cancer (n = 98). Boxes extend from the 25th to 75th percentiles and the horizontal line in the box represent median. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001.
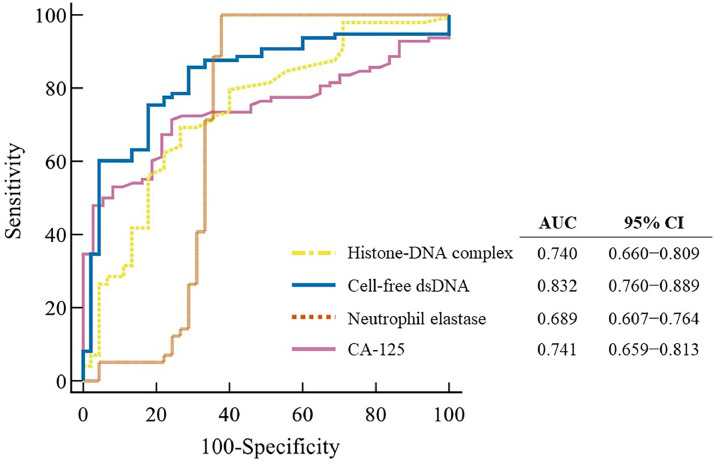
The diagnostic performances of the three circulating NET markers for identifying endometrial cancer are shown in Fig. 2. All three markers showed significant areas under the ROC curve (AUC) values in the following order: cell-free dsDNA (0.832; 95 % CI, 0.760–0.889), histone-DNA complex (0.740; 95 % CI, 0.660–0.809), and neutrophil elastase (0.689; 95 % CI, 0.607–0.764). The AUC values of the three circulating NET markers were comparable to those of CA-125 (0.741; 95 % CI, 0.659–0.813). Among the three circulating NET markers, neutrophil elastase showed the best sensitivity (100 %; 95 % CI, 96.3–100) and accuracy (88.1 %; 95 % CI, 81.6–92.9), which were superior to those of CA-125 (Supplementary Table 1). Also, cell-free dsDNA showed the best specificity (82.2 %; 95 % CI, 67.9–92.0) than that of CA-125 (73.0 %; 95 % CI, 55.9–86.2).
Fig. 2.
Diagnostic performance of the markers for detection of endometrial cancer using receiver operating characteristic curve analysis.
In patients with endometrial cancer, the levels of the histone-DNA complex were higher in FIGO stage IV disease than those in FIGO stage I-III disease, but the difference was not statistically significant (Supplementary Fig. 1A). Also, no differences in the levels of cell-free dsDNA and neutrophil elastase were observed according to FIGO stage (Supplementary Fig. 1B, C). In contrast, serum CA-125 levels were significantly higher in patients with FIGO stages III–IV than in those with FIGO stages I–II (Supplementary Fig. 1D). The levels of the three NET markers and CA-125 did not differ among patients according to the histologic subtype, histologic grade, or ESGO-ESP-ESTRO risk classification (Supplementary Figs. 2–4).
Prognostic values on the circulating NET markers in endometrial cancer
For survival analyses, the optimal cutoff levels for the histone-DNA complex and cell-free dsDNA were determined using the Youden index from the AUC analysis. Cutoff levels for neutrophil elastase were determined arbitrarily as Q3 values because the cutoff level obtained by the Youden index was not appropriate.
No differences in PFS were observed between the patients with high and low NET marker levels (Supplementary Fig. 5). The levels of the three circulating NET markers were not associated with PFS in univariate analysis (Supplementary Table 2). However, in multivariate analysis adjusting for FIGO stage, histology, and lymphovascular space invasion, and lymph node involvement, high cell-free dsDNA (>95.2 ng/mL) was identified as an independent prognostic marker (adjusted HR, 2.75; 95 % CI, 1.09–6.92; P = 0.032).
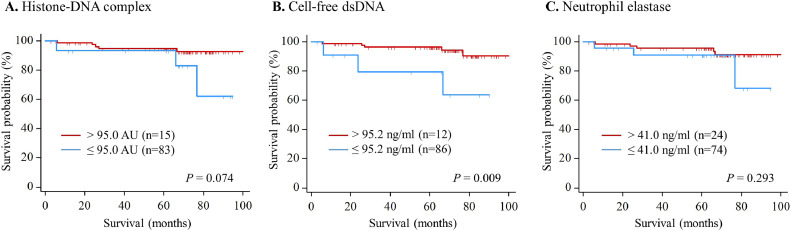
No differences in OS were observed between the high and low histone-DNA complexes (cutoff: 95.0 AU) and neutrophil elastase (cutoff: 41.0 ng/mL) (Fig. 3). However, patients with high cell-free dsDNA (>95.2 ng/mL) showed significantly worse OS than those with low high cell-free dsDNA (≤95.2 ng/mL) (HR, 5.51; 95 % CI, 1.31–23.16; P = 0.020) (Table 2). In the multivariate analysis adjusted for FIGO stage, histology, lymphovascular space invasion, and lymph node involvement, cell-free dsDNA was identified as an independent prognostic marker (adjusted HR, 11.51; 95 % CI, 2.06–64.22; P = 0.005).
Fig. 3.
Kaplan–Meier analysis for overall survival (A) Histone-DNA complex; (B) Cell-free double-stranded DNA (dsDNA); (C) Neutrophil elastase.
Table 2.
Cox regression analysis for prediction of overall survival in endometrial cancer.
| Variables |
Univariate |
Multivariate |
||
|---|---|---|---|---|
| HR (95 % CI) | P value | Adjusted HR (95 % CI) | P value | |
| FIGO stage III-IV vs. I-II | 2.30 (0.57—9.20) | 0.241 | 4.58 (0.25—83.41) | 0.304 |
| Non-endometrioid vs. endometrioid | 12.06 (2.92—49.84) | <0.001 | 14.50 (2.59—81.05) | 0.002 |
| Lymphovascular space invasion yes vs. no | 1.53 (0.38—6.12) | 0.548 | 1.32 (0.10—18.02) | 0.835 |
| Lymph node metastasis yes vs. no | 1.11 (0.22—5.54) | 0.894 | 0.16 (0.02—1.58) | 0.118 |
| Histone-DNA complex >95.0 vs. ≤95.0 AU | 3.42 (0.82—14.31) | 0.093 | ||
| Cell-free dsDNA >95.2 vs. ≤95.2 ng/ml | 5.51 (1.31—23.16) | 0.020 | 11.51(2.06—64.22) | 0.005 |
| Neutrophil elastase >41.0 vs. ≤41.0 ng/ml | 2.13 (0.51—8.94) | 0.304 | ||
Abbreviations: HR, hazard ratio; CI, confidence interval; dsDNA, double-stranded DNA; FIGO, International Federation of Gynecology and Obstetrics.
Discussion
This study showed a significant increase in circulating NET markers in patients with endometrial cancer. Cancer cells recruit neutrophils into tissues to produce NET by releasing cytokines and generating reactive oxygen species [19,20]. NET formed in the tumor microenvironment can release their components into circulation, which may induce elevated NET markers in endometrial cancer. In addition, peripheral neutrophils, which are susceptible to various inflammatory stimuli, showed elevated levels of NET markers in our study.
Recently, the pathophysiology of NET has been studied in relation to cancers of the gastrointestinal tract, genital system, and hematopoietic system [21]. We previously reported increased levels of circulating NET markers in patients with different types of cancers [18,[22], [23], [24]]. Regarding endometrial cancer, one study reported elevated levels of histones and cell-free dsDNA in both endometrial tissue and serum [15]. Our study showed three types of circulating NET markers were elevated in patients with endometrial cancer.
Notably, our results demonstrated substantial detection sensitivity for cell-free dsDNA in endometrial cancer. In a recent meta-analysis, the diagnostic sensitivity of CA-125 pooled from several studies with healthy controls or patients with benign uterine diseases was 35.0 % [25], and 72.4 % in this study. The diagnostic accuracy of human epididymis protein 4 (HE4) alone or in combination with CA-125 is better than that of CA-125, but neither was translated into routine clinical practice to date [26,27].
HE4 and CA-125 have prognostic features in various studies [28]. Neutrophilia and inflammatory markers, such as C-reactive protein at the time of initial diagnosis were identified as independent prognostic factors for endometrial cancer [29]. Our results demonstrate that the cell-free dsDNA level is a prognostic marker in endometrial cancer. To the best of our knowledge, this is the first study to demonstrate that NET markers are prognostic factors in endometrial cancer. In our previous study, neutrophil elastase was shown to be a prognostic factor for high-grade serous ovarian cancer [18]. Ovarian cancers usually exhibit relatively large tumors, whereas endometrial cancers usually exhibit small tumors. Nonetheless, in our study, circulating cell-free dsDNA was shown to be a significant prognostic marker for endometrial cancer.
NET formed in the tumor microenvironment are abundant in neutrophil proteases that decompose laminin in the extracellular matrix and activate dormant cancer cells [7]. NET contribute to cancer cell proliferation via immunosuppression and angiogenesis [[8], [9], [10]]. In addition, NET wrap tumor cells in the circulatory system and aid in their adhesion to distant organs [11]. Therefore, NET formation in tumor tissues could potentiate cancer progression, which may indicate poor survival in cancer patients. In our study, cell-free dsDNA was the only prognostic marker among the three NET markers. Cell-free dsDNA levels measured at baseline have prognostic value for OS and PFS in several types of cancers, such as colorectal, lung, and ovarian cancer [[30], [31], [32]]. A recent report demonstrated that the levels of cell-free dsDNA in the serum of patients with endometrial cancer were considerably elevated in high-grade endometrial cancer (grade 2 or 3) compared with those with benign lesions [33]. During NET formation, cell-free dsDNA is released after loss of the nuclear envelope and mixing of karyosomes and cytoplasmic granules in human neutrophils [34]. It can be speculated that cell-free dsDNA, which is representative of NET formation, is a significant prognostic marker owing to its sensitive nature.
The strength of this study is that it is the first study elucidating the diagnostic performance and prognostic impact of circulating NET markers in endometrial cancer. Additionally, the study benefited from the confirmation of circulating NET marker levels using the same experimental methods as in our previous studies on different types of malignancy [18,[22], [23], [24]]. Patients with endometrial cancer in our cohort study were managed by expert gynecologic oncologists and radiation oncologists who are faculty in our institutions, ensuring consistency in treatment and follow-up.
The current study had several limitations. First, this was a small retrospective case-control study conducted at a single center. Second, the control group in our study included only healthy controls; patients with non-cancerous endometrial diseases, such as endometrial hyperplasia or endometritis, were not included. Third, patients with endometrial cancer were considerably older than the healthy controls in our study. Age-related decline in NET formation has been reported [35]; therefore, it is speculated that there is no possibility of age-related NET elevation in patients.
In this study, high levels of circulating NET markers were observed in patients with endometrial cancer. Diagnostic performance of circulating NET markers needs to be validated with a larger cohort size and multiple centers, including patients with varying ages and non-cancerous endometrial diseases. Further studies, in combination with other potential biomarkers and non-invasive screening strategies, will improve the detection of patients eligible for invasive endometrial biopsy to confirm endometrial cancer. In this respect, the considerable elevation of cell-free dsDNA in our results may have potential as screening markers for endometrial cancer, after clinical validation in future prospective studies.
Our findings also suggest that circulating cell-free dsDNA levels may be useful prognostic markers for endometrial cancer. Moreover, its prognostic value was independent of FIGO stage, histology, lymphovascular space invasion, and lymph node involvement, which implies its potential use as a beneficial prognostic marker in the clinical field. Future prospective studies are warranted to validate the prognostic value of cell-free dsDNA levels.
In conclusion, we demonstrated that three circulating NET markers were elevated in endometrial cancer. Moreover, cell-free dsDNA has been identified as an independent prognostic marker for PFS and OS.
Funding
This research was supported by a grant of Korean Cell-Based Artificial Blood Project funded by the Korean government (The Ministry of Science and ICT, The Ministry of Trade, Industry and Energy, the Ministry of Health & Welfare, the Ministry of Food and Drug Safety; No. HX23C1718) and by a grant no 0320220430 from the Seoul National University Hospital Research Fund (No. 0320220430).
Data availability statement
External researchers can make written requests to the corresponding authors (HKK and ML) for sharing of all data relevant to the study.
CRediT authorship contribution statement
Yeonju Seo: Writing – review & editing, Writing – original draft, Visualization, Software, Investigation, Formal analysis, Data curation, Conceptualization. Se Ik Kim: Writing – review & editing, Writing – original draft, Visualization, Investigation, Formal analysis, Data curation, Conceptualization. Sang Hoon Song: Writing – review & editing, Formal analysis, Data curation. Jisoo G. Kim: Writing – review & editing, Writing – original draft, Visualization, Validation. Ja-Yoon Gu: Writing – review & editing, Data curation. Hye Won Jeon: Writing – review & editing, Validation, Investigation. Maria Lee: Writing – review & editing, Validation, Supervision, Resources, Investigation, Conceptualization. Hyun Kyung Kim: Writing – review & editing, Validation, Supervision, Resources, Conceptualization.
Declaration of competing interest
The authors declare that they have no competing interests.
Acknowledgments
The biospecimens and data used in this study were provided by the Biobank of Seoul National University Hospital, a member of Korea Biobank Network.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.tranon.2024.102072.
Contributor Information
Maria Lee, Email: marialee@snu.ac.kr.
Hyun Kyung Kim, Email: lukekhk@snu.ac.kr.
Appendix. Supplementary materials
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R.L., Miller K.D., Wagle N.S., Jemal A. Cancer statistics, 2023. CA Cancer J. Clin. 2023;73(1):17–48. doi: 10.3322/caac.21763. [DOI] [PubMed] [Google Scholar]
- 3.Lim M.C., Won Y.J., Ko M.J., Kim M., Shim S.H., Suh D.H., et al. Incidence of cervical, endometrial, and ovarian cancer in Korea during 1999-2015. J. Gynecol. Oncol. 2019;30(1):e38. doi: 10.3802/jgo.2019.30.e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greten F.R., Grivennikov S.I. Inflammation and cancer: triggers, mechanisms, and consequences. Immunity. 2019;51(1):27–41. doi: 10.1016/j.immuni.2019.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brinkmann V., Reichard U., Goosmann C., Fauler B., Uhlemann Y., Weiss D.S., et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303(5663):1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 6.Yang H., Biermann M.H., Brauner J.M., Liu Y., Zhao Y., Herrmann M. New insights into neutrophil extracellular traps: mechanisms of formation and role in inflammation. Front. Immunol. 2016;7:302. doi: 10.3389/fimmu.2016.00302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Albrengues J., Shields M.A., Ng D., Park C.G., Ambrico A., Poindexter M.E., et al. Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. Science. 2018;361(6409) doi: 10.1126/science.aao4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chirivì M., Maiullari F., Milan M., Presutti D., Cordiglieri C., Crosti M., et al. tumor extracellular matrix stiffness promptly modulates the phenotype and gene expression of infiltrating T lymphocytes. Int. J. Mol. Sci. 2021;22(11) doi: 10.3390/ijms22115862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mantovani A., Cassatella M.A., Costantini C., Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat. Rev. Immunol. 2011;11(8):519–531. doi: 10.1038/nri3024. [DOI] [PubMed] [Google Scholar]
- 10.Poto R., Cristinziano L., Modestino L., de Paulis A., Marone G., Loffredo S., et al. Neutrophil extracellular traps, angiogenesis and cancer. Biomedicines. 2022;10(2) doi: 10.3390/biomedicines10020431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cools-Lartigue J., Spicer J., McDonald B., Gowing S., Chow S., Giannias B., et al. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J. Clin. Investig. 2013;123(8):3446–3458. doi: 10.1172/JCI67484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ronchetti L., Terrenato I., Ferretti M., Corrado G., Goeman F., Donzelli S., et al. Circulating cell free DNA and citrullinated histone H3 as useful biomarkers of NETosis in endometrial cancer. J. Exp. Clin. Cancer Res. 2022;41(1):151. doi: 10.1186/s13046-022-02359-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tilg H., Moschen A.R. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat. Rev. Immunol. 2006;6(10):772–783. doi: 10.1038/nri1937. [DOI] [PubMed] [Google Scholar]
- 14.Mu N., Zhu Y., Wang Y., Zhang H., Xue F. Insulin resistance: a significant risk factor of endometrial cancer. Gynecol. Oncol. 2012;125(3):751–757. doi: 10.1016/j.ygyno.2012.03.032. [DOI] [PubMed] [Google Scholar]
- 15.MacKintosh M.L., Derbyshire A.E., McVey R.J., Bolton J., Nickkho-Amiry M., Higgins C.L., et al. The impact of obesity and bariatric surgery on circulating and tissue biomarkers of endometrial cancer risk. Int. J. Cancer. 2019;144(3):641–650. doi: 10.1002/ijc.31913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Concin N., Matias-Guiu X., Vergote I., Cibula D., Mirza M.R., Marnitz S., et al. ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Int. J. Gynecol. Cancer. 2021;31(1):12–39. doi: 10.1136/ijgc-2020-002230. [DOI] [PubMed] [Google Scholar]
- 17.Eisenhauer E.A., Therasse P., Bogaerts J., Schwartz L.H., Sargent D., Ford R., et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur. J. Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 18.Kim J.G., Kim S.I., Song S.H., Gu J.Y., Lee M., Kim H.K. Diagnostic and prognostic role of circulating neutrophil extracellular trap markers and prekallikrein in patients with high-grade serous ovarian cancer. Front. Oncol. 2022;12 doi: 10.3389/fonc.2022.992056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Demers M., Krause D.S., Schatzberg D., Martinod K., Voorhees J.R., Fuchs T.A., et al. Cancers predispose neutrophils to release extracellular DNA traps that contribute to cancer-associated thrombosis. Proc. Natl. Acad. Sci. U. S. A. 2012;109(32):13076–13081. doi: 10.1073/pnas.1200419109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen Y., Hu H., Tan S., Dong Q., Fan X., Wang Y., et al. The role of neutrophil extracellular traps in cancer progression, metastasis and therapy. Exp. Hematol. Oncol. 2022;11(1):99. doi: 10.1186/s40164-022-00345-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao J., Jin J. Neutrophil extracellular traps: new players in cancer research. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.937565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seo J.D., Gu J.Y., Jung H.S., Kim Y.J., Kim H.K. Contact system activation and neutrophil extracellular trap markers: risk factors for portal vein thrombosis in patients with hepatocellular carcinoma. Clin. Appl. Thromb. Hemost. 2019;25 doi: 10.1177/1076029618825310. 1076029618825310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoo H.J., Lee J.S., Kim J.E., Gu J., Koh Y., Kim I., et al. Extracellular histone released from leukemic cells increases their adhesion to endothelium and protects them from spontaneous and chemotherapy-induced leukemic cell death. PLoS One. 2016;11(10) doi: 10.1371/journal.pone.0163982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jung H.S., Gu J., Kim J.E., Nam Y., Song J.W., Kim H.K. Cancer cell-induced neutrophil extracellular traps promote both hypercoagulability and cancer progression. PLoS One. 2019;14(4) doi: 10.1371/journal.pone.0216055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li J., Wang X., Qu W., Wang J., Jiang S.W. Comparison of serum human epididymis protein 4 and CA125 on endometrial cancer detection: a meta-analysis. Clin. Chim. Acta. 2019;488:215–220. doi: 10.1016/j.cca.2018.11.011. [DOI] [PubMed] [Google Scholar]
- 26.Crosbie E.J., Kitson S.J., McAlpine J.N., Mukhopadhyay A., Powell M.E., Singh N. Endometrial cancer. Lancet. 2022;399(10333):1412–1428. doi: 10.1016/S0140-6736(22)00323-3. [DOI] [PubMed] [Google Scholar]
- 27.Angioli R., Plotti F., Capriglione S., Montera R., Damiani P., Ricciardi R., et al. The role of novel biomarker HE4 in endometrial cancer: a case control prospective study. Tumour Biol. 2013;34(1):571–576. doi: 10.1007/s13277-012-0583-0. [DOI] [PubMed] [Google Scholar]
- 28.Njoku K., Barr C.E., Crosbie E.J. Current and emerging prognostic biomarkers in endometrial cancer. Front. Oncol. 2022;12 doi: 10.3389/fonc.2022.890908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takahashi R., Mabuchi S., Kuroda H., Kozasa K., Yokoi E., Matsumoto Y., et al. The significance of pretreatment thrombocytosis and its association with neutrophilia in patients with surgically treated endometrial cancer. Int. J. Gynecol. Cancer. 2017;27(7):1399–1407. doi: 10.1097/IGC.0000000000001019. [DOI] [PubMed] [Google Scholar]
- 30.Spindler K.L., Appelt A.L., Pallisgaard N., Andersen R.F., Brandslund I., Jakobsen A. Cell-free DNA in healthy individuals, noncancerous disease and strong prognostic value in colorectal cancer. Int. J. Cancer. 2014;135(12):2984–2991. doi: 10.1002/ijc.28946. [DOI] [PubMed] [Google Scholar]
- 31.Tissot C., Toffart A.C., Villar S., Souquet P.J., Merle P., Moro-Sibilot D., et al. Circulating free DNA concentration is an independent prognostic biomarker in lung cancer. Eur. Respir. J. 2015;46(6):1773–1780. doi: 10.1183/13993003.00676-2015. [DOI] [PubMed] [Google Scholar]
- 32.Kamat A.A., Baldwin M., Urbauer D., Dang D., Han L.Y., Godwin A., et al. Plasma cell-free DNA in ovarian cancer: an independent prognostic biomarker. Cancer. 2010;116(8):1918–1925. doi: 10.1002/cncr.24997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cicchillitti L., Corrado G., De Angeli M., Mancini E., Baiocco E., Patrizi L., et al. Circulating cell-free DNA content as blood based biomarker in endometrial cancer. Oncotarget. 2017;8(70):115230–115243. doi: 10.18632/oncotarget.23247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fuchs T.A., Abed U., Goosmann C., Hurwitz R., Schulze I., Wahn V., et al. Novel cell death program leads to neutrophil extracellular traps. J. Cell Biol. 2007;176(2):231–241. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hazeldine J., Harris P., Chapple I.L., Grant M., Greenwood H., Livesey A., et al. Impaired neutrophil extracellular trap formation: a novel defect in the innate immune system of aged individuals. Aging Cell. 2014;13(4):690–698. doi: 10.1111/acel.12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
External researchers can make written requests to the corresponding authors (HKK and ML) for sharing of all data relevant to the study.