Abstract
Prostate cancer rarely metastasizes to the stomach and kidneys. We report a 73-year-old male with such spread, highlighting significant clinical challenges. Initially diagnosed via biopsy and imaging, he received hormone therapy and cytoreductive radical prostatectomy. Despite initial management, the cancer progressed to metastatic castration-resistant prostate cancer, with gastric and renal metastases confirmed by imaging and biopsy. This case emphasizes the need for awareness of rare metastatic sites, comprehensive diagnostic evaluations, and further research into these atypical metastases to improve patient outcomes and develop better treatment strategies for managing advanced prostate cancer effectively.
Keywords: Metastasis, Prostate cancer, Kidney, Stomach
1. Introduction
Prostate cancer, which affects 5 % of men younger than 30 years of age and 59 % of men older than 79 years of age, is one of the most prevalent malignancies among men worldwide.1 Prostate cancer is typically confined to the prostate gland during its early stages, but it is capable of metastasizing to the bones, lymph nodes, lungs, and other organs.2 Although the majority of prostate cancer metastases are found in proximate organs and tissues, rarer sites of metastases, including the anal canal, and testis, have been reported.3,4 These types of metastases not only highlight the heterogeneity of the metastatic behavior of prostate cancer but also create unique challenges for the clinical diagnosis and treatment. Similar cases have been relatively under-reported in the literature. We performed an in-depth examination of this case to provide a better understanding of the unusual patterns of prostate cancer metastases and their potential implications for clinical practice. In this report, we describe the uncommon progression of prostate cancer with metastases to the stomach and kidneys.
2. Case presentation
A 73-year-old male patient with a history of type 2 diabetes mellitus that was managed with medication presented with poor oral intake, general weakness, and significant weight loss (15 kg over the course of 2 months) after receiving the Moderna vaccine for coronavirus disease 2019 (COVID-19). The patient reported persistent urinary frequency, incomplete bladder emptying, and nocturia twice per night for several years. Computed tomography revealed no abdominal abnormalities; however, a 7.2- × 5.3-cm soft tissue mass in the pelvic cavity that involved the prostate gland and the base of the urinary bladder was identified. An evaluation of his prostate-specific antigen (PSA) level indicated that it had increased to >1000 ng/mL. A digital rectal examination revealed multiple firm nodules in both prostate lobes. Magnetic resonance imaging showed advanced prostate cancer (5.6× 4.4 × 4.6 cm) with extension to the seminal vesicles, bladder invasion, bilateral pelvic lymphadenopathy, and diffuse bone metastases.
On November 9, 2021, transrectal ultrasonography and prostate biopsy confirmed adenocarcinoma of the prostate with Gleason pattern 5 + 4 that involved both lobes. A bone scan on November 11, 2021, showed diffusely increased uptake across the spine, pelvis, thoracic cage, shoulders, and femurs, indicating diffuse bone metastases. The clinical diagnosis was metastatic castration-sensitive prostate cancer with bone metastases (cT2cN0M1b, stage IVB). Because of the COVID-19 pandemic in Taiwan, the patient received androgen deprivation therapy for 6 months, which resulted in improved voiding function and a significant decrease in the PSA level to 0.523 ng/mL. A subsequent magnetic resonance imaging examination on June 2, 2022, showed reduction of the prostate and no significant abnormalities.
On June 14, 2024, the patient underwent robotic-assisted cytoreductive prostatectomy and bilateral pelvic lymph node dissection because of persistent lower urinary tract symptoms. A pathological examination confirmed adenocarcinoma of the prostate (solid and acinar types) with 2.86 % involvement of the prostatic tissue and metastatic adenocarcinoma in the left obturator lymph nodes (ypT2N1cM1b, stage IVB). Postoperatively, the PSA levels were maintained at less than 4 ng/mL with continued androgen deprivation therapy.
In March 2023, the PSA levels increased to 8.1 ng/mL, thus prompting a switch to enzalutamide for metastatic castration-resistant prostate cancer. The patient also received targeted radiotherapy of the left rib and right scapula. Subsequent imaging revealed a new disease focus at the right sacrum and a new 1.1-cm hypoenhancing liver nodule. On December 1, 2023, the PSA level increased to 257 ng/mL, and chemotherapy comprising docetaxel was initiated. The second cycle was postponed because the patient experienced general malaise; therefore, supportive care was prioritized.
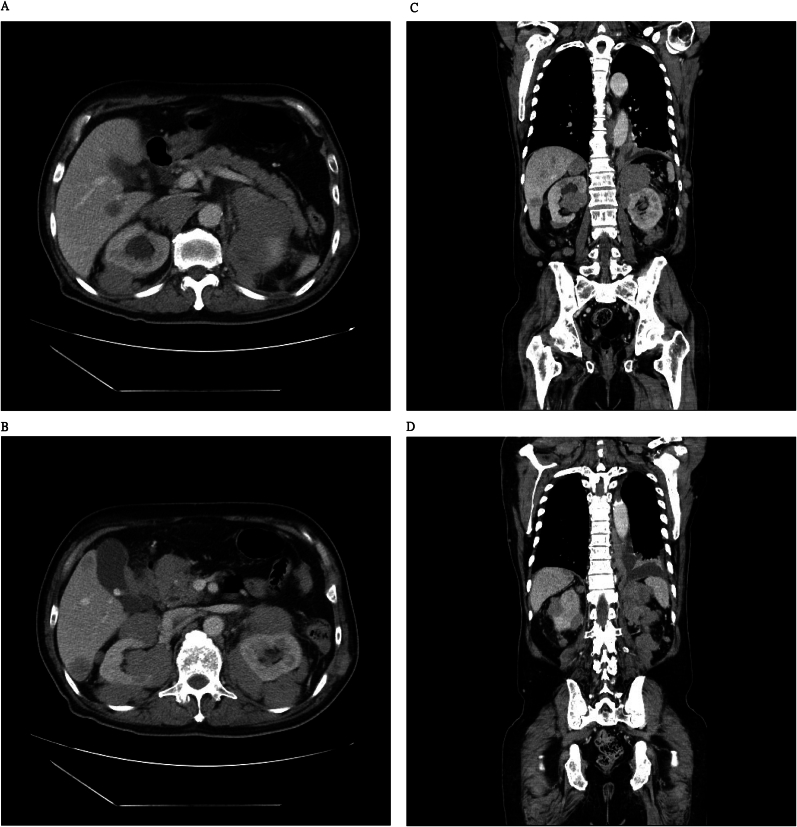
On February 10, 2024, the patient experienced fever and malaise. Computed tomography revealed left lower lobe pneumonia, liver metastasis, enlarged hypoenhancing renal and adrenal nodules, and osteoblastic lesions (Fig. 1). The patient was treated with levofloxacin, resulting in fever resolution and leukocytosis improvement. However, the PSA levels increased significantly to 1431.5 ng/mL.
Fig. 1.
Enlarged bilateral hypoenhanced renal nodules and masses in the abdomen observed using computed tomography (CT) with contrast.
(A and B) Axial view. (C and D) Coronal view.
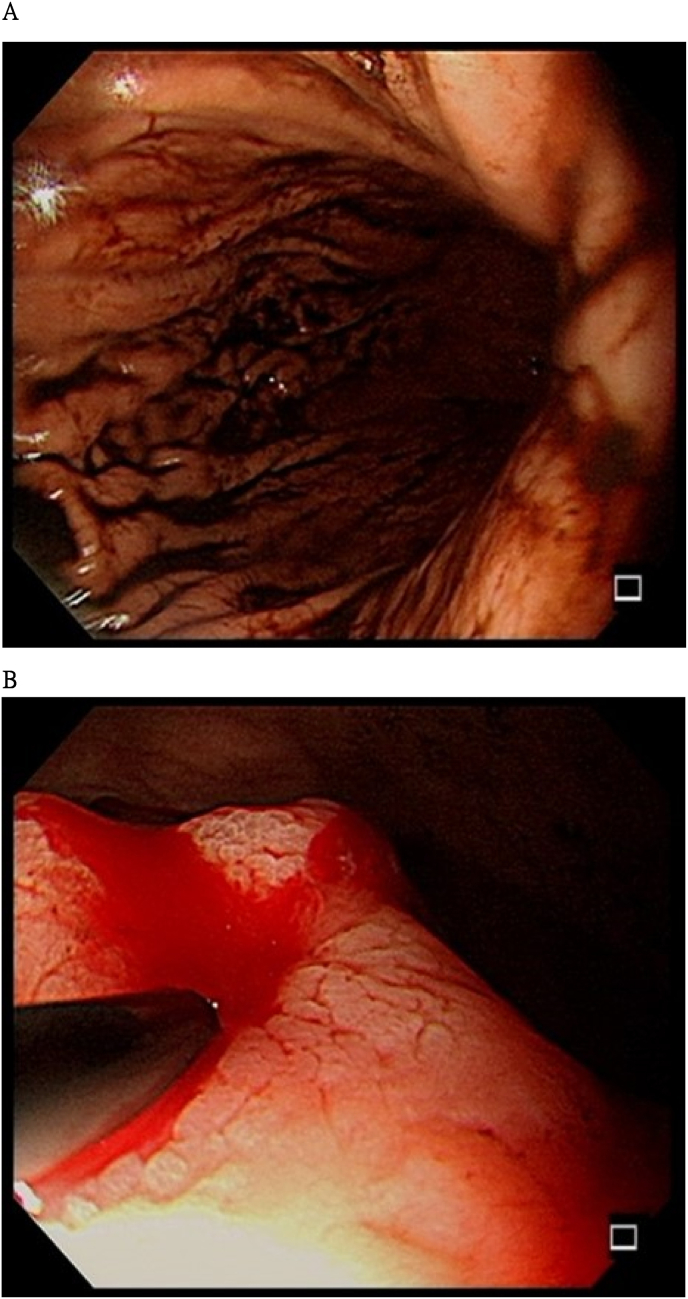
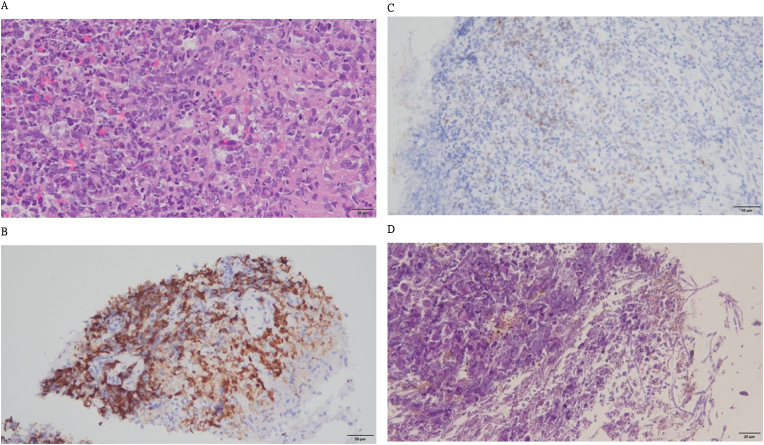
On February 19, 2024, anemia, hemoptysis, and massive coffee ground emesis were observed. Esophagogastroduodenoscopy revealed a 1-cm ulcerative gastric mass; therefore, a biopsy was performed (Fig. 2). Histopathological features of the gastric biopsy sample included scattered pleomorphic and hyperchromatic tumor cells that infiltrated the stroma (Fig. 3A) and positive immunohistochemical expressions of CK18 (Fig. 3B) and NKX3.1 (Fig. 3C), consistent with poorly differentiated metastatic adenocarcinoma of prostatic origin. Focal ulceration with necrosis and Candida infection were also observed (Fig. 3D). Against medical advice, the family of the patient requested his discharge, and he died at home as a result of hypovolemic shock and metastatic castration-resistant prostate cancer.
Fig. 2.
Esophagogastroduodenoscopy was performed by a gastroenterologist because massive coffee ground emesis occurred.
(A) Esophagitis with a short mucosal break at the esophagus–cardiac junction is observed in the esophagus. (B) A 1-cm ulcerative mass in the stomach with recent bleeding after endoscopic injection sclerotherapy. Four biopsies and a Campylobacter-like organism test were performed.
Fig. 3.
Histopathological features and immunohistochemical examination results of the biopsy.
(A) Poorly differentiated carcinoma composed of hyperchromatic nuclei and prominent nucleoli arranged as discohesive cells and solid nested patterns with stromal necrosis invasion (hematoxylin and eosin [H&E]; original magnification, × 400). (B) CK18 immunohistochemical staining highlights tumor invasion (CK18; original magnification, × 200). (C) NKX3.1 immunohistochemical staining shows focally positive tumor cells compatible with metastatic adenocarcinoma of prostatic origin (NKX3.1; original magnification, × 200). (D) Scattered Candida pseudohyphae within the cell debris (H&E; original magnification, × 400).
3. Discussion
This case report is the first to highlight the rare and clinically significant incidence of simultaneous gastric and renal metastases stemming from prostate cancer. An exhaustive literature review revealed only 17 documented cases of gastric metastases attributable to prostate cancer, and no instances of renal metastases from this primary source have been reported.5 This case provides an important understanding of the metastatic potential and patterns of prostate cancer, thus underscoring the need for ongoing research and awareness within the clinical community.
The mechanism of prostate cancer metastasis is a complex process that involves various molecular, cellular, and environmental factors and remains a crucial area of ongoing research. Transcription factors, such as Stat 3, play a significant role in the metastatic progression of prostate cancer; therefore, transcription factors are necessary for progression from a primary tumor to metastatic prostate cancer.6 Prostate cancer metastasis can manifest in diverse locations, including regional tissue, lymph nodes, and bones, because of the mechanisms of lymph node metastasis, including lymphangiogenesis and the formation of new lymphatic vessels induced by tumor-secreted factors such as vascular endothelial growth factor-C. However, the requirement of lymphangiogenesis for lymph node metastasis remains a subject of debate.7,8 Additionally, the expression of the chemokine receptor CCR7, which promotes cancer progression through lymph node metastasis, is induced by tumor necrosis factor-α (TNF-α) in prostate cancer cells, suggesting that TNF-α and the CCL21/CCR7 axis might increase the metastatic potential of prostate cancer cells in lymph node metastasis.9 Prostate cancer metastasis to the bone is induced by interactions between prostate cancer exosomes, growth factors, and the cell microenvironment, thus highlighting the critical role of the tumor microenvironment in metastasis.10 An understanding of the mechanisms that lead to the development of bone metastasis is of significant interest because the bones are common metastasis sites.11
Primary metastases of prostate cancer occur in the bones and lymph nodes, and visceral metastasis is less common. Although some case reports have described less common sites of metastasis, our case of prostate cancer that metastasized to the kidney and stomach elucidated a rare phenomenon.12 This subject is limited to case reports and clinical observations in the literature. Case reports have described individuals with prostate cancer who later experienced stomach metastasis and presented with symptoms such as abdominal discomfort, nausea, vomiting, and upper gastrointestinal bleeding years after the initial diagnosis of prostate cancer.13 The diagnosis of stomach metastasis is often confirmed by endoscopy and histopathological examinations that reveal adenocarcinoma of prostatic origin. These metastases can present as ulcerations in the gastric body, and their diagnosis is crucial for appropriate management.14 Based on a previous study that suggested that lymphatics may facilitate metastases to the gastrointestinal tract because the prostate is well-supplied with lymphatic channels,15 we proposed the following possible mechanism of stomach metastasis: alterations in the tumor microenvironment, such as changes in adhesion molecules and the extracellular matrix, may facilitate the dissemination of cancer cells to distant sites. However, this potential mechanism requires further investigation. Because there is no worldwide consensus regarding the treatment of prostate cancer that has metastasized to the stomach, empirical therapy, including chemotherapy, hormonal therapy, and palliative care, is required because of the lack of systematic evaluations of this specific metastasis site. We reviewed 17 case reports and found that the average patient age was 68 years, and that most prostate cancer was in the metastatic stage at the time of the initial diagnosis. Most patients presented with gastroenteritis symptoms, such as nausea, vomiting, epigastric pain, or anemia, and all histological subtypes were adenocarcinoma. According to the literature, the treatment of gastric metastasis is diverse. Five patients underwent endocrine therapy, two patients underwent surgery, three patients underwent chemotherapy, and two patients underwent radiotherapy.5
In the context of prostate cancer, the occurrence of kidney metastases, although rare, represents a significant deviation from the common metastatic pathways such as the bones and lymph nodes. The mechanisms underlying this atypical metastatic behavior remain poorly understood, suggesting a complex interplay of tumor biology and host factors. The clinical implications of such metastases are profound and affect therapeutic decisions and the prognosis. Typically, prostate cancer that metastasizes to the kidney may present with non-specific symptoms such as flank pain or hematuria, thus necessitating a differential diagnosis to exclude primary renal pathologies.16 The diagnostic approach often involves imaging studies and biopsy. Immunohistochemical staining plays a crucial role in confirming the prostatic origin of the renal lesion. Recent studies have highlighted the rarity of renal metastases from prostate cancer and emphasized the need for increased clinical suspicion and thorough diagnostic investigations of patients presenting with renal abnormalities. For instance, a comprehensive analysis of 35 cases revealed that renal metastases are not only uncommon but also associated with an advanced stage of prostate cancer and poor outcomes.17 This is consistent with the findings of another study that documented a case of prostate adenocarcinoma that metastasized to the renal and retroperitoneal regions, further complicating the clinical management and treatment strategy.18
Because there have been few detailed studies of the mechanism of prostate cancer metastasis, specifically metastases to the stomach and kidneys, further research is required to elucidate the unique pathways involved. The use of new blood markers and imaging tools has greatly improved early diagnoses.19,20 However, further genetic research and molecular research should be performed. An understanding of the molecular and cellular mechanisms of this unusual metastatic route could allow for new targeted therapies and improve management strategies. Therefore, ongoing research and case studies are essential to the elucidation of this rare phenomenon and enhancement of the overall therapeutic setting for metastatic prostate cancer.
Prostate cancer is characterized by its potential to metastasize to the bones and lymph nodes. However, although rare, atypical metastatic pathways, including the kidney and stomach, present significant clinical challenges and underscore the complex nature of disease progression. An understanding of these rare pathways is crucial to improving the diagnostic accuracy, tailoring treatment strategies, and enhancing patient outcomes.
4. Conclusion
Prostate cancer metastases to the kidneys and stomach are rare. Further research is necessary to understand the mechanisms underlying cancer cell dissemination and organ tropism associated with prostate cancer metastases to the kidneys and stomach. Studies should focus on the molecular biology and cellular biology of metastases to enable the development of targeted therapies.
Funding
Not applicable.
Informed consent
Informed written consent was obtained from the patient for publication of this report and any accompanying images.
Supported by
Nil.
Informed consent statement
Informed written consent was obtained from the patient for publication of this report and any accompanying images.
Conflict-of-interest statement
The authors declare that they have no conflict of interest to disclose.
CARE checklist (2016) statement
The authors have read the CARE Checklist (2016), and the manuscript was prepared and revised according to the CARE Checklist (2016).
CRediT authorship contribution statement
Jhe-Yuan Hsu: Data curation, Formal analysis, Writing – original draft, Writing – review & editing. Yi-Sheng Lin: Conceptualization, Formal analysis, Validation, Writing – original draft, Writing – review & editing. Li-Hua Huang: Conceptualization, Investigation, Methodology. Wei-Chun Wen: Conceptualization, Investigation, Methodology. Hong-Wei Gao: Validation, Visualization, Writing – review & editing. Chao-Yu Hsu: Conceptualization, Supervision. Yen-Chuan Ou: Conceptualization, Supervision, Methodology. Min-Che Tung: Conceptualization, Supervision.
Declaration of competing interest
The authors declare that they have no conflict of interest to disclose.
Acknowledgements
We extend our heartfelt appreciation to the patient for consenting to the publication of his case. Additionally, we would like to express our special thanks to the staff of Tungs' Taichung MetroHarbor Hospital for their exemplary care and dedication to patient service.
References
- 1.Bell K.J.L., Del Mar C., Wright G., Dickinson J., Glasziou P. Prevalence of incidental prostate cancer: a systematic review of autopsy studies. Int J Cancer. 2015;137(7):1749–1757. doi: 10.1002/ijc.29538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bubendorf L., Schöpfer A., Wagner U., et al. Metastatic patterns of prostate cancer: an autopsy study of 1,589 patients. Hum Pathol. 2000;31(5):578–583. doi: 10.1053/HP.2000.6698. [DOI] [PubMed] [Google Scholar]
- 3.Dulskas A., Cereska V., Zurauskas E., Stratilatovas E., Jankevicius F. Prostate cancer solitary metastasis to anal canal: case report and review of literature. BMC Cancer. 2019 April 23;19(1):374. doi: 10.1186/s12885-019-5573-9. PMID: 31014272, PMCID: PMC6480615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smelzo S., Mantica G., Tenace N.P., et al. Prostate cancer testicular metastasis: are they underestimated? Case report and analysis of the literature. Urologia. 2022 November;89(4):645–647. doi: 10.1177/03915603211009118. Epub April 8 2021. PMID: 33832367. [DOI] [PubMed] [Google Scholar]
- 5.Moshref L., Abidullah M., Czaykowski P., Chowdhury A., Wightman R., Hebbard P. Prostate cancer metastasis to stomach: a case report and review of literature. Curr Oncol. 2023 March 30;30(4):3901–3914. doi: 10.3390/curroncol30040295. PMID: 37185408, PMCID: PMC10137262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nevalainen M.T., Thomas Jefferson Univ, Pa P. Metastatic Progression of Prostate Cancer. 2007. Transcription factor. [Google Scholar]
- 7.Datta K., Muders M., Zhang H., Tindall D.J. Mechanism of lymph node metastasis in prostate cancer. Future Oncol. 2010;6(5):823–836. doi: 10.2217/fon.10.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen C.H., Chung H.J., Lin A.T.L., Chen K.K. Is it worth removing prostatic anterior fat pad to detect lymph node metastasis of prostate cancer during robotic-assisted radical prostatectomy? Urol Sci. November-December 2018;29(6):284–287. doi: 10.4103/UROS.UROS_73_18. [DOI] [Google Scholar]
- 9.Maolake A., Izumi K., Natsagdorj A., et al. Tumor necrosis factor-α induces prostate cancer cell migration in lymphatic metastasis through CCR7 upregulation. Cancer Sci. 2018;109(5):1524–1531. doi: 10.1111/cas.13586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Famuyiwa T.O. JoubinJebelli. Prostate cancer cells produce exosomes modulating metastasis to the bones. J Cancer Prev Curr Res. 2017;8(2) doi: 10.15406/jcpcr.2017.08.00269. [DOI] [Google Scholar]
- 11.Ye L., Kynaston H.G., Jiang W.G. Bone metastasis in prostate cancer: molecular and cellular mechanisms. Int J Mol Med. 2007;20(1):103–111. doi: 10.3892/ijmm.20.1.103. [DOI] [PubMed] [Google Scholar]
- 12.Zhong S.R., Lai Y.W., Wu Y.Y. Accidently found metastatic adenocarcinoma of prostate in an incised inguinal hernia sac. Urol Sci. May-June 2020;31(3):136–138. doi: 10.4103/UROS.UROS_94_19. [DOI] [Google Scholar]
- 13.Hong K.P., Lee S.J., Hong G.S., Yoon H., Shim B.S. Prostate cancer metastasis to the stomach. Korean J Urol. 2010;51(6):431–433. doi: 10.4111/kju.2010.51.6.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel H., Kumar A., Shaaban H., et al. Synchronous metastasis of prostate adenocarcinoma to the stomach and colon: a case report. N Am J Med Sci. 2014;6(3):152–154. doi: 10.4103/1947-2714.128478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Green L.K. Hematogenous metastases to the stomach. A review of 67 cases. Cancer. 1990;65(7):1596–1600. doi: 10.1002/1097-0142(19900401)65:7<1596::aid-cncr2820650724>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 16.Munshi F., Shinder B.M., Sadimin E., Mayer T.M., Singer E.A. Metastatic prostate cancer to the renal pelvis and proximal ureter: a case report and review of the literature. Cancer Stud Ther. 2019 September;4(4):119. Epub August 11 2019. PMID: 32148662, PMCID: PMC7059776. [PMC free article] [PubMed] [Google Scholar]
- 17.Jinchao C., Nienie Q., Shaoxing Z. Metastases to the kidney: an analysis of 35 cases and a review of literature. Front Oncol. 2021;10 doi: 10.3389/fonc.2020.632221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen C., He H., Yu Z., Qiu Y., Wang X. Renal and retroperitoneal metastasis from prostate adenocarcinoma: a case report. World J Surg Oncol. 2016;14:74. doi: 10.1186/s12957-016-0834-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin K.H., Hsieh T.Y., Chen C.H., Pu Y.S. Digital rectal examination still plays a crucial role of predicting outcomes in the prostate cancer patients undergoing primary total prostate cryoablation. Urol Sci. October-December 2023;34(4):187–193. doi: 10.4103/UROS.UROS_139_22. [DOI] [Google Scholar]
- 20.Chiu S.T., Chen Y.C., Huang C.Y., et al. Prostate biopsy strategy integrating prostate health index and multiparametric magnetic resonance imaging optimizes the predictive value of clinically significant prostate cancer in prostate imaging reporting and data system gray-zone imaging. Urol Sci. April-June 2023;34(2):86–92. doi: 10.4103/UROS.UROS_33_22. [DOI] [Google Scholar]