Abstract
Generalized lymphangiomatosis (GLA) is a very rare condition in adults, characterized by diffused proliferation of lymphatic vessels that requires differential diagnosis from other vascular disorders such as cavernous or capillary hemangioma. This is because of overlapping characteristics on histopathological examination. Therefore, imaging features such as CT and MRI are useful to evaluate morphological characteristics, location, and the extent of the spread as well as differential diagnosis with other pathologies. We report a case of a 22-year-old female patient with left hemothorax after thoracoscopic sympathectomy for the treatment of hand sweating. The patient underwent drainage and cleaning of the left pleura. Chest computed tomography and lumbar spine magnetic resonance imaging showed multiple fat infiltration foci of the lumbar spine and pelvis. A wing bone biopsy of the pelvis was initially performed for the diagnosis of chronic osteomyelitis. Afterwards, the patient continued to have pleural drainage and developed hemothorax and chylothorax, amounting to 3000 mL. The chest tube was blocked with a mixture of biological glue and lipiodol (2 mL of glue, ratio of glue to lipiodol: 1:4) and a 3 i-ED coil complex. After the intervention, the pleural fluid decreased; the left pleural fluid was still 15 mm thick, and the amount of fluid drained after 1 week was 100 mL. Aspiration of the chest wall lesion showed fluid rich in fat droplets. Combined with the results of lumbar spine magnetic resonance imaging and the old biopsy, this was consistent with generalized lymphangiomatosis.
Keywords: Generalized lymphangiomatosis, Magnetic resonance imaging, Thoracic duct, Interventional radiology, Embolization therapeutic
Introduction
Lymphangiomatosis is a rare benign disease characterized by the proliferation and dilatation of lymphatic vessels, most commonly found in the head and neck region [1]. The disease can occur in soft tissue, bones, and various organs in the body, such as the liver, spleen, mediastinum, and lungs, leading to diverse symptoms due to damages to the corresponding organs, such as pleural effusion and pathological bone fractures.…. [2]. The underlying pathogenesis remains unclear but is generally considered to be a congenital malformation of the lymphatic system due to changes in lymphatic circulation at 14-20 weeks of fetal development [3]. These lymph vessels are dilated and filled with chyle. This disease is common in children and young people under 20 years old [4]. Its incidence is unknown due to its rarity, which poses challenges in diagnosis and treatment [5]. However, many cases of lymphangiomatosis can be confused with other vascular disorders such as cavernous or capillary hemangioma due to the overlap in the histological findings of these conditions. Therefore, imaging features play an important role in diagnosing this disease. We report the case of a 22-year-old female patient with generalized lymphatic anomaly (GLA) associated with lesions of the vertebrae, mediastinum, spleen, and chest wall soft tissue, which was diagnosed by histology.
Case report
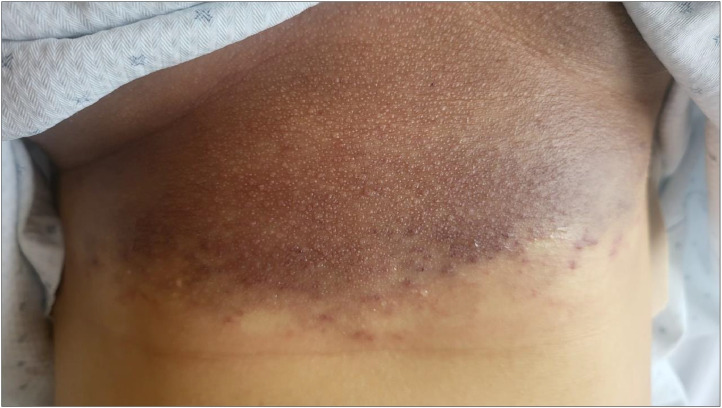
The patient is a 22-year-old female with no unusual medical history. The patient presented with a left hemothorax after a thoracoscopic sympathectomy for the treatment of hand sweating and a subcutaneous hematoma of the chest wall (Fig. 1).
Fig. 1.
Purple spots on the chest.
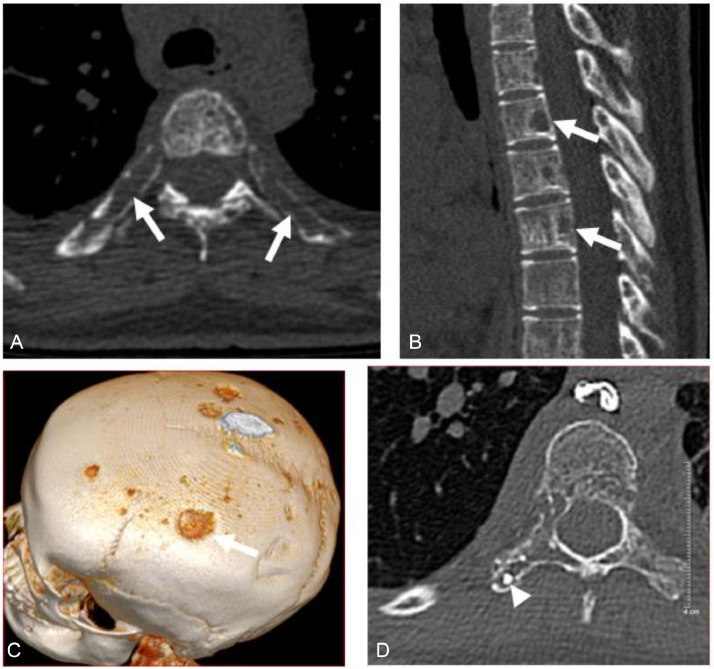
The patient had 3000 mL/day of pleural fluid drained; however, after 2 weeks of drainage, the patient still had pleural effusion. The patient was monitored by chest CT without contrast injection, which showed many foci of bone loss with unclear borders at the vertebrae and ribs (Fig 2A-D). A CT scan of the skull without contrast showed similar lesions in the skull (Fig. 2C).
Fig. 2.
(A-C) CT scan before intervention, showing multiple foci of bone loss in the posterior ribs and vertebrae (arrows). (D) CT postintervention shows lipiodol deposits in bone lytic lesions (head arrow).
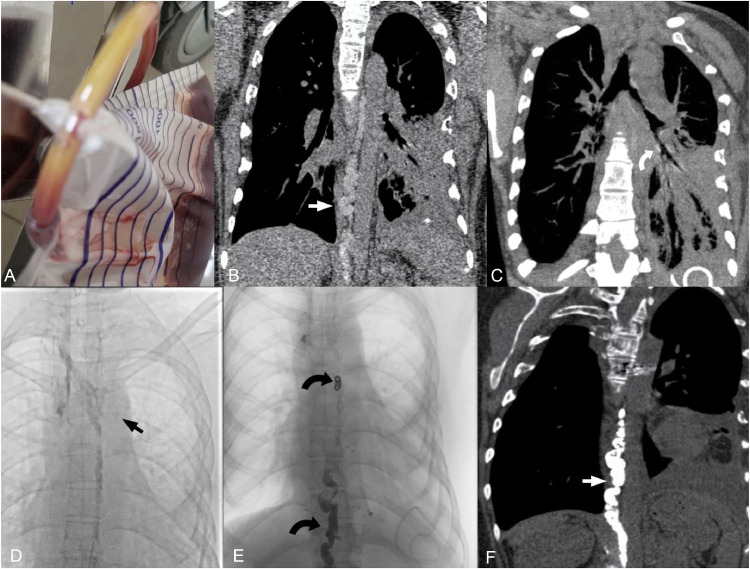
Continued monitoring of pleural fluid drainage showed a decrease in the amount of blood, however, the drainage fluid appeared milky white, resembling chylous fluid (Fig. 3A). The patient underwent testing to measure triglyceride levels in the pleural fluid, which were found to be 5.73 units. Subsequently, the patient underwent a chest computed tomography scan with contrast medium injected through the inguinal lymph nodes, revealing tortuous dilation of the thoracic duct (Fig. 3B) and contrast medium drainage through the lymphatic system into the left pleural space (Fig. 3C).
Fig. 3.
CT images and lymphatic DSA. (A) Thin red pleural fluid with cloudy yellow deposits. (B, C) CT lymphatic images show tortuous dilation of the thoracic duct (straight white arrow, B), with contrast leaking into the left hilum (curved white arrow, C). (D, E) Prenodal lymphatic DSA image with contrast leakage into the left lung (straight black arrow, D), postnodal chest tube with coils and Histoacryl and Lipiodol mixture on DSA (curved black arrow, E). Chest tube image after vascular intervention on CT scan (white arrow, F).
Following these findings, the decision was made to intervene by embolizing the chest tube with digital subtraction angiography (DSA). A Chiba 21 Gauge needle was inserted into the chest tube, and access was gained with a Progreat 2.0 microcatheter and a Boston V-18 control wire guidewire. Contrast agent injection revealed the significant dilation of chest tube and an abnormality at the entry point of the right subclavian vein, with contrast leakage into the hilum and left pleural space (Fig. 3D). The intervention first involved embolizing the chest tube filling site near the right subclavian vein entry point with 3 i-ED coils (5 × 15 mm, 5 × 15 mm, and 4 × 12 mm). Subsequently, the remaining portion of the chest tube was embolized with a mixture of Histoacryl and Lipiodol glue in a 1:4 ratio (approximately 8 mL of Lipiodol) (Fig. 3E).
One day after the intervention, a chest computed tomography scan without contrast injection revealed complete blockage of the thoracic duct by the glue (Fig. 3F).
After the intervention, the amount of pleural drainage fluid gradually decreased to 100 mL after 1 week, and the fluid became clear. Triglycerides were not detected (-) in the pleural fluid, and the patient was discharged.
Discussion
Since 2014, the term “diffuse systemic lymphangiomatosis” has been replaced by GLA to distinguish it from many other congenital lymphatic malformations [6]. This group of lymphatic anomalies includes Gorham–Stout disease, which is distinguished from GLA by progressive osteolysis and cortical invasion [7]. GLA is a benign lesion arising from proliferative lymphatic vessels, most commonly found in the head and neck region. Up to 65% of patients are children and infants, and nearly 90% of cases are diagnosed within the first 2 years of life. It is very rare in adults. The disease has an equal sex ratio and no reported correlation with familial factors [8]. The reported case involved a 22-year-old female patient, which is not a common age group for this condition.
GLA arises from abnormal development of lymphatic vessels that result in thin-walled and abnormally interconnected lymphatic vessels and can lead to symptoms related to obstruction and invasion of these structures. Multiple organs such as lungs, liver, spleen, and especially bones may be involved simultaneously in about 75% of cases [9]. Intra-abdominal lymphangiomas account for less than 1% of all cases and usually affect the retroperitoneum [10]. Clinical features are atypical and depend on the anatomical location and the extent of organ involvement. Damaged organs contain endothelial spaces surrounded by a connective tissue layer of variable thickness containing lymphoid tissue, smooth muscle, and round cells. Areas of accumulation of adipose tissue, lymphocytes, and venous stones may also be detected [11]. Therefore, systemic manifestations are very broad and can include chylothorax and pericardial effusion, diffused damage to the lungs, subcutaneous soft tissue, mediastinum, lymph nodes, bone loss, abdominal viscera, and fascial thickening, as well as the involvement of suspension, skin, cervical lymph nodes, and may be accompanied by disseminated intravascular coagulation [12]. The patient was reported to have no unusual medical history, and the finding of hemorrhagic chylothorax after thoracoscopic sympathectomy for the treatment of hand sweating may have been due to damages to the thoracic duct during surgery.
GLA patients are often asymptomatic and diagnosed incidentally based on imaging methods. Ultrasound is a useful imaging tool in the initial assessment of lesion characteristics such as morphology, location, and blood vessels. Typically, cystic lesions are multiseptated with thin walls. Depending on the nature of the fluid inside, it may show an anechoic or hyperechoic pattern representing blood, pus, or chyle [11]. CT scans and MRIs provide better information about the sizes, extent, and relationship to adjacent structures. CT scanning can detect multilocular lesions with a density roughly equivalent to that of water and aid in determining the presence of fatty and calcified components. It has been reported that small, band-like calcifications along the septum are more frequent than in the abdominal cavity [11]. Chest CT can detect interstitial thickening along with pleural effusion and mediastinal lymphadenopathy [13]. The MRI characteristics of lesions in the viscera and mesentery are very different. Depending on the nature of the lesion, the signal intensity may not be uniform, but in general, it has the nature of an exudate with decreased signal on T1-weighted images and increased signal on T2-weighted images [14]. For GLA with bone involvement, a differential diagnosis is required, including Gorham-Stout disease (GSD) and Kaposiform lymphangiomatosis (KLA) [15]. For GSD, cortical loss and progressive osteolysis are the main features [16]. On the other hand, the osteolytic lesions in lymphangioma are round, noncontiguous, and do not progress [11]. Vertebral lesions are more common in GLA than in GSD [17]. In KLA, the lesions are multiple, noncontiguous, lytic, and cortex-sparing lesions, and thoracic lesions are more common in KLA than in GLA. Thickening along the bronchovascular bundle and interlobular septal thickening are uncommon in GLA. Therefore, KLA is a more severe disease with episodes of infection and hemorrhage in the pleura and pericardium and incomplete recovery [16]. In the presented case, the patient had diffuse osteolytic lesions in the axial bones, including the vertebrae, pelvis, and ribs. These lesions were round, with no cortical destruction and no progressive edematous lesions, consistent with bone lesions in GLA. These bone lesions can lead to pathological fractures and joint deformities due to excessive bone resorption [17].
Microscopic examination of mediastinal and pulmonary lesions of GLA shows proliferation of lymphatic channels lining a complex anastomosing endothelium, causing lymphatic dilation [12]. Immunostaining of endothelial cells in the GLA was positive for D2-40, CD31, factor VIII-related antigen, and SMA, but negative for HMB-45, characteristic of lympholeiomyomatosis (in blue). Lymph node biopsy often reveals lymphoid hyperplasia that is difficult to distinguish from nonspecific chronic inflammation [4]. The patient was reported to have had a biopsy of the iliac bone and a bone marrow examination showing fibrosis and infiltration of mononuclear cells, suggesting osteomyelitis. Aspiration of soft tissue lesions under the skin of the chest wall showed subcutaneous fat granules. These findings, combined with imaging, features consistency with GLA pathology.
Lymphatic leak is a rare but serious complication that can occur after a variety of surgical procedures, such as pancreaticoduodenectomy, abdominothoracic esophagectomy, thoracic sympathectomy, and kidney surgery… [18]. The patient was admitted to the hospital due to hemorrhagic chylothorax after thoracoscopic sympathectomy. Subsequently, the chest tube was embolized with coils, histoacryl biological glue, and lipiodol. This is a highly safe and effective method for treating postoperative chylous fistula, with a success rate of up to 73%, compared to a success rate of 27% for surgical methods combined with conservative treatment [19]. The patient reported that after the intervention, the chylothorax resolved, with the pleural fluid triglyceride level remaining at 0.58, and the amount of pleural fluid collected 1 week after the intervention was 100 mL.
Studies of reported cases of GLA suggest that there is no cure for GLA, and all treatments are mainly supportive and symptomatic. Recurrent pleural effusion can be treated with pleural drainage or pleurodesis. Medical treatments including propranolol, corticosteroids, immunosuppressants, and radiation therapy can reduce parenchymal involvement and effusion. Bevacizumab has been shown to reduce lymphatic channel proliferation and pleural effusion. The main causes of death in patients with GLA are respiratory failure, infection, and chylothorax [2].
Conclusion
Generalized lymphangiomatosis is a rare disease with nonspecific symptoms and can be a diagnostic challenge. A comprehensive medical history, physical examination, laboratory testing, and imaging are required to make a diagnosis. Characteristic imaging findings are essential to rule out other possible diagnoses and help guide the diagnosis of GLA. For GLA patients with thoracic duct damage after surgery, thoracic duct embolization is a safe and effective method to treat chylous fistula.
Patient consent
The patient has consented to the release of all information to be published in this article.
Footnotes
Competing Interests: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Tabrizi Z, Dadkhah A, soleimani S, Moaddab M. Generalized cystic lymphangiomatosis incidentally diagnosed in an asymptomatic adult: imaging findings of a very rare case. Radiol Case Rep. 2023;19(1):130–135. doi: 10.1016/j.radcr.2023.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mehrnahad M, Kord A, Rezaei Z, Kord R. Late diagnosis of generalized lymphangiomatosis in a woman presenting with respiratory distress. Radiol Case Rep. 2020;15(8):1189–1193. doi: 10.1016/j.radcr.2020.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ozel A, Uysal E, Dokucu AI, Erturk SM, Basak M, Cantisani V. US, CT and MRI findings in a case of diffuse lymphangiomatosis and cystic hygroma. J Ultrasound. 2008;11(1):22–25. doi: 10.1016/j.jus.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thoracic lymphangiomatosis in a child : journal of pediatric hematology/oncology. Accessed March 23, 2024. https://journals.lww.com/jpho-online/abstract/2004/02000/thoracic_lymphangiomatosis_in_a_child.18.aspx.
- 5.Liu T, Basseri S, Mussari B, DaBreo D, SenGupta S, Villalobos D, et al. Generalized lymphatic anomalies and review of the current management landscape: a case report and review of the literature. J Med Case Rep. 2021;15(1):398. doi: 10.1186/s13256-021-02953-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wassef M, Blei F, Adams D, Alomari A, Baselga E, Berenstein A, et al. Vascular anomalies classification: recommendations from the International Society for the Study of Vascular Anomalies. Pediatrics. 2015;136(1):e203–e214. doi: 10.1542/peds.2014-3673. [DOI] [PubMed] [Google Scholar]
- 7.Kwag E, Shim SS, Kim Y, Chang JH, Kim KC. CT features of generalized lymphangiomatosis in adult patients. Clin Imaging. 2013;37(4):723–727. doi: 10.1016/j.clinimag.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 8.Abdominal lymphatic malformations | Die Radiologie. Accessed March 24, 2024. 10.1007/s00117-017-0337-5.
- 9.Faul JL, Berry GJ, Colby TV, Ruoss SJ, Walter MB, Rosen GD, et al. Thoracic lymphangiomas, lymphangiectasis, lymphangiomatosis, and lymphatic dysplasia syndrome. Am J Respir Crit Care Med. 2000;161(3 Pt 1):1037–1046. doi: 10.1164/ajrccm.161.3.9904056. [DOI] [PubMed] [Google Scholar]
- 10.Wunderbaldinger P, Paya K, Partik B, Turetschek K, Hörmann M, Horcher E, et al. CT and MR imaging of generalized cystic lymphangiomatosis in pediatric patients. AJR Am J Roentgenol. 2000;174(3):827–832. doi: 10.2214/ajr.174.3.1740827. [DOI] [PubMed] [Google Scholar]
- 11.Scopus preview - Scopus - Document details - Intraabdominal lymphatic malformations: pearls and pitfalls of diagnosis and differential diagnoses in pediatric patients. 10.2214/AJR.16.17008. (Accessed December 22, 2016). [DOI] [PubMed]
- 12.Putta T, Irodi A, Thangakunam B, Oliver A, Gunasingam R. Young patient with generalized lymphangiomatosis: differentiating the differential. Indian J Radiol Imaging. 2016;26(03):411–415. doi: 10.4103/0971-3026.190416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Le HDT, Vo DS, Le DD, Dang CT, Nguyen Thanh T. Generalized lymphangiomatosis: a rare manifestation of lymphatic malformation. Radiol Case Rep. 2020;16(1):66–71. doi: 10.1016/j.radcr.2020.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herruela-Suffee C, Warin M, Castier-Amouyel M, Dallery F, Bonnaire B, Constans JM. Whole-body MRI in generalized cystic lymphangiomatosis in the pediatric population: diagnosis, differential diagnoses, and follow-up. Skeletal Radiol. 2016;45(2):177–185. doi: 10.1007/s00256-015-2280-8. [DOI] [PubMed] [Google Scholar]
- 15.Lala S, Mulliken JB, Alomari AI, Fishman SJ, Kozakewich HP, Chaudry G. Gorham-Stout disease and generalized lymphatic anomaly–clinical, radiologic, and histologic differentiation. Skeletal Radiol. 2013;42(7):917–924. doi: 10.1007/s00256-012-1565-4. [DOI] [PubMed] [Google Scholar]
- 16.Joshi M, Phansalkar DS. Simple lymphangioma to generalized lymphatic anomaly: role of imaging in disclosure of a rare and morbid disease. Case Rep Radiol. 2015;2015 doi: 10.1155/2015/603859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ozeki M, Fukao T. Generalized lymphatic anomaly and gorham–stout disease: overview and recent insights. Adv Wound Care (New Rochelle) 2019;8(6):230–245. doi: 10.1089/wound.2018.0850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee EW, Shin JH, Ko HK, Park J, Kim SH, Sung KB. Lymphangiography to treat postoperative lymphatic leakage: a technical review. Korean J Radiol. 2014;15(6):724–732. doi: 10.3348/kjr.2014.15.6.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maldonado F, Cartin-Ceba R, Hawkins FJ, Ryu JH. Medical and surgical management of chylothorax and associated outcomes. Am J Med Sci. 2010;339(4):314–318. doi: 10.1097/MAJ.0b013e3181cdcd6c. [DOI] [PubMed] [Google Scholar]