Abstract
Extensive research has made significant progress in exploring the potential application of extracellular vesicles (EV) in the diagnosis and treatment of osteoarthritis (OA). However, there is current a lack of study on bibliometrics. In this study, we completed a novel bibliometric analysis of EV research in OA over the past two decades. Specifically, we identified a total of 354 relevant publications obtained between January 1, 2003 and December 31, 2022. We also provided a description of the distribution information regarding the countries or regions of publication, institutions involved, journals, authors, citations, and keywords. The primary research focuses encompassed the role of extracellular vesicles in the diagnosis of OA, delivery of active ingredients, treatment strategies, and cartilage repair. These findings highlight the latest research frontiers and emerging areas, providing valuable insights for further investigations on the application of extracellular vesicles in the context of osteoarthritis.
Keywords: Extracellular vesicles, Bibliometrics, Data visualization, Osteoarthritis, Research hotspot
1. Introduction
Osteoarthritis (OA) is a prevalent chronic joint disease worldwide, affecting around 10 % of men and 18 % of women over the age of 60 in the global population [1]. This condition is characterized by systemic chronic inflammation, damage and degeneration of articular cartilage, subchondral bone sclerosis, and degeneration of the joint capsule, among other features [[2], [3], [4]]. Currently, the treatment options for end-stage osteoarthritis primarily focus on pain management, including the use of analgesics and non-steroidal anti-inflammatory drugs, as well as joint replacement surgeries. However, these approaches do not address the early symptoms of osteoarthritis or the limitations associated with joint replacement. Additionally, the cost of treating osteoarthritis is substantial, accounting for approximately 1.0 %–2.5 % of the GDP in some developed countries [5]. Therefore, it is crucial to identify convenient and effective alternatives for the treatment of OA.
Mounting evidence indicates that extracellular vesicles (EV) possess the capacity to surpass the constraints imposed by conventional therapies in the realm of osteoarthritis treatment. Particularly in the fields of regenerative medicine and nanomedicine, EV has emerged as a research hotspot, attracting significant attention in recent years. EV offers tremendous potential due to its unique ability to transport bioactive molecules and facilitate intercellular communication. This characteristic positions them as a promising therapeutic avenue for addressing the intricate pathology of osteoarthritis [[6], [7], [8]]. Extracellular vesicles are nanoscale small vesicles that include exosomes, apoptotic bodies, and microvesicles. The characteristics of EV include possessing phospholipid bilayers and the ability to mediate intercellular communication. These attributes position extracellular vesicles as a promising modality for the treatment and diagnosis of osteoarthritis [[9], [10], [11]]. Extracellular vesicles have become a potential treatment method for many diseases, such as diabetes [12], cancer [13], and osteoarthritis [14], and have made contributions in the fields of alleviating inflammation and immune regulation [15], and cartilage defects [16]. Hence, it is imperative to gain a comprehensive understanding of the trends and hotspots surrounding extracellular vesicles in the context of osteoarthritis.
Although extracellular vesicles have received increasing attention in the field of osteoarthritis, there is current a lack of study on bibliometrics specifically focused on EV-related articles in the context of osteoarthritis. The purpose of our research is to systematically evaluate the distribution information by publications, countries, institutions, journals, authors, citations, keywords. The main objectives are to identify the primary contributors and research hotspots in this field and propose future prospects for development. Bibliometrics is a widely recognized statistical method based on public literature databases. It provides several advantages, including the ability to quantitatively analyze and evaluate publications within specific fields domains [17,18], aiding in the examination of trends and potential research hotspots in scientific literature [19]. Moreover, bibliometrics also assists in identifying collaborative relationships among research institutions in emerging fields [20], and facilitate a more profound comprehension of research trends and hotspots in associated fields [21]. Given the escalating quantity and impact of publications, bibliometrics will persist in playing a pivotal role in their assessment. Furthermore, bibliometric tools such as CiteSpace [22], VoSvivewer [23,24], and R package “bibliometrix” [23,25]. These bibliometric visualization tools have been commonly used to explore research in specific fields, and more and more scholars are realizing the importance of bibliometrics in their work. Through the application of mathematical and statistical techniques, bibliometrics enables the macro-level analysis of published literature, unveiling the evolution of specific topics. This enables researchers to obtain a grasp of research trends and hotspot [26]. For instance, Ya-Wen Pen et al. utilized bibliometric analysis to examine the research trends and frontiers related to extracellular vesicles and fibrosis from 2013 to 2022 [27]. Furthermore, this methodology can also assist clinical researchers in aligning their research direction and pursuing relevant areas of interest [28]. Gu et al. [29] utilized the bibliometric approach to visualize clinical trials in the field of knee osteoarthritis. Their findings revealed that various clinical treatment modalities for knee osteoarthritis, including pharmacological interventions, intra-articular treatments, non-pharmacological interventions such as exercise or dietary interventions, and knee arthroplasty, were prominently represented in the clinical trials pertaining to osteoarthritis. Through the utilization of bibliometric analysis and visualization techniques to investigate the characteristics of these clinical trials, we can uncover potential future research hotspots and assist researchers in refining their research direction. In our study, we utilized bibliometric analysis and visualization techniques to analyze the current trends and potential future hotspots in osteoarthritis and extracellular vesicles, providing reference for researchers and clinicians.
2. Method details
Bibliometric analysis entails the following procedures: data retrieval and collection, followed by detailed checks using established software tools. This article delves into these program steps in depth.
2.1. Data retrieval and collection
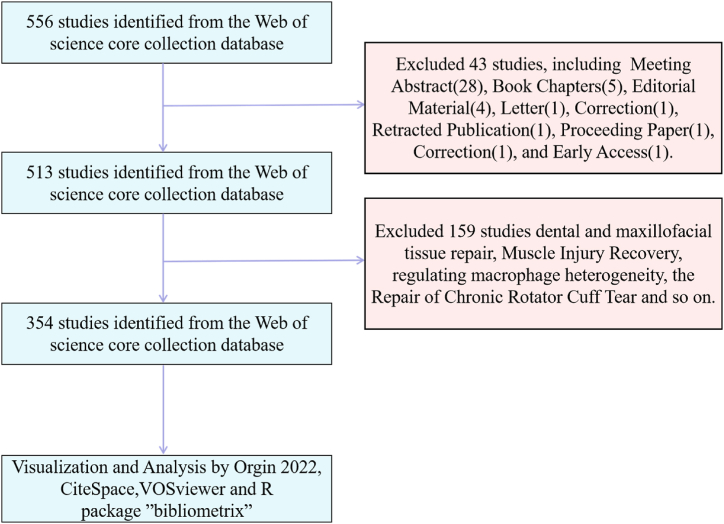
Collect all publications using the Web of Science Core Collection (WoSCC) database, which provides a general statistical source for bibliometric software [30,31]. To prevent bias induced by database updates, all publications to our study be acquired on November 13, 2023. The collected literature was published from January 1, 2003, to December 31, 2022. We searched for the following topics: TS= ("extracellular vesicles") AND TS= ("osteoarthritis"). Since the search topics in WoSCC can be viewed as keyword search field information, we have chosen specific search topics to improve the accuracy of search results. In addition, we also used the MeSH method in the Institutions of Health to extract all search keywords. The complete search method is as follows: The Topic=("extracellular vesicles" OR "extracellular vesicle" OR "vesicle, extracellular" OR "vesicles, extracellular" OR "exosomes" OR "exovesicle" OR "exovesicles" OR "apoptotic bodies" OR "apoptotic body" OR "bodies, apoptotic" OR "body, apoptotic") AND TS=("osteoarthritis" OR "osteoarthritides" OR "osteoarthrosis" OR "osteoarthrosis" OR "arthritis, degenerative" OR "arthritides, degenerative" OR "degenerative arthritides" OR "degenerative arthritis" OR "arthrosis" OR "arthroses" OR "osteoarthrosis deformans") AND LA=("English"). The publication types are set to "articles" and "review". After the initial search, we further excluded Meeting Abstract, book chapters, editorial materials, letters, proceeding paper, retracted publication, correction, and early access to guarantee the consistency and precision of the gathered information. A team consisting of two reviewers was established to screen titles and abstracts based on inclusion and exclusion criteria. If necessary, reviewers had the option to read the full texts to conduct a more thorough assessment of the eligibility of the screened publications. Any differing viewpoints between the two reviewers were subject to discussion and resolution. The screening process is shown in Fig. 1.
Fig. 1.
Flow chart of data collection.
To ensure the selection of research that best aligns with the research objectives, we have adopted the following exclusion criteria.
-
1.
For the study focusing on OA and EV, any literature that does not concurrently investigate both OA and EV should be excluded. In other words, publications that are not relevant to the research topic should be excluded.
-
2.
Meeting abstract, book chapters, editorial materials, letters, corrections, retracted publications, proceeding papers, and early access should be excluded. In other words, publications that do not meet the criteria of being categorized as "articles" or "comments" should be excluded.
-
3.
Publications related to osteoarthritis and extracellular vesicles that are not in English should be excluded.
-
4.
Literature published outside of January 1, 2003, to November 13, 2023, is excluded.
2.2. Quantitative and statistical analysis
The included publications and references are exported in plain text for bibliometric analysis and visualization in the field of literature research. In the past two decades, with the progress of data analysis and text mining, several software tools have been developed for more effective bibliometric analysis, including CiteSpace, VOSviewer and R package "bibliometrix". VOSviewer is a computer software for bibliometric mapping jointly developed by Professor van Eck and Waltman, which can mine information from numerous publications for intuitive analysis [32]. It can mine information from many publications for intuitive analysis, and is usually used to build and visualize co-authorship, co-citation, and co-occurrence networks. In this study, in the map generated by VOSviewer, each node represents a country, institution, author or keyword. It assigns many closely related nodes to clusters of different colors, and the strength of relationships between nodes is represented by the density of lines [33].
CiteSpace is another scientific econometric analysis software developed by Professor Chen C. By calculating intermediary centrality and constructing visual networks, it can illustrate the relationship between distribution, patterns, and scientific knowledge, offering a fresh perspective for exploring trends and frontiers [22]. In our study, we used CiteSpace to accurately locate references and keywords, plotted the dual-map overlay of journals and keyword timeline map, and used burst detection references to analyze references and keywords that show sudden and significant changes in frequency within a certain period of time and are popular.
The R package "bibliometric" is often used as a tool for performing bibliometric analysis to construct the global geographic distribution of publications [34]. We also conducted a quantitative analysis of publications year by year using Origin 2022, and drew Veen diagrams for highly cited papers, co-cited references, and burst reference using the Draw Venn outline online website.
3. Result
3.1. Overview
We have retrieved keywords in the WoSCC and found a total of 354 studies on extracellular vesicles in osteoarthritis, including 214 articles and 140 reviews. 354 papers come from 1893 authors from 583 institutions in 47 countries or regions, published in 178 journals, citing 2600 journals and 16,625 references.
3.2. The countries or regions contributions to publications
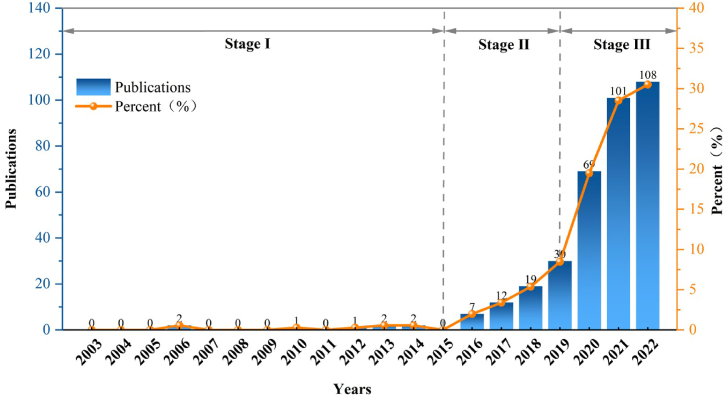
We can observe that the annual growth in the number of publications can be divided into three stages, as illustrated in Fig. 2. Published a total of 8 papers from 2002 to 2015. The initial publication in this field dates back to 2006, serving as the starting point for subsequent articles published in this area [35]. In 2010, the first review article was published [36]. There were very few studies on extracellular vesicles in osteoarthritis in the first phase. From 2016 to 2019, there was a slight increase in publications starting, with the average annual papers being approximately 17. The publications increased significantly from 2020 to 2022, with an average of approximately 92.7 publications per year, reaching a peak in 2022 (a total of 108 papers, accounting for 30.5 %).
Fig. 2.
Number of annual publications on extracellular vesicles in osteoarthritis from 2003 to 2022.
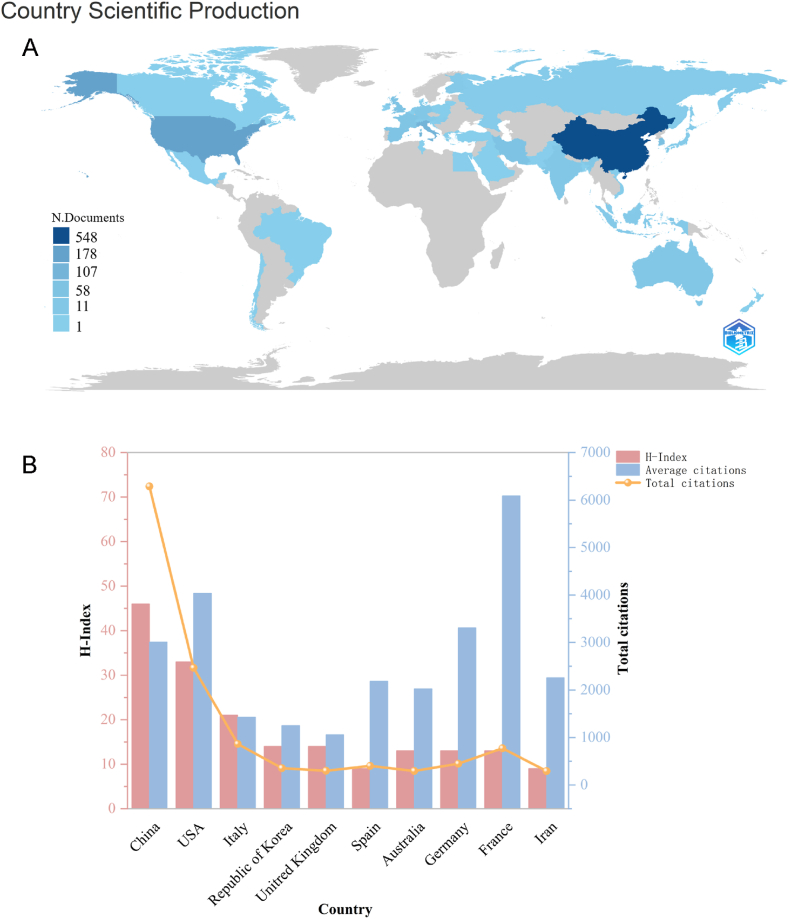
We analyzed the distribution of the 10 most productive countries/regions. The research output of these countries takes 85.13 % (355/417) of the total number of publications, these countries mainly in Europe (n = 5) and Asia (n = 3) in Fig. 3A and Table 1. As shown in Fig. 3B, China has the most publications (40.29 %), followed by USA (12.23 %), India (10.07 %), Republic of Korea (4.56 %) and the United Kingdom (4.32 %). In addition, China has the most citations (6287), which shows that China has world-leading capabilities in this field. USA is the second largest producer, with 51 publications, 2467 citations. Therefore, this indicates that China and USA have world leading strength and have extremely important global influence in the field of research on extracellular vesicles in osteoarthritis.
Fig. 3.
Contributions of various countries to the study of extracellular vesicles in osteoarthritis (A) National scientific production and geographical distribution. (B) Total citations, average citations and H-index of the top 10 countries contributing to publications.
Table 1.
The top 10 countries or regions that published information about extracellular vesicles in osteoarthritis.
| Rank | Country | Publications | Percentage (%) | Total citations | Average citations |
|---|---|---|---|---|---|
| 1 | China (Asia) | 168 | 40.29 % | 6287 | 37.42 |
| 2 | USA (North America) | 51 | 12.23 % | 2467 | 48.37 |
| 3 | Italy (Europe) | 42 | 10.07 % | 864 | 20.57 |
| 4 | Republic of Korea (Asia) | 19 | 4.56 % | 355 | 18.68 |
| 5 | England (Europe) | 18 | 4.32 % | 299 | 16.611 |
| 6 | Spain (Europe) | 14 | 3.36 % | 401 | 28.64 |
| 7 | Australia (Oceania) | 11 | 2.64 % | 296 | 26.91 |
| 8 | Germany (Europe) | 11 | 2.64 % | 447 | 40.63 |
| 9 | France (Europe) | 11 | 2.64 % | 773 | 70.27 |
| 10 | Iran (Asia) | 10 | 2.40 % | 294 | 29.40 |
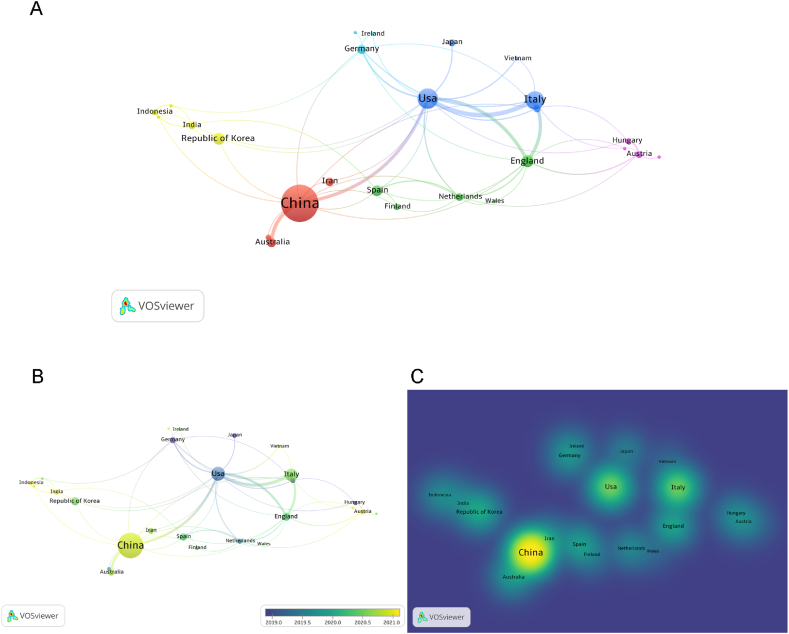
Then we visualized the cooperation of countries with ≥2 publications. As shown in Fig. 4A, China has high intensity of cooperation with USA, Germany, Britain and Republic of Korea; USA has high intensity of cooperation with Italy, China, Republic of Korea and Germany. Since 2019, the research of extracellular vesicle osteoarthritis in USA and Germany has increased, while the research in China, India, Austria and other countries has increased since 2021 (Fig. 4B). The density visualization map also shows that China plays a leading role in the field of extracellular vesicle OA internationally (Fig. 4C).
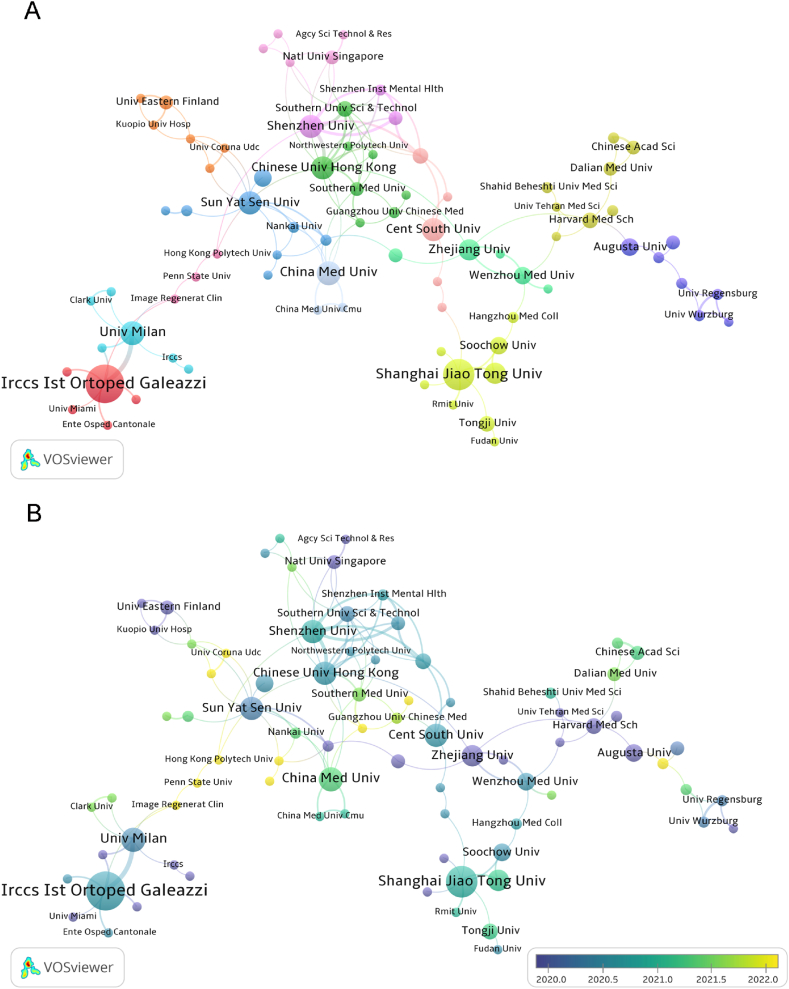
Fig. 4.
Network map from different countries or regions regarding extracellular vesicles in osteoarthritis. (A) Cooperation between countries or regions based on VOSviewer. (B) Dynamics and trends of countries over time. (C) Density map of country distribution of published articles based on VOSviewer.
3.3. The institutions contributions to publications
From 2003 to 2022, a total of 958 papers were published by 583 institutions. Table 2 summarizes the top 10 production institutions from different countries in osteoarthritis and extracellular vesicles research, including institution name, country sources, publications, total citations and average citations. Among them, there are 7 institutions from China, 2 from USA, and 1 from Italy. And IRCCS Istituto Ortopedico Galeazzi is a leading institution (publishing 22 papers), followed by Shanghai Jiao Tong University (publishing 15 papers), which is in a leading position in terms of total citations (923).
Table 2.
Top 10 institutions for publications on extracellular vesicles in osteoarthritis.
| Rank | Institution | Publications | Percentage (%) | Total citations | Average citations |
|---|---|---|---|---|---|
| 1 | IRCCS Istituto Ortopedico Galeazzi (Italia) | 22 | 2.30 % | 412 | 18.73 |
| 2 | Shanghai Jiao Tong University (China) | 15 | 1.57 % | 923 | 61.53 |
| 3 | The University of Milan (Italia) | 10 | 1.04 % | 167 | 16.70 |
| 4 | China Medical University (China) | 10 | 1.04 % | 233 | 23.30 |
| 5 | The Chinese University of Hong Kong (China) | 9 | 0.94 % | 561 | 62.33 |
| 6 | Shenzhen University (China) | 9 | 0.94 % | 488 | 54.22 |
| 7 | Sun Yat-Sen University (China) | 9 | 0.94 % | 623 | 69.22 |
| 8 | Central South University (China) | 9 | 0.94 % | 198 | 22.00 |
| 9 | Future Biologics (USA) | 8 | 0.84 % | 111 | 13.88 |
| 10 | Nanjing Medical University (China) | 8 | 0.84 % | 230 | 28.75 |
In Fig. 5A, we find active cooperation among various institutions from around the world. For example, Shanghai Jiao Tong University has close cooperation with Nanjing Medical University, Suzhou University, and the Tongji University. Fig. 5B shows that since 2022, institutions such as Image Regenerative Clinic and Guangzhou University of Chinese Medicine have increased their research in this field. However, the role of most institutions is not adequate.
Fig. 5.
Visualization of extracellular vesicle research institutions in osteoarthritis. (A) Analysis of institutional cooperation network based on VOSviewer. (B) Over time, the dynamics and trends of institutions.
3.4. Journal and co-cited journal analysis
We listed the top 15 journals with 125 published papers (Table 3). As shown in Table 3, International Journal of Molecular Sciences and Cells are the most productive journals, with 18 and 15 papers published respectively. Significantly, Theranostics has the highest average number of citations. In addition, the value of journals and the value of their included publications can be evaluated by the IF of journals [19]. Biomaterials has the highest IF (14), followed by Theranostics (12.4). In summary, the International Journal of Molecular Sciences, Biomaterials and Theranostics may show the greatest impact.
Table 3.
Top 15 journals for research of extracellular vesicle in OA.
| Rank | Journal | Country | Publications | Total citations | Average citations | IF (2022) | JCR |
|---|---|---|---|---|---|---|---|
| 1 | International Journal of Molecular Sciences | Switzerland | 18 | 343 | 19.06 | 5.6 | Q1 |
| 2 | Cells | Switzerland | 15 | 224 | 14.93 | 6.0 | Q2 |
| 3 | Stem Cell Research & Therapy | England | 14 | 363 | 25.93 | 7.5 | Q1 |
| 4 | Frontiers in Bioengineering and Biotechnology | Switzerland | 14 | 1350 | 96.43 | 5.7 | Q1 |
| 5 | Frontiers in Cell and Developmental Biology | Switzerland | 10 | 213 | 21.30 | 5.5 | Q1 |
| 6 | Arthritis Research & Therapy | England | 8 | 519 | 64.88 | 4.9 | Q2 |
| 7 | Journal of Cellular and Molecular Medicine | England | 6 | 204 | 34.00 | 5.3 | Q2 |
| 8 | Stem Cells International | USA | 6 | 109 | 18.17 | 4.3 | Q2 |
| 9 | Biomaterials | Netherlands | 5 | 731 | 146.20 | 14 | Q1 |
| 10 | Frontiers in Immunology | Switzerland | 5 | 38 | 7.60 | 7.3 | Q1 |
| 11 | Journal of Nanobiotechnology | England | 5 | 116 | 23.20 | 10.2 | Q1 |
| 12 | Journal of Orthopaedic Surgery and Research | England | 5 | 160 | 32.00 | 2.6 | Q2 |
| 13 | Scientific Reports | England | 5 | 527 | 105.40 | 4.6 | Q2 |
| 14 | Theranostics | Australia | 5 | 948 | 189.60 | 12.4 | Q1 |
| 15 | Biomedicines | Switzerland | 4 | 28 | 7.00 | 4.7 | Q1 |
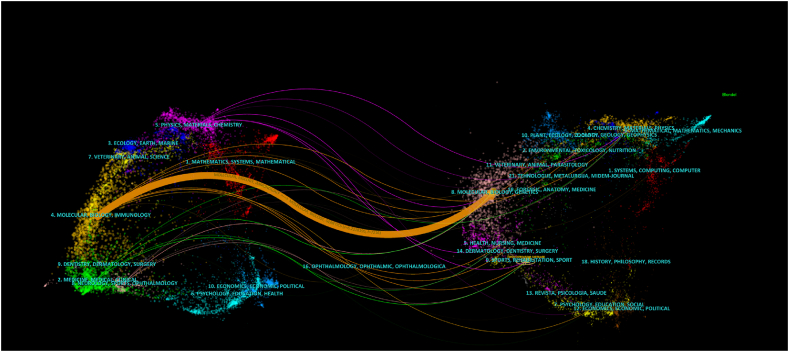
The cited relationship between journals and co-cited journals can be displayed by the dual-map of journals [37]. The cited journals are on the left, and the co-cited journals are on the right in Fig. 6. The colorful path is the primary citation path, indicating that papers published in Molecular/Biology/Genetics journals are commonly cited by papers published in Molecular/Biology/Immunology journals. Clinically relevant studies have been published in a few journals, indicating that most of the current studies are still in the basic stage of preclinical research.
Fig. 6.
The dual-map overlay of journals on research of exosomes in osteoarthritis.
3.5. Authors and co-cited authors analysis
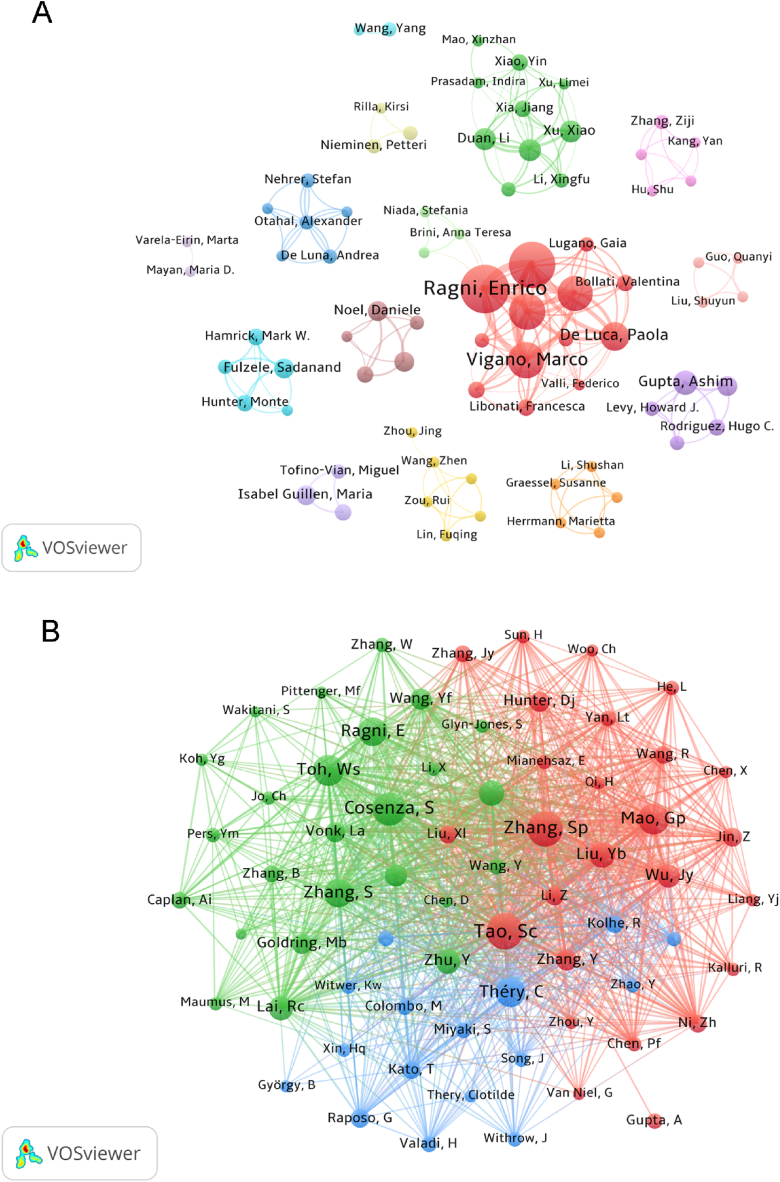
A total of 1893 authors published 354 of all analyzed publications between 2003 and 2022. In Table 4, we summarize the most productive top 10 authors. It is not difficult to see that the authors with the highest productivity are Ragin and Enrico (20), followed by de Girolamo, Laura (19), Colombini, Alessandra (14), vigano, Marco (14), orfei, Carlotta perucca (13), these highly productive authors are all from IRCCS Istituto ortopedico Galeazzi (Italy). The H-index is considered as an evaluation index of the scientific output of researchers [34], interestingly, Liang and Yujie are the authors with the highest average number of citations and h index. We established a co-authorship network based on the authors whose number of publications is greater than or equal to 3. As shown in Fig. 7A, the authors with the most cooperative relationships with other authors are Ragin and Enrico.
Table 4.
The most productive top 10 authors on research of extracellular vesicle in osteoarthritis.
| Rank | Authors | Publications | Total citations | Average citations | H-index |
|---|---|---|---|---|---|
| 1 | Ragin, Enrico(Italia) | 20 | 384 | 19.20 | 27 |
| 2 | De Girolamo, laura(Italia) | 19 | 359 | 18.89 | 33 |
| 3 | Colombini, Alessandra(Italia) | 14 | 341 | 24.36 | 28 |
| 4 | Vigano, Marco(Italia) | 14 | 339 | 24.21 | 22 |
| 5 | Orfei, Carlotta Perucca(Italia) | 13 | 240 | 18.46 | 12 |
| 6 | De Luca, Paola(Italia) | 10 | 279 | 27.90 | 21 |
| 7 | Gupta, Ashim(Italia) | 8 | 111 | 13.88 | 15 |
| 8 | Duan, Li(China) | 7 | 430 | 61.43 | 22 |
| 9 | Liang, Yujie(China) | 7 | 430 | 61.43 | 41 |
| 10 | Xu, Xiao(China) | 7 | 430 | 61.43 | 14 |
Fig. 7.
Visualization of authors and co-cited authors involved in extracellular vesicles of osteoarthritis. (A) The network map of productive authors. (B) Co-citation network of authors.
Co-cited authors refer to at least two authors who are cited by one or more publications, and the research results between these authors are cited in multiple literatures, thus forming contact and communication [19]. As shown in Table 5, Tao SC are the most frequently cited authors (169 times), followed by Zhang, SP (160 times) and Cosenza, S (147 times). As shown in Fig. 7B, different authors have positive cooperative relationships. Zhang and SP have the most cooperative relationships with other authors, such as Zhang, SP and Tao SC, Cosenza, s, théry, C.
Table 5.
Top 10 co-cited authors on research of extracellular vesicle in OA.
| Rank | Co-Cited Authors | Citations | H-index |
|---|---|---|---|
| 1 | Tao, Sc | 169 | 20 |
| 2 | Zhang, Sp | 160 | 10 |
| 3 | Cosenza, S | 147 | 4 |
| 4 | Toh, Ws | 134 | 34 |
| 5 | Mao, Gp | 128 | 13 |
| 6 | Théry, C | 121 | 67 |
| 7 | Ragni, E | 116 | 27 |
| 8 | Zhang, S | 114 | 15 |
| 9 | Liu, Yb | 100 | 27 |
| 10 | Tofiño-vian, M | 98 | 5 |
3.6. Highly cited papers, references burst and co-cited references analysis
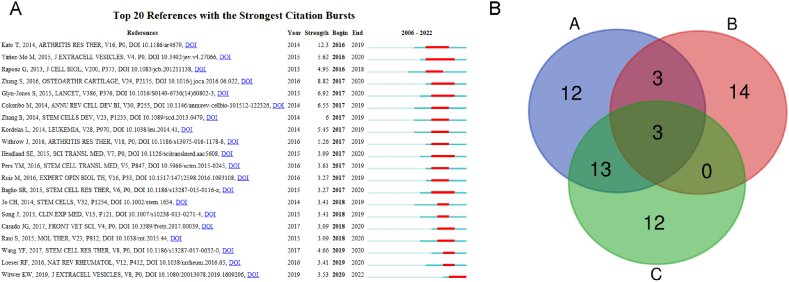
Highly cited papers can help identify important breakthrough research in a certain research area [32]. The purpose of the citation explosion is to confirm whether the literature published in a specific field has experienced a significant increase in a period of time [38]. Co-cited references analysis is an important part of bibliometric analysis. The paper with the most co-citations usually means that it has a representative role in a certain academic field [39]. The highly cited papers have been cited more than 200 times in Table 6, and the paper published by Tao SC has the highest number of citations (427 times). Table 7 lists the 10 most frequently co-cited references. The top 10 co-cited papers have been cited at least 70 times, and Tao SC et al. have the most citations (132 times), which reflects that Tao SC has a certain influence in the research field. In addition, 20 references of bursts were identified (Fig. 8A), and the data evidenced that the references burst in 2017 was very obvious and lasted until 2020. The reference with the highest burstness (strength: 12.3) was published by Kato T40, followed by Zhang S (strength: 8.82) [41].
Table 6.
Top10 references are highly cited in the study of extracellular vesicle in osteoarthritis.
| Rank | Title | Institution | First Authors | Journal | Citations | Year |
|---|---|---|---|---|---|---|
| 1 | Exosomes derived from miR-140-5p-overexpressing human synovial mesenchymal stem cells enhance e cartilage tissue regeneration and prevent osteoarthritis of the knee in a rat model [36] | Shanghai Jiao Tong University | Tao Sc | Theranostics | 427 | 2017 |
| 2 | Mesenchymal stem cells derived exosomes and microparticles protect cartilage and bone from degradation in osteoarthritis [37] | Montpellier University | Cosenza S | Scientific Reports | 360 | 2017 |
| 3 | MSC exosome as a cell-free MSC therapy for cartilage regeneration: Implications for osteoarthritis treatment [38] | National University of Singapore | Toh Ws | Seminars in Cell & Developmental Biology | 297 | 2017 |
| 4 | MSC exosomes alleviate temporomandibular joint osteoarthritis by attenuating inflammation and restoring matrix homeostasis [39] | National University of Singapore | Zhang Sp | Biomaterials | 275 | 2019 |
| 5 | miR-100-5p-abundant exosomes derived from infrapatellar fat pad MSCs protect articular cartilage and ameliorate gait abnormalities via inhibition of mTOR in osteoarthritis [40] | Third Military Medical University | Wu Jy | Biomaterials | 274 | 2019 |
| 6 | Comparison of exosomes secreted by induced pluripotent stem cell-derived mesenchymal stem cells and synovial membrane-derived mesenchymal stem cells for the treatment of osteoarthritis [41] | Shanghai Jiao Tong University Affiliated Sixth People's Hospital | Zhu Y | Stem Cell Research & Therapy | 259 | 2017 |
| 7 | Exosomes derived from miR-92a-3p-overexpressing human mesenchymal stem cells enhance chondrogenesis and suppress cartilage degradation via targeting WNT5A [42] | First Affiliated Hospital of Sun Yat-sen University | Mao Gp | Stem Cell Research & Therapy | 257 | 2018 |
| 8 | Desktop-stereolithography 3D printing of a radially oriented extracellular matrix/mesenchymal stem cell exosome bioink for osteochondral defect regeneration [43] | Medical College of Zhejiang University | Chen, Pf | Theranostics | 235 | 2019 |
| 9 | Mesenchymal stromal/stem cell-derived extracellular vesicles promote human cartilage regeneration in vitro [44] | Utrecht University | Vonk, L | Theranostics | 216 | 2018 |
| 10 | A membrane form of TNF-α presented by exosomes delays T cell activation-induced cell death [27] | University of Alabama at Birmingham | Zhang, H | Journal of Immunology | 206 | 2006 |
Table 7.
The top 10 co-cited references related to the study of extracellular vesicles in osteoarthritis.
| Rank | Title | Institution | First Authors | Journal | Citations | Year |
|---|---|---|---|---|---|---|
| 1 | Exosomes derived from miR-140-5p-overexpressing human synovial mesenchymal stem cells enhance cartilage tissue regeneration and prevent osteoarthritis of the knee in a rat model [36] | Shanghai Jiao Tong University | Tao Sc | Theranostics | 132 | 2017 |
| 2 | Mesenchymal stem cells derived exosomes and microparticles protect cartilage and bone from degradation in osteoarthritis [37] | Montpellier University | Cosenza S | Scientific Reports | 108 | 2017 |
| 3 | Exosomes derived from human embryonic mesenchymal stem cells promote osteochondral regeneration [35] | National University of Singapore | Zhang S | Osteoarthr and Cartilage | 105 | 2016 |
| 4 | MSC exosomes mediate cartilage repair by enhancing proliferation, attenuating apoptosis and modulating immune reactivity [45] | National University of Singapore | Zhang Sp | Biomaterials | 98 | 2018 |
| 5 | Exosomes derived from miR-92a-3p-overexpressing human mesenchymal stem cells enhance chondrogenesis and suppress cartilage degradation via targeting WNT5A [42] | First Affiliated Hospital of Sun Yat-sen University | Mao Gp | Stem Cell Research & Therapy | 87 | 2018 |
| 6 | Comparison of exosomes secreted by induced pluripotent stem cell-derived mesenchymal stem cells and synovial membrane-derived mesenchymal stem cells for the treatment of osteoarthritis [41] | Shanghai Jiao Tong University Affiliated Sixth People's Hospital | Zhu Y | Stem Cell Research & Therapy | 87 | 2017 |
| 7 | miR-100-5p-abundant exosomes derived from infrapatellar fat pad MSCs protect articular cartilage and ameliorate gait abnormalities via inhibition of mTOR in osteoarthritis [40] | Third Military Medical University | Wu Jy | Biomaterials | 83 | 2019 |
| 8 | MSC exosome as a cell-free MSC therapy for cartilage regeneration: Implications for osteoarthritis treatment [38] | National University of Singapore | Toh Ws | Seminars in Cell & Developmental Biology | 92 | 2017 |
| 9 | Exosomes from embryonic mesenchymal stem cells alleviate osteoarthritis through balancing synthesis and degradation of cartilage extracellular matrix [46] | Zhejiang University | Wang Yf | Stem Cell Research & Therapy | 72 | 2017 |
| 10 | Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines [47] | Institut Curie | Théry C | Journal of Extravellular Vesicles | 70 | 2018 |
Fig. 8.
References with citation burst and veen outline (A) 20 references with the strongest citation burst. (B) Veen outline of highly cited, co cited, and explosive cited papers.
For convenience, we will name the co-citations papers not less than 100, the 20 most cited references, and the highly cited papers as A, B, and C, respectively, and then analyze their common papers. According to the Venn analysis (Fig. 8B), there are three papers in A, B, and C simultaneously, including one paper published by Withrow J, one paper published by Wang Y and Yu D, and one paper published by Kato T and Miyaki S, reflecting the influence of Withrow J, Wang Y and Yu D, Kato T and Miyaki S in the field of extracellular vesicle research in osteoarthritis. A and C have 13 papers together, and a and B have 3 papers together.
3.7. keyword analysis
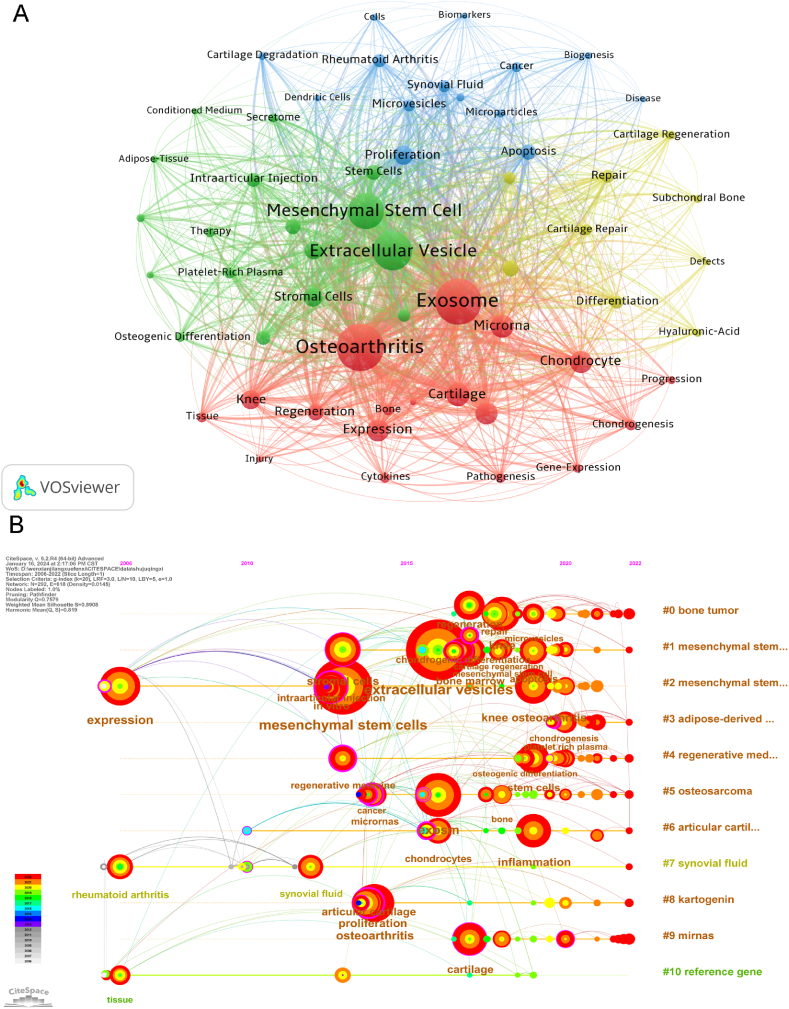
Keyword co-occurrence analysis can enable researchers to quickly understand the hot topics in a certain research field [34]. We used VOSviewer to analyze keywords extracted from 354 papers, and Table 8 shows that the most frequently occurring keywords are Osteoarthritis (235 times), Exosome (221 times), Extracellular vesicle (171 times), Mesenchymal Stem Cell (151 times), Cartilage (72 times), and MicroRNA (72 times). The above keywords are the research direction of extracellular vesicles in osteoarthritis. Keywords with fewer than 10 occurrences were excluded. These keywords are divided into four clusters of different colors, representing four different research topics (Fig. 9A). The red cluster is related to the delivery of active ingredients in osteoarthritis, and the main keywords are: "Osteoarthritis", "Exosome", "microRNA", "Regeneration", "Expression". The green cluster is related to the therapeutic effect of extracellular vesicle in osteoarthritis and the main keywords are: "Extracellular vehicle", "Mesenchymal stem cell", "Intraarticular injection", "Therapy". The Blue clustering is related to the diagnosis of osteoarthritis, and the main keywords are: "Apoptosis", "Proliferation", "Microvesicles", "Biomarkers". Yellow clustering is related to cartilage repair in osteoarthritis and the main keywords are: "Repair", "Cartilage Repair", "Hyaluronic acid".
Table 8.
The top 20 high-frequency keywords about extracellular vesicles in osteoarthritis.
| Rank | Keywords | Occurrences | Total link strength | Rank | Keyword | Occurrences | Total link strength |
|---|---|---|---|---|---|---|---|
| 1 | Osteoarthritis | 235 | 1038 | 11 | Knee | 55 | 283 |
| 2 | Exosome | 221 | 933 | 12 | Stromal Cells | 52 | 273 |
| 3 | Extracellular Vesicle | 171 | 816 | 13 | Knee Osteoarthritis | 51 | 221 |
| 4 | Mesenchymal Stem Cell | 151 | 734 | 14 | Regeneration | 41 | 230 |
| 5 | Cartilage | 72 | 359 | 15 | Articular-Cartilage | 39 | 188 |
| 6 | Microrna | 72 | 340 | 16 | Apoptosis | 37 | 176 |
| 7 | Chondrocyte | 69 | 330 | 17 | In-Vitro | 36 | 160 |
| 8 | Expression | 69 | 294 | 18 | Stem Cells | 35 | 165 |
| 9 | Inflammation | 67 | 345 | 19 | Intraarticular Injection | 34 | 165 |
| 10 | Proliferation | 56 | 273 | 20 | Bone-Marrow | 32 | 178 |
Fig. 9.
Keyword localization in the study of extracellular vesicles in osteoarthritis. (B) Visualization map of keywords clustering on engineered exosomes This map constituted by 58 items, including red clusters (18 items), green clusters (17 items) and blue clusters (14 items) and yellow clusters (9 items). (B) Timeline visualization of high-frequency topic keywords from 2006 to 2022. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Furthermore, we presented a visual map of keywords changing over time (Fig. 9B), node size represents frequency. In this timeline map, Q-value is 0.7579 and S-value is 0.8908. From 2006 to 2015, mesenchymal stem cells, osteoarthritis and microRNAs were the main hot research keywords in this period. The popular keywords from 2016 to 2019 are extracellular vesicles, exosomes, knee osteoarthritis, and mesenchymal stem cells. The hot keywords from 2020 to 2022 are still mesenchymal stem cells, microRNAs, extracellular vesicles, osteoarthritis, cartilage and repair. From 2006 to 2015, mesenchymal stem cells, osteoarthritis and microRNAs were the main hot research keywords in this period. The popular keywords from 2016 to 2019 are extracellular vesicles, exosomes, knee osteoarthritis, and mesenchymal stem cells. The hot keywords from 2020 to 2022 are still mesenchymal stem cells, microRNAs, extracellular vesicles, osteoarthritis, cartilage and repair.
4. Discussion
4.1. General description
This project collected 354 original articles and reviews in the past 20 years on the WoSCC database to discover hot topics and future development of extracellular vesicles in osteoarthritis.
Our research has yielded valuable insights that are worthy of further study and consideration. In general, the number of publications can serve as an indicator of productivity [42], while the number of citations received by these publications can reflect their influence in the field [43]. Our research findings indicate a limited number of articles published before 2016, suggesting that the study of extracellular vesicles in osteoarthritis is still in its early stages. However, in the past four years, there has been a rapid growth of relevant papers, with 108 publications in 2022 alone. This substantial increase accounts for nearly one-third of the publications in the past 20 years. These results indicate a significant rise in global attention towards studying the role of extracellular vesicles in the context of osteoarthritis. Therefore, literature pertinent to this field may experience growth in the upcoming years. From the perspective of countries or regions, China and USA are the main countries to carry out research on extracellular vesicles in osteoarthritis. Among the top 10 countries/regions, China ranks first. Similarly, about 70 % of research institutions come from China in Table 2, demonstrating rapid growth over the past two decades, China exhibits strong scientific research potential. Among the top 10 scholars with the most influential papers, 7 are from Italy and 3 are from China, indicating that although the productivity of publications in China is large, the quality of study still needs to be improved. It is obvious that other countries, including India and Spain, are also facing the same problems. Based on the results and discussions here, we recommend encouraging active cooperation between different countries and institutions. From the perspective of the journal, International Journal of Molecular Sciences (IF = 5.6, Q1), Biomaterials (IF = 14, Q1) and Theranostics (IF = 12.4, Q1) with the most publications, co-cited, and average citations respectively. Demonstrating that these journals have higher influence and innovation in the study of extracellular vesicles in osteoarthritis, and they provide support for the academic research of extracellular vesicles in osteoarthritis. In addition, at present, the research on extracellular vesicles in osteoarthritis is mainly published in molecular, biological and genetic journals, and the clinical research is published in a few journals, signifying the necessity to further broaden pertinent clinical trial research.
From the perspective of the author, we found that Ragin and Enrico are the most effective authors. His team mainly studies extracellular vesicles, mesenchymal stem cells, and the improvement and treatment of arthritis. In recent years, his research team has developed an emerging technology of time-lapse quantitative microscopy to monitor EV migration in specific tissues [44]. In addition, they identified key regulatory molecules and microRNAs in extracellular vesicles with anti-inflammatory and regenerative properties in skeletal muscle diseases [45]. Significantly, Although Liang, Yujie ranks low, his H-index, average citations per paper and total citations rank first, indicating that his published papers have attracted the attention of many scholars in this field. Liang, Yujie focused on the research of targeted therapy and pathogenesis of osteoarthritis. Two of them pointed out that targeting exosomes as chondrocyte specific nanocarriers to deliver drugs could alleviate the related symptoms of osteoarthritis [46,47]. In addition, he also found that fusing mesenchymal stem cell binding peptide E7 with exosomal membrane protein lamp 2b to produce exosomes with E7 peptide on the surface delivered kartogenin to synovial fluid derived mesenchymal stem cells, thereby alleviating cartilage degradation in osteoarthritis [48]. As far as the co-cited authors are concerned, Tao, Sc has been cited by other scholars the most. In 2017, Tao, Sc found that exosomes derived from synovial mesenchymal stem cells overexpressing mir-140-5p could regenerate cartilage tissue of osteoarthritis rats and prevent the occurrence of osteoarthritis [49]. Subsequently, Tao, Sc proposed to use PDLLA-PEG-PDLLA targeted copolymer gel as a carrying object of small extracellular vesicles combined with circRNA3503 to prevent and specifically treat OA [50]. The above shows that some scholars have provided innovative influences in the prevention and treatment of osteoarthritis based on extracellular vesicles in the future.
4.2. Trends and frontiers
We selected the 10 publications with the highest number of co-cited references to determine the research basis of extracellular vesicles in osteoarthritis. As shown in Table 6, as early as 2006, it was first pointed out that exosomes produced by synovial fibroblasts from patients with rheumatoid arthritis contain membrane-bound forms of TNF-a, increased the apoptosis resistance of activated T cells [35]. Up to now, the cellular communication between extracellular vesicles and osteoarthritis related cells is still a hot spot, both in cited references and co-cited references. Tao,Sc published a paper in 2017 with the largest number of citations. This paper studies the prevention and treatment of osteoarthritis by exosomal delivery of microRNA, which provide the significance for the study of extracellular vesicle delivery of active ingredients in osteoarthritis. In a word, the research contents of the top 10 cited literatures include: the isolation, purification and identification of exosomes, the biological functions of exosomes, and delivery of active ingredients, which are the basic research of exosomes. References bursts are employed to signify emerging topics within a research domain [51], In Fig. 8A, the main topics references with citation bursts are the biological functions and formation mechanisms of extracellular vesicles and exosomes, the treatment of osteoarthritis based on mesenchymal stem cells, and the cell-free therapy of osteoarthritis based on exogenous extracellular vesicles.
Keyword analysis can assist in rapidly identifying the hotspots and trends of osteoarthritis extracellular vesicles. Among the keywords within the scope of our study, except "Osteoarthritis" and "Extracellular vesicle", the keywords "Exosome" and "Mesenchymal stem cell" appeared most frequently, 221 and 151 times respectively, indicating that they are the most widely studied subclasses and may have great therapeutic potential. In the keyword co-occurrence visualization network diagram, all keywords were divided into the following four clusters: diagnosis of osteoarthritis, active ingredient delivery, therapeutic effect, cartilage repair. These four clusters show the main exploration themes in this research field. From the timeline map of keyword, we found that the keywords related to the diagnosis of osteoarthritis appeared earlier, and the active ingredient delivery of osteoarthritis is a nearby research hotspot.
Extracellular vesicles have many advantages in osteoarthritis diagnosis, active ingredient delivery, treatment and cartilage repair. Foremost, extracellular vesicles are a promising new biomarker because they can encapsulate information from specific cells and have good delivery ability in vivo [52]. The development of biomarkers for EV shows great potential not only included nucleic acids, proteins, lipids and other macromolecules, but also in cancer, cardiovascular disease and metabolic disease [[53], [54], [55]]. In terms of treatment, EV can circumvent the risks associated with cell therapy. In addition, extracellular vesicles have good modifiability. Through the application of biological or chemical engineering technology can enhance the targeting of extracellular vesicles toward receptor cells, thus contributing to address issues such as limited residence time and challenges in achieving effective concentrations due to the unique structure of the joint cavity, to improve the curative effect. In addition, because EV can effectively cross biological barriers and transmit signaling molecules in recipient cells [56], they also have the potential to become drug delivery vehicles.
At present, based on the symptoms exhibited by osteoarthritis patients, including pain, reduced functionality, and restricted morning stiffness, additional X-rays can aid in the diagnostic process [[57], [58], [59]]. It is worth noting that there is no gold standard for early diagnosis of osteoarthritis patients, so extracellular vesicles based on different sources can play a role as early diagnostic biomarkers of osteoarthritis. There have been a couple of studies suggesting that plasma derived extracellular vesicles and synovial derived extracellular vesicles can diagnose and distinguish different stages of osteoarthritis [60,61]. In addition, there exists a notable disparity in the protein expression of serum exosomes between osteoarthritis patients and healthy donors was first found in 2018 [62]. It is suggested that plasma derived exosomes have potential as biomarkers for OA diagnosis. However, the therapeutic potential of natural exosomes is limited, and engineered exosomes meet the level of targeting, long-term performance and biocompatibility required for therapeutic use. In addition, extracellular vesicles provide an efficient and precise therapeutic platform for osteoarthritis by serving as drug delivery vehicles. For example, Zhirong Wang found that exosomes derived from synovial mesenchymal stem cells overexpressing miR-155-5p could alleviate the progression of osteoarthritis [63]. However, the therapeutic potential of natural exosomes is limited, and engineered exosomes have potential advantages in targeting, long-acting and biocompatibility levels required for therapeutic effects. For example, Shi Cong Tao et al. combined circrna3503 associated with sleep with small extracellular vesicle (sEV) derived from synovial mesenchymal stem cells, then PDLLA-PEG-PDLLA, (PLEL) was used as a carrying object of sEV to form PLEL-circRNA3503-OE-sEV targeted therapeutic agent, which was found to interact with chondrocytes and have a significant impact on preventing extracellular matrix degradation [50]. Shu Zhao et al. used exosomes from subcutaneous adipose derived mesenchymal stem cells (ADMSCs-Exos) as a delivery platform for mir-199a-3p to increase the level of mTOR autophagy in the cartilage of rat OA model to promote the repair of damaged cartilage. After the chondrocyte binding peptide (CAP) labeling exosomes, CAP-ADMSCs-Exos can be targeted to reach the depth of joint tissue, which has further advantages for the treatment of cartilage defects in OA mice [64], Yujie Liang et al. used engineering technology to fuse cap with membrane glycoprotein 2b protein on the surface of dendritic cell-derived exosomes, targeted miR-140 to chondrocytes deep in cartilage tissue, and prolonged retention at the cartilage site alleviated the progression of OA [46]. These academic achievements have promoted researchers' research on EV and OA, and provided new directions for researchers.
Based on the aforementioned analysis, several potential future research areas in this field can be identified. These include: utilizing extracellular vesicles as drug delivery vehicles to administer bioactive small molecules for the treatment of osteoarthritis, developing extracellular vesicle-based biomarkers for the early diagnosis of osteoarthritis, and modifying the surface of extracellular vesicles to enhance their selectivity for target organs and cells. However, there are several challenges that need to be addressed for the clinical transformation of extracellular vesicles. These challenges encompass the development of efficient methods for the separation, purification, and storage of extracellular vesicles [65], the optimization of drug delivery efficiency using extracellular vesicles for osteoarthritis treatment, the limited targeting ability of extracellular vesicles [66], and strategies to extend their in vivo circulating half-life [67]. Overcoming these obstacles is crucial to ensure the effectiveness of extracellular vesicles for patients. Future research endeavors are anticipated to primarily concentrate on addressing these issues and conducting clinical translation utilizing extracellular vesicle therapy for osteoarthritis.
5. Conclusion
With the increasing number of publications on extracellular vesicles in the field of osteoarthritis, it is of significant importance to retrospectively examine the major research achievements to identify the research hotspots and future directions in this field. Our study aims to fill this gap by conducting a comprehensive bibliometric analysis of articles related to EV in OA for the first time. Our research findings indicate a rapid growth in interest in this field, specifically from 2006 to 2022, with a substantial increase in relevant publications. These publications predominantly originate from China and USA, although there is room for improvement in fostering active collaborations among other countries and institutions. On one hand, investigating the mechanisms of EV in the progression of OA contributes to understanding the disease process and aids in its diagnosis. Further, when compared to traditional therapies, EV offer greater advantages in the treatment of OA, including lower toxicity, higher biocompatibility, and enhanced efficiency. Consequently, cell-free therapeutic strategies based on EV hold significant potential for the treatment of OA. It is worth noting that future attention should also be directed towards the translation of basic research findings into clinical applications of EV in OA patients. In summary, our study provides a systematic visualization of the research literature on EV in osteoarthritis, offering guidance and references for understanding the current trend of research and exploring novel research directions of extracellular vesicles in osteoarthritis.
Limitations of the study
There are some limitations to this work. First, this study only used the papers in WoSCC for analysis, limiting the scope of the study to English literature only. Although our research process has undergone strict procedures and good structure, the selection of papers that can only be found in WoSCC as analysis data may lead to the omission of some studies. Secondly, due to insufficient data, the publication situation in 2023 could not be reflected.
Funding
This study was supported by National Natural Science Foundation of China (82060354), Natural Science Foundation of Inner Mongolia (2023MS02001, 2020MS08166, 2021LHMS08028, 2021MS08043), Grassland Talent Project of Inner Mongolia (2022CYYC1C-88), Science and Technology Plan Project of Inner Mongolia (2020GG0292).
Ethics declarations
This study does not involve human and/or animal subjects, therefore it does not require approval from the institutional ethics review committee, nor does it require informed consent.
Data availability statement
Data will be made available on request.
Additional information
There is no supplementary information in this article.
CRediT authorship contribution statement
Yongkang Ding: Writing – original draft, Visualization, Software, Resources, Project administration, Methodology, Data curation. Lu Liang: Methodology, Investigation. Ye Guo: Writing – review & editing. Bing Zhu: Writing – review & editing, Methodology, Investigation.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Ye Guo, Email: guoye158@126.com.
Bing Zhu, Email: zhubing@alumni.tongji.edu.cn.
References
- 1.Glyn-Jones S., Palmer A.J., Agricola R., Price A.J., Vincent T.L., Weinans H., Carr A.J. Osteoarthritis. Lancet. 2015;386:376–387. doi: 10.1016/s0140-6736(14)60802-3. [DOI] [PubMed] [Google Scholar]
- 2.Dou H., Wang S., Hu J., Song J., Zhang C., Wang J., Xiao L. Osteoarthritis models: from animals to tissue engineering. J. Tissue Eng. 2023;14 doi: 10.1177/20417314231172584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abramoff B., Caldera F.E. Osteoarthritis: pathology, diagnosis, and treatment options. Med Clin North Am. 2020;104:293–311. doi: 10.1016/j.mcna.2019.10.007. [DOI] [PubMed] [Google Scholar]
- 4.Schulze-Tanzil G. Intraarticular ligament degeneration is interrelated with cartilage and bone destruction in osteoarthritis. Cells 8. 2019 doi: 10.3390/cells8090990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leifer V.P., Katz J.N., Losina E. The burden of OA-health services and economics. Osteoarthritis Cartilage. 2022;30:10–16. doi: 10.1016/j.joca.2021.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li M., Fang F., Sun M., Zhang Y., Hu M., Zhang J. Extracellular vesicles as bioactive nanotherapeutics: an emerging paradigm for regenerative medicine. Theranostics. 2022;12:4879–4903. doi: 10.7150/thno.72812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jin Y., Xu M., Zhu H., Dong C., Ji J., Liu Y., Deng A., Gu Z. Therapeutic effects of bone marrow mesenchymal stem cells-derived exosomes on osteoarthritis. J. Cell Mol. Med. 2021;25:9281–9294. doi: 10.1111/jcmm.16860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vandergriff A., Huang K., Shen D., Hu S., Hensley M.T., Caranasos T.G., Qian L., Cheng K. Targeting regenerative exosomes to myocardial infarction using cardiac homing peptide. Theranostics. 2018;8:1869–1878. doi: 10.7150/thno.20524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jeppesen D.K., Zhang Q., Franklin J.L., Coffey R.J. Extracellular vesicles and nanoparticles: emerging complexities. Trends Cell Biol. 2023;33:667–681. doi: 10.1016/j.tcb.2023.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y., Liu Q., Zhang X., Huang H., Tang S., Chai Y., Xu Z., Li M., Chen X., Liu J., Yang C. Recent advances in exosome-mediated nucleic acid delivery for cancer therapy. J. Nanobiotechnol. 2022;20:279. doi: 10.1186/s12951-022-01472-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matsuzaka Y., Yashiro R. Therapeutic strategy of mesenchymal-stem-cell-derived extracellular vesicles as regenerative medicine. Int. J. Mol. Sci. 2022;23 doi: 10.3390/ijms23126480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu W., Song X., Yu H., Sun J., Zhao Y. Therapeutic potentials of extracellular vesicles for the treatment of diabetes and diabetic complications. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21145163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weng Z., Zhang B., Wu C., Yu F., Han B., Li B., Li L. Therapeutic roles of mesenchymal stem cell-derived extracellular vesicles in cancer. J. Hematol. Oncol. 2021;14:136. doi: 10.1186/s13045-021-01141-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Z., Zhuang Y., Fang L., Yuan C., Wang X., Lin K. Breakthrough of extracellular vesicles in pathogenesis, diagnosis and treatment of osteoarthritis. Bioact. Mater. 2023;22:423–452. doi: 10.1016/j.bioactmat.2022.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li K., Yan G., Huang H., Zheng M., Ma K., Cui X., Lu D., Zheng L., Zhu B., Cheng J., Zhao J. Anti-inflammatory and immunomodulatory effects of the extracellular vesicles derived from human umbilical cord mesenchymal stem cells on osteoarthritis via M2 macrophages. J. Nanobiotechnol. 2022;20:38. doi: 10.1186/s12951-021-01236-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li S., Liu J., Liu S., Jiao W., Wang X. Chitosan oligosaccharides packaged into rat adipose mesenchymal stem cells-derived extracellular vesicles facilitating cartilage injury repair and alleviating osteoarthritis. J. Nanobiotechnol. 2021;19:343. doi: 10.1186/s12951-021-01086-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi J., Gao Y., Ming L., Yang K., Sun Y., Chen J., Shi S., Geng J., Li L., Wu J., Tian J. A bibliometric analysis of global research output on network meta-analysis. BMC Med. Inf. Decis. Making. 2021;21:144. doi: 10.1186/s12911-021-01470-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eraslan F.N., Bhat M.A., Gaga E.O., Gedik K. In: Ecological and Health Effects of Building Materials. Malik J.A., Marathe S., editors. Springer International Publishing; 2022. Comprehensive analysis of research trends in volatile organic compounds emitted from building materials: a bibliometric analysis; pp. 87–112. [DOI] [Google Scholar]
- 19.Zhang J., Lin M., Huang Y., Wang Y., Huang T., Wu Z., Li Z., Xu J., Zhao R., Luo X. Harnessing hyaluronic acid for the treatment of osteoarthritis: a bibliometric analysis. Front. Bioeng. Biotechnol. 2022;10 doi: 10.3389/fbioe.2022.961459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mukherjee D., Lim W.M., Kumar S., Donthu N. Guidelines for advancing theory and practice through bibliometric research. J. Bus. Res. 2022;148:101–115. doi: 10.1016/j.jbusres.2022.04.042. [DOI] [Google Scholar]
- 21.Wang Y., Bai J., Zhang L., Liu H., Wang W., Liu Z., Zhang G. Advances in studies on the plant rhizosphere microorganisms in wetlands: a visualization analysis based on CiteSpace. Chemosphere. 2023;317 doi: 10.1016/j.chemosphere.2023.137860. [DOI] [PubMed] [Google Scholar]
- 22.Chen C. CiteSpace II: detecting and visualizing emerging trends and transient patterns in scientific literature. J. Am. Soc. Inf. Sci. Technol. 2006;57:359–377. doi: 10.1002/asi.20317. [DOI] [Google Scholar]
- 23.Tang J.Q., Shen Q.H., Han Y.Y., Wu Y., He X.F., Li D.W., Huang Y. Analysis of research status and trends on marine benthic dinoflagellate toxins: a bibliometric study based on web of science database and VOSviewer. Environ. Res. 2023;238 doi: 10.1016/j.envres.2023.117179. [DOI] [PubMed] [Google Scholar]
- 24.Bhat M.A. Indoor microplastics: a comprehensive review and bibliometric analysis. Environ. Sci. Pollut. Res. Int. 2023;30:121269–121291. doi: 10.1007/s11356-023-30902-0. [DOI] [PubMed] [Google Scholar]
- 25.Bhat M.A., Eraslan F.N., Gaga E.O., Gedik K. Microplastics in the Ecosphere. 2023. Scientometric analysis of microplastics across the globe; pp. 1–13. [DOI] [Google Scholar]
- 26.Donthu N., Kumar S., Mukherjee D., Pandey N., Lim W.M. How to conduct a bibliometric analysis: an overview and guidelines. J. Bus. Res. 2021;133:285–296. doi: 10.1016/j.jbusres.2021.04.070. [DOI] [Google Scholar]
- 27.Peng Y.W., Tang R., Xu Q.Y., Mei S.Y., Zhou Y., Feng J.H., Zhang S.Y., He Z.Y. Worldwide productivity and research trend of publications concerning extracellular vesicles role in fibrosis: a bibliometric study from 2013 to 2022. Heliyon. 2024;10 doi: 10.1016/j.heliyon.2024.e24357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang W., Li M., Li X., Wang X., Liu Y., Yang J. Global trends and research status in ankylosing spondylitis clinical trials: a bibliometric analysis of the last 20 years. Front. Immunol. 2023;14 doi: 10.3389/fimmu.2023.1328439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gu J.Y., Han F., Chen S.Y., Zhang Q. Bibliometric analysis of publications in clinical trials on knee osteoarthritis between 2001 and 2022. J. Pain Res. 2023;16:1961–1977. doi: 10.2147/jpr.S392840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wen P., Liu R., Wang J., Wang Y., Song W., Zhang Y. Bibliometric insights from publications on subchondral bone research in osteoarthritis. Front. Physiol. 2022;13 doi: 10.3389/fphys.2022.1095868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang Z., Lin J., Li H., He Z., Wang K., Lei L., Li H., Xing D., Lin J. Bibliometric and visualization analysis of macrophages associated with osteoarthritis from 1991 to 2021. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.1013498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li D., Yu D., Li Y., Yang R. A bibliometric analysis of PROTAC from 2001 to 2021. Eur. J. Med. Chem. 2022;244 doi: 10.1016/j.ejmech.2022.114838. [DOI] [PubMed] [Google Scholar]
- 33.Wu F., Gao J., Kang J., Wang X., Niu Q., Liu J., Zhang L. Knowledge mapping of exosomes in autoimmune diseases: a bibliometric analysis (2002-2021) Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.939433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiang S., Liu Y., Zheng H., Zhang L., Zhao H., Sang X., Xu Y., Lu X. Evolutionary patterns and research frontiers in neoadjuvant immunotherapy: a bibliometric analysis. Int. J. Surg. 2023;109:2774–2783. doi: 10.1097/js9.0000000000000492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang H.G., Liu C., Su K., Yu S., Zhang L., Zhang S., Wang J., Cao X., Grizzle W., Kimberly R.P. A membrane form of TNF-alpha presented by exosomes delays T cell activation-induced cell death. J. Immunol. 2006;176:7385–7393. doi: 10.4049/jimmunol.176.12.7385. [DOI] [PubMed] [Google Scholar]
- 36.Anderson H.C., Mulhall D., Garimella R. Role of extracellular membrane vesicles in the pathogenesis of various diseases, including cancer, renal diseases, atherosclerosis, and arthritis. Lab. Invest. 2010;90:1549–1557. doi: 10.1038/labinvest.2010.152. [DOI] [PubMed] [Google Scholar]
- 37.Chen C. Science mapping: a systematic review of the literature. Journal of Data and Information Science. 2017;2:1–40. doi: 10.1515/jdis-2017-0006. [DOI] [Google Scholar]
- 38.Ghasemi A., Yun S., Li X. Fractal structures arising from interfacial instabilities in bio-oil atomization. Sci. Rep. 2021;11:411. doi: 10.1038/s41598-020-80059-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Z., Yang Y., Kong W., Huang S., Tan Y., Huang S., Zhang M., Lu H., Li Y., Li X., et al. A bibliometric and visual analysis of single nucleotide polymorphism studies in depression. Curr. Neuropharmacol. 2024;22:302–322. doi: 10.2174/1570159x21666230815125430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kato T., Miyaki S., Ishitobi H., Nakamura Y., Nakasa T., Lotz M.K., Ochi M. Exosomes from IL-1β stimulated synovial fibroblasts induce osteoarthritic changes in articular chondrocytes. Arthritis Res. Ther. 2014;16 doi: 10.1186/ar4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang S., Chu W.C., Lai R.C., Lim S.K., Hui J.H., Toh W.S. Exosomes derived from human embryonic mesenchymal stem cells promote osteochondral regeneration. Osteoarthritis Cartilage. 2016;24:2135–2140. doi: 10.1016/j.joca.2016.06.022. [DOI] [PubMed] [Google Scholar]
- 42.Durieux V., Gevenois P.A. Bibliometric indicators: quality measurements of scientific publication. Radiology. 2010;255:342–351. doi: 10.1148/radiol.09090626. [DOI] [PubMed] [Google Scholar]
- 43.Muniz F., Celeste R.K., Oballe H.J.R., Rösing C.K. Citation Analysis and Trends in review articles in dentistry. J. Evid. Base Dent. Pract. 2018;18:110–118. doi: 10.1016/j.jebdp.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 44.Mortati L., de Girolamo L., Perucca Orfei C., Viganò M., Brayda-Bruno M., Ragni E., Colombini A. In vitro study of extracellular vesicles migration in cartilage-derived osteoarthritis samples using real-time quantitative multimodal nonlinear optics imaging. Pharmaceutics. 2020;12 doi: 10.3390/pharmaceutics12080734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ragni E., Papait A., Perucca Orfei C., Silini A.R., Colombini A., Viganò M., Libonati F., Parolini O., de Girolamo L. Amniotic membrane-mesenchymal stromal cells secreted factors and extracellular vesicle-miRNAs: anti-inflammatory and regenerative features for musculoskeletal tissues. Stem Cells Transl Med. 2021;10:1044–1062. doi: 10.1002/sctm.20-0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liang Y., Xu X., Li X., Xiong J., Li B., Duan L., Wang D., Xia J. Chondrocyte-targeted MicroRNA delivery by engineered exosomes toward a cell-free osteoarthritis therapy. ACS Appl. Mater. Interfaces. 2020;12:36938–36947. doi: 10.1021/acsami.0c10458. [DOI] [PubMed] [Google Scholar]
- 47.Liang Y., Xu X., Xu L., Iqbal Z., Ouyang K., Zhang H., Wen C., Duan L., Xia J. Chondrocyte-specific genomic editing enabled by hybrid exosomes for osteoarthritis treatment. Theranostics. 2022;12:4866–4878. doi: 10.7150/thno.69368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu X., Liang Y., Li X., Ouyang K., Wang M., Cao T., Li W., Liu J., Xiong J., Li B., et al. Exosome-mediated delivery of kartogenin for chondrogenesis of synovial fluid-derived mesenchymal stem cells and cartilage regeneration. Biomaterials. 2021;269 doi: 10.1016/j.biomaterials.2020.120539. [DOI] [PubMed] [Google Scholar]
- 49.Tao S.C., Yuan T., Zhang Y.L., Yin W.J., Guo S.C., Zhang C.Q. Exosomes derived from miR-140-5p-overexpressing human synovial mesenchymal stem cells enhance cartilage tissue regeneration and prevent osteoarthritis of the knee in a rat model. Theranostics. 2017;7:180–195. doi: 10.7150/thno.17133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tao S.C., Huang J.Y., Gao Y., Li Z.X., Wei Z.Y., Dawes H., Guo S.C. Small extracellular vesicles in combination with sleep-related circRNA3503: a targeted therapeutic agent with injectable thermosensitive hydrogel to prevent osteoarthritis. Bioact. Mater. 2021;6:4455–4469. doi: 10.1016/j.bioactmat.2021.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tan L., Wang X., Yuan K., Yin T., Du R., Shen L., Zhu Z., Yu S., Zhang H., Wang G. Structural and temporal dynamics analysis on drug-eluting stents: history, research hotspots and emerging trends. Bioact. Mater. 2023;23:170–186. doi: 10.1016/j.bioactmat.2022.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maehara M., Toyoda E., Takahashi T., Watanabe M., Sato M. Potential of exosomes for diagnosis and treatment of joint disease: towards a point-of-care therapy for osteoarthritis of the knee. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms22052666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee Y., Ni J., Beretov J., Wasinger V.C., Graham P., Li Y. Recent advances of small extracellular vesicle biomarkers in breast cancer diagnosis and prognosis. Mol. Cancer. 2023;22:33. doi: 10.1186/s12943-023-01741-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Du Y., Wu L., Wang L., Reiter R.J., Lip G.Y.H., Ren J. Extracellular vesicles in cardiovascular diseases: from pathophysiology to diagnosis and therapy. Cytokine Growth Factor Rev. 2023;74:40–55. doi: 10.1016/j.cytogfr.2023.09.006. [DOI] [PubMed] [Google Scholar]
- 55.Hu S., Hu Y., Yan W. Extracellular vesicle-mediated interorgan communication in metabolic diseases. Trends Endocrinol. Metabol. 2023;34:571–582. doi: 10.1016/j.tem.2023.06.002. [DOI] [PubMed] [Google Scholar]
- 56.Elsharkasy O.M., Nordin J.Z., Hagey D.W., de Jong O.G., Schiffelers R.M., Andaloussi S.E., Vader P. Extracellular vesicles as drug delivery systems: why and how? Adv. Drug Deliv. Rev. 2020;159:332–343. doi: 10.1016/j.addr.2020.04.004. [DOI] [PubMed] [Google Scholar]
- 57.Zhang W., Doherty M., Peat G., Bierma-Zeinstra M.A., Arden N.K., Bresnihan B., Herrero-Beaumont G., Kirschner S., Leeb B.F., Lohmander L.S., et al. EULAR evidence-based recommendations for the diagnosis of knee osteoarthritis. Ann. Rheum. Dis. 2010;69:483–489. doi: 10.1136/ard.2009.113100. [DOI] [PubMed] [Google Scholar]
- 58.Altman R., Asch E., Bloch D., Bole G., Borenstein D., Brandt K., Christy W., Cooke T.D., Greenwald R., Hochberg M., et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986;29:1039–1049. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 59.Hunter D.J., Bierma-Zeinstra S. Osteoarthritis. Lancet. 2019;393:1745–1759. doi: 10.1016/s0140-6736(19)30417-9. [DOI] [PubMed] [Google Scholar]
- 60.Zhao Y., Xu J. Synovial fluid-derived exosomal lncRNA PCGEM1 as biomarker for the different stages of osteoarthritis. Int. Orthop. 2018;42:2865–2872. doi: 10.1007/s00264-018-4093-6. [DOI] [PubMed] [Google Scholar]
- 61.Matejova J., Fecskeova L.K., Slovinska L., Harvanova D., Spakova T., Bzdilova J. Plasma-derived extracellular vesicle surface markers CD45, CD326 and CD56 correlate with the stage of osteoarthritis: a primary study of a novel and promising diagnostic tool of the disease. Sci. Rep. 2023;13 doi: 10.1038/s41598-023-47074-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tsuno H., Arito M., Suematsu N., Sato T., Hashimoto A., Matsui T., Omoteyama K., Sato M., Okamoto K., Tohma S., et al. A proteomic analysis of serum-derived exosomes in rheumatoid arthritis. BMC Rheumatol. 2018;2:35. doi: 10.1186/s41927-018-0041-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang Z., Yan K., Ge G., Zhang D., Bai J., Guo X., Zhou J., Xu T., Xu M., Long X., et al. Exosomes derived from miR-155-5p-overexpressing synovial mesenchymal stem cells prevent osteoarthritis via enhancing proliferation and migration, attenuating apoptosis, and modulating extracellular matrix secretion in chondrocytes. Cell Biol. Toxicol. 2021;37:85–96. doi: 10.1007/s10565-020-09559-9. [DOI] [PubMed] [Google Scholar]
- 64.Zhao S., Xiu G., Wang J., Wen Y., Lu J., Wu B., Wang G., Yang D., Ling B., Du D., Xu J. Engineering exosomes derived from subcutaneous fat MSCs specially promote cartilage repair as miR-199a-3p delivery vehicles in Osteoarthritis. J. Nanobiotechnol. 2023;21:341. doi: 10.1186/s12951-023-02086-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hao Y., Song H., Zhou Z., Chen X., Li H., Zhang Y., Wang J., Ren X., Wang X. Promotion or inhibition of extracellular vesicle release: emerging therapeutic opportunities. J Control Release. 2021;340:136–148. doi: 10.1016/j.jconrel.2021.10.019. [DOI] [PubMed] [Google Scholar]
- 66.Herrmann I.K., Wood M.J.A., Fuhrmann G. Extracellular vesicles as a next-generation drug delivery platform. Nat. Nanotechnol. 2021;16:748–759. doi: 10.1038/s41565-021-00931-2. [DOI] [PubMed] [Google Scholar]
- 67.Wei B., Huang H., Cao Q., Song X., Zhang Z. Bibliometric and visualized analysis of the applications of exosomes based drug delivery. Biomed. Pharmacother. 2024;176 doi: 10.1016/j.biopha.2024.116803. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.