Abstract
Aim
There is an ongoing search for novel biomarkers of diabetes. We conducted a systematic review and meta-analysis of the serum concentrations of ischemia-modified albumin (IMA), a candidate biomarker of oxidative stress, acidosis, and ischemia, in patients with pre-diabetes, different types of diabetes mellitus (type 1, T1DM, type 2, T2DM, and gestational, GDM), and healthy controls.
Methods
We searched for case-control studies published in PubMed, Web of Science, and Scopus from inception to December 31, 2023. The risk of bias and the certainty of evidence were assessed using the Joanna Briggs Institute Critical Appraisal Checklist and GRADE, respectively.
Results
In 29 studies, T2DM patients had significantly higher IMA concentrations when compared to controls (standard mean difference, SMD = 1.83, 95 % CI 1.46 to 2.21, p˂0.001; I2 = 95.7 %, p < 0.001; low certainty of evidence). Significant associations were observed between the SMD and glycated hemoglobin (p = 0.007), creatinine (p = 0.003), triglycerides (p = 0.029), and the presence of diabetes complications (p = 0.003). Similar trends, albeit in a smaller number of studies, were observed in T1DM (two studies; SMD = 1.59, 95 % CI -0.09 to 3.26, p˂0.063; I2 = 95.8 %, p < 0.001), GDM (three studies; SMD = 3.41, 95 % CI 1.14 to 5.67, p = 0.003; I2 = 97.0 %, p < 0.001) and pre-diabetes (three studies; SMD = 15.25, 95 % CI 9.86 to 20.65, p˂0.001; I2 = 99.3 %, p < 0.001).
Conclusion
Our study suggests that IMA is a promising biomarker for determining the presence of oxidative stress, acidosis, and ischemia in pre-diabetes and T1DM, T2DM, and GDM. However, the utility of measuring circulating IMA warrants confirmation in prospective studies investigating clinical endpoints in pre-diabetes and in different types of diabetes (PROSPERO registration number: CRD42024504690).
Keywords: Ischemia-modified albumin, IMA, Type 1 diabetes, Type 2 diabetes, Gestational diabetes, Pre-diabetes, Biomarkers
Highlights
-
•
T2DM patients had significantly higher IMA concentrations than controls.
-
•
Similar trends were observed in T1DM, GDM, and pre-diabetes.
-
•
IMA is a promising biomarker of pre-diabetes and T1DM, T2DM, and GDM.
1. Introduction
Diabetes mellitus, particularly type 2 (T2DM), remains a global public health burden [1,2]. In 2021, T2DM accounted for 96 % of total cases of diabetes (529 million people living with diabetes worldwide) and 95.4 % of disability-adjusted life years due to diabetes. It is estimated that more than 1.31 billion people will have diabetes by 2050 [3]. Despite these figures undisputedly support the notion that T2DM represents the bulk of the cases of diabetes and their associated negative impact on quality of life and burden on healthcare systems, the global and/or local incidence of other types of diabetes mellitus, particularly type 1 (T1DM) and gestational (GDM), as well as pre-diabetes also continues to grow at an alarming rate [[4], [5], [6], [7], [8], [9], [10], [11]].
While the increasing availability of safe and effective hypoglycemic agents and insulins has revolutionized the management of different types of diabetes [[12], [13], [14], [15], [16], [17], [18]], a significant body of research has increasingly focused on the identification of robust disease biomarkers to enhance early diagnosis and risk stratification and predict response to therapy and clinical outcomes [19]. Circulating, e.g., glycated hemoglobin [20], and imaging, e.g., left ventricular global longitudinal strain on echocardiography [21], biomarkers are available in clinical practice. Other biomarkers, e.g., adiponectin, fetuin A, alpha-hydroxybutyrate, C-reactive protein (CRP), interleukin-6, white blood cell count, and fibrinogen have also been investigated with mixed results [[22], [23], [24]]. In addition to glycation, albumin, one of the most abundant circulating proteins, can also undergo a series of chemical modifications targeting the N-terminal sequence in the presence of ischemic conditions, which lead to the formation of ischemia-modified albumin (IMA) [25]. It has been suggested that these chemical modifications are triggered by oxidative stress, increased production of reactive oxygen species, and acidosis, which are typically associated with ischaemic events. Notably, such modifications reduce the binding capacity of IMA for metals, particularly copper, nickel, and cobalt [25]. Serum concentrations of IMA have been shown to increase within 24 h of the occurrence of an acute ischemic stroke and gradually decrease over the following days [26]. Similarly, several other studies have investigated the pathophysiological role of IMA in conditions of cardiac ischemia [[27], [28], [29]]. Given that the formation of IMA reflects, in addition to ischemia, the presence of oxidative stress and acidosis, common alterations observed in patients with diabetes [[30], [31], [32], [33], [34]], we conducted a systematic review and meta-analysis of serum IMA concentrations in patients with pre-diabetes, TD2M, TD1M, and GDM, and in healthy controls. We hypothesised that patients with pre-diabetes and diabetes have significantly higher concentrations of serum IMA compared to healthy controls, highlighting the potential role of IMA as a biomarker of diabetes.
2. Materials and methods
2.1. Search strategy and study selection
We conducted a systematic search for articles published in PubMed, Web of Science, and Scopus from inception to December 31, 2023 using the following terms: “IMA” OR “ischemia modified albumin” OR “ischemia-modified albumin” AND “diabetes”. Two investigators independently screened each abstract and, if relevant, the full text of the publication according to the following inclusion criteria: (i) measurement of serum IMA, (ii) comparison of patients with diabetes (type 1 diabetes mellitus, T1DM, type 2 diabetes mellitus, T2DM, gestational diabetes mellitus, GDM, or pre-diabetes) and healthy controls in case-control studies, (iii) use of English language, and (iv) availability of the full-text of the publication. The references of individual articles were hand-searched for additional studies.
The following variables were independently extracted and transferred into an electronic spreadsheet for further analysis: year of publication, first author, study country, type of diabetes, sample size, age, male to female ratio, mean disease duration, body mass index (BMI), C-reactive protein (CRP), glucose, glycated hemoglobin, albumin, creatinine, cholesterol (total, LDL and HDL), triglycerides, systolic and diastolic pressure, and presence of diabetic complications (e.g., diabetic retinopathy, neuropathy, nephropathy, diabetic foot, cardiovascular disease, and albuminuria) [35].
We calculated the risk of bias and the certainty of evidence using established methods [36,37] and used the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) 2020 statement to accurately report the relevant methods for identifying, selecting, appraising, and synthesizing studies (Supplementary Tables 1 and 2) [38]. We registered the study protocol in an international registry (PROSPERO registration number CRD42024504690).
2.2. Statistical analysis
We calculated standardized mean differences (SMDs) and 95 % confidence intervals (CIs) to generate forest plots to investigate differences in serum IMA concentrations between patients with diabetes and healthy controls (a p-value <0.05 was considered statistically significant). If required, means and standard deviations were extrapolated from medians and interquartile ranges or medians and ranges according to published methods [39]. SMD heterogeneity was assessed using the Q statistic (significance level at p < 0.10) and was interpreted as low when I2 ≤ 25 %, moderate when 25 % < I2 < 75 %, and high when I2 ≥ 75 % [40,41]. High heterogeneity warranted the use of random-effect models based on the inverse-variance method.
Sensitivity analysis was performed to confirm the stability of the meta-analysis results [42]. The presence of publication bias was assessed using the Begg's and the Egger's test and the “trim and fill” method [[43], [44], [45]].
Univariate meta-regression analyses were conducted to investigate associations between the effect size and year of publication, study country, sample size, age, male-to-female ratio, mean disease duration, BMI, CRP, glucose, glycated hemoglobin, albumin, creatinine, cholesterol (total, LDL and HDL), triglycerides, systolic and diastolic pressure, and presence of complications (e.g., diabetic retinopathy, neuropathy, nephropathy, diabetic foot, cardiovascular disease, and albuminuria). Statistical analyses were performed using Stata 14 (Stata Corp., College Station, TX, USA).
3. Results
3.1. Study selection
From 870 articles initially identified, 819 were excluded after the initial screening because they were duplicates or irrelevant. After a full-text review of the remaining 51 articles, two were excluded because they provided duplicate data, three because of missing data, and eight because they were not a case-control study, leaving 38 studies for analysis [[46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74], [75], [76], [77], [78], [79], [80], [81], [82], [83]] (Fig. 1 and Table 1). The risk of bias was low in 33 studies [46,47,[49], [50], [51], [52], [53], [54], [55], [56], [57], [58],[60], [61], [62], [63], [64], [65],[67], [68], [69], [70], [71], [72], [73], [74], [75], [76], [77], [78], [79], [80], [81]] and moderate in the remaining five [48,59,66,82,83] (Table 2). The initial level of certainty was considered low in all studies, given their cross-sectional design (rating 2).
Fig. 1.
PRISMA 2020 flow diagram.
Table 1.
Characteristics of the studies reporting ischemia-modified albumin concentrations in patients with diabetes and healthy controls.
| Study | Healthy controls |
Patients with diabetes |
Type of diabetes | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Age (Years) | M/F | IMA (Mean ± SD) | n | Age (Years) | M/F | IMA (Mean ± SD) | ||
| Piwowar A et al., 2008, Poland [46] | 25 | 57 | 6/19 | 0.33 ± 0.09^ | 76 | 65 | 11/65 | 0.56 ± 0.11^ | T2DM |
| Ukinc K et al., 2009, Turkey [47] | 30 | 51 | 15/15 | 0.26 ± 0.04^ | 50 | 52 | 22/28 | 0.33 ± 0.05^ | T2DM |
| Dahiya K et al., 2010, India [48] | 30 | matched | matched | 54.60 ± 15.17^ | 60 | matched | matched | 61.20 ± 21.80^ | T2DM |
| Kaefer M et al., 2011, Brazil [49] | 26 | 52 | 7/18 | 0.41 ± 0.09^ | 80 | 59 | 31/49 | 0.53 ± 0.12^ | T2DM |
| Ma SG et al. (a) 2011, China [50] | 45 | 56 | 21/24 | 97.35 ± 5.25^ | 50 | 56 | 22/28 | 103.30 ± 7.43^ | T2DM |
| Ma SG et al. (b) 2011, China [50] | 45 | 56 | 21/24 | 97.35 ± 5.25^ | 47 | 58 | 19/28 | 139.80 ± 20.00^ | T2DM |
| Turk A et al. (a) 2011, Turkey [51] | 36 | NR | 17/19 | 0.62 ± 0.04^ | 35 | 56 | 13/22 | 0.66 ± 0.13^ | T2DM |
| Turk A et al. (b) 2011, Turkey [51] | 36 | NR | 17/19 | 0.62 ± 0.04^ | 35 | 60 | 15/20 | 0.77 ± 0.07^ | T2DM |
| Ma SG et al. (a) 2012, China [52] | 33 | 52 | 23/10 | 46.31 ± 11.42^ | 56 | 53 | 36/20 | 61.47 ± 10.93^ | T2DM |
| Ma SG et al. (b) 2012, China [52] | 33 | 52 | 23/10 | 46.31 ± 11.42^ | 48 | 54 | 36/12 | 78.15 ± 15.39^ | T2DM |
| Ma SG et al. (a) 2012, China [53] | 40 | 49 | 16/24 | 43.48 ± 10.67^ | 30 | 49 | 12/18 | 60.82 ± 17.41^ | T1DM |
| Ma SG et al. (b) 2012, China [53] | 40 | 49 | 16/24 | 43.48 ± 10.67^ | 27 | 47 | 11/16 | 96.22 ± 20.45^ | T1DM |
| Ma SG et al., 2012, China [54] | 30 | 24 | 0/30 | 65.84 ± 10.36^ | 40 | 29 | 0/40 | 83.77 ± 11.45^ | GDM |
| Dayanand CD et al., 2013, India [55] | 70 | NR | NR | 0.07 ± 0.07^ | 70 | NR | NR | 0.30 ± 0.13^ | T2DM |
| Korkmaz GG et al. (a) 2013, Turkey [56] | 35 | 55 | 15/20 | 8.10 ± 0.91^ | 55 | 55 | 25/30 | 9.11 ± 0.65^ | Pre-diabetes |
| Korkmaz GG et al. (b) 2013, Turkey [56] | 35 | 55 | 15/20 | 8.10 ± 0.91^ | 20 | 60 | 11/9 | 9.39 ± 0.95^ | T2DM |
| Kirboga K et al., 2014, Turkey [58] | 22 | 66 | matched | 39.20 ± 28.80# | 22 | 66 | matched | 55.30 ± 26.80# | T2DM |
| Refaat G et al. (a) 2014, Egypt [59] | 10 | 55 | 5/5 | 0.39 ± 0.10^ | 20 | 52 | 12/8 | 0.51 ± 0.21^ | T2DM |
| Refaat G et al. (b) 2014, Egypt [59] | 10 | 55 | 5/5 | 0.39 ± 0.10^ | 20 | 53 | 11/9 | 0.65 ± 0.09^ | T2DM |
| Erdem SS et al. (a) 2015, Turkey [57] | 24 | 44 | 7/17 | 0.54 ± 0.16^ | 29 | 44 | 9/30 | 0.59 ± 0.11^ | Pre-diabetes |
| Erdem SS et al. (b) 2015, Turkey [57] | 24 | 44 | 7/17 | 0.54 ± 0.16^ | 30 | 47 | 11/29 | 0.69 ± 0.22^ | T2DM |
| Reddy VS et al. (a) 2015, India [60] | 23 | 31 | NR | 0.30 ± 0.17^ | 16 | 64 | NR | 0.43 ± 0.19^ | T2DM |
| Reddy VS et al. (b) 2015, India [60] | 23 | 31 | matched | 0.30 ± 0.17^ | 18 | 57 | matched | 0.57 ± 0.12^ | T2DM |
| Ahamad A et al. (a) 2016, India [61] | 30 | 47 | 16/14 | 45.70 ± 23.90# | 20 | 48 | 12/8 | 109.40 ± 50.30# | T2DM |
| Ahamad A et al. (b) 2016, India [61] | 30 | 47 | 16/14 | 45.70 ± 23.90# | 20 | 50 | 12/8 | 154.50 ± 43.10# | T2DM |
| Ahamad A et al. (c) 2016, India [61] | 30 | 47 | 16/14 | 45.70 ± 23.90# | 20 | 50 | 9/11 | 178.10 ± 67.90# | T2DM |
| D'Souza JMP et al. (a) 2016, India [62] | 50 | 49 | matched | 0.42 ± 0.12^ | 50 | 51 | matched | 0.52 ± 0.14^ | T2DM |
| D'Souza JMP et al. (b) 2016, India [62] | 50 | 49 | matched | 0.42 ± 0.12^ | 50 | 53 | matched | 0.60 ± 0.17^ | T2DM |
| Inci A et al., 2016, Turkey [63] | 32 | 50 | 12/20 | 0.45 ± 0.06^ | 109 | 62 | 62/47 | 0.47 ± 0.07^ | T2DM |
| Miric DJ et al. (a) 2016, Serbia [64] | 30 | 60 | 13/17 | 28.50 ± 8.70^ | 51 | 62 | 20/31 | 34.70 ± 12.40^ | T2DM |
| Miric DJ et al. (b) 2016, Serbia [64] | 30 | 60 | 13/17 | 28.50 ± 8.70^ | 29 | 63 | 13/16 | 40.30 ± 11.80^ | T2DM |
| Muhtaroğlu S et al. (a) 2016, Turkey [65] | 30 | 53 | 16/14 | 0.39 ± 0.05^ | 30 | 57 | 15/15 | 0.48 ± 0.09^ | T2DM |
| Muhtaroğlu S et al. (b) 2016, Turkey [65] | 30 | 53 | 16/14 | 0.39 ± 0.05^ | 30 | 59 | 17/13 | 0.72 ± 0.12^ | T2DM |
| Ghosh K et al., 2017, India [66] | 30 | NR | NR | 0.30 ± 0.05^ | 100 | 56 | 57/43 | 0.58 ± 0.09^ | T2DM |
| Sadik L et al., 2017, Sudan [67] | 70 | 59 | 30/40 | 3.21 ± 10.73# | 70 | 58 | 30/40 | 10.78 ± 10.25# | T2DM |
| Balamir I et al., 2018, Turkey [68] | 88 | 53 | 33/55 | 0.46 ± 0.11^ | 88 | 55 | 30/58 | 0.55 ± 0.13^ | T2DM |
| Bhaskhar KU et al., 2018, India [69] | 40 | matched | matched | 0.66 ± 0.15^ | 40 | matched | matched | 1.15 ± 0.14^ | T2DM |
| Sudha K et al. (a) 2018, India [70] | 30 | 49 | NR | 0.86 ± 0.31^ | 30 | 52 | NR | 0.92 ± 0.33^ | T2DM |
| Sudha K et al. (b) 2018, India [70] | 30 | 49 | NR | 0.86 ± 0.31^ | 30 | 47 | NR | 0.66 ± 0.21^ | T2DM |
| Yazici MU et al., 2019, Turkey [71] | 30 | 8.9 | 15/15 | 0.59 ± 0.10^ | 24 | 10.4 | 11/13 | 0.61 ± 0.10^ | T1DM |
| Beyazit F et al., 2020, Turkey [72] | 45 | 28 | NR | 0.01 ± 0.05^ | 45 | 31 | NR | 0.44 ± 0.08^ | GDM |
| El-Eshmawy MM et al., 2020, Egypt [73] | 50 | 43 | 25/25 | 0.16 ± 0.01^ | 100 | 41 | 50/50 | 1.04 ± 0.01^ | Pre-diabetes |
| Sushith S et al. (a) 2020, India [74] | 35 | 49 | NR | 0.23 ± 0.03^ | 35 | 54 | NR | 0.48 ± 0.69^ | T2DM |
| Sushith S et al. (b) 2020, India [74] | 35 | 49 | NR | 0.23 ± 0.03^ | 35 | 55 | NR | 0.59 ± 0.51^ | T2DM |
| Alay H et al. (a) 2021, Turkey [75] | 30 | 63 | 17/13 | 16.90 ± 5.30# | 30 | 63 | 17/13 | 28.10 ± 5.40# | T2DM |
| Alay H et al. (b) 2021, Turkey [75] | 30 | 63 | 17/13 | 16.90 ± 5.30# | 30 | 63 | 19/11 | 35.15 ± 7.70# | T2DM |
| Chaudhry SR et al. (a) 2021, Pakistan [76] | 20 | NR | NR | 0.49 ± 0.09^ | 20 | NR | NR | 0.58 ± 0.06^ | T2DM |
| Chaudhry SR et al. (b) 2021, Pakistan [76] | 20 | NR | NR | 0.49 ± 0.09^ | 20 | NR | NR | 0.64 ± 0.01^ | T2DM |
| Mertoglu C et al. (a) 2021, Turkey [77] | 50 | 55 | NR | 0.80 ± 0.30* | 21 | 56 | NR | 0.50 ± 0.10* | T2DM |
| Mertoglu C et al. (b) 2021, Turkey [77] | 50 | 55 | NR | 0.80 ± 0.30* | 22 | 61 | NR | 0.80 ± 0.40* | T2DM |
| Mertoglu C et al. (c) 2021, Turkey [77] | 50 | 55 | NR | 0.80 ± 0.30* | 69 | 65 | NR | 1.20 ± 0.20* | T2DM |
| Mertoglu C et al. (d) 2021, Turkey [77] | 50 | 55 | NR | 0.80 ± 0.30* | 126 | 58 | NR | 1.10 ± 0.30* | T2DM |
| Ozkan S et al., 2021, Turkey [78] | 60 | 43 | 30/30 | 1.14 ± 0.02^ | 120 | 43 | 75/45 | 2.61 ± 0.26^ | T2DM |
| Xiang L et al. (a) 2021, China [79] | 45 | 54 | 23/22 | 20.11 ± 4.69# | 61 | 54 | 31/30 | 95.41 ± 5.63# | T2DM |
| Xiang L et al. (b) 2021, China [79] | 45 | 54 | 23/22 | 20.11 ± 4.69# | 34 | 54 | 18/16 | 77.95 ± 3.81# | T2DM |
| Arslan A et al., 2022, Turkey [80] | 37 | 27 | 0/37 | 0.86 ± 0.09^ | 40 | 27 | 0/40 | 1.53 ± 0.41^ | GDM |
| Feng F et al., 2022, China [81] | 110 | 59 | 61/49 | 60.32 ± 12.70^ | 110 | 58 | 59/51 | 90.87 ± 19.43^ | T2DM |
| Kurt HA et al., 2022, Turkey [82] | 40 | 55 | 40/0 | 0.26 ± 0.05^ | 46 | 52 | 46/0 | 0.35 ± 0.04^ | T2DM |
| Zainal IG et al., 2022, Iraq [83] | 32 | NR | NR | 0.68 ± 0.04^ | 28 | NR | NR | 0.85 ± 0.10^ | T2DM |
Legend: M/F, male to female ratio; IMA, ischemia-modified albumin; NR, not reported; T2DM, type 2 diabetes mellitus; T1DM, type 1 diabetes mellitus; GDM, gestational diabetes mellitus; ^, albumin cobalt binding test; #, enzyme-linked immunosorbent assay; *, analytical method not reported. IMA values are reported as absorbance units, ng/mL, or U/L.
Table 2.
Assessment of the risk of bias using the Joanna Briggs Institute critical appraisal checklist.
| Study | Were the inclusion criteria clearly defined? | Were the subjects and the setting described in detail? | Was the exposure measured in a reliable way? | Were standard criteria used to assess the condition? | Were confounding factors identified? | Were strategies to deal with confounding factors stated? | Were the outcomes measured in a reliable way? | Was appropriate statistical analysis used? | Risk of bias |
|---|---|---|---|---|---|---|---|---|---|
| Piwowar A et al. [46] | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Low |
| Ukinc K et al. [47] | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Low |
| Dahiya K et al. [48] | No | No | Yes | Yes | No | No | Yes | Yes | Moderate |
| Kaefer M et al. [49] | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Low |
| Ma SG et al. [50] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| Turk A et al. [51] | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Low |
| Ma SG et al. [52] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| Ma SG et al. [53] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| Ma SG et al. [54] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| Dayanand CD et al. [55] | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Low |
| Korkmaz GG et al. [56] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| Kirboga K et al. [58] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| Refaat G et al. [59] | No | No | Yes | Yes | No | No | Yes | Yes | Moderate |
| Erdem SS et al. [57] | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Low |
| Reddy VS et al. [60] | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Low |
| Ahamad A et al. [61] | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Low |
| D'Souza JMP et al. [62] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| Inci A et al. [63] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| Miric DJ et al. [64] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| Muhtaroğlu S et al. [65] | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Low |
| Ghosh K et al. [66] | No | No | Yes | Yes | No | No | Yes | Yes | Moderate |
| Sadik L et al. [67] | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Low |
| Balamir I et al. [68] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| Bhaskhar KU et al. [69] | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Low |
| Sudha K et al. [70] | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Low |
| Yazici MU et al. [71] | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Low |
| Beyazit F et al. [72] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| El-Eshmawy MM et al. [73] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| Sushith S et al. [74] | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Low |
| Alay H et al. [75] | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Low |
| Chaudhry SR et al. [76] | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Low |
| Mertoglu C et al. [77] | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Low |
| Ozkan S et al. [78] | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Low |
| Xiang L et al. [79] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| Arslan A et al. [80] | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Low |
| Feng F et al. [81] | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Low |
| Kurt HA et al. [82] | No | No | Yes | Yes | No | No | Yes | Yes | Moderate |
| Zainal IG et al. [83] | No | No | Yes | Yes | No | No | Yes | Yes | Moderate |
3.2. Ischemia-modified albumin and type 2 diabetes mellitus
Twenty-nine studies, including 47 group comparisons, investigated serum IMA in 2143 T2DM patients (mean age 56 years, 52 % females) and 1706 healthy controls (mean age 52 years, 53 % females) [[46], [47], [48], [49], [50], [51], [52],[55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70],[74], [75], [76], [77], [78], [79]]. Eleven studies were conducted in Turkey [47,51,[56], [57], [58],63,65,68,75,77,78], nine in India [48,55,[60], [61], [62],66,69,70,74], three in China [50,52,79], one in Serbia [64], one in Poland [46], one in Brazil [49], one in Egypt [59], one in Sudan [67], and one in Pakistan [76]. Five studies measured IMA using an enzyme-linked immunosorbent assay (ELISA) [58,61,67,75,79], 23 an albumin cobalt binding (ACB) test [[46], [47], [48], [49], [50], [51], [52],[55], [56], [57],59,60,[62], [63], [64], [65], [66],[68], [69], [70],74,76,78], whereas the remaining one did not provide relevant information regarding the test used [77]. The mean T2DM disease duration ranged between 0 and 15 years.
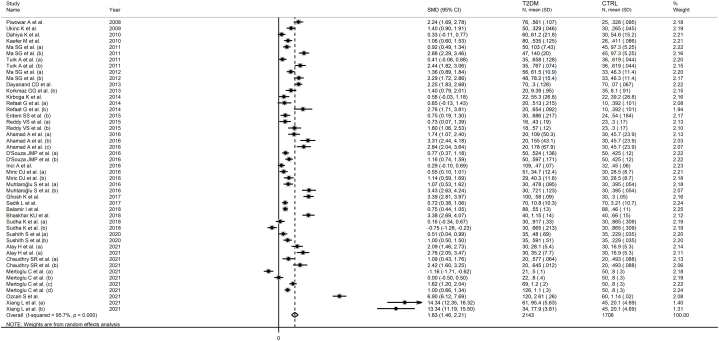
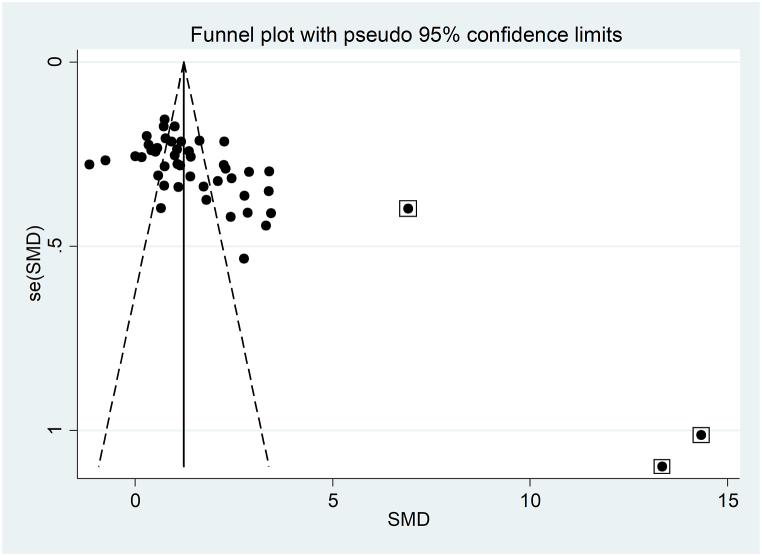
The forest plot showed that serum IMA concentrations were significantly higher in T2DM patients when compared to controls (SMD = 1.83, 95 % CI 1.46 to 2.21, p˂0.001; I2 = 95.7 %, p < 0.001; Fig. 2). Sensitivity analysis confirmed the stability of the meta-analysis results, with the corresponding pooled SMD values ranging between 1.64 and 1.69 (Fig. 3). However, the last three group comparisons [78,79] exerted a slightly distortive effect on the effect size, and therefore, their combined omission should be considered to evaluate the effect on the SMD. This is also evident in the funnel plot analysis, which confirmed the distortive effect of the three group comparisons [78,79] (enclosed circles in Fig. 4). Their removal reduced the magnitude of the effect size, which, however, remained significant (SMD = 1.37, 95 % CI 1.08 to 1.65, p˂0.001; I2 = 92.5 %, p < 0.001).
Fig. 2.
Forest plot of studies investigating serum ischemia-modified albumin concentrations in patients with type 2 diabetes and healthy controls.
Fig. 3.
Sensitivity analysis of the association between serum ischemia-modified albumin and type 2 diabetes.
Fig. 4.
Funnel plot of studies investigating the association between serum ischemia-modified albumin and type 2 diabetes.
The analysis of small studies' effect on the remaining 44 study groups showed significant publication bias according to Begg's (p = 0.001) and Egger's (p = 0.001) test. Accordingly, the “trim-and-fill” method identified nine missing studies to be added to the left side of the funnel plot to ensure symmetry (Fig. 5). The effect size was further reduced to 0.95 (95 % CI 1.08 to 1.65, p˂0.001) but remained significant.
Fig. 5.
Funnel plot of studies investigating the association between serum ischemia-modified albumin and type 2 diabetes after “trimming and filling”. Dummy studies and genuine studies are represented by enclosed circles and free circles, respectively.
In meta-regression analysis, there were no significant associations between the effect size and age (t = −0.08, p = 0.940), male to female ratio (t = −0.53, p = 0.600), participant number (t = −0.42, p = 0.676), publication year (t = −0.89, p = 0.381), mean disease duration (t = 1.33, p = 0.194), BMI (t = −1.48, p = 0.156), CRP (t = 1.46, p = 0.178), albumin (t = −1.26, p = 0.223), total cholesterol (t = 0.31, p = 0.759), LDL-cholesterol (t = −0.56, p = 0.581), HDL-cholesterol (t = −0.98, p = 0.341), systolic (t = 0.70, p = 0.496), or diastolic pressure (t = 0.14, p = 0.894). By contrast, significant and positive associations were observed between the effect size and glycated hemoglobin (t = 2.87, p = 0.007), creatinine (t = 2.35, p = 0.003), and triglycerides (t = 2.35, p = 0.029) (Fig. 6). In addition, a non-significant trend was observed with serum glucose (t = 1.74, p = 0.092).
Fig. 6.
Bubble plot reporting univariate meta-regression analysis between the effect size and glycated haemoglobin (A), creatinine (B), and triglycerides (C).
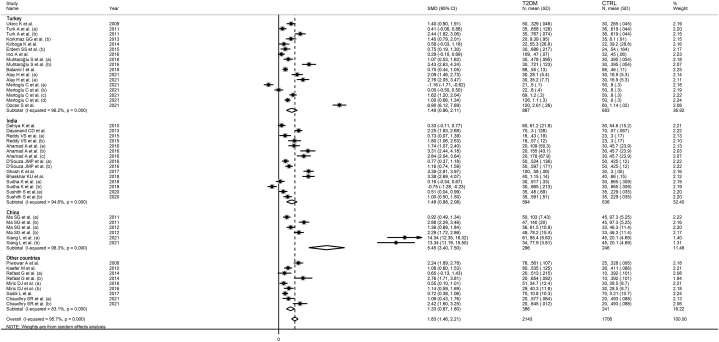
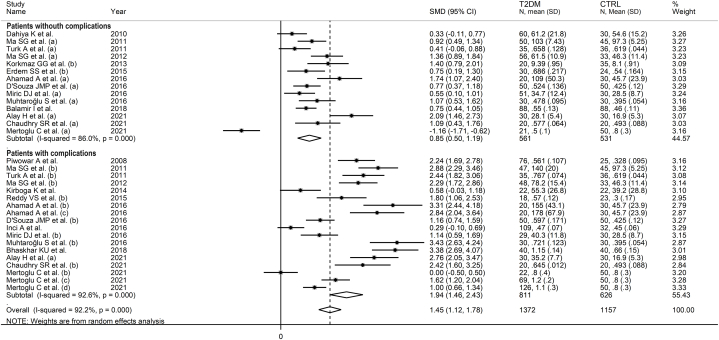
In subgroup analysis, the pooled SMD was statistically significant in studies conducted in Turkey (SMD = 1.49, 95 % CI 0.86 to 2.11, p˂0.001; I2 = 96.2 %, p˂0.001), India (SMD = 1.48, 95 % CI 0.88 to 2.08, p˂0.001; I2 = 94.6 %, p˂0.001), China (SMD = 5.45, 95 % CI 3.40 to 7.50, p˂0.001; I2 = 98.3 %, p˂0.001), and other countries (SMD = 1.33, 95 % CI 0.87 to 1.80, p˂0.001; I2 = 83.1 %, p˂0.001, Fig. 7). The effect size in Chinese studies was significantly higher when compared to other countries (p = 0.001). In addition, a significant difference (p = 0.006) was observed between the pooled SMD of studies using ACB (SMD = 1.53, 95 % CI 1.15 to 1.91, p˂0.001; I2 = 94.5 %, p˂0.001) and ELISA (SMD = 4.30, 95 % CI 1.58 to 2.38, p˂0.001; I2 = 97.7 %, p˂0.001, Fig. 8). Finally, there was a significant difference (p = 0.003) in the pooled SMD between studies conducted in T2DM patients with complications (SMD = 1.94, 95 % CI 1.46 to 2.43, p˂0.001; I2 = 92.6 %, p˂0.001) and without complications (SMD = 0.85, 95 % CI 0.50 to 1.19, p˂0.001; I2 = 86.0 %, p˂0.001, Fig. 9). The reported complications included diabetic retinopathy, neuropathy, nephropathy, diabetic foot, cardiovascular disease, and albuminuria [46,[50], [51], [52],58,[60], [61], [62], [63], [64], [65],69,[75], [76], [77]].
Fig. 7.
Forest plot of studies investigating serum ischemia-modified albumin concentrations in patients with type 2 diabetes and healthy controls according to the study country.
Fig. 8.
Forest plot of studies investigating serum ischemia-modified albumin concentrations in patients with type 2 diabetes and healthy controls according to the assay used to detect ischemia-modified albumin.
Fig. 9.
Forest plot of studies investigating serum ischemia-modified albumin concentrations in patients with type 2 diabetes and healthy controls according to the presence of diabetic complications.
The overall level of certainty remained low (rating 2) after considering the low-moderate risk of bias in all studies (no change), the high and unexplainable heterogeneity (downgrade one level), the lack of indirectness (no change), the large effect size (SMD = 1.83, upgrade one level) [84], and the presence of publication bias which was partially addressed by the “trim and fill” method (no change).
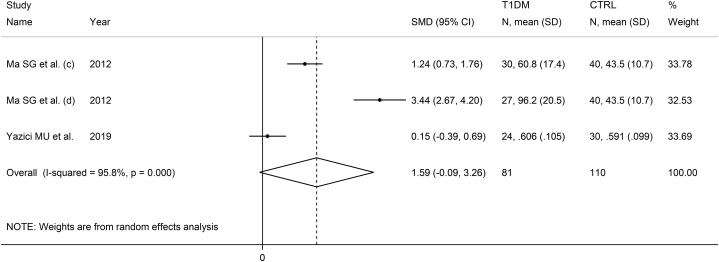
3.3. Ischemia-modified albumin and type 1 diabetes mellitus
Two studies with three group comparisons investigated serum IMA in a total of 81 T1DM patients (mean age 37 years, 58 % females) and 110 healthy controls (mean age 38 years, 57 % females) [53,71]. One study was conducted in Turkey [71], and one in China [53], and both used the ACB assay to measure serum IMA.
The forest plot showed that IMA concentrations were non-significantly higher in T1DM patients when compared to controls (SMD = 1.59, 95 % CI -0.09 to 3.26, p˂0.063; I2 = 95.8 %, p < 0.001; Fig. 10).
Fig. 10.
Forest plot of studies investigating serum ischemia-modified albumin concentrations in patients with type 1 diabetes and healthy controls.
Because of the small number of studies, sensitivity, publication bias, meta-regression, and sub-group analyses could not be assessed. Consequently, the certainty of evidence was downgraded to very low (rating 1).
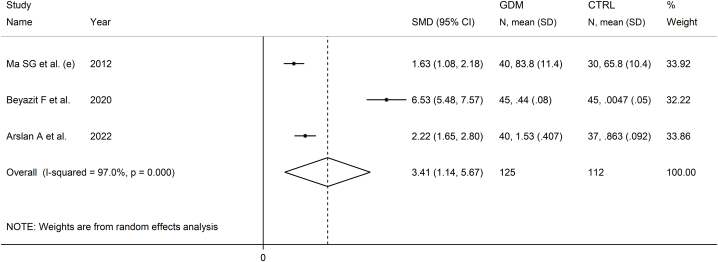
3.4. Ischemia-modified albumin and gestational diabetes mellitus
Three studies investigated serum IMA in 125 patients with GDM (mean age 29 years) and 112 healthy controls (mean age 27 years) [54,72,80]. Two studies were conducted in Turkey [72,80], and one in China [54], and all used the ACB assay to measure serum IMA.
The forest plot showed that serum IMA concentrations were significantly higher in patients with GDM when compared to controls (SMD = 3.41, 95 % CI 1.14 to 5.67, p = 0.003; I2 = 97.0 %, p < 0.001; Fig. 11).
Fig. 11.
Forest plot of studies investigating serum ischemia-modified albumin concentrations in patients with gestational diabetes mellitus and healthy controls.
Because of the small number of studies, sensitivity, publication bias, meta-regression, and sub-group analyses could not be assessed. Consequently, the certainty of evidence was downgraded to very low (rating 1).
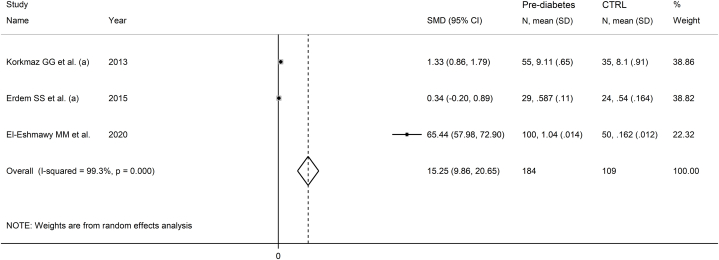
3.5. Ischemia-modified albumin and pre-diabetes
Three studies investigated serum IMA in 184 patients with pre-diabetes (mean age 46 years, 57 % females) and 109 healthy controls (mean age 47 years, 57 % females) [56,57,73]. Two studies were performed in Turkey [56,57], one in Egypt [73], and all used the ACB assay to measure serum IMA.
The forest plot showed that serum IMA concentrations were significantly higher in patients with pre-diabetes than controls (SMD = 15.25, 95 % CI 9.86 to 20.65, p˂0.001; I2 = 99.3 %, p < 0.001; Fig. 12).
Fig. 12.
Forest plot of studies investigating serum ischemia-modified albumin concentrations in patients with pre-diabetes and healthy controls.
Because of the small number of studies, sensitivity, publication bias, meta-regression, and sub-group analyses could not be assessed. Consequently, the certainty of evidence was downgraded to very low (rating 1).
4. Discussion
A significant proportion of the articles captured in our systematic literature search involved patients with TD2M. Our analyses showed that T2DM patients had significantly higher IMA concentrations when compared to healthy controls. In meta-regression and subgroup analysis, significant associations were observed between the effect size and glycated hemoglobin, creatinine, triglycerides, and the presence of diabetes complications. Another interesting finding in subgroup analysis was that the SMD was significantly different in studies of different geographical location, which supports the generalizability of our findings. Similar observations of higher IMA concentrations, albeit in a smaller number of studies, were observed in patients with T1DM, GDM, and pre-diabetes. Collectively, these results suggest the potential role of IMA as a biomarker of oxidative stress, acidosis, and ischemia in different types of diabetes as well as pre-diabetes.
Although the exact chemical reactions involved in forming IMA are not fully established, IMA generally reverts to albumin following an ischaemic event. For example, a balloon occlusion during a percutaneous coronary intervention was shown to increase serum IMA concentrations acutely. This increase persisted for up to 12 h before returning to baseline after a further 12 h [85]. It has been suggested that the magnitude of the formation of IMA is positively associated with the duration of the ischaemic process [25].
Several methods have been developed to measure IMA. Whilst some are relatively simple and have high sensitivity and specificity, particularly the ACB assay, the ELISA, and the surface plasmon resonance immunosensor, their use is currently limited to research studies [25]. In our systematic review and meta-analysis, most of the selected studies used the ABC method based on measuring the binding of cobalt to albumin in serum [86]. Whilst extensively used, this method is not exempt from limitations as conformational changes in albumin due to fluctuations in pH or the presence of denaturing agents, chemicals, or medications can influence the results [25]. Another issue is the lack of standardization, as most authors express the results as absorbance units, which might depend on the experience of the investigator and/or the sensitivity of the equipment [25]. Furthermore, some investigators have used IMA internal standards obtained in their laboratories [25]. Such limitations might explain, at least partly, the between-study variance in our meta-analysis. However, the lack of consensus regarding the exact mechanisms involved in the formation of IMA should also be emphasized. To address these issues, methods based on immunological reactions using antibodies to modified albumin have been proposed, although their use remains relatively limited. In our analyses, the use of ELISA was associated with a significantly larger effect size when compared to ACB spectrophotometric assays.
The role of IMA as a biomarker has been traditionally investigated in clinically overt ischaemic states, e.g., acute coronary syndrome and ischemic stroke. However, a significant increase in IMA concentrations has also been reported in heart failure [87], neurodegenerative disorders [88], pregnancy disorders [89], and cancer [90]. The results of these studies suggest that the likely common denominator for the acute increase in IMA accompanying a wide range of conditions is a state of oxidative stress and, perhaps, acidosis, rather than ischemia per se [91,92]. This hypothesis is further corroborated by a study investigating IMA as a biomarker of lower-extremity artery disease, a condition characterized by a significant pro-inflammatory and pro-oxidant state in T2DM patients. In this study, IMA concentrations were independently associated with the risk of peripheral revascularization or lower-limb amputation after 5.6 years of follow-up [93]. Appropriately designed prospective studies are warranted to investigate the capacity of IMA to predict diabetes complications and other adverse clinical outcomes in order to justify the use of this biomarker in routine practice.
Strengths of our study include the assessment of serum IMA in pre-diabetes and different types of diabetes, i.e., T2DM, T1DM, and GDM, the assessment of possible associations between the effect size and several study and patient characteristics (only possible for T2DM because of the sufficient number of studies), and a rigorous evaluation of the risk of bias and the certainty of evidence. Furthermore, sensitivity analysis ruled out the effect of individual studies on the overall effect size. Important limitations include the lack of assessment of publication bias and the assessment of meta-regression and subgroup analysis in studies of T1DM, GDM, and pre-diabetes because of the limited number of studies identified. Furthermore, it is important to highlight that all the identified studies were cross-sectional. This study design does not allow establishing a cause-effect relationship between serum IMA concentrations and pre-diabetes or different types of diabetes. As previously discussed, longitudinal studies are now required to appropriately investigate whether alterations in IMA can causally lead to alterations in glycaemic control and specific clinical complications. Only then, can the role of IMA as a biomarker of diabetes be fully established.
5. Conclusion
In conclusion, our systematic review and meta-analysis has shown the potential utility of serum IMA as a biomarker of pre-diabetes and T2DM, T1DM, and GDM and of diabetes complications in T2DM patients. However, additional research is required to confirm these observations and determine whether IMA can enhance risk stratification and the capacity to predict diabetes complications and other adverse clinical outcomes in prospective studies in order to justify its routine use in clinical practice.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability
The data that support the findings of this systematic review and meta-analysis are available from AZ upon reasonable request.
CRediT authorship contribution statement
Angelo Zinellu: Writing – review & editing, Methodology, Formal analysis, Data curation, Conceptualization. Arduino A. Mangoni: Writing – review & editing, Writing – original draft, Validation, Methodology, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e35953.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Lin X., Xu Y., Pan X., Xu J., Ding Y., Sun X., et al. Global, regional, and national burden and trend of diabetes in 195 countries and territories: an analysis from 1990 to 2025. Sci. Rep. 2020;10(1) doi: 10.1038/s41598-020-71908-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khan M.A.B., Hashim M.J., King J.K., Govender R.D., Mustafa H., Al Kaabi J. Epidemiology of type 2 diabetes - global burden of disease and forecasted trends. J Epidemiol Glob Health. 2020;10(1):107–111. doi: 10.2991/jegh.k.191028.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collaborators G.B.D.D. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: a systematic analysis for the Global Burden of Disease Study 2021. Lancet. 2023;402(10397):203–234. doi: 10.1016/S0140-6736(23)01301-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guo S.J., Shao H. Growing global burden of type 1 diabetes needs multitiered precision public health interventions. Lancet Diabetes Endocrinol. 2022;10(10):688–689. doi: 10.1016/S2213-8587(22)00257-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gregory G.A., Robinson T.I.G., Linklater S.E., Wang F., Colagiuri S., de Beaufort C., et al. Global incidence, prevalence, and mortality of type 1 diabetes in 2021 with projection to 2040: a modelling study. Lancet Diabetes Endocrinol. 2022;10(10):741–760. doi: 10.1016/S2213-8587(22)00218-2. [DOI] [PubMed] [Google Scholar]
- 6.Mnatzaganian G., Woodward M., McIntyre H.D., Ma L., Yuen N., He F., et al. Trends in percentages of gestational diabetes mellitus attributable to overweight, obesity, and morbid obesity in regional Victoria: an eight-year population-based panel study. BMC Pregnancy Childbirth. 2022;22(1):95. doi: 10.1186/s12884-022-04420-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bahl S., Dhabhai N., Taneja S., Mittal P., Dewan R., Kaur J., et al. Burden, risk factors and outcomes associated with gestational diabetes in a population-based cohort of pregnant women from North India. BMC Pregnancy Childbirth. 2022;22(1):32. doi: 10.1186/s12884-022-04389-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laurie J.G., McIntyre H.D. A review of the current status of gestational diabetes mellitus in Australia-the clinical impact of changing population demographics and diagnostic criteria on prevalence. Int J Environ Res Public Health. 2020;17(24) doi: 10.3390/ijerph17249387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rooney M.R., Fang M., Ogurtsova K., Ozkan B., Echouffo-Tcheugui J.B., Boyko E.J., et al. Global prevalence of prediabetes. Diabetes Care. 2023;46(7):1388–1394. doi: 10.2337/dc22-2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Echouffo-Tcheugui J.B., Kengne A.P., Ali M.K. Issues in defining the burden of prediabetes globally. Curr Diab Rep. 2018;18(11):105. doi: 10.1007/s11892-018-1089-y. [DOI] [PubMed] [Google Scholar]
- 11.Hostalek U. Global epidemiology of prediabetes - present and future perspectives. Clin Diabetes Endocrinol. 2019;5:5. doi: 10.1186/s40842-019-0080-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sattar N. Advances in the clinical management of type 2 diabetes: a brief history of the past 15 years and challenges for the future. BMC Med. 2019;17(1):46. doi: 10.1186/s12916-019-1281-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Su J., Luo Y., Hu S., Tang L., Ouyang S. Advances in research on type 2 diabetes mellitus targets and therapeutic agents. Int. J. Mol. Sci. 2023;24(17) doi: 10.3390/ijms241713381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aathira R., Jain V. Advances in management of type 1 diabetes mellitus. World J. Diabetes. 2014;5(5):689–696. doi: 10.4239/wjd.v5.i5.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akil A.A., Yassin E., Al-Maraghi A., Aliyev E., Al-Malki K., Fakhro K.A. Diagnosis and treatment of type 1 diabetes at the dawn of the personalized medicine era. J. Transl. Med. 2021;19(1):137. doi: 10.1186/s12967-021-02778-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benhalima K. Recent advances in gestational diabetes mellitus. J. Clin. Med. 2021;10(10) doi: 10.3390/jcm10102202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bukhari I., Iqbal F., Thorne R.F. Research advances in gestational, neonatal diabetes mellitus and metabolic disorders. Front. Endocrinol. 2022;13 doi: 10.3389/fendo.2022.969952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Echouffo-Tcheugui J.B., Perreault L., Ji L., Dagogo-Jack S. Diagnosis and management of prediabetes: a review. JAMA. 2023;329(14):1206–1216. doi: 10.1001/jama.2023.4063. [DOI] [PubMed] [Google Scholar]
- 19.Lyons T.J., Basu A. Biomarkers in diabetes: hemoglobin A1c, vascular and tissue markers. Transl. Res. 2012;159(4):303–312. doi: 10.1016/j.trsl.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sherwani S.I., Khan H.A., Ekhzaimy A., Masood A., Sakharkar M.K. Significance of HbA1c test in diagnosis and prognosis of diabetic patients. Biomark. Insights. 2016;11:95–104. doi: 10.4137/BMI.S38440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sonaglioni A., Bordoni T., Naselli A., Nicolosi G.L., Grasso E., Bianchi S., et al. Influence of gestational diabetes mellitus on subclinical myocardial dysfunction during pregnancy: a systematic review and meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2024;292:17–24. doi: 10.1016/j.ejogrb.2023.11.007. [DOI] [PubMed] [Google Scholar]
- 22.Dorcely B., Katz K., Jagannathan R., Chiang S.S., Oluwadare B., Goldberg I.J., et al. Novel biomarkers for prediabetes, diabetes, and associated complications. Diabetes Metab Syndr Obes. 2017;10:345–361. doi: 10.2147/DMSO.S100074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ortiz-Martinez M., Gonzalez-Gonzalez M., Martagon A.J., Hlavinka V., Willson R.C., Rito-Palomares M. Recent developments in biomarkers for diagnosis and screening of type 2 diabetes mellitus. Curr Diab Rep. 2022;22(3):95–115. doi: 10.1007/s11892-022-01453-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laakso M. Biomarkers for type 2 diabetes. Mol Metab. 2019;27S(Suppl):S139–S146. doi: 10.1016/j.molmet.2019.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shevtsova A., Gordiienko I., Tkachenko V., Ushakova G. Ischemia-modified albumin: origins and clinical implications. Dis. Markers. 2021;2021 doi: 10.1155/2021/9945424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abboud H., Labreuche J., Meseguer E., Lavallee P.C., Simon O., Olivot J.M., et al. Ischemia-modified albumin in acute stroke. Cerebrovasc. Dis. 2007;23(2–3):216–220. doi: 10.1159/000097644. [DOI] [PubMed] [Google Scholar]
- 27.Jawade P., Khillare K.M., Mangudkar S., Palange A., Dhadwad J., Deshmukh M. A comparative study of ischemia-modified albumin: a promising biomarker for early detection of acute coronary syndrome (ACS) Cureus. 2023;15(8) doi: 10.7759/cureus.44357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gaze D.C. Ischemia modified albumin: a novel biomarker for the detection of cardiac ischemia. Drug Metab Pharmacokinet. 2009;24(4):333–341. doi: 10.2133/dmpk.24.333. [DOI] [PubMed] [Google Scholar]
- 29.Worster A., Devereaux P.J., Heels-Ansdell D., Guyatt G.H., Opie J., Mookadam F., et al. Capability of ischemia-modified albumin to predict serious cardiac outcomes in the short term among patients with potential acute coronary syndrome. CMAJ (Can. Med. Assoc. J.) 2005;172(13):1685–1690. doi: 10.1503/cmaj.045194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ighodaro O.M. Molecular pathways associated with oxidative stress in diabetes mellitus. Biomed. Pharmacother. 2018;108:656–662. doi: 10.1016/j.biopha.2018.09.058. [DOI] [PubMed] [Google Scholar]
- 31.Zhang P., Li T., Wu X., Nice E.C., Huang C., Zhang Y. Oxidative stress and diabetes: antioxidative strategies. Front. Med. 2020;14(5):583–600. doi: 10.1007/s11684-019-0729-1. [DOI] [PubMed] [Google Scholar]
- 32.An Y., Xu B.T., Wan S.R., Ma X.M., Long Y., Xu Y., et al. The role of oxidative stress in diabetes mellitus-induced vascular endothelial dysfunction. Cardiovasc. Diabetol. 2023;22(1):237. doi: 10.1186/s12933-023-01965-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Giha H.A. Hidden chronic metabolic acidosis of diabetes type 2 (CMAD): clues, causes and consequences. Rev. Endocr. Metab. Disord. 2023;24(4):735–750. doi: 10.1007/s11154-023-09816-2. [DOI] [PubMed] [Google Scholar]
- 34.Souto G., Donapetry C., Calvino J., Adeva M.M. Metabolic acidosis-induced insulin resistance and cardiovascular risk. Metab. Syndr. Relat. Disord. 2011;9(4):247–253. doi: 10.1089/met.2010.0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nellaiappan K., Preeti K., Khatri D.K., Singh S.B. Diabetic complications: an update on pathobiology and therapeutic strategies. Curr. Diabetes Rev. 2022;18(1) doi: 10.2174/1573399817666210309104203. [DOI] [PubMed] [Google Scholar]
- 36.Moola S., Munn Z., Tufanaru C., Aromataris E., Sears K., Sfetcu R., et al. In: Joanna Briggs Institute Reviewer's Manual. Aromataris E., Munn Z., editors. Johanna Briggs Institute; Adelaide, Australia: 2017. Systematic reviews of etiology and risk. [Google Scholar]
- 37.Balshem H., Helfand M., Schunemann H.J., Oxman A.D., Kunz R., Brozek J., et al. GRADE guidelines: 3. Rating the quality of evidence. J. Clin. Epidemiol. 2011;64(4):401–406. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 38.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372 doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wan X., Wang W., Liu J., Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Higgins J.P., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 41.Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tobias A. Assessing the influence of a single study in the meta-analysis estimate. Stata Technical Bulletin. 1999;47:15–17. [Google Scholar]
- 43.Begg C.B., Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101. [PubMed] [Google Scholar]
- 44.Sterne J.A., Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J. Clin. Epidemiol. 2001;54(10):1046–1055. doi: 10.1016/s0895-4356(01)00377-8. [DOI] [PubMed] [Google Scholar]
- 45.Duval S., Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 46.Piwowar A., Knapik-Kordecka M., Warwas M. Ischemia-modified albumin level in type 2 diabetes mellitus - preliminary report. Dis. Markers. 2008;24(6):311–317. doi: 10.1155/2008/784313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ukinc K., Eminagaoglu S., Ersoz H.O., Erem C., Karahan C., Hacihasanoglu A.B., et al. A novel indicator of widespread endothelial damage and ischemia in diabetic patients: ischemia-modified albumin. Endocrine. 2009;36(3):425–432. doi: 10.1007/s12020-009-9236-5. [DOI] [PubMed] [Google Scholar]
- 48.Dahiya K., Aggarwal K., Seth S., Singh V., Sharma T.K. Type 2 diabetes mellitus without vascular complications and ischemia modified albumin. Clin. Lab. 2010;56(5–6):187–190. [PubMed] [Google Scholar]
- 49.Kaefer M., Piva S.J., De Carvalho J.A., Da Silva D.B., Becker A.M., Coelho A.C., et al. Association between ischemia modified albumin, inflammation and hyperglycemia in type 2 diabetes mellitus. Clin. Biochem. 2010;43(4–5):450–454. doi: 10.1016/j.clinbiochem.2009.11.018. [DOI] [PubMed] [Google Scholar]
- 50.Ma S.G., Xu W., Wei C.L., Wu X.J., Hong B., Wang Z.J., et al. Role of ischemia-modified albumin and total homocysteine in estimating symptomatic lacunar infarction in type 2 diabetic patients. Clin. Biochem. 2011;44(16):1299–1303. doi: 10.1016/j.clinbiochem.2011.08.1136. [DOI] [PubMed] [Google Scholar]
- 51.Turk A., Nuhoglu I., Mentese A., Karahan S.C., Erdol H., Erem C. The relationship between diabetic retinopathy and serum levels of ischemia-modified albumin and malondialdehyde. Retina. 2011;31(3):602–608. doi: 10.1097/IAE.0b013e3181ed8cd1. [DOI] [PubMed] [Google Scholar]
- 52.Ma S.G., Jin Y., Hu W., Bai F., Xu W., Yu W.N. Evaluation of ischemia-modified albumin and C-reactive protein in type 2 diabetics with and without ketosis. Biomark. Insights. 2012;7:19–26. doi: 10.4137/BMI.S9060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ma S.G., Jin Y., Xu W., Hu W., Bai F., Wu X.J. Increased serum levels of ischemia-modified albumin and C-reactive protein in type 1 diabetes patients with ketoacidosis. Endocrine. 2012;42(3):570–576. doi: 10.1007/s12020-012-9652-9. [DOI] [PubMed] [Google Scholar]
- 54.Ma S.G., Yu W.N., Jin Y., Hong B., Hu W. Evaluation of serum ischemia-modified albumin levels in pregnant women with and without gestational diabetes mellitus. Gynecol. Endocrinol. 2012;28(11):837–840. doi: 10.3109/09513590.2012.683069. [DOI] [PubMed] [Google Scholar]
- 55.Dayanand C.D., Vegi P.K., Lakshmaiah V., Kutty A.V.M. Association of ischemia modified albumin in terms of hypoxic risk with carbonylated protein, glycosylated hemoglobin and plasma insulin in type 2 diabetes mellitus. Int. J. Biotechnol. Biochem. 2013;9(3):275–284. [Google Scholar]
- 56.Korkmaz G.G., Konukoglu D., Kurtulus E.M., Irmak H., Bolayirli M., Uzun H. Total antioxidant status and markers of oxidative stress in subjects with normal or impaired glucose regulation (IFG, IGT) in diabetic patients. Scand. J. Clin. Lab. Invest. 2013;73(8):641–649. doi: 10.3109/00365513.2013.846477. [DOI] [PubMed] [Google Scholar]
- 57.Erdem S.S., Toker A., Kayrak M., Çiçekler H., Gönülalan G., Abdulhalikov T., et al. Oxidant and antioxidant parameters in prediabetes and diabetes. Int. J. Diabetes Dev. Ctries. 2014;35(S3):465–470. [Google Scholar]
- 58.Kirboga K., Ozec A.V., Kosker M., Dursun A., Toker M.I., Aydin H., et al. The association between diabetic retinopathy and levels of ischemia-modified albumin, total thiol, total antioxidant capacity, and total oxidative stress in serum and aqueous humor. J Ophthalmol. 2014;2014 doi: 10.1155/2014/820853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Refaat S., El-Ghaffar N.A., Khalil A. The relationship between ischemia modified albumin and lipids in type 2 Egyptian diabetic patients. Adv. Biol. Res. 2014;8(1):18–22. [Google Scholar]
- 60.Reddy V.S., Agrawal P., Sethi S., Gupta N., Garg R., Madaan H., et al. Associations of FPG, A1C and disease duration with protein markers of oxidative damage and antioxidative defense in type 2 diabetes and diabetic retinopathy. Eye. 2015;29(12):1585–1593. doi: 10.1038/eye.2015.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ahmad A., Manjrekar P., Yadav C., Agarwal A., Srikantiah R.M., Hegde A. Evaluation of ischemia-modified albumin, malondialdehyde, and advanced oxidative protein products as markers of vascular injury in diabetic nephropathy. Biomark. Insights. 2016;11:63–68. doi: 10.4137/BMI.S39053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.D'Souza J.M., D'Souza R.P., Vijin V.F., Shetty A., Arunachalam C., Pai V.R., et al. High predictive ability of glycated hemoglobin on comparison with oxidative stress markers in assessment of chronic vascular complications in type 2 diabetes mellitus. Scand. J. Clin. Lab. Invest. 2016;76(1):51–57. doi: 10.3109/00365513.2015.1092048. [DOI] [PubMed] [Google Scholar]
- 63.Inci A., Olmaz R., Sari F., Coban M., Ellidag H.Y., Sarikaya M. Increased oxidative stress in diabetic nephropathy and its relationship with soluble Klotho levels. Hippokratia. 2016;20(3):198–203. [PMC free article] [PubMed] [Google Scholar]
- 64.Miric D.J., Kisic B.M., Filipovic-Danic S., Grbic R., Dragojevic I., Miric M.B., et al. Xanthine oxidase activity in type 2 diabetes mellitus patients with and without diabetic peripheral neuropathy. J. Diabetes Res. 2016;2016 doi: 10.1155/2016/4370490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Muhtaroglu S., Barlak Keti D., Unluhizarci K. Investigation of ischemia-modified albumin levels and some atherosclerosis-related serum parameters in patients with diabetic foot. Turk. J. Med. Sci. 2016;46(1):126–132. doi: 10.3906/sag-1406-38. [DOI] [PubMed] [Google Scholar]
- 66.Ghosh K., Muddeshwar M.G., Ghosh K. Ischemia modified albumin test to detect early diabetic complications. Am. J. Med. Sci. 2017;354(5):467–470. doi: 10.1016/j.amjms.2017.06.017. [DOI] [PubMed] [Google Scholar]
- 67.Sadik I., Yagoub Z., Sayed N., El Nour A., Abide El Hameed M., Satee A.B. The level of ischemic modified albumin (IMA) as risk marker for cardio vascular disease (CVD) among some diabetic patients (type II) in khartoum state -Sudan. Sudan Journal of Medical Sciences. 2017;12(4) [Google Scholar]
- 68.Balamir I., Ates I., Topcuoglu C., Turhan T. Association of Endocan, ischemia-modified albumin, and hsCRP levels with endothelial dysfunction in type 2 diabetes mellitus. Angiology. 2018;69(7):609–616. doi: 10.1177/0003319717740781. [DOI] [PubMed] [Google Scholar]
- 69.Bhaskhar K.U., Devİ H.N., Bitla A.R., Rao S., Kumar S.V., Sachan A.B. Ischemia modified albumin levels in patients with diabetic nephropathy. The Turkish Journal of Endocrinology and Metabolism. 2018;22(3):145–150. [Google Scholar]
- 70.Sudha K., Reshma K., Ahmad A., Marathe A. Correlation of atherogenic indices and IMA with glycaemic control in diabetic patients with and without dyslipidemia. Indian Journal of Public Health Research & Development. 2018;9(1) [Google Scholar]
- 71.Yazici M.U., Ayar G., Savas-Erdeve S., Azapagasi E., Neselioglu S., Erel O., et al. Role of ischemia modified albumin serum levels as an oxidative stress marker in children with diabetic ketoacidosis. Comb. Chem. High Throughput Screen. 2019;22(8):577–581. doi: 10.2174/1386207322666191008214919. [DOI] [PubMed] [Google Scholar]
- 72.Beyazit F., Karacaer K.Ö., Turkon H. The association of oxidative stress with serum irisin and betatrophin in pregnant women with gestational diabetes mellitus. Clin. Diabetol. 2020;9(5):328–334. [Google Scholar]
- 73.El-Eshmawy M.M., Gad D.F., El-Baiomy A.A. Elevated serum levels of ischemia modified albumin and malondialdehyde are related to atherogenic index of plasma in a cohort of prediabetes. Endocr., Metab. Immune Disord.: Drug Targets. 2020;20(8):1347–1354. doi: 10.2174/1871530320666200503052226. [DOI] [PubMed] [Google Scholar]
- 74.Sushith S., Krishnamurthy H.N., Reshma S., Janice D., Madan G., Ashok K.J., et al. Serum ischemia-modified albumin, fibrinogen, high sensitivity C- reactive proteins in type-2 diabetes mellitus without hypertension and diabetes mellitus with hypertension: a case-control study. Rep Biochem Mol Biol. 2020;9(2):241–249. doi: 10.29252/rbmb.9.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Alay H., Laloglu E., Kesmez Can F. An evaluation of ischemia-modified albumin levels in the development of diabetic foot ulcer. Int. J. Clin. Pract. 2021;75(9) doi: 10.1111/ijcp.14589. [DOI] [PubMed] [Google Scholar]
- 76.Chaudhry S.R., Saeed M., Chaudhry Z.R., Iqbal K., Rashid E., Rasheed F. Ischemia modified albumin levels in diabetes mellitus patients with and without diabetic retinopathy. J Islam Int Med Coll. 2021;16(3):161–164. [Google Scholar]
- 77.Mertoglu C., Siranli G., Coban T.A., Karakurt Y., Ersoy A., Ozcicek A., et al. The role of protein oxidation in the development of diabetic microvascular complications. North Clin Istanb. 2021;8(5):500–506. doi: 10.14744/nci.2021.33341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ozkan S., Adanas C., Alp H.H. Is ischemia-modified albumin a biomarker in wagner classification in diabetic foot ulcers? Int. J. Clin. Pract. 2021;75(12) doi: 10.1111/ijcp.14830. [DOI] [PubMed] [Google Scholar]
- 79.Xiang L., Zhang M., Wu H., Xie D. The expression and prognostic value of ischemia modified albumin (IMA), red blood cell distribution width (RDW), and lipoprotein (LP) in patients with diabetes mellitus complicated with coronary heart disease. Ann. Palliat. Med. 2021;10(4):4463–4471. doi: 10.21037/apm-21-425. [DOI] [PubMed] [Google Scholar]
- 80.Arslan A. Serum ischemia modified albumin and oxidative stress levels in patients with gestational diabetes mellitus. E. J. Med. 2022;27(4):507–512. [Google Scholar]
- 81.Feng F., Chen Y., Wang G., Huang P., Zhu Q., Zhou B. Correlation of serum CysC, IMA, and LP-PLA2 levels with type 2 diabetes mellitus patients with lower extremity atherosclerotic occlusive disease. Front Surg. 2022;9 doi: 10.3389/fsurg.2022.846470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kurt H.A., Demirci E., Alan C. Endothelial dysfunction and ischemia-modified albumin levels in males with diabetic and nondiabetic erectile dysfunction. Dis. Markers. 2022;2022 doi: 10.1155/2022/3661822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zainal I.G. Study the profile of some antioxidant markers in diabetic mellitus and non-diabetic patients with cardiovascular disease. Med J Babylon. 2022;19(4):653–658. [Google Scholar]
- 84.Cohen J. Statistical power analysis. Curr. Dir. Psychol. Sci. 1992;1(3):98–101. [Google Scholar]
- 85.Sinha M.K., Vazquez J.M., Calvino R., Gaze D.C., Collinson P.O., Kaski J.C. Effects of balloon occlusion during percutaneous coronary intervention on circulating Ischemia Modified Albumin and transmyocardial lactate extraction. Heart. 2006;92(12):1852–1853. doi: 10.1136/hrt.2005.078089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bar-Or D., Lau E., Winkler J.V. A novel assay for cobalt-albumin binding and its potential as a marker for myocardial ischemia-a preliminary report. J. Emerg. Med. 2000;19(4):311–315. doi: 10.1016/s0736-4679(00)00255-9. [DOI] [PubMed] [Google Scholar]
- 87.Ellidag H.Y., Eren E., Yilmaz N., Cekin Y. Oxidative stress and ischemia-modified albumin in chronic ischemic heart failure. Redox Rep. 2014;19(3):118–123. doi: 10.1179/1351000213Y.0000000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Altunoglu E., Guntas G., Erdenen F., Akkaya E., Topac I., Irmak H., et al. Ischemia-modified albumin and advanced oxidation protein products as potential biomarkers of protein oxidation in Alzheimer's disease. Geriatr. Gerontol. Int. 2015;15(7):872–880. doi: 10.1111/ggi.12361. [DOI] [PubMed] [Google Scholar]
- 89.Rossi A., Bortolotti N., Vescovo S., Romanello I., Forzano L., Londero A.P., et al. Ischemia-modified albumin in pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013;170(2):348–351. doi: 10.1016/j.ejogrb.2013.06.037. [DOI] [PubMed] [Google Scholar]
- 90.Kundaktepe B.P., Sozer V., Durmus S., Kocael P.C., Kundaktepe F.O., Papila C., et al. The evaluation of oxidative stress parameters in breast and colon cancer. Medicine (Baltim.) 2021;100(11) doi: 10.1097/MD.0000000000025104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liguori I., Russo G., Curcio F., Bulli G., Aran L., Della-Morte D., et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging. 2018;13:757–772. doi: 10.2147/CIA.S158513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hajam Y.A., Rani R., Ganie S.Y., Sheikh T.A., Javaid D., Qadri S.S., et al. Oxidative stress in human pathology and aging: molecular mechanisms and perspectives. Cells. 2022;11(3) doi: 10.3390/cells11030552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nativel M., Schneider F., Saulnier P.J., Gand E., Ragot S., Meilhac O., et al. Prognostic values of inflammatory and redox status biomarkers on the risk of major lower-extremity artery disease in individuals with type 2 diabetes. Diabetes Care. 2018;41(10):2162–2169. doi: 10.2337/dc18-0695. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this systematic review and meta-analysis are available from AZ upon reasonable request.