Abstract
The rapid loss of skeletal-muscle protein during starvation and after denervation occurs primarily through increased rates of protein breakdown and activation of a non-lysosomal ATP-dependent proteolytic process. To investigate whether protein flux through the ubiquitin (Ub)-proteasome pathway is enhanced, as was suggested by related studies, we measured, using specific polyclonal antibodies, the levels of Ub-conjugated proteins in normal and atrophying muscles. The content of these critical intermediates had increased 50-250% after food deprivation in the extensor digitorum longus and soleus muscles 2 days after denervation. Like rates of proteolysis, the amount of Ub-protein conjugates and the fraction of Ub conjugated to proteins increased progressively during food deprivation and returned to normal within 1 day of refeeding. During starvation, muscles of adrenalectomized rats failed to increase protein breakdown, and they showed 50% lower levels of Ub-protein conjugates than those of starved control animals. The changes in the pools of Ub-conjugated proteins (the substrates for the 26S proteasome) thus coincided with and can account for the alterations in overall proteolysis. In this pathway, large multiubiquitinated proteins are preferentially degraded, and the Ub-protein conjugates that accumulated in atrophying muscles were of high molecular mass (> 100 kDa). When innervated and denervated gastrocnemius muscles were fractionated, a significant increase in ubiquitinated proteins was found in the myofibrillar fraction, the proteins of which are preferentially degraded on denervation, but not in the soluble fraction. Thus activation of this proteolytic pathway in atrophying muscles probably occurs initially by increasing Ub conjugation to cell proteins. The resulting accumulation of Ub-protein conjugates suggests that their degradation by the 26S proteasome complex subsequently becomes rate-limiting in these catabolic states.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bond U., Agell N., Haas A. L., Redman K., Schlesinger M. J. Ubiquitin in stressed chicken embryo fibroblasts. J Biol Chem. 1988 Feb 15;263(5):2384–2388. [PubMed] [Google Scholar]
- Driscoll J., Goldberg A. L. Skeletal muscle proteasome can degrade proteins in an ATP-dependent process that does not require ubiquitin. Proc Natl Acad Sci U S A. 1989 Feb;86(3):787–791. doi: 10.1073/pnas.86.3.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll J., Goldberg A. L. The proteasome (multicatalytic protease) is a component of the 1500-kDa proteolytic complex which degrades ubiquitin-conjugated proteins. J Biol Chem. 1990 Mar 25;265(9):4789–4792. [PubMed] [Google Scholar]
- Eytan E., Ganoth D., Armon T., Hershko A. ATP-dependent incorporation of 20S protease into the 26S complex that degrades proteins conjugated to ubiquitin. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7751–7755. doi: 10.1073/pnas.86.20.7751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan J. M., Waxman L., Goldberg A. L. Skeletal muscle and liver contain a soluble ATP + ubiquitin-dependent proteolytic system. Biochem J. 1987 Apr 15;243(2):335–343. doi: 10.1042/bj2430335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finley D., Chau V. Ubiquitination. Annu Rev Cell Biol. 1991;7:25–69. doi: 10.1146/annurev.cb.07.110191.000325. [DOI] [PubMed] [Google Scholar]
- Furuno K., Goldberg A. L. The activation of protein degradation in muscle by Ca2+ or muscle injury does not involve a lysosomal mechanism. Biochem J. 1986 Aug 1;237(3):859–864. doi: 10.1042/bj2370859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuno K., Goodman M. N., Goldberg A. L. Role of different proteolytic systems in the degradation of muscle proteins during denervation atrophy. J Biol Chem. 1990 May 25;265(15):8550–8557. [PubMed] [Google Scholar]
- García-Martínez C., Agell N., Llovera M., López-Soriano F. J., Argilés J. M. Tumour necrosis factor-alpha increases the ubiquitinization of rat skeletal muscle proteins. FEBS Lett. 1993 Jun 1;323(3):211–214. doi: 10.1016/0014-5793(93)81341-v. [DOI] [PubMed] [Google Scholar]
- Goldberg A. L. Protein turnover in skeletal muscle. II. Effects of denervation and cortisone on protein catabolism in skeletal muscle. J Biol Chem. 1969 Jun 25;244(12):3223–3229. [PubMed] [Google Scholar]
- Goldberg A. L. The mechanism and functions of ATP-dependent proteases in bacterial and animal cells. Eur J Biochem. 1992 Jan 15;203(1-2):9–23. doi: 10.1111/j.1432-1033.1992.tb19822.x. [DOI] [PubMed] [Google Scholar]
- Goldberg A. L., Tischler M., DeMartino G., Griffin G. Hormonal regulation of protein degradation and synthesis in skeletal muscle. Fed Proc. 1980 Jan;39(1):31–36. [PubMed] [Google Scholar]
- Goodman M. N., Ruderman N. B. Starvation in the rat. I. Effect of age and obesity on organ weights, RNA, DNA, and protein. Am J Physiol. 1980 Oct;239(4):E269–E276. doi: 10.1152/ajpendo.1980.239.4.E269. [DOI] [PubMed] [Google Scholar]
- Haas A. L., Bright P. M. The immunochemical detection and quantitation of intracellular ubiquitin-protein conjugates. J Biol Chem. 1985 Oct 15;260(23):12464–12473. [PubMed] [Google Scholar]
- Haas A. L., Bright P. M. The resolution and characterization of putative ubiquitin carrier protein isozymes from rabbit reticulocytes. J Biol Chem. 1988 Sep 15;263(26):13258–13267. [PubMed] [Google Scholar]
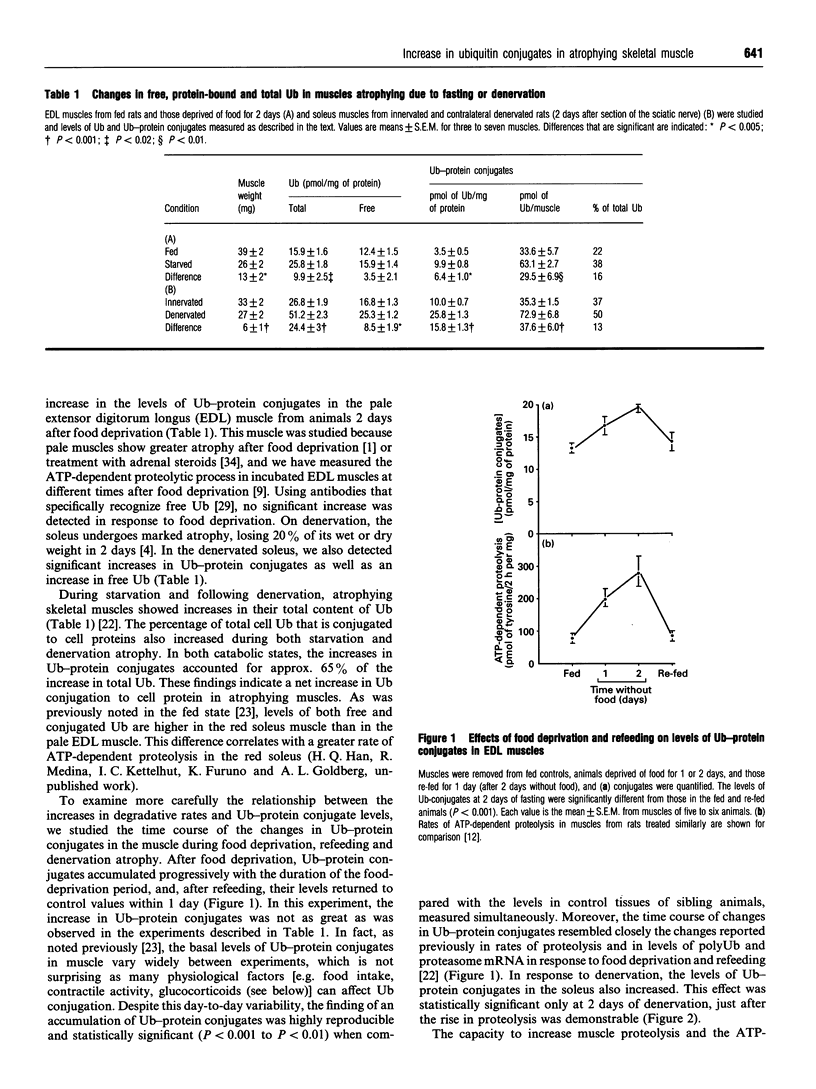
- Haas A. L., Murphy K. E., Bright P. M. The inactivation of ubiquitin accounts for the inability to demonstrate ATP, ubiquitin-dependent proteolysis in liver extracts. J Biol Chem. 1985 Apr 25;260(8):4694–4703. [PubMed] [Google Scholar]
- Haas A. L., Rose I. A. The mechanism of ubiquitin activating enzyme. A kinetic and equilibrium analysis. J Biol Chem. 1982 Sep 10;257(17):10329–10337. [PubMed] [Google Scholar]
- Hadari T., Warms J. V., Rose I. A., Hershko A. A ubiquitin C-terminal isopeptidase that acts on polyubiquitin chains. Role in protein degradation. J Biol Chem. 1992 Jan 15;267(2):719–727. [PubMed] [Google Scholar]
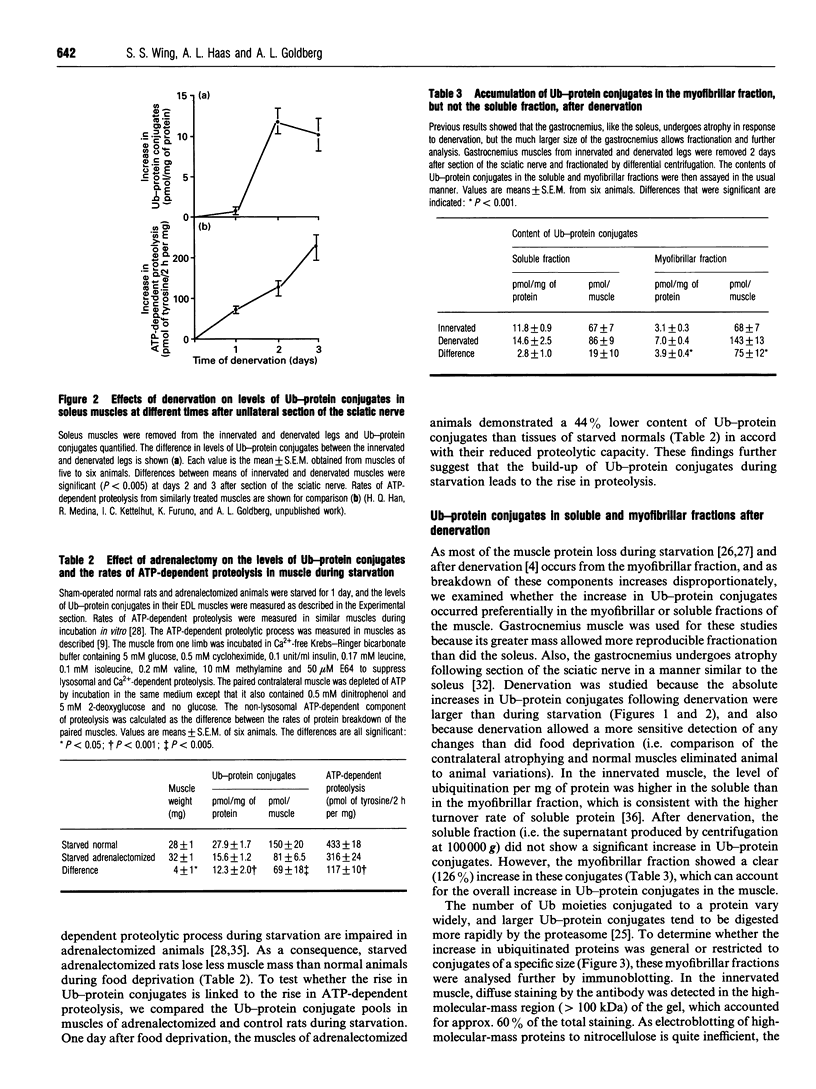
- Hershko A., Ciechanover A. The ubiquitin system for protein degradation. Annu Rev Biochem. 1992;61:761–807. doi: 10.1146/annurev.bi.61.070192.003553. [DOI] [PubMed] [Google Scholar]
- Hershko A., Heller H., Elias S., Ciechanover A. Components of ubiquitin-protein ligase system. Resolution, affinity purification, and role in protein breakdown. J Biol Chem. 1983 Jul 10;258(13):8206–8214. [PubMed] [Google Scholar]
- Hershko A., Leshinsky E., Ganoth D., Heller H. ATP-dependent degradation of ubiquitin-protein conjugates. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1619–1623. doi: 10.1073/pnas.81.6.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hough R., Pratt G., Rechsteiner M. Purification of two high molecular weight proteases from rabbit reticulocyte lysate. J Biol Chem. 1987 Jun 15;262(17):8303–8313. [PubMed] [Google Scholar]
- Jentsch S. The ubiquitin-conjugation system. Annu Rev Genet. 1992;26:179–207. doi: 10.1146/annurev.ge.26.120192.001143. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Li J. B., Goldberg A. L. Effects of food deprivation on protein synthesis and degradation in rat skeletal muscles. Am J Physiol. 1976 Aug;231(2):441–448. doi: 10.1152/ajplegacy.1976.231.2.441. [DOI] [PubMed] [Google Scholar]
- Li J. B., Higgins J. E., Jefferson L. S. Changes in protein turnover in skeletal muscle in response to fasting. Am J Physiol. 1979 Mar;236(3):E222–E228. doi: 10.1152/ajpendo.1979.236.3.E222. [DOI] [PubMed] [Google Scholar]
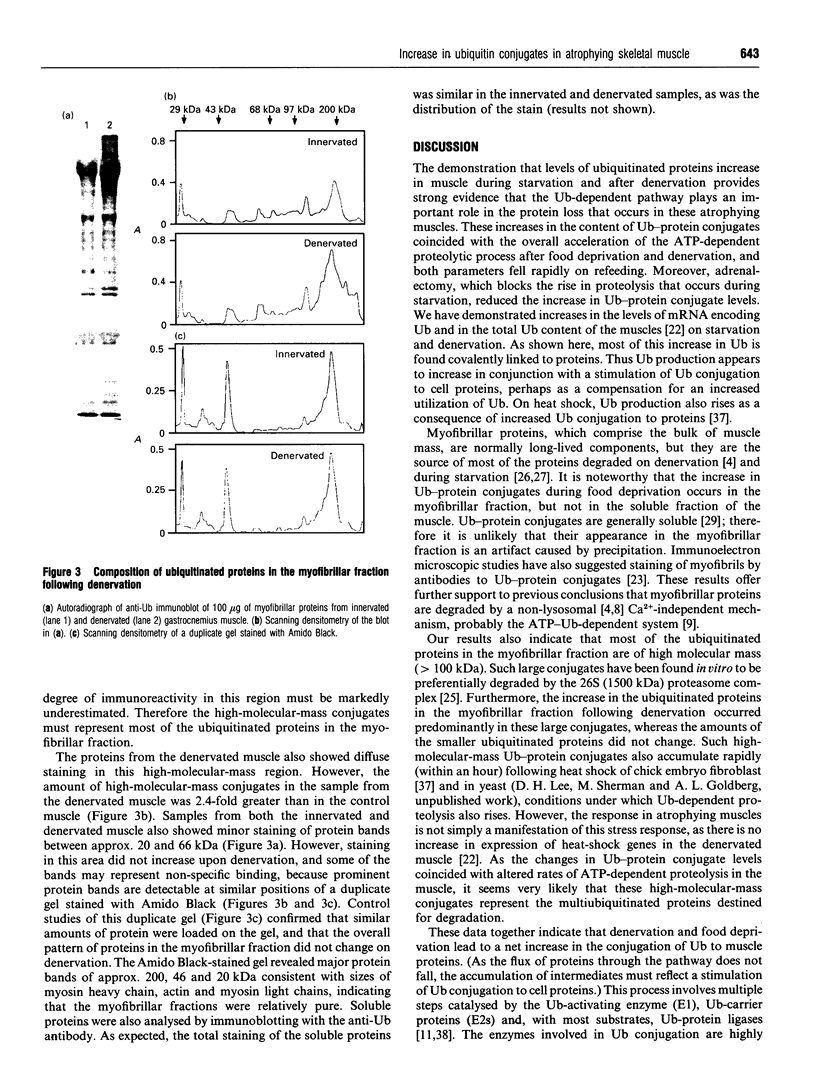
- Li J. B., Wassner S. J. Effects of food deprivation and refeeding on total protein and actomyosin degradation. Am J Physiol. 1984 Jan;246(1 Pt 1):E32–E37. doi: 10.1152/ajpendo.1984.246.1.E32. [DOI] [PubMed] [Google Scholar]
- Lowell B. B., Ruderman N. B., Goodman M. N. Evidence that lysosomes are not involved in the degradation of myofibrillar proteins in rat skeletal muscle. Biochem J. 1986 Feb 15;234(1):237–240. doi: 10.1042/bj2340237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowell B. B., Ruderman N. B., Goodman M. N. Regulation of myofibrillar protein degradation in rat skeletal muscle during brief and prolonged starvation. Metabolism. 1986 Dec;35(12):1121–1127. doi: 10.1016/0026-0495(86)90025-9. [DOI] [PubMed] [Google Scholar]
- Medina R., Wing S. S., Goldberg A. L. Increase in levels of polyubiquitin and proteasome mRNA in skeletal muscle during starvation and denervation atrophy. Biochem J. 1995 May 1;307(Pt 3):631–637. doi: 10.1042/bj3070631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina R., Wing S. S., Haas A., Goldberg A. L. Activation of the ubiquitin-ATP-dependent proteolytic system in skeletal muscle during fasting and denervation atrophy. Biomed Biochim Acta. 1991;50(4-6):347–356. [PubMed] [Google Scholar]
- Pickart C. M., Vella A. T. Levels of active ubiquitin carrier proteins decline during erythroid maturation. J Biol Chem. 1988 Aug 25;263(24):12028–12035. [PubMed] [Google Scholar]
- Preedy V. R., Sugden P. H. The effects of fasting or hypoxia on rates of protein synthesis in vivo in subcellular fractions of rat heart and gastrocnemius muscle. Biochem J. 1989 Jan 15;257(2):519–527. doi: 10.1042/bj2570519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley D. A., Bain J. L., Ellis S., Haas A. L. Quantitation and immunocytochemical localization of ubiquitin conjugates within rat red and white skeletal muscles. J Histochem Cytochem. 1988 Jun;36(6):621–632. doi: 10.1177/36.6.2835410. [DOI] [PubMed] [Google Scholar]
- Waxman L., Fagan J. M., Goldberg A. L. Demonstration of two distinct high molecular weight proteases in rabbit reticulocytes, one of which degrades ubiquitin conjugates. J Biol Chem. 1987 Feb 25;262(6):2451–2457. [PubMed] [Google Scholar]
- Wilkinson K. D., Lee K. M., Deshpande S., Duerksen-Hughes P., Boss J. M., Pohl J. The neuron-specific protein PGP 9.5 is a ubiquitin carboxyl-terminal hydrolase. Science. 1989 Nov 3;246(4930):670–673. doi: 10.1126/science.2530630. [DOI] [PubMed] [Google Scholar]
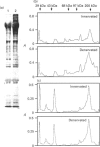
- Wing S. S., Banville D. 14-kDa ubiquitin-conjugating enzyme: structure of the rat gene and regulation upon fasting and by insulin. Am J Physiol. 1994 Jul;267(1 Pt 1):E39–E48. doi: 10.1152/ajpendo.1994.267.1.E39. [DOI] [PubMed] [Google Scholar]
- Wing S. S., Goldberg A. L. Glucocorticoids activate the ATP-ubiquitin-dependent proteolytic system in skeletal muscle during fasting. Am J Physiol. 1993 Apr;264(4 Pt 1):E668–E676. doi: 10.1152/ajpendo.1993.264.4.E668. [DOI] [PubMed] [Google Scholar]
- Zeman R. J., Kameyama T., Matsumoto K., Bernstein P., Etlinger J. D. Regulation of protein degradation in muscle by calcium. Evidence for enhanced nonlysosomal proteolysis associated with elevated cytosolic calcium. J Biol Chem. 1985 Nov 5;260(25):13619–13624. [PubMed] [Google Scholar]
- van der Westhuyzen D. R., Matsumoto K., Etlinger J. D. Easily releasable myofilaments from skeletal and cardiac muscles maintained in vitro. Role in myofibrillar assembly and turnover. J Biol Chem. 1981 Nov 25;256(22):11791–11797. [PubMed] [Google Scholar]