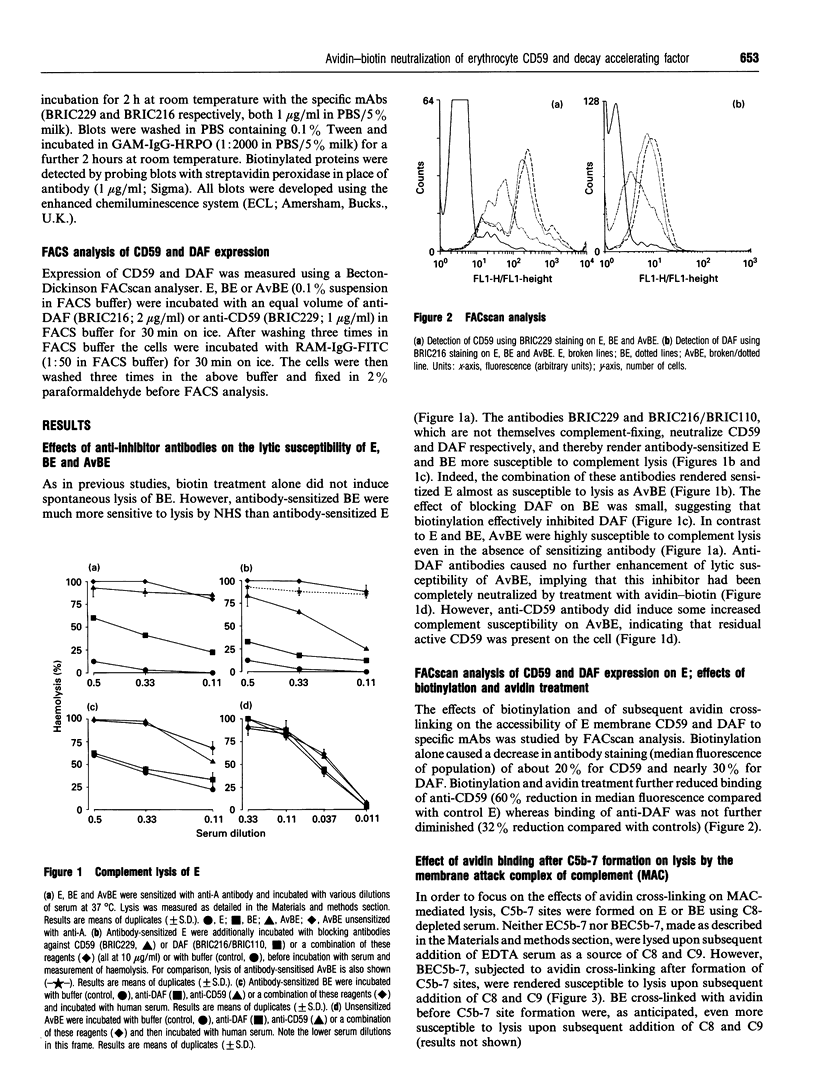
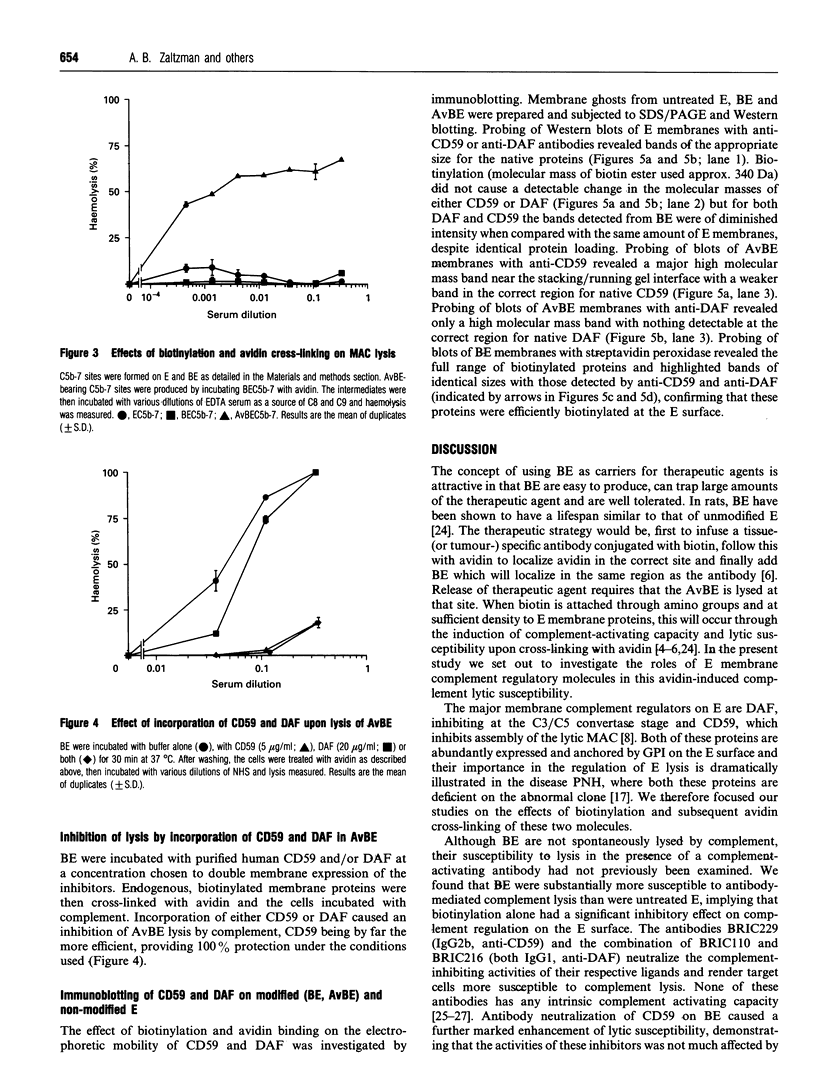
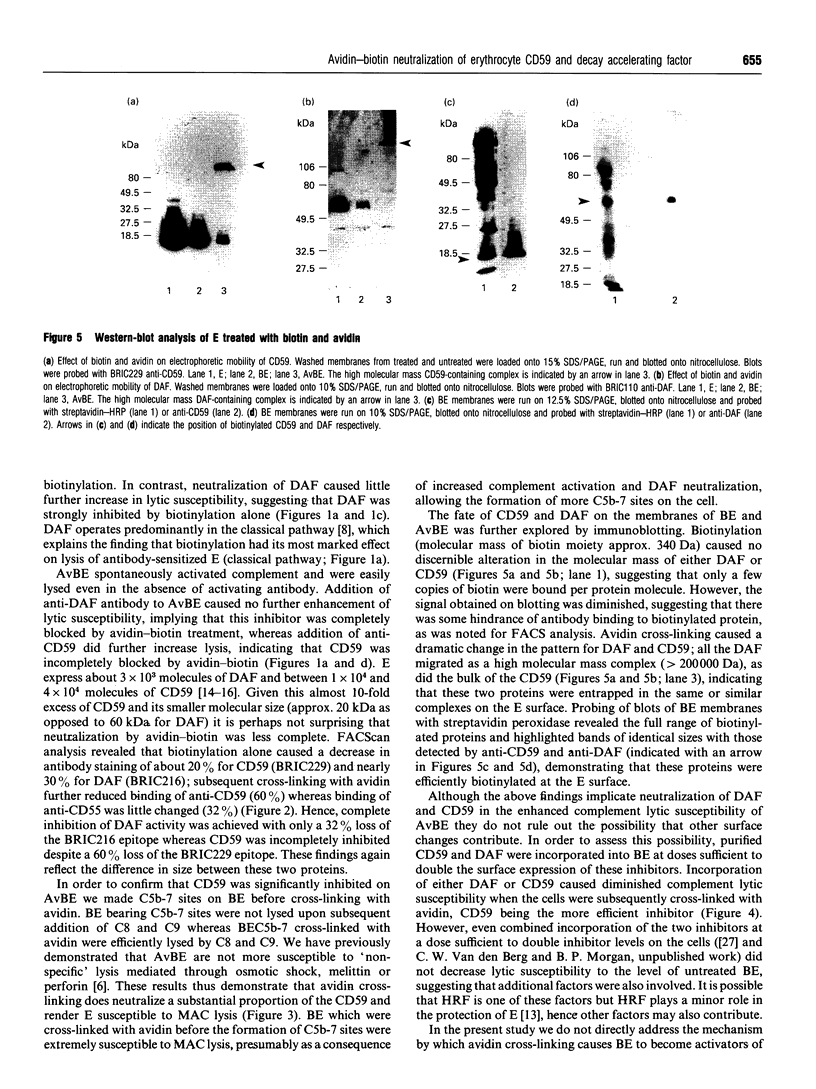
Abstract
Biotinylation of erythrocytes (E) followed by avidin cross-linking at specific sites has been suggested as a novel means of drug delivery. Upon avidin cross-linking, biotinylated E become complement-activating and highly susceptible to complement lysis, thus bringing about release of entrapped drug. We set out to examine the mechanisms of this biotin-avidin-induced lytic susceptibility, focusing on the effects of biotinylation and avidin cross-linking on the major E complement regulatory molecules, decay accelerating factor (DAF) and CD59. We demonstrate here that biotinylation of E, which does not render them complement activating, partially inhibits DAF but has little effect on CD59. Subsequent cross-linking with avidin causes complete inhibition of DAF and near complete loss of CD59 activity. Following cross-linking, DAF and CD59 become associated in high molecular mass avidin-containing complexes on the membrane. Incorporation of physiological amounts of CD59 into the membranes of biotinylated and avidin cross-linked E is sufficient to render these cells resistant to complement lysis whereas incorporation of DAF has relatively little effect. An understanding of the molecular mechanisms underlying complement susceptibility of biotin-avidin treated E should allow a rational design of strategies for drug delivery using E or other large, potentially complement-activating carriers.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraha A., Morgan B. P., Luzio J. P. The preparation and characterization of monoclonal antibodies to human complement component C8 and their use in purification of C8 and C8 subunits. Biochem J. 1988 Apr 1;251(1):285–292. doi: 10.1042/bj2510285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne K. E., Hall S. E., Thompson S., Arce M. A., Kinoshita T., Fujita T., Anstee D. J., Rosse W., Lublin D. M. Mapping of epitopes, glycosylation sites, and complement regulatory domains in human decay accelerating factor. J Immunol. 1992 Nov 1;149(9):2906–2913. [PubMed] [Google Scholar]
- Fearon D. T. Identification of the membrane glycoprotein that is the C3b receptor of the human erythrocyte, polymorphonuclear leukocyte, B lymphocyte, and monocyte. J Exp Med. 1980 Jul 1;152(1):20–30. doi: 10.1084/jem.152.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher A., Bryant J. A., Gardner B., Judson P. A., Spring F. A., Parsons S. F., Mallinson G., Anstee D. J. New monoclonal antibodies in CD59: use for the analysis of peripheral blood cells from paroxysmal nocturnal haemoglobinuria (PNH) patients and for the quantitation of CD59 on normal and decay accelerating factor (DAF)-deficient erythrocytes. Immunology. 1992 Mar;75(3):507–512. [PMC free article] [PubMed] [Google Scholar]
- Kinoshita T., Medof M. E., Silber R., Nussenzweig V. Distribution of decay-accelerating factor in the peripheral blood of normal individuals and patients with paroxysmal nocturnal hemoglobinuria. J Exp Med. 1985 Jul 1;162(1):75–92. doi: 10.1084/jem.162.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Meri S., Morgan B. P., Davies A., Daniels R. H., Olavesen M. G., Waldmann H., Lachmann P. J. Human protectin (CD59), an 18,000-20,000 MW complement lysis restricting factor, inhibits C5b-8 catalysed insertion of C9 into lipid bilayers. Immunology. 1990 Sep;71(1):1–9. [PMC free article] [PubMed] [Google Scholar]
- Morgan B. P., Daw R. A., Siddle K., Luzio J. P., Campbell A. K. Immunoaffinity purification of human complement component C9 using monoclonal antibodies. J Immunol Methods. 1983 Nov 25;64(3):269–281. doi: 10.1016/0022-1759(83)90434-9. [DOI] [PubMed] [Google Scholar]
- Morgan B. P. Effects of the membrane attack complex of complement on nucleated cells. Curr Top Microbiol Immunol. 1992;178:115–140. doi: 10.1007/978-3-642-77014-2_8. [DOI] [PubMed] [Google Scholar]
- Morgan B. P., Meri S. Membrane proteins that protect against complement lysis. Springer Semin Immunopathol. 1994;15(4):369–396. doi: 10.1007/BF01837366. [DOI] [PubMed] [Google Scholar]
- Muzykantov V. R., Seregina N., Smirnov M. D. Fast lysis by complement and uptake by liver of avidin-carrying biotinylated erythrocytes. Int J Artif Organs. 1992 Oct;15(10):622–627. [PubMed] [Google Scholar]
- Muzykantov V. R., Smirnov M. D., Klibanov A. L. Avidin attachment to red blood cells via a phospholipid derivative of biotin provides complement-resistant immunoerythrocytes. J Immunol Methods. 1993 Feb 3;158(2):183–190. doi: 10.1016/0022-1759(93)90212-p. [DOI] [PubMed] [Google Scholar]
- Muzykantov V. R., Smirnov M. D., Samokhin G. P. Avidin attachment to biotinylated erythrocytes induces homologous lysis via the alternative pathway of complement. Blood. 1991 Nov 15;78(10):2611–2618. [PubMed] [Google Scholar]
- Muzykantov V. R., Smirnov M. D., Zaltzman A. B., Samokhin G. P. Tannin-mediated attachment of avidin provides complement-resistant immunoerythrocytes that can be lysed in the presence of activator of complement. Anal Biochem. 1993 Feb 1;208(2):338–342. doi: 10.1006/abio.1993.1057. [DOI] [PubMed] [Google Scholar]
- Pangburn M. K., Müller-Eberhard H. J. The alternative pathway of complement. Springer Semin Immunopathol. 1984;7(2-3):163–192. doi: 10.1007/BF01893019. [DOI] [PubMed] [Google Scholar]
- Pangburn M. K., Schreiber R. D., Müller-Eberhard H. J. Deficiency of an erythrocyte membrane protein with complement regulatory activity in paroxysmal nocturnal hemoglobinuria. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5430–5434. doi: 10.1073/pnas.80.17.5430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney I. A., Morgan B. P. Characterization of the membrane attack complex inhibitory protein CD59 antigen on human amniotic cells and in amniotic fluid. Immunology. 1992 Aug;76(4):541–547. [PMC free article] [PubMed] [Google Scholar]
- Rosse W. F. The glycolipid anchor of membrane surface proteins. Semin Hematol. 1993 Jul;30(3):219–231. [PubMed] [Google Scholar]
- Rothman I. K., Gelfand J. A., Fauci A. S., Frank M. M. The immune adherence receptor: dissociation between the expression of erythrocyte and mononuclear cell C3b receptors. J Immunol. 1975 Nov;115(5):1312–1315. [PubMed] [Google Scholar]
- Tandon N., Morgan B. P., Weetman A. P. Expression and function of membrane attack complex inhibitory proteins on thyroid follicular cells. Immunology. 1992 Feb;75(2):372–377. [PMC free article] [PubMed] [Google Scholar]
- Vogel C. W., Müller-Eberhard H. J. Cobra venom factor: improved method for purification and biochemical characterization. J Immunol Methods. 1984 Oct 12;73(1):203–220. doi: 10.1016/0022-1759(84)90045-0. [DOI] [PubMed] [Google Scholar]
- Watts M. J., Dankert J. R., Morgan E. P. Isolation and characterization of a membrane-attack-complex-inhibiting protein present in human serum and other biological fluids. Biochem J. 1990 Jan 15;265(2):471–477. doi: 10.1042/bj2650471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilchek M., Bayer E. A. The avidin-biotin complex in bioanalytical applications. Anal Biochem. 1988 May 15;171(1):1–32. doi: 10.1016/0003-2697(88)90120-0. [DOI] [PubMed] [Google Scholar]
- van den Berg C. W., Morgan B. P. Complement-inhibiting activities of human CD59 and analogues from rat, sheep, and pig are not homologously restricted. J Immunol. 1994 Apr 15;152(8):4095–4101. [PubMed] [Google Scholar]