Abstract
Dyslipidemia, characterized by abnormal lipid levels in the blood, significantly escalates the risk of atherosclerotic cardiovascular disease and requires effective treatment strategies. While existing therapies can be effective, long-term adherence is often challenging. There has been an interest in developing enduring and more efficient solutions. In this context, gene editing, particularly clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated protein 9 (Cas9) technology, emerges as a groundbreaking approach, offering potential long-term control of dyslipidemia by directly modifying gene expression. This review delves into the mechanistic insights of various gene-editing tools. We comprehensively analyze various pre-clinical and clinical studies, evaluating the safety, efficacy, and therapeutic implications of gene editing in dyslipidemia management. Key genetic targets, such as low-density lipoprotein receptor (LDLR), proprotein convertase subtilisin/kexin type 9 (PCSK9), angiopoietin-like protein 3 (ANGPTL3), apolipoprotein C3 (APOC3), and lipoprotein (a) (Lp(a)), known for their pivotal roles in lipid metabolism, are scrutinized. The paper highlights the promising outcomes of gene editing in achieving sustained lipid homeostasis, discusses the challenges and ethical considerations in genome editing, and envisions the future of gene therapy in revolutionizing dyslipidemia treatment and cardiovascular risk reduction.
Keywords: dyslipidemia, gene editing, CRISPR/Cas9, LDLR, PCSK9, ANGPTL3, APOC3, Lp(a)
1. Introduction
Multiple lines of evidence, including animal studies, human genetics, epidemiology and randomized controlled studies, support the link between dyslipidemia and increased risk and severity of cardiovascular diseases (CVD) [1, 2]. Despite ongoing research to identify causes and treatments, dyslipidemia often remains underdiagnosed and inadequately treated [3]. It is often manageable through lifestyle modifications and medication, particularly statins. For patients at high risk, combining statins with other drugs like ezetimibe and proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors may enhance treatment efficacy [4]. However, maintaining long-term medication adherence is challenging for many, leading to suboptimal outcomes [5]. Studies indicate that only about half of dyslipidemia patients continue with statin therapy, resulting in increased CVD incidents and healthcare costs [6]. Moreover, additionally, a significant number of patients with severe or refractory hypercholesterolemia, especially among certain women and ethnic groups, continue to exhibit high cholesterol levels despite maximum tolerated medical therapy, often due to drug side effects, non-adherence to treatment, or poor drug response [7, 8].
Advances in lipid therapeutics offer substantial hope for the future. The focus is increasingly shifting towards gene editing techniques [9]. Numerous in vivo studies have successfully modified the expression of liver-specific genes using these methods, and recent clinical trials have shown encouraging results in conditions like transthyretin amyloidosis. For instance, the use of NTLA-2001, a gene editing-based therapeutic, significantly slowed the progression of transthyretin amyloidosis in six patients [10]. While these findings are promising, the journey to integrate these therapies into clinical practice is long, considering the unknown effects and potential side effects. This review will delve into gene editing and its applications in dyslipidemia treatment, offering clinicians and researchers new insights to enhance patient care.
2. Gene Editing
The roles of genetic factors in a variety of diseases, such as cancers, infections, and inherited diseases, are well-established. In response, scientists have been diligently working to develop new genetic tools. These tools aim to bolster immune reactions against harmful cancers, deactivate the genetic material of disease-causing pathogens, and rectify genetic mutations. While introducing new genes has shown promise, gene editing is often crucial for achieving therapeutic objectives.
Genome editing has become a focal point of interest due to its ability to create a range of genetic modifications across different settings. Recent scientific and technological advancements have led to the development of various gene editing methods. These include transcription activator-like effector nucleases (TALEN), homologous recombination, zinc-finger nucleases (ZFNs), and, most notably, the recent innovation of clustered regularly interspaced short palindromic repeats-clustered regularly interspaced short palindromic repeats (CRISPR)-associated protein 9 (CRISPR-Cas9).
CRISPR-Cas9 has emerged as the most popular method due to its effectiveness and versatility. However, despite its numerous advantages, CRISPR-Cas9 is not without limitations.
3. Genome Editing Technologies
Base editing, epigenome editing, and prime editing are molecular techniques that utilize the high precision of the CRISPR/Cas9 system to target specific genomic sites accurately. The capacity to target specific regions of DNA stays intact even after alterations are made to the Cas9 cleavage domains. These modifications give rise to two variants of Cas9: nickase Cas9 (nCas9), which cleaves just one strand of DNA, and dead Cas9 (dCas9), which does not cleave DNA. The introduction of extra domains to nCas9 or dCas9 can enhance the capabilities of the CRISPR/Cas9 system by including a range of activities.
3.1 Nuclease Editing
The CRISPR/Cas systems, originating in bacteria, consist of two main varieties: CRISPR/Cas9 and CRISPR/Cas12. These variants function as platforms for editing genomes. The fundamental constituents of these systems consist of a Cas protein and a guide RNA [11]. The guide RNA exhibits inherent search and binding capabilities built into its RNA sequence. On the other hand, the Cas protein is responsible for coordinating the initiation of a DNA double-strand break, utilizing one or two cleavage domains to cleave both strands of DNA. The primary utilization of the CRISPR/Cas system for genome manipulation in mammalian cells entailed the utilization of Streptococcus pyogenes Cas9, generally denoted as SpCas9 [12, 13, 14]. The guide RNA, approximately 100 nucleotides long, contains information that determines the specificity of DNA targeting. This information is encoded within the first 20 nucleotides of the guide RNA, referred to as the spacer region. The SpCas9 protein establishes a molecular complex with RNA, specifically binding to the remaining 80 nucleotides. The protein-RNA complex engages in the process of scanning double-stranded DNA molecules that it comes into contact with. During DNA scanning, the SpCas9 enzyme exhibits transient pauses at particular areas with NGG patterns, where the letter N denotes any nucleotide. Currently, the guide RNA’s spacer region exhibits alignment with the DNA strand that does not possess the NGG pattern, commonly called the target strand. The occurrence of a strong affinity between the target strand and spacer sequences leads to a notable formation of Watson-Crick base pairs between DNA and RNA, thereby initiating the activation of SpCas9. As a result, a double-strand break arises close to the third base pair preceding the NGG motif. The DNA sequence located on the non-target strand corresponding to the RNA spacer sequence is called the protospacer. This protospacer includes a 20-nucleotide region positioned directly upstream of the NGG motif, known as the protospacer-adjacent motif (PAM).
3.2 Base Editing
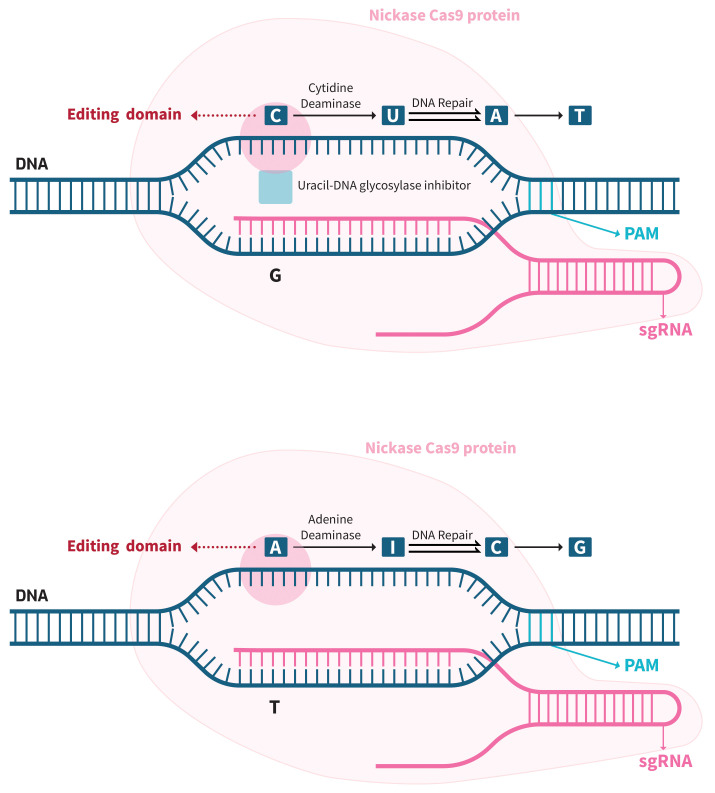
There are two main classifications of base editors: cytosine base editors, which assist the conversion of a cytosine (C) base to another base, often thymine (T) or occasionally guanine (G) [15, 16], on a DNA strand, and adenine base editors, which enable the replacement of an adenine (A) base with a guanine (G) [17]. The capability to catalyze cytidine residue deamination inside a specified editing window on the non-target DNA strand can be achieved by combining nCas9 with various cytidine deaminase domains sourced from natural origins, such as apolipoprotein B mRNA editing enzyme catalytic subunit 1 (APOBEC1) or activation-induced cytidine deaminase (AID) proteins. The DNA strand separation facilitated by Cas9, followed by the binding of the target strand to the guide RNA, results in an R-loop structure. The arrangement of this structure leads to the creation of a DNA bubble that consists of a single strand inside a certain region of the non-hybridized and non-targeted strand. This region is easily accessible to the deaminase domain, which determines the editing window. The dimensions of the editing window are contingent upon the particular Cas9 ortholog utilized, as deamination results in the conversion of C to U (uracil). The enzyme uracil-DNA glycosylase is commonly involved in the reversal of this conversion. However, the introduction of an inhibitor domain to nCas9 hinders the occurrence of this repair process. The nCas9 enzyme exhibits high specificity in its ability to cleave the target strand. Simultaneously, the repair mechanism entails the removal of nucleotides surrounding the nick site and their subsequent replacement by complementary base pairing with the non-target strand. The presence of a U nucleotide in the non-target strand induces the placement of an A nucleotide in the corresponding position of the target strand, as a result of the complementary base pairing between A and U. The process of cellular repair involves the replacement of the non-standard U nucleotide, typically present in RNA, with the ordinary T nucleotide. As a result, a cytosine-guanine (C-G) base pair is converted into a thymine-adenine (T-A) base pair (Fig. 1, Ref. [18]) [19].
Fig. 1.
CRISPR DNA base-editing tools. A: DNA base-editors have two main components, which are a Cas enzyme for programmable DNA binding and a base modification enzyme for targeted nucleotide change. Two types of DNA base-editors have been developed: cytosine base-editors and adenine base-editors. Cytosine base editing: cytosine deaminase produces uracil, that base pairs as thymidine; uracil-DNA glycosylase inhibitor enhances the efficacy of cytosine base-editing by binding and inhibiting uracil N-glycosylate: adenine base editing: adenine deaminase generates inosine, that base pairs as thymidine (modified from Kantor et al. [18]). PAM, protospacer adjacent motif; sgRNA, small guide RNA; A, adenine; C, cytosine; T, thymine; G, guanine; U, uracil; I, inosine; Cas9, clustered regularly interspaced short palindromic repeats (CRISPR)-associated protein 9.
3.3 Prime Editing
Prime editing involves the combination of Cas9 nickase with a specifically engineered reverse transcriptase enzyme. This enzyme utilizes an RNA template to aid in incorporating new DNA sequences. This methodology functions autonomously from DNA double-strand breaks or the presence of a donor DNA template, hence enabling precise incorporation of targeted point mutations or indels across a wider range of editing possibilities. The application of prime editing has been utilized in laboratory settings to correct a variety of genetic disease-causing factors in human cell lines. This has led to the development of specific therapeutic methods tailored to each target gene [20]. Although there is currently minimal in vivo testing of this approach, it has the potential to effectively target a wide range of mutations, such as deletions, duplications, and inversions. As a result, it presents intriguing opportunities for correcting most disease-causing variations. In addition, a primary editing guide RNA (pegRNA) facilitates the accurate identification and targeting of vulnerable gene areas, hence enabling the correction of closely located genetic variations within such regions. As mentioned above, the capability surpasses the limitations of single-base editing. It demonstrates superior efficacy compared to homology-directed repair (HDR), significantly expanding the range of sequence targets and therapeutic approaches [21].
3.4 Epigenome Editing
Epigenome editing distinguishes itself from other editing modalities by confining alterations solely to DNA sequences. In contrast, it influences gene expression by modulating protein-DNA interactions. When a compound consisting of dCas9 and guide RNA (gRNA) is targeted towards a specific region located within a gene promoter or transcriptional enhancer, it indirectly influences the DNA molecule. However, it can impede the expression of genes through a process known as CRISPR interference, which hinders the interactions between certain components that would normally bind to the specific sequence being targeted [22]. The knockdown effectiveness can be augmented by fusing dCas9 with a gene expression-inhibiting domain, such as the Krüppel-associated box (KRAB) domain. The fusion event leads to a restructuring of the chromatin structure in the vicinity, resulting in a modification of the accessibility of the DNA sequence for the transcriptional machinery [23]. In contrast, the augmentation of gene expression through CRISPR activation can be achieved by combining dCas9 with a transcriptional activator domain, such as VP16, or by connecting activators to the gRNA via the manipulation of RNA aptamers located at its 3’ terminus [24]. It is worth noting that the observed changes in gene expression are temporary, occurring only while the dCas9 fusion protein is present at the designated location. To obtain long-term stability in the process of epigenome editing, it is possible to lengthen the duration of activity by combining dCas9 with a methyltransferase or demethylase domain [25]. Enhanced methylation in the vicinity of the promoter region of a certain gene, particularly at cytosine bases within CpG dinucleotide sequences, typically results in the inhibition of gene expression. In contrast, a reduction in methylation generally increases gene expression. Persistent methylation alterations have been seen to endure during numerous cellular divisions. However, it is possible to undo these modifications by utilizing a fusion protein of dCas9 that has an opposing influence on the same genomic location [26].
4. Gene Editing for the Management of Dyslipidemia
Gene editing techniques have been employed to either disrupt or repair targeted genes, garnering significant attention for their potential in managing dyslipidemia. So far, this approach has yielded encouraging results. While gene repair is primarily applicable to the treatment of monogenic diseases, gene disruption offers broader possibilities in this context. A variety of genes that play pivotal roles in lipid metabolism, such as low-density lipoprotein receptor (LDLR), PCSK9, angiopoietin-like protein 3 (ANGPTL3), apolipoprotein C3 (APOC3), and lipoprotein(a) (LPA), have been strategically targeted for the treatment of dyslipidemia, showcasing the versatility and promise of gene editing in this field.
4.1 LDLR
Familial hypercholesterolemia (FH) is the most common monogenic disorder in humans. It follows an autosomal dominant inheritance pattern andis characterized by tendinous xanthomas, arcus juvenilis, and premature coronary heart disease (CHD) [27]. FH is due to mutations in three canonical genes, which encode LDLR, apolipoprotein B (apoB), or PCSK9, accounting for approximately 90%, 5%, and 1% of all patients, respectively. The LDLR, which is located on the cell surface, is expressed in hepatocytes and contributed to regulating LDL levels [28].
Heterozygous mutations can cause CVD in middle age, and currently, available treatments, such as statins, work well in these patients, however low density lipoprotein cholesterol (LDL-C) is often suboptimally lowered with statin monotherapy. However, treatments are minimally effective in those with homozygous mutations with FH (HoFH) [29]. Statins decrease the blood cholesterol and LDL-C via inhibiting hydroxy-methyl-glutaryl coenzyme-A (HMG-CoA) reductase and increasing the expression of LDLR, leading to enhance in LDL-C removal from the circulation [30]; thus, the therapeutic effects of statins hugely depend on the LDLR function and those with non-functional LDLR do not respond on statin therapy [31]. Homozygous individuals are prone to develop CVDs at early stages of life, contributing to early premature death [32, 33]. LDLR is virtually absent in HoFH patients. Therefore, available treatments cannot reduce LDL levels efficiently [34]. To prolong the survival of these patients, we should use more aggressive approaches, including combination lipid lowering therapy. Apheresis is also an option but is demanding for patients, expensive, and availability is limited [35].
Carlson et al. [36] used TALENs to inactivate both alleles of the LDLR gene in the pig fibroblast genome, which was obtained in 10 out of 11 piglets following somatic cell nuclear transfer. Hence, they concluded that in the biomedical field, TALENs, which can be accessed very readily in all labs, have the potential for generating lipid metabolic disease [36]. In another experimental research, to mimic lipid metabolic disease, Huang et al. [37] targeted both LDLR and ApoE genes of porcine embryonic fibroblasts at the same time through applying the CRISPR/Cas9 method. All six founder pigs displayed biallelic inactivation of LDLR without finding off-target incidents and serum values of cholesterol, LDL, and ApoB were enhanced remarkably, indicating that CRISPR/Cas9 approach can be utilized to simulate lipid metabolic disease and atherosclerotic models.
In recent years, gene therapy has been considered a potential solution for the management of HoFH patients. Omer et al. [38] cultured skin fibroblasts from a HoFH patient with 3-base pair deletion in exon four of LDLR and thereafter, to correct mutations permanently, the investigators used Cas9 nickase (Cas9n) with paired single-guide RNAs (sgRNA). The insertion corrected mutations successfully and receptor function was restored. It was found that both alleles of 83% (10 out of 12) of enriched clones were corrected. Moreover, these reprogrammed cells were capable of differentiating into hepatocyte-like cells, which were shown to have a comparable LDL endocytosis compared to hepatocytes cells with normal LDLR function. Therefore, by applying this method, we can have access to countless source of autologous hepatocytes that either can be delivered to the liver directly or engineered into a functional liver-like tissue, helping us to restore the normal function of LDLR and reduce LDL levels [38].
In another research by Jarrett et al. [39], an in vivo model of metabolic diseases was generated using the adeno-associated virus (AAV)-CRISPR approach. In order to generate the disease, the authors introduced guide RNAs, which were able to target exon 14 of LDLR or exon 5 of ApoB. LDLR disruption contributed to severe hypercholesterolemia and atherosclerosis. On the contrary, mice with disruption of both LDLR and ApoB showed a rapid decrease in plasma cholesterol, which lasted for 20 weeks, indicating that ApoB disruption can decrease cholesterol levels significantly. In addition, while atherosclerotic plaques were easily detectable in mice with LDLR disruption, concomitant disruption of ApoB and LDLR exerted anti-atherosclerotic effects. Although ApoB disruption showed promising outcomes in terms of reducing plasma cholesterol, hepatic fat accumulation was observed, which can be detrimental and limit the applicability of this method in clinical practices. Another limitation of this approach was the high rates of off-target events [39].
Very recently, Zhao et al. [40] designed an in vivo experiment to evaluate CRISPR/Cas9 delivered by AAV as a method of targeted genome editing to restore the function of LDLR and improve atherosclerosis in cases with HoFH. They produced a mouse model of atherosclerosis, LdlrE208X, by inserting a nonsense mutation. They sought to correct the mutation in the LDLR gene using CRISPR/Cas9 and subsequently, injected these cells into mice. The researchers demonstrated that the function of LDLR protein was restored partially (6.7%) (the restoration was defined by homology-directed repair-mediated correction of the T-G mutation), while the LDLR protein level was restored to roughly 18% in six wild type mice. Treated mice had lower serum levels of triglyceride, cholesterol (65%), and LDL, as well as lower macrophage infiltration and smaller atherosclerotic plaques compared to the control group. Thus, AAV-CRISPR/Cas9–mediated LDLR gene correction can be taken into account for HoHF treatment [40].
Satisfactory findings regarding targeting LDLR have been achieved (Table 1, Ref. [36, 37, 38, 39, 40]), which can make it a potential target for the treatment of HoHF patients; however, there is a main challenge that should be mentioned. Nearly 2000 pathogenic variants of LDLR have been identified so far [41], and it seems profoundly difficult to evaluate the value and safety of current personalized approaches in clinical settings using available technologies. Therefore, it is better to take into account other available options to change the values of LDL and ameliorate CVDs.
Table 1.
Comparative overview of gene editing interventions on LDLR.
| Author | Study design | Method | Findings | Treatment-related adverse effects |
| Carlson et al. [36] | Pig | TALEN | Biallelic inactivation of LDLR in 10 out of 11 animals | - |
| Huang et al. [37] | Pig | CRISPR/Cas9 | Biallelic inactivation of ApoE and LDLR in all animals | No off-target mutations were found. |
| Elevated plasma levels of LDL-C, ApoB, and TC | ||||
| Omer et al. [38] | Human iPSC | CRISPR/Cas9 | Both alleles of 83% of enriched clones were corrected | No off-target mutations were detected. |
| Jarrett et al. [39] | Cas9 transgenic mice | AAV-CRISPR | LDLR disruption led to hypercholesteremia and atherosclerosis | Off-target sites were found for the LDLR gene, but not ApoB. |
| ApoB disruption led to lower levels of cholesterol | Mice received LDLR + ApoB gRNAs showed evidence of microvesicular steatosis. | |||
| Zhao et al. [40] | LdlrE208X mouse | AAV-CRISPR/Cas9 | LDLR expression restored partly | Most of off-target mutations were found in introns of different genes. |
| Lowered plasma levels of cholesterol, TG, and LDL-C |
LDLR, low density lipoprotein receptor; CRISPR, clustered regularly interspaced short palindromic repeats; Cas9, CRISPR-associated protein 9; TALEN, transcription activator-like effector nuclease; ApoB, apolipoprotein B; ApoE, apolipoprotein E; LDL-C, low density lipoprotein cholesterol; TG, triglyceride; iPSC, induced-pluripotent stem cell; AAV, adeno-associated virus; gRNAs, guide RNAs; TC, total cholesterol.
4.2 PCSK9
It has been demonstrated that PCSK9 has crucial roles in degrading LDLR in hepatocytes. It can bind to LDLR and this complex prevents LDLR from entering recycle vesicles, preventing LDL from returning to the cell surface. Formation of this complex in the Golgi apparatus can enhance the transfer of the complex from the Golgi apparatus toward the lysosome, leading to a remarkable increase in LDLR degradation [42].
There is accumulated evidence in support of the fact that PCSK9 can be considered a promising potential therapeutic target for dyslipidemia and CVDs [43]. It has been repeatedly demonstrated that the loss-of-function mutations in PCSK9 are associated with lower LDL and coronary events and no adverse effects [44, 45]. Consequently, several medications with the potential of inhibiting PCSK9 have yielded hopeful results. Evolocumab was introduced as a monoclonal antibody that prohibits PCSK9 and reduces LDL up to 60%, resulting in some benefits for those with CVDs [46]. Another human monoclonal antibody is alirocumab which can reduce the risk of CVD events in people with a history of acute coronary syndrome [47]. Several studies have investigated the applicability of genome editing for managing dyslipidemia via targeting PCSK9 and here we discuss some of these investigations.
Up to now, several studies have assessed the applicability of gene editing to target the PCSK9 gene (Table 2, Ref. [48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60]). The first in vivo study that sought to disrupt the expression of PCSK9 in mice was conducted by Ding et al. [48]. The authors applied adenovirus to deliver CRISPR/SpCas9 and a guide RNA to target PCSK9 accurately. Four days after the injection, they found over 50% mutagenesis in PCSK9 in all mice, which led to a substantial reduction in plasma PCSK9, augmented LDLR levels, and a 35%–40% decrease in plasma cholesterol values. Interestingly, off-target incidents were not observed in ten sites and another study found no off-target incidents in up to 180 sites using the CRISPR/SpCas9 approach [48]. While adenovirus can deliver SpCas9 efficiently, it is noteworthy to mention that it can induce an immune response [61]. Moreover, delivering SpCas9 due to its size, limits its usage in experimental research. Consequently, scientists introduced AAV as a more proper tool because it provokes immune response less [62] and has a wider range of serotype specificity [63]. In this regard, Ran et al. [49] designed six Cas9 from Staphylococcus aureus (SaCas9), which is 1 kb shorter than SpCas9, enabling the packing of sgRNA into an AAV with a limited cargo size. The investigators made a package consisting of sgRNA, SaCas9, and AAV in vitro, and injected that package to target the PCSK9 of mice liver. After 7 days, they found over 40% of disruption in the PCSK9 gene, contributing to approximately 95% and 40% reduction in the serum values of PCKS9 and cholesterol, respectively. In addition, they evaluated the sensitivity and specificity of SaCas9, which were promising [49]. In order to address the main drawback of using viruses as a delivery tool, Yin et al. [50] incorporated the combination of SpCas9 and modified sgRNA into a lipid nanoparticle. Modification of sgRNA did not prohibit the interaction between sgRNA and SpCas9, and increased genome editing activity. Five days following the intravenous injection, it was observed that up to 80% of the PCSK9 gene was disrupted, serum PCSK9 protein values were undetectable, and cholesterol levels were reduced by 35%–40% in mice [50].
Table 2.
Comparative overview of gene editing interventions on PCSK9 and cholesterol levels.
| Author | Study design | Method | Findings | Treatment-related adverse effects |
| Ding et al. [48] | C57BL/6 mice | CRISPR-SpCas9 | 50% mutagenesis in PCSK9 | No off-target mutations were detected. |
| Decreased plasma levels of PCSK9 and cholesterol | ||||
| Increased hepatic levels of LDLR | ||||
| Ran et al. [49] | C57BL/6 mice | CRISPR/SaCas9 | Decreased serum levels of PCSK9 and total cholesterol | No off-target mutations were detected. |
| Yin et al. [50] | C57BL/6 mice | Nanoparticle-CRISPR-SpCas9 | 80% editing in PCSK9 gene | No off-target mutations were detected. |
| Very low serum levels of PCSK9 | ||||
| 35–40% reduction in serum levels of cholesterol | ||||
| Wang et al. [51] | Chimeric liver-humanized mice | Chimeric liver-humanized mice model | 52% reduction in PCSK9 protein levels | No off-target mutations were detected. |
| Chadwick et al. [52] | C57BL/6 mice | Adenoviral base editing (BE3) | Decreased plasma levels of PCKS9 protein (50%) and cholesterol (30%) | No off-target mutations were detected. |
| Levy et al. [53] | C57BL/6 mice | AAV-cytosine and adenine base editing | 38% genome editing in the liver | A single off-target site. |
| Musunuru et al. [54] | Cynomolgus monkeys | ABE8.8-m nanoparticle- base editing | Decreased plasma levels of PCSK9 protein (90%) and LDL-C (60%) | No off-target editing with a dose of 0.5 mg/kg LNP and a low level of target editing (mean 1%) with a dose of 1.5 mg/kg LNP. |
| Wang et al. [55] | Rhesus macaque | AAV-meganuclease editing | Decreased plasma levels of PCSK9 protein (84%) and LDL-C (60%) | First generation: 487–629 off-target cleavage sites were identified. |
| Second generation: 192–257 off-target cleavage sites were identified. | ||||
| Breton et al. [56] | Rhesus macaque | AAV-meganuclease editing | Reduction in plasma levels of PCSK9 (40–76% of baseline) and LDL-C (64–89% of baseline) | Off-target editing, which was lower in the second generation of AAV-meganuclease editing compared to the first generation. |
| Wang et al. [57] | Rhesus macaque | AAV-meganuclease editing | Decreased plasma levels of PCSK9 and LDL-C, which consistently lasted for three years | Mild-to-moderate liver capsular and subcapsular fibrosis and minimal-to-mild mononuclear cell infiltrates in the liver tissue. |
| A low frequency of off-target editing. | ||||
| Rothgangl et al. [58] | C57BL/6 and Alb-Cre Trp53flox/flox, cynomolgus macaques | ABE base editing | Mice: Plasma levels of PCSK and LDL-C decreased 95% and 58%, respectively | No off-target genome editing in DNA. |
| Macaques: Plasma levels of PCSK and LDL-C decreased 32% and 14%, respectively | Very mild lobular mixed cell infiltration in the liver. | |||
| Lee et al. [59] | Cynomolgus monkeys | ABE8.8-m nanoparticle- base editing | Mean PCSK9 editing of 46% and 70% following treatment with VERVE-101 at 0.75 and 1.5 mg/kg, respectively | A transient rise in alanine aminotransferase and aspartate aminotransferase, which resolved after two weeks. |
| 67% and 83% reductions in the blood levels of PCSK9, and 49% and 69% reductions in blood levels of LDL-C, which lasted for 476 days, following treatment with 0.75 and 1.5 mg/kg VERVE-101, respectively | No change in total bilirubin. | |||
| Bellinger [60] | 10 HeFH patients with a history of coronary revascularization and a mean LDL-C of 193 mg/dL | ABE8.8-m nanoparticle- base editing | PCSK9 levels were decreased by 59% and 84% in two patients received 0.45 mg/kg dose and 45% in the patient received 0.6 mg/kg | One patient experienced a fatal cardiac arrest 5 weeks after infusion. |
| LDL-C level was reduced by 39% and 48% in participants received 0.45 mg/kg dose and 55% in the participant received 0.6 mg/kg dose | One patient experienced myocardial infarction one day after drug infusion and non-sustained ventricular tachycardia 4 weeks after infusion. | |||
| A 55% reduction in LDL-C of the patient received 0.6 mg/kg persisted for six months |
LDLR, low-density lipoprotein receptor; PCSK9, proprotein convertase subtilisin/kexin type 9; CRISPR, clustered regularly interspaced short palindromic repeats; SpCas9, Streptococcus pyogenes Cas9; Cas9, CRISPR-associated protein 9; LDL-C, low density lipoprotein cholesterol; AAV, adeno-associated virus; ABE, adenine base editors; HeFH, heterozygous familial hypercholesterolemia; LNP, lipid nanoparticle.
Base editing enables scientists to accurately introduce single-nucleotide variants into disease-causing genes and modify their function [54]. For the first time, Chadwick et al. [52] examined the therapeutic potential of base editor 3 (BE3), as the most commonly used base editor, to generate nonsense variants in PCSK9 liver tissues of mice. This approach led to decreased plasma PCSK9 protein and cholesterol by approximately 50% and 30%, respectively, and no off-target incidents were found. Although its efficacy was lower than genome editing, it was associated with a lower risk of finding on-target indels and off-target incidents because this process does not need to break double-strand DNA. One of the main drawbacks of this approach is using adenovirus for delivering base editor because BE3 is large [52]. To tackle the size limitation, Levy et al. [53] used a split base-editor dual-AAV approach as a more suitable vector and revealed the efficacy of base editing in the mice liver was 38%.
In another experimental study, adenine base editor was delivered via lipid nanoparticles to disrupt the expression of PCSK9. First, on hepatocytes, the levels of splice site editing were measured up to 60% and PCSK9 declined about 55%. Thereafter, 70% based editing at the splice site was detected in the liver of mice. In the final step, the authors assessed the efficacy of this approach on monkeys. It was shown that PCSK9 protein and cholesterol levels decreased by about 81% and 65%, respectively, suggesting that the approach could edit both PCSK9 alleles of virtually all hepatocytes. Using lipid nanoparticles provoked a mild to moderate rise in liver function tests (aspartate aminotransferase (AST) and alanine aminotransferase (ALT)) which lasted for only 1–2 weeks, whereas using AAV meganuclease can cause a moderate rise in these enzymes, which lasts for months. Off-target editing was found at one site of monkeys, which shows no similar homology to the human genome, which is consistent with the fact that no off-target editing was found in human hepatocytes [54]. The efficacy of the approach in terms of reducing LDL was similar to or better than lipid-lowering agents, and a 65% reduction in LDL can reduce the risk of CVD substantially [64]. Similar to the last study, Rothgangl et al. [58] utilized an adenine base editor that was delivered by a lipid nanoparticle, comprising of a gRNA and the base editor messenger RNA (mRNA). The authors used adenine base editing to target splice donor site of PCSK9 intron 1. First, they tested the efficacy of RNA on mice, and found a significant decrease in the levels of PCSK9 and LDL-C. Thereafter, 0.75 mg/kg or 1.5 mg/kg RNA was injected in four groups of macaques. Each dose was given as a single dose or as two doses after two weeks from the first dose. After a month, base editing was occurred in 2.03 0.85% and 27.6 5.87% of groups received a single dose of 0.75 and 1.5 mg/kg, respectively. The corresponding values for groups receive two doses of 0.75 and 1.5 mg/kg were 3.31 1.73% and 24.14 1.52%, respectively. The second injection did not enhance the rate of base editing, because after the first injection, IgG antibody against base editor was formed. In animals received 1.5 mg/kg RNA, PCSK9 levels were reduced about 26% and 39% in the single- and repeated-dose group, respectively, which was associated with a 9% and 19% reductions in the levels of LDL-C in the single- and repeated-dose group, respectively. No off-target mutations were detected [58].
Although these research have demonstrated the effect and safety of PCSK9 editing in mice, the translation of these findings into human patients is limited due to several reasons. First of all, off-target incidents observed in mice models probably not hold up in the human genome since there are notable differences between genomes of a mouse and a human. Secondly, if the same site in the mouse and human genome being targeted using the same sgRNA, the outcomes of PCSK9 editing will be hugely different as human and mouse hepatocytes are physiologically different [26]. In order to overcome this obstacle, Wang et al. [51] designed chimeric liver-humanized mice model in which mice hepatocytes were replaced by human hepatocytes in order to target the human PCSK9 gene. Blood values of PCSK9 protein were reduced on average by 52% and no off-target incidents were reported [51].
To translate the efficacy of genome editing into clinical settings, it is mandatory to assess their performance on animal models other than mice. Wang et al. [55] revealed that meganuclease targeting of PCSK9 in the liver of primates brings about 84% and 60% reduction in serum levels of PCSK9 and LDL, respectively. Of note, this approach had some consequences that should be mentioned. First, off-target editing was remarkably high, which can lead to the translocation of chromosomes. Second, a mild rise in AST and ALT was found, which lasted for approximately 40 days [55]. In order to limit off-target mutations, the same authors performed two different strategies: (1) a shorter version of thyroid hormone-binding globulin (TBG) to decrease nuclease expression, (2) inserting the M2PCSK9 target sequence into the AAV genome that expresses the nuclease and/or fusing the nuclease to a specific peptide to facilitates its degradation. The investigators observed the same efficacy with the less off-target mutations [56]. In the later study, they found that the impact of this method on PCSK9 and LDL-C can last for three years consistently [57]. These findings highlight the importance of this approach for the treatment of those with genetic disorders because these patients need treatments with life-long effects. Little off-target editing was found and they were stable during the follow-up.
Promising findings concerning the efficacy, safety, and durability of a new drug, called VERVE-101, for reducing LDL-C levels has brought hope for future management of dyslipidemia using genome editing. VERVE-101, an investigational CRISPR base-editing medicine, was designated to reduce LDL-C in patients with FH. This medicine consists of an engineered lipid nanoparticle that encapsulates an adenine base editor messenger RNA that Verve has licensed from Beam Therapeutics, and a guide RNA targeting the PCSK9 gene [65]. The drug comprises of a guide RNA that targets a splice donor at the boundary of intron 1 and exon 1 of PCSK9 and introduce a stop codon adjacent to the beginning of intron 1, which would terminate the generation of a PCSK9 protein and the mRNA with the ability to encode the adenine base editor 8.8-m [66]. Musunuru et al. [54] found a near-complete knockdown of PCSK9 in the livers of cynomolgus monkeys following a single infusion of VERVE-101. They observed reductions in blood levels of PCSK9 and LDL-C by about 90% and 60%, respectively, which were remained for at least 8 months following the administration. Its efficacy in terms of reducing LDL-C levels equals or surpasses the efficacy of existing treatments and moreover, in contrast to other treatments, VERVE-101 can offer once-and-done treatment of enhanced levels of LDL-C. A very recent study conducted by Lee et al. [59] sought to evaluate the efficacy, durability, and safety of VERVE-101 among cynomolgus monkeys. Authors decided to choose 1.5 mg/kg VERVE-101 for the experiment since they are closely related to humans in terms of genetic and physiology. Additionally, authors did not perform any modification on the drug since the target DNA location for the drug was similar between humans and cynomolgus monkeys. 36 cynomolgus monkeys were employed of which 10 received a vehicle control, four received 0.75 mg/kg VERVE-101, and 22 received 1.5 mg/kg VERVE-101. The mean editing of PCSK9 was measured two weeks after the dosing using targeted amplicon sequencing method and was 0.1%, 46%, and 70% in the control group, the group received 0.75 mg/kg, and the group received 1.5 mg/kg, respectively. Animal treated with 0.75 mg/kg experienced 67% and 49% reductions in the levels of PCSK9 and LDL-C, respectively. The corresponding values for animals treated with 1.5 mg/kg were 83% and 69%, respectively. Dose-dependent and durable (up to 476 days) decrease in the levels of PCSK9 and LDL-C was detected; percentage changes for PCSK9 from baseline values were 8%, 49%, and 69% for the control group, animals received 0.75 mg/kg, and animals received 1.5 mg/kg, respectively; the corresponding values for LDL-C were 10%, 50%, and 68%, respectively. The investigators found a strong correlation between the levels of PCSK9 and LDL-C 12 months after dosing. Regarding safety, a transient rise in aspartate AST and ALT following infusion, which were resolved after two weeks. 12 months after dosing, histopathological evaluations of liver and other organs of subjects did not show any microscopic and macroscopic changes related to the drug. No significant change in the level of PCSK9 editing before and 3 months after dosing was found. The PCSK9 edit was not transmit to any offsprings [59].
These preclinical efficacy and safety data contributed to the initiation of an ongoing human clinical trial, namely heart-1. Heart-1, an open-label, single ascending dose trial, was designed to examine the efficacy and safety of VERVE-101 in 10 patients with heterozygous FH, history of coronary revascularization, and a mean LDL-C of 193 mg/dL despite receiving maximally tolerated lipid lowering treatments. 10 patients (8 men and 2 women) received a single intravenous infusion of VERVE-101 with the dosages of 0.1 (n = 3), 0.3 (n = 3), 0.45 (n = 3), and 0.6 mg/kg (n = 1). Nine patients were followed for 28 days and blood values of PCSK9 were decreased by 59% and 84% in those received 0.45 mg/kg dose and 45% in the patient received 0.6 mg/kg. LDL-C level was reduced by 39% and 48% in participants received 0.45 mg/kg dose and 55% in the participant received 0.6 mg/kg dose. A 55% reduction in LDL-C of the patient received 0.6 mg/kg persisted for six months. Transient rise in liver enzymes and transient infusion reactions were observed. During the follow-up, three cardiovascular events in two patients were observed. 5 weeks following dosing, one patient who received 0.3 mg/kg dose experienced a fatal cardiac arrest, which was not related to the drug since both patients had extensive atherosclerotic cardiovascular disease (ASCVD) and the independent safety bord claimed that these events probably related to underlying ASCVD diseases [67]. The other patient (received 0.45 mg/kg dose) experienced acute myocardial infarction one day after infusion and non-sustained ventricular tachycardia after a month after infusion. The independent safety claimed that these adverse events were not related to the drug and are consistent with their underlying cardiovascular diseases; therefore, they decided to continue the trial [60, 67].
4.3 ANGPTL3
In addition to PCSK9, targeting ANGPTL3 can be considered as a valuable therapeutic approach for dyslipidemia management. A number of experimental research has revealed that ANGPTL3, which is secreted mainly in the liver, is able to inhibit lipoprotein lipase (LPL) and endothelial lipase (EL) activity, and its inactivation by mutations is associated with enhanced activity of LPL, causing lower levels of lipids and conveying protection against CVD [68, 69]. A monoclonal antibody drug, named evinacumab, with the ability to target ANGPTL3 has developed, and it has been demonstrated that this drug can reduce lipid levels through several mechanisms [68, 70], suggesting that targeting ANGPTL3 may be a compelling therapeutic option for the management of HoFH patients [71] and has an effect on LDL-C independent of LDLR [72].
Qiu et al. [73] utilized a lipid nanoparticle to deliver CRISPR-Cas9 mRNA to mice liver to knock down ANGPTL3. The efficacy of this method was appeared 38%, and the levels of ANGPTL3, LDL, and triglyceride (TG) were decreased 65%, 56%, and 29%, respectively, and these effects were lasted for 100 days. The authors found no off-target incidents in nine sites and moreover, no toxicity impact was found in the liver [73]. Lipid nanoparticles deliver gene editing through liver LDLR; therefore, it is not efficient to use standard lipid nanoparticles as a delivery system in patients with HoFH; nevertheless, Kasiewicz et al. [74] designed a new lipid nanoparticle system included N-acetylgalactosamine (GalNAc) with the ability to bind to the asialoglycoprotein receptor (ASGPR) to target ANGPTL3 in nonhuman primates with HoFH. The efficacy of this new technology was much higher than standard lipid nanoparticles [74].
Adenovirus vectors with the capacity to encode EB3 and guide RNA targeting ANGPTL3 were generated by Chadwick et al. [75] so as to manage patients with dyslipdemia. After a week following the injection, 49%, 31%, and 19% reduction in levels of ANGPTL3 protein, blood TC, and cholesterol were detected, respectively. The investigators also found out that targeting ANGPTLl3 could lower the blood levels of TG more than targeting PCSK9 or the combination of ANGPTL3 and PCSK9. At last, the authors evaluated the impact of targeting ANGPTL3 in LDLR-negative mice, which resembles a proper model of HoFH and found a 56% and 51% reduction in TG and cholesterol, respectively, after two weeks after the injection. Interestingly, no reduction in bone marrow hematopoietic stem cells was appeared [75].
It is of importance to note that using drugs with the ability to target ANGPTL3 may be accompanied by complications that should be paid attention to. Vupanorsen is an antisense oligonucleotide that target ANGPTL3 in the liver. A randomized clinical trial reported an increase in liver function tests up to three times and also, hepatic fat fraction up to 76%. It is totally unclear that these complications are attributed to metabolic effects of the drug or off-target incidents due to ANGPTL3 targeting [76]. Thus, adverse effects of drugs enabling to target ANGPTL3 should be assessed more carefully. Of note, a very recent study conducted by Pennisi et al. [77], tried to evaluate whether intracellular ANGPTL3 down-regulation can contribute to enhance in lipid contents within the hepatocyte. The authors inhibited ANGPTL3 expression by silencing RNA in primary human hepatocytes and HepG2/LX-2 3-dimensional spheroids, and in Huh7, HepG2, and Hep3B2 cultured in 2 dimensions. Intracellular ANGPTL3 down-regulation was associated with enhanced hepatic TG contents in all models. It was shown that ANGPTL3 provoked lower values of intracellular deiodinase type 1 protein, leading to a decrease in beta-oxidation and as a result, enhance in TG content. These findings suggest that that the intra- and extracellular inhibition of ANGPTL3 will have different effects, a feature of importance when applying gene editing for ANGPTL3 compared to antibodies against ANGPTL3 [77].
4.4 APOC3
APOC3 is known as a glycoprotein mainly synthesized in the liver. It is able to inhibit hydrolysis and catabolism of triglyceride-rich lipoproteins (TRLs), which contribute to higher values of plasma TG [78]. A cohort study revealed that those with homozygous loss of function of APOC3 experienced a lower rise in post-prandial TG [79]. Guo et al. [80] developed a human-like model to assess the effects of APOC3 inactivation on the lipid profile. The plasma levels of TG were reduced significantly in APOC3 knockout hamsters on chow diet; nonetheless, high-density lipoprotein cholesterol (HDL-C) and total cholesterol values did not change significantly. Lipoprotein disc electrophoresis demonstrated an enhancement in LDL fractions and a decrease in very low-density lipoprotein (VLDL) fractions, pointing out that APOC3 inhibition can increase the conversion of VLDL to LDL. The authors put hamsters on a high-cholesterol/high-fat diet to assess the association between APOC3 and atherosclerosis and observed reduced levels of TG, total cholesterol, ApoB, and ApoE and enhanced levels of HDL-C and ApoA1. In summary, they concluded that APOC3 inhibition can exert cardioprotective effects through modifying lipid levels [80]. In another in vivo research, APOC3 protein expression was inhibited by CRISPR/Cas9 approach. While under a normal chow diet, only plasma levels of TG were decreased significantly, under a high-fat diet, plasma levels of TG, LDL-C, and total cholesterol (TC) were decreased significantly, highlighting that APOC3 inactivation could impact cholesterol transport, and this impact is pronounced under a high-fat diet [81]. Collectively, APOC3 can be considered as a suitable target for management of dyslipidemia.
4.5 Lipoprotein(a) [Lp(a)]
Accumulated evidence illustrated that lipoprotein (a) (Lp(a)) can act as an independent risk factor for CVDs [82]. Additionally, Lp(a) is carrier of oxidize phospholipids, contributing to the progression of atherosclerotic plaque [83]. Lp(a) is encoded by LPA gene, which produces in the liver and can bind to ApoB100 [84]. It has been reported that up to 90% variations in Lp(a) levels can be explained by variations in LPA gene [85]. Lp(a) concentration cannot be modified by lifestyle modifications or diet changes; hence, interventions should be implemented to change it [86]. An in vivo study sought to assess the capability of promoting ApoB mRNA editing for decreasing the risk of atherosclerosis. It was demonstrated that transfer of APOBEC1, which was encoded by recombinant adenovirus, enhanced ApoB mRNA editing in transgenic mice, leading to a remarkable decrease in plasma Lp(a) concentrations. The same findings were appeared when ApoB mRNA was edited in rabbits. ApoB gene editing in rabbits was correlated with decreased LDL concentrations [87]. A very recent study applied AAV-CRISPR to disrupt LPA transgene in the mice livers in order to decrease Lp(a) concentration. The outcome was satisfactory and Lp(a) levels decreased to a great extent and importantly, this approach was completely safe since no changes in liver function tests, overall body weight, cholesterol, and liver histology were observed [88].
5. Therapeutic Considerations
We should optimize genome editing technologies efficacy and safety in order to bring them into clinical practices and assess their impacts on human. In this regard, choosing the best delivery tool is of great importance. Adenoviral vectors should not be considered to be used in clinical settings due to their safety problems and instead, AAVs are preferred [89, 90]; however, there are several limitations that should be tackled first. The main problem is that the size limitation of AAVs hinders the efficient delivery of base editors or SpCas9. The second problem is that using AAVs vectors will bring about extended expression of the delivered gene. Although this feature may seem beneficial, it can increase the incidence of off-target mutagenesis [75]. Using lipid nanoparticles as tools for the delivery can overcome the size and degradation limitations properly [91, 92].
The experimental studies that have investigated the applicability of genome editing for the management of dyslipidemia have reported low off-target mutagenesis incidents, but unfortunately, they are not fully reliable because next-generation DNA sequencing has some obstacles. First, its sensitivity is not high to detect rare mutations and these rare mutations may cause some problems. Second, DNA sequencing cannot assess the whole genome and only detects off-target incidents at particular sites. Several unbiased methods, in particular whole genome sequencing (WGS), have been proposed that are able to detect off-target mutagenesis precisely. WGS has been applied in several studies to find off-target incidents introduced by CRISPR/Cas9 in animals, plant, and human primary cells and the outcomes were promising. However, the main limitation of this method is that it is highly expensive [93].
6. Conclusions
Exploration of lipid-related genes contributed to the invention of new treatments with the ability to target these genes for the prevention of atherosclerosis; nonetheless, these medications should be administered frequently effective concentrations. Gene editing is potent to alter the genes expression for a long duration and exerts its long-term impacts on dyslipidemia. Noteworthy, due to permanent changes in genome, it is of great importance to evaluate its safety in the long run.
Acknowledgment
Not applicable.
Abbreviations
CVD, cardiovascular disease; PCSK9, proprotein convertase subtilisin/kexin type 9; TALEN, transcription activator-like effector nuclease; ZFNs, zinc-finger nucleases; CRISPR-Cas9, clustered regularly interspaced short palindromic repeats-CRISPR-associated protein 9; nCas9, nickase Cas9; dCas9, dead Cas9; PAM, protospacer-adjacent motif; C, cytosine; T, thymine; G, guanine; A, adenine; pegRNA, primary editing guide RNA; HDR, homology-directed repair; KRAB, Krüppel-associated box; LDLR, LDL receptor; ANGPTL3, Angiopoietin-like protein 3; APOC3, apolipoprotein C3; FH, familial hypercholesteremia; ApoB, apolipoprotein B; CHD, coronary heart disease; HoFH, homozygous familial hypercholesteremia; Cas9n, Cas9 nickase; sgRNA, paired single-guide RNAs; AAV, adeno-associated virus; SaCas9, Staphylococcus aureus Cas9; TBG, thyroid hormone-binding globulin; AST, aspartate aminotransferase; ALT, alanine aminotransferase; LPL, lipoprotein lipase; EL, endothelial lipase; GalNAc, N-acetylgalactosamine; ASGPR, asialoglycoprotein receptor; TRLs, triglyceride-rich lipoproteins; Lp(a), lipoprotein (a); WGS, whole genome sequencing.
Footnotes
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author Contributions
SSTZ and MDS designed and performed the research study, wrote the manuscript, contributed to editorial changes in the manuscript, and approved the final manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
Ethics Approval and Consent to Participate
Not applicable.
Funding
This research received no external funding.
Conflict of Interest
The author declares no conflict of interest. Michael D. Shapiro is serving as one of the Editorial Board members and Guest editors of this journal. We declare that Michael D. Shapiro had no involvement in the peer review of this article and has no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to Leonardo De Luca and Brian Tomlinson. Michael D. Shapiro is supported by institutional grants from Amgen, Boehringer Ingelheim, 89Bio, Esperion, Genentech, Novartis, Ionis, Merck, New Amsterdam. He has participated in Scientific Advisory Boards with Amgen, Agepha, Ionis, Novartis, Precision BioScience, New Amsterdam, and Merck. He has served as a consultant for Ionis, Novartis, Regeneron, Aidoc, Shanghai Pharma Biotherapeutics, Kaneka, Novo Nordisk.
References
- [1].Ference BA, Ginsberg HN, Graham I, Ray KK, Packard CJ, Bruckert E, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. European Heart Journal . 2017;38:2459–2472. doi: 10.1093/eurheartj/ehx144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Stein R, Ferrari F, Scolari F. Genetics, Dyslipidemia, and Cardiovascular Disease: New Insights. Current Cardiology Reports . 2019;21:68. doi: 10.1007/s11886-019-1161-5. [DOI] [PubMed] [Google Scholar]
- [3].Halcox JP, Banegas JR, Roy C, Dallongeville J, De Backer G, Guallar E, et al. Prevalence and treatment of atherogenic dyslipidemia in the primary prevention of cardiovascular disease in Europe: EURIKA, a cross-sectional observational study. BMC Cardiovascular Disorders . 2017;17:160. doi: 10.1186/s12872-017-0591-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Dybiec J, Baran W, Dąbek B, Fularski P, Młynarska E, Radzioch E, et al. Advances in Treatment of Dyslipidemia. International Journal of Molecular Sciences . 2023;24:13288. doi: 10.3390/ijms241713288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kengne AP, Brière JB, Zhu L, Li J, Bhatia MK, Atanasov P, et al. Impact of poor medication adherence on clinical outcomes and health resource utilization in patients with hypertension and/or dyslipidemia: systematic review. Expert Review of Pharmacoeconomics & Outcomes Research . 2024;24:143–154. doi: 10.1080/14737167.2023.2266135. [DOI] [PubMed] [Google Scholar]
- [6].Bosworth HB, Ngouyombo B, Liska J, Zullig LL, Atlani C, Beal AC. The importance of cholesterol medication adherence: the need for behavioral change intervention programs. Patient Preference and Adherence . 2018;12:341–348. doi: 10.2147/PPA.S153766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ahmed ST, Akeroyd JM, Mahtta D, Street R, Slagle J, Navar AM, et al. Shared Decisions: A Qualitative Study on Clinician and Patient Perspectives on Statin Therapy and Statin-Associated Side Effects. Journal of the American Heart Association . 2020;9:e017915. doi: 10.1161/JAHA.120.017915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Labos C, Brophy JM, Smith GD, Sniderman AD, Thanassoulis G. Evaluation of the Pleiotropic Effects of Statins: A Reanalysis of the Randomized Trial Evidence Using Egger Regression-Brief Report. Arteriosclerosis, Thrombosis, and Vascular Biology . 2018;38:262–265. doi: 10.1161/ATVBAHA.117.310052. [DOI] [PubMed] [Google Scholar]
- [9].Carroll D. Genome Editing: Past, Present, and Future. The Yale Journal of Biology and Medicine . 2017;90:653–659. [PMC free article] [PubMed] [Google Scholar]
- [10].Gillmore JD, Gane E, Taubel J, Kao J, Fontana M, Maitland ML, et al. CRISPR-Cas9 In Vivo Gene Editing for Transthyretin Amyloidosis. The New England Journal of Medicine . 2021;385:493–502. doi: 10.1056/NEJMoa2107454. [DOI] [PubMed] [Google Scholar]
- [11].Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science (New York, N.Y.) . 2012;337:816–821. doi: 10.1126/science.1225829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Cho SW, Kim S, Kim JM, Kim JS. Targeted genome engineering in human cells with the Cas9 RNA-guided endonuclease. Nature Biotechnology . 2013;31:230–232. doi: 10.1038/nbt.2507. [DOI] [PubMed] [Google Scholar]
- [13].Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, et al. Multiplex genome engineering using CRISPR/Cas systems. Science (New York, N.Y.) . 2013;339:819–823. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Mali P, Yang L, Esvelt KM, Aach J, Guell M, DiCarlo JE, et al. RNA-guided human genome engineering via Cas9. Science (New York, N.Y.) . 2013;339:823–826. doi: 10.1126/science.1232033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Koblan LW, Arbab M, Shen MW, Hussmann JA, Anzalone AV, Doman JL, et al. Efficient C•G-to-G•C base editors developed using CRISPRi screens, target-library analysis, and machine learning. Nature Biotechnology . 2021;39:1414–1425. doi: 10.1038/s41587-021-00938-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Komor AC, Kim YB, Packer MS, Zuris JA, Liu DR. Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature . 2016;533:420–424. doi: 10.1038/nature17946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Gaudelli NM, Komor AC, Rees HA, Packer MS, Badran AH, Bryson DI, et al. Programmable base editing of A•T to G•C in genomic DNA without DNA cleavage. Nature . 2017;551:464–471. doi: 10.1038/nature24644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kantor A, McClements ME, MacLaren RE. CRISPR-Cas9 DNA Base-Editing and Prime-Editing. International Journal of Molecular Sciences . 2020;21:6240. doi: 10.3390/ijms21176240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Musunuru K. Moving toward genome-editing therapies for cardiovascular diseases. The Journal of Clinical Investigation . 2022;132:e148555. doi: 10.1172/JCI148555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Anzalone AV, Randolph PB, Davis JR, Sousa AA, Koblan LW, Levy JM, et al. Search-and-replace genome editing without double-strand breaks or donor DNA. Nature . 2019;576:149–157. doi: 10.1038/s41586-019-1711-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Scholefield J, Harrison PT. Prime editing - an update on the field. Gene Therapy . 2021;28:396–401. doi: 10.1038/s41434-021-00263-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Qi LS, Larson MH, Gilbert LA, Doudna JA, Weissman JS, Arkin AP, et al. Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell . 2013;152:1173–1183. doi: 10.1016/j.cell.2013.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Gilbert LA, Larson MH, Morsut L, Liu Z, Brar GA, Torres SE, et al. CRISPR-mediated modular RNA-guided regulation of transcription in eukaryotes. Cell . 2013;154:442–451. doi: 10.1016/j.cell.2013.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Gilbert LA, Horlbeck MA, Adamson B, Villalta JE, Chen Y, Whitehead EH, et al. Genome-Scale CRISPR-Mediated Control of Gene Repression and Activation. Cell . 2014;159:647–661. doi: 10.1016/j.cell.2014.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Nuñez JK, Chen J, Pommier GC, Cogan JZ, Replogle JM, Adriaens C, et al. Genome-wide programmable transcriptional memory by CRISPR-based epigenome editing. Cell . 2021;184:2503–2519.e17. doi: 10.1016/j.cell.2021.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Musunuru K. CRISPR and cardiovascular diseases. Cardiovascular Research . 2023;119:79–93. doi: 10.1093/cvr/cvac048. [DOI] [PubMed] [Google Scholar]
- [27].Raal FJ, Rosenson RS, Reeskamp LF, Hovingh GK, Kastelein JJP, Rubba P, et al. Evinacumab for Homozygous Familial Hypercholesterolemia. The New England Journal of Medicine . 2020;383:711–720. doi: 10.1056/NEJMoa2004215. [DOI] [PubMed] [Google Scholar]
- [28].Hobbs HH, Brown MS, Goldstein JL. Molecular genetics of the LDL receptor gene in familial hypercholesterolemia. Human Mutation . 1992;1:445–466. doi: 10.1002/humu.1380010602. [DOI] [PubMed] [Google Scholar]
- [29].Rader DJ, Cohen J, Hobbs HH. Monogenic hypercholesterolemia: new insights in pathogenesis and treatment. The Journal of Clinical Investigation . 2003;111:1795–1803. doi: 10.1172/JCI18925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Yu Q, Chen Y, Xu CB. Statins and New-Onset Diabetes Mellitus: LDL Receptor May Provide a Key Link. Frontiers in Pharmacology . 2017;8:372. doi: 10.3389/fphar.2017.00372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Gelissen IC, McLachlan AJ. The pharmacogenomics of statins. Pharmacological Research . 2014;88:99–106. doi: 10.1016/j.phrs.2013.12.002. [DOI] [PubMed] [Google Scholar]
- [32].Raal F, Panz V, Immelman A, Pilcher G. Elevated PCSK9 levels in untreated patients with heterozygous or homozygous familial hypercholesterolemia and the response to high-dose statin therapy. Journal of the American Heart Association . 2013;2:e000028. doi: 10.1161/JAHA.112.000028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Soutar AK, Naoumova RP. Mechanisms of disease: genetic causes of familial hypercholesterolemia. Nature Clinical Practice. Cardiovascular Medicine . 2007;4:214–225. doi: 10.1038/ncpcardio0836. [DOI] [PubMed] [Google Scholar]
- [34].Oka K, Pastore L, Kim IH, Merched A, Nomura S, Lee HJ, et al. Long-term stable correction of low-density lipoprotein receptor-deficient mice with a helper-dependent adenoviral vector expressing the very low-density lipoprotein receptor. Circulation . 2001;103:1274–1281. doi: 10.1161/01.cir.103.9.1274. [DOI] [PubMed] [Google Scholar]
- [35].Al-Ashwal A, Alnouri F, Sabbour H, Al-Mahfouz A, Al-Sayed N, Razzaghy-Azar M, et al. Identification and Treatment of Patients with Homozygous Familial Hypercholesterolaemia: Information and Recommendations from a Middle East Advisory Panel. Current Vascular Pharmacology . 2015;13:759–770. doi: 10.2174/1570161113666150827125040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Carlson DF, Tan W, Lillico SG, Stverakova D, Proudfoot C, Christian M, et al. Efficient TALEN-mediated gene knockout in livestock. Proceedings of the National Academy of Sciences of the United States of America . 2012;109:17382–17387. doi: 10.1073/pnas.1211446109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Huang L, Hua Z, Xiao H, Cheng Y, Xu K, Gao Q, et al. CRISPR/Cas9-mediated ApoE-/- and LDLR-/- double gene knockout in pigs elevates serum LDL-C and TC levels. Oncotarget . 2017;8:37751–37760. doi: 10.18632/oncotarget.17154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Omer L, Hudson EA, Zheng S, Hoying JB, Shan Y, Boyd NL. CRISPR Correction of a Homozygous Low-Density Lipoprotein Receptor Mutation in Familial Hypercholesterolemia Induced Pluripotent Stem Cells. Hepatology Communications . 2017;1:886–898. doi: 10.1002/hep4.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Jarrett KE, Lee CM, Yeh YH, Hsu RH, Gupta R, Zhang M, et al. Somatic genome editing with CRISPR/Cas9 generates and corrects a metabolic disease. Scientific Reports . 2017;7:44624. doi: 10.1038/srep44624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Zhao H, Li Y, He L, Pu W, Yu W, Li Y, et al. In Vivo AAV-CRISPR/Cas9-Mediated Gene Editing Ameliorates Atherosclerosis in Familial Hypercholesterolemia. Circulation . 2020;141:67–79. doi: 10.1161/CIRCULATIONAHA.119.042476. [DOI] [PubMed] [Google Scholar]
- [41].Landrum MJ, Lee JM, Benson M, Brown G, Chao C, Chitipiralla S, et al. ClinVar: public archive of interpretations of clinically relevant variants. Nucleic Acids Research . 2016;44:D862–D868. doi: 10.1093/nar/gkv1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Burke AC, Dron JS, Hegele RA, Huff MW. PCSK9: Regulation and Target for Drug Development for Dyslipidemia. Annual Review of Pharmacology and Toxicology . 2017;57:223–244. doi: 10.1146/annurev-pharmtox-010716-104944. [DOI] [PubMed] [Google Scholar]
- [43].Chan DC, Watts GF. The Promise of PCSK9 and Lipoprotein(a) as Targets for Gene Silencing Therapies. Clinical Therapeutics . 2023;45:1034–1046. doi: 10.1016/j.clinthera.2023.07.008. [DOI] [PubMed] [Google Scholar]
- [44].Cohen JC, Boerwinkle E, Mosley TH, Jr, Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. The New England Journal of Medicine . 2006;354:1264–1272. doi: 10.1056/NEJMoa054013. [DOI] [PubMed] [Google Scholar]
- [45].Zhao Z, Tuakli-Wosornu Y, Lagace TA, Kinch L, Grishin NV, Horton JD, et al. Molecular characterization of loss-of-function mutations in PCSK9 and identification of a compound heterozygote. American Journal of Human Genetics . 2006;79:514–523. doi: 10.1086/507488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, et al. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. The New England Journal of Medicine . 2017;376:1713–1722. doi: 10.1056/NEJMoa1615664. [DOI] [PubMed] [Google Scholar]
- [47].Schwartz GG, Steg PG, Szarek M, Bhatt DL, Bittner VA, Diaz R, et al. Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome. The New England Journal of Medicine . 2018;379:2097–2107. doi: 10.1056/NEJMoa1801174. [DOI] [PubMed] [Google Scholar]
- [48].Ding Q, Strong A, Patel KM, Ng SL, Gosis BS, Regan SN, et al. Permanent alteration of PCSK9 with 5RISPR-Cas9 genome editing. Circulation Research . 2014;115:488–492. doi: 10.1161/CIRCRESAHA.115.304351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Ran FA, Cong L, Yan WX, Scott DA, Gootenberg JS, Kriz AJ, et al. In vivo genome editing using Staphylococcus aureus Cas9. Nature . 2015;520:186–191. doi: 10.1038/nature14299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Yin H, Song CQ, Suresh S, Wu Q, Walsh S, Rhym LH, et al. Structure-guided chemical modification of guide RNA enables potent non-viral in vivo genome editing. Nature Biotechnology . 2017;35:1179–1187. doi: 10.1038/nbt.4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Wang X, Raghavan A, Chen T, Qiao L, Zhang Y, Ding Q, et al. CRISPR-Cas9 Targeting of PCSK9 in Human Hepatocytes In Vivo-Brief Report. Arteriosclerosis, Thrombosis, and Vascular Biology . 2016;36:783–786. doi: 10.1161/ATVBAHA.116.307227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Chadwick AC, Wang X, Musunuru K. In Vivo Base Editing of PCSK9 (Proprotein Convertase Subtilisin/Kexin Type 9) as a Therapeutic Alternative to Genome Editing. Arteriosclerosis, Thrombosis, and Vascular Biology . 2017;37:1741–1747. doi: 10.1161/ATVBAHA.117.309881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Levy JM, Yeh WH, Pendse N, Davis JR, Hennessey E, Butcher R, et al. Cytosine and adenine base editing of the brain, liver, retina, heart and skeletal muscle of mice via adeno-associated viruses. Nature Biomedical Engineering . 2020;4:97–110. doi: 10.1038/s41551-019-0501-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Musunuru K, Chadwick AC, Mizoguchi T, Garcia SP, DeNizio JE, Reiss CW, et al. In vivo CRISPR base editing of PCSK9 durably lowers cholesterol in primates. Nature . 2021;593:429–434. doi: 10.1038/s41586-021-03534-y. [DOI] [PubMed] [Google Scholar]
- [55].Wang L, Smith J, Breton C, Clark P, Zhang J, Ying L, et al. Meganuclease targeting of PCSK9 in macaque liver leads to stable reduction in serum cholesterol. Nature Biotechnology . 2018;36:717–725. doi: 10.1038/nbt.4182. [DOI] [PubMed] [Google Scholar]
- [56].Breton C, Furmanak T, Avitto AN, Smith MK, Latshaw C, Yan H, et al. Increasing the Specificity of AAV-Based Gene Editing through Self-Targeting and Short-Promoter Strategies. Molecular Therapy: the Journal of the American Society of Gene Therapy . 2021;29:1047–1056. doi: 10.1016/j.ymthe.2020.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Wang L, Breton C, Warzecha CC, Bell P, Yan H, He Z, et al. Long-term stable reduction of low-density lipoprotein in nonhuman primates following in vivo genome editing of PCSK9. Molecular Therapy: the Journal of the American Society of Gene Therapy . 2021;29:2019–2029. doi: 10.1016/j.ymthe.2021.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Rothgangl T, Dennis MK, Lin PJC, Oka R, Witzigmann D, Villiger L, et al. In vivo adenine base editing of PCSK9 in macaques reduces LDL cholesterol levels. Nature Biotechnology . 2021;39:949–957. doi: 10.1038/s41587-021-00933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Lee RG, Mazzola AM, Braun MC, Platt C, Vafai SB, Kathiresan S, et al. Efficacy and Safety of an Investigational Single-Course CRISPR Base-Editing Therapy Targeting PCSK9 in Nonhuman Primate and Mouse Models. Circulation . 2023;147:242–253. doi: 10.1161/CIRCULATIONAHA.122.062132. [DOI] [PubMed] [Google Scholar]
- [60].Bellinger AM. VERVE-101: CRISPR-Based Gene Editing Therapy Shows Promise in Reducing LDL-C and PCSK9 Levels in Patients With HeFH. 2023. [(Accessed: 21 January 2024)]. Available at: https://www.acc.org/Latest-in-Cardiology/Articles/2023/11/08/20/14/sun-445pm-heart1-aha-2023.
- [61].Akcakaya P, Bobbin ML, Guo JA, Malagon-Lopez J, Clement K, Garcia SP, et al. In vivo CRISPR editing with no detectable genome-wide off-target mutations. Nature . 2018;561:416–419. doi: 10.1038/s41586-018-0500-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Vasileva A, Jessberger R. Precise hit: adeno-associated virus in gene targeting. Nature Reviews. Microbiology . 2005;3:837–847. doi: 10.1038/nrmicro1266. [DOI] [PubMed] [Google Scholar]
- [63].Mingozzi F, High KA. Therapeutic in vivo gene transfer for genetic disease using AAV: progress and challenges. Nature Reviews. Genetics . 2011;12:341–355. doi: 10.1038/nrg2988. [DOI] [PubMed] [Google Scholar]
- [64].Walker HE, Rizzo M, Fras Z, Jug B, Banach M, Penson PE. CRISPR Gene Editing in Lipid Disorders and Atherosclerosis: Mechanisms and Opportunities. Metabolites . 2021;11:857. doi: 10.3390/metabo11120857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Tyumentseva M, Tyumentsev A, Akimkin V. CRISPR/Cas9 Landscape: Current State and Future Perspectives. International Journal of Molecular Sciences . 2023;24:16077. doi: 10.3390/ijms242216077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Gaudelli NM, Lam DK, Rees HA, Solá-Esteves NM, Barrera LA, Born DA, et al. Directed evolution of adenine base editors with increased activity and therapeutic application. Nature Biotechnology . 2020;38:892–900. doi: 10.1038/s41587-020-0491-6. [DOI] [PubMed] [Google Scholar]
- [67].Lewis BS. First-in-human trial of PCSK9 gene editing therapy for lowering cholesterol: a new frontier in cardiovascular pharmacotherapy? News from AHA. European Heart Journal. Cardiovascular Pharmacotherapy . 2024;10:87–88. doi: 10.1093/ehjcvp/pvad095. [DOI] [PubMed] [Google Scholar]
- [68].Musunuru K, Pirruccello JP, Do R, Peloso GM, Guiducci C, Sougnez C, et al. Exome sequencing, ANGPTL3 mutations, and familial combined hypolipidemia. The New England Journal of Medicine . 2010;363:2220–2227. doi: 10.1056/NEJMoa1002926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Romeo S, Yin W, Kozlitina J, Pennacchio LA, Boerwinkle E, Hobbs HH, et al. Rare loss-of-function mutations in ANGPTL family members contribute to plasma triglyceride levels in humans. The Journal of Clinical Investigation . 2009;119:70–79. doi: 10.1172/JCI37118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Reeskamp LF, Millar JS, Wu L, Jansen H, van Harskamp D, Schierbeek H, et al. ANGPTL3 Inhibition With Evinacumab Results in Faster Clearance of IDL and LDL apoB in Patients With Homozygous Familial Hypercholesterolemia-Brief Report. Arteriosclerosis, Thrombosis, and Vascular Biology . 2021;41:1753–1759. doi: 10.1161/ATVBAHA.120.315204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Stefanutti C, Chan DC, Di Giacomo S, Morozzi C, Watts GF. Long-Term Efficacy and Safety of Evinacumab in Patients with Homozygous Familial Hypercholesterolemia: Real-World Clinical Experience. Pharmaceuticals (Basel, Switzerland) . 2022;15:1389. doi: 10.3390/ph15111389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Geladari E, Tsamadia P, Vallianou NG. ANGPTL3 Inhibitors - Their Role in Cardiovascular Disease Through Regulation of Lipid Metabolism. Circulation Journal: Official Journal of the Japanese Circulation Society . 2019;83:267–273. doi: 10.1253/circj.CJ-18-0442. [DOI] [PubMed] [Google Scholar]
- [73].Qiu M, Glass Z, Chen J, Haas M, Jin X, Zhao X, et al. Lipid nanoparticle-mediated codelivery of Cas9 mRNA and single-guide RNA achieves liver-specific in vivo genome editing of Angptl3. Proceedings of the National Academy of Sciences of the United States of America . 2021;118:e2020401118. doi: 10.1073/pnas.2020401118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Kasiewicz LN, Biswas S, Beach A, Ren H, Dutta C, Mazzola AM, et al. GalNAc-Lipid nanoparticles enable non-LDLR dependent hepatic delivery of a CRISPR base editing therapy. Nature Communications . 2023;14:2776. doi: 10.1038/s41467-023-37465-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Chadwick AC, Evitt NH, Lv W, Musunuru K. Reduced blood lipid levels with in vivo CRISPR-Cas9 base editing of ANGPTL3. Circulation . 2018;137:975–977. doi: 10.1161/CIRCULATIONAHA.117.031335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Bergmark BA, Marston NA, Bramson CR, Curto M, Ramos V, Jevne A, et al. Effect of Vupanorsen on Non-High-Density Lipoprotein Cholesterol Levels in Statin-Treated Patients With Elevated Cholesterol: TRANSLATE-TIMI 70. Circulation . 2022;145:1377–1386. doi: 10.1161/CIRCULATIONAHA.122.059266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Pennisi G, Maurotti S, Ciociola E, Jamialahmadi O, Bertolazzi G, Mirarchi A, et al. ANGPTL3 Downregulation Increases Intracellular Lipids by Reducing Energy Utilization. Arteriosclerosis, Thrombosis, and Vascular Biology . 2024;44:1086–1097. doi: 10.1161/ATVBAHA.123.319789. [DOI] [PubMed] [Google Scholar]
- [78].Gong T, Zhou R. ApoC3: an ‘alarmin’ triggering sterile inflammation. Nature Immunology . 2020;21:9–11. doi: 10.1038/s41590-019-0562-3. [DOI] [PubMed] [Google Scholar]
- [79].Saleheen D, Natarajan P, Armean IM, Zhao W, Rasheed A, Khetarpal SA, et al. Human knockouts and phenotypic analysis in a cohort with a high rate of consanguinity. Nature . 2017;544:235–239. doi: 10.1038/nature22034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Guo M, Xu Y, Dong Z, Zhou Z, Cong N, Gao M, et al. Inactivation of ApoC3 by CRISPR/Cas9 Protects Against Atherosclerosis in Hamsters. Circulation Research . 2020;127:1456–1458. doi: 10.1161/CIRCRESAHA.120.317686. [DOI] [PubMed] [Google Scholar]
- [81].Zha Y, Lu Y, Zhang T, Yan K, Zhuang W, Liang J, et al. CRISPR/Cas9-mediated knockout of APOC3 stabilizes plasma lipids and inhibits atherosclerosis in rabbits. Lipids in Health and Disease . 2021;20:180. doi: 10.1186/s12944-021-01605-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Erqou S, Thompson A, Di Angelantonio E, Saleheen D, Kaptoge S, Marcovina S, et al. Apolipoprotein(a) isoforms and the risk of vascular disease: systematic review of 40 studies involving 58,000 participants. Journal of the American College of Cardiology . 2010;55:2160–2167. doi: 10.1016/j.jacc.2009.10.080. [DOI] [PubMed] [Google Scholar]
- [83].Leibundgut G, Scipione C, Yin H, Schneider M, Boffa MB, Green S, et al. Determinants of binding of oxidized phospholipids on apolipoprotein (a) and lipoprotein (a) Journal of Lipid Research . 2013;54:2815–2830. doi: 10.1194/jlr.M040733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Koschinsky ML, Marcovina SM. Structure-function relationships in apolipoprotein(a): insights into lipoprotein(a) assembly and pathogenicity. Current Opinion in Lipidology . 2004;15:167–174. doi: 10.1097/00041433-200404000-00009. [DOI] [PubMed] [Google Scholar]
- [85].Boerwinkle E, Leffert CC, Lin J, Lackner C, Chiesa G, Hobbs HH. Apolipoprotein(a) gene accounts for greater than 90% of the variation in plasma lipoprotein(a) concentrations. The Journal of Clinical Investigation . 1992;90:52–60. doi: 10.1172/JCI115855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Nordestgaard BG, Langsted A. Lipoprotein (a) as a cause of cardiovascular disease: insights from epidemiology, genetics, and biology. Journal of Lipid Research . 2016;57:1953–1975. doi: 10.1194/jlr.R071233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Hughes SD, Rouy D, Navaratnam N, Scott J, Rubin EM. Gene transfer of cytidine deaminase apoBEC-1 lowers lipoprotein(a) in transgenic mice and induces apolipoprotein B editing in rabbits. Human Gene Therapy . 1996;7:39–49. doi: 10.1089/hum.1996.7.1-39. [DOI] [PubMed] [Google Scholar]
- [88].Doerfler AM, Park SH, Assini JM, Youssef A, Saxena L, Yaseen AB, et al. LPA disruption with AAV-CRISPR potently lowers plasma apo(a) in transgenic mouse model: A proof-of-concept study. Molecular Therapy. Methods & Clinical Development . 2022;27:337–351. doi: 10.1016/j.omtm.2022.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Gregory SM, Nazir SA, Metcalf JP. Implications of the innate immune response to adenovirus and adenoviral vectors. Future Virology . 2011;6:357–374. doi: 10.2217/fvl.11.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Muruve DA. The innate immune response to adenovirus vectors. Human Gene Therapy . 2004;15:1157–1166. doi: 10.1089/hum.2004.15.1157. [DOI] [PubMed] [Google Scholar]
- [91].Wang M, Zuris JA, Meng F, Rees H, Sun S, Deng P, et al. Efficient delivery of genome-editing proteins using bioreducible lipid nanoparticles. Proceedings of the National Academy of Sciences of the United States of America . 2016;113:2868–2873. doi: 10.1073/pnas.1520244113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Yin H, Song CQ, Dorkin JR, Zhu LJ, Li Y, Wu Q, et al. Therapeutic genome editing by combined viral and non-viral delivery of CRISPR system components in vivo. Nature Biotechnology . 2016;34:328–333. doi: 10.1038/nbt.3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Chen S, Yao Y, Zhang Y, Fan G. CRISPR system: Discovery, development and off-target detection. Cellular Signalling . 2020;70:109577. doi: 10.1016/j.cellsig.2020.109577. [DOI] [PubMed] [Google Scholar]