Abstract
Amphibian populations are declining globally due to climate change. However, the impacts on the geographic distribution of amphibians on the Qinghai-Tibetan Plateau (QTP), a global biodiversity hotspot with 112 species of amphibians that is sensitive to global climate change, remains unclear. In this study, MaxEnt and barycentre shift analyses were performed to reveal the impact of climate change on the potential future habitats of amphibians on the QTP using the BCC-CSM2-MR global climate model of the Coupled Model Intercomparison Projects Phase 6 (CMIP6) climate pattern with three shared socioeconomic pathways (SSP). In contrast to the widespread decline in the amphibian population, the future scenarios projected an increase in most amphibian habitats on the QTP, accompanied by migration to higher elevations or latitudes under three climatic projections (SSP 1–2.6, 3–7.0, and 5–8.5). Average annual precipitation was the most crucial environmental variable impacting the future distribution of amphibians. The findings indicate that amphibians would flourish under climate change on the QTP, which is of great significance for the protection of amphibians and biodiversity on the QTP.
Keywords: Amphibian, Climate change, Flourishing, Future distribution, The Qinghai-Tibetan Plateau
Highlights
-
•
Amphibians rise to flourishing under climate change on the QTP.
-
•
Amphibians trend to migrate to higher altitudes or latitudes under climate change.
-
•
The average annual precipitation was the most important environmental variable.
1. Introduction
The Intergovernmental Panel on Climate Change has forecasted a potential increase in global average temperature from 1.5 to 5.8 °C throughout the 21st century. This phenomenon is expected to be accompanied by an increased occurrence of aberrant and extreme weather events, including heatwaves, floods, and droughts [1,2]. The significant warming trend in the global climate notably affects the distribution of species. The geographical range of a species is influenced by its ability to withstand harsh environments, limitations in its ability to disperse, and its biological links to other species [3]. Climate change affects species distribution through various mechanisms, including range shifts, alterations in relative abundance within regions, and subtle shifts in the timing and utilisation of microhabitats [[4], [5], [6]]. In the face of climatic shifts, to avoid extinction, species must adapt, migrate, or acclimatise. Therefore, predicting the influence of future climate change on species habitats is of significant importance.
The Qinghai-Tibetan Plateau (QTP), known as the “Third Pole,” “Roof of the World,” and “Water Tower of Asia,” is the highest and most expansive plateau in the world, with an average elevation of over 4000 m [7,8]. Extreme climatic and geographic regions host diverse ecosystems, making the QTP a hotspot for high-altitude biodiversity, including numerous endemic and endangered species [9]. Thus, the QTP has emerged as a global centre for biodiversity research. The QTP is characterised by harsh, cold, and arid climatic conditions, resulting in delicate ecosystems that are susceptible to human activity [10]. The QTP experiences more pronounced effects of global warming than other regions, with significant impacts on its natural systems and biodiversity [11]. Past research has predominantly concentrated on assessing the effects of precipitation and temperature on biodiversity [12,13]. Studies found that increased temperature and precipitation in the QTP since the 1980s [14] have significantly improved biodiversity [11,13].
China is home to approximately 654 amphibian species, with approximately 112 found on the QTP (Table S1), belonging to two orders, nine families, and 24 genera [15], of which the orders Caudata and Anura are particularly abundant. Among them, 52 species are unique to the QTP and six species are listed on the key protected wild animal list of China and designated as second-level nationally protected wildlife [16]. These species included Andrias davidianus (Caudata: Cryptobranchidae), Batrachuperus karlschmidti (Caudata: Hynobiidae), Batrachuperus tibetanus (Caudata: Hynobiidae), Tylototriton verrucosus (Caudata: Salamandridae), Ranodon sibiricus (Caudata: Hynobiidae), and Scutiger chintingensis (Anura: Megophryidae). A total of 68 species are listed on the Red List of Chinese Biodiversity, 67 on the International Union for Conservation of Nature Red List, and 2 on the Convention on International Trade in Endangered Species of Wild Fauna and Flora [[15], [16], [17], [18], [19], [20], [21]]. Habitat degradation or loss, capture, and environmental pollution are the top three endangerment factors for endangered amphibians [17,22].
The biodiversity of the QTP is mostly influenced by the climate [9] and studies have shown that increased precipitation on the QTP greatly triggers biodiversity changes in terms of physiological, ecological, and biogeographic traits [11,23]. Together, these influences lead to considerable susceptibility to alterations in the corresponding environment, spread of diseases, and habitat exploitation of amphibian species [24,25]. This vulnerability is particularly evident under climate change, as climate shifts significantly affect amphibians. According to an international evaluation conducted by the International Union for Conservation of Nature, numerous amphibian populations are facing a decline on a global scale, with over one-third of species being endangered [26]. However, the impact of climate change on the geographic distribution of amphibians in the QTP is unclear. It is necessary to reveal the impact of climate change on the geographic distribution of amphibians in the QTP for amphibian biodiversity conservation.
Species distribution modelling (SDM) is a statistical technique that employs observed data to deduce the ecological needs of species and delineate their possible distribution [27]. SDM is an important tool in basic ecology and biogeography and has been widely used to study the relationship between species distribution and climate in the context of global change [28]. Among the various SDM algorithms, MaxEnt is an exceptionally versatile modelling algorithm that shows enhanced prediction capacity compared to other modelling algorithms, even with smaller sample sizes. It is applicable to presence-only data, simple to operate, and exhibits high predictive accuracy [[29], [30], [31]]. MaxEnt can apply environmental parameters, including land cover, distance, and geographical factors, to perform a detailed assessment of the contribution of each variable to species distribution [32]. Considering the vast expanse of the QTP and the associated challenges in accurately collecting amphibian data, MaxEnt provides substantial advantages over other ecological niche modelling techniques.
This study evaluated the spatial and temporal scales of amphibian habitat distribution in the QTP. MaxEnt was employed to predict the distributions under current climatic conditions and three future scenarios (2041–2060, 2061–2080, and 2081–2100) corresponding to three climatic projections (SSP 1–2.6, SSP 3–7.0, and SSP 5–8.5). For the first time, we studied the possible influence of future climate change on amphibians on the QTP. The study aimed to (1) obtain the potential habitat for amphibians under current climatic conditions on the QTP and analyse the major environmental factors affecting amphibian distribution on the QTP, (2) predict potential distribution areas of amphibians on the QTP under the conditions of future climate change, (3) identify the directional offsets of appropriate habitat for QTP amphibians, and (4) analyse shifts and patterns in future habitats suitable for each family. Our research provides basic data for the investigation and distribution of amphibian germplasm resources on the QTP, which is important for the biodiversity conservation of the species.
2. Material and methods
2.1. Species distribution data
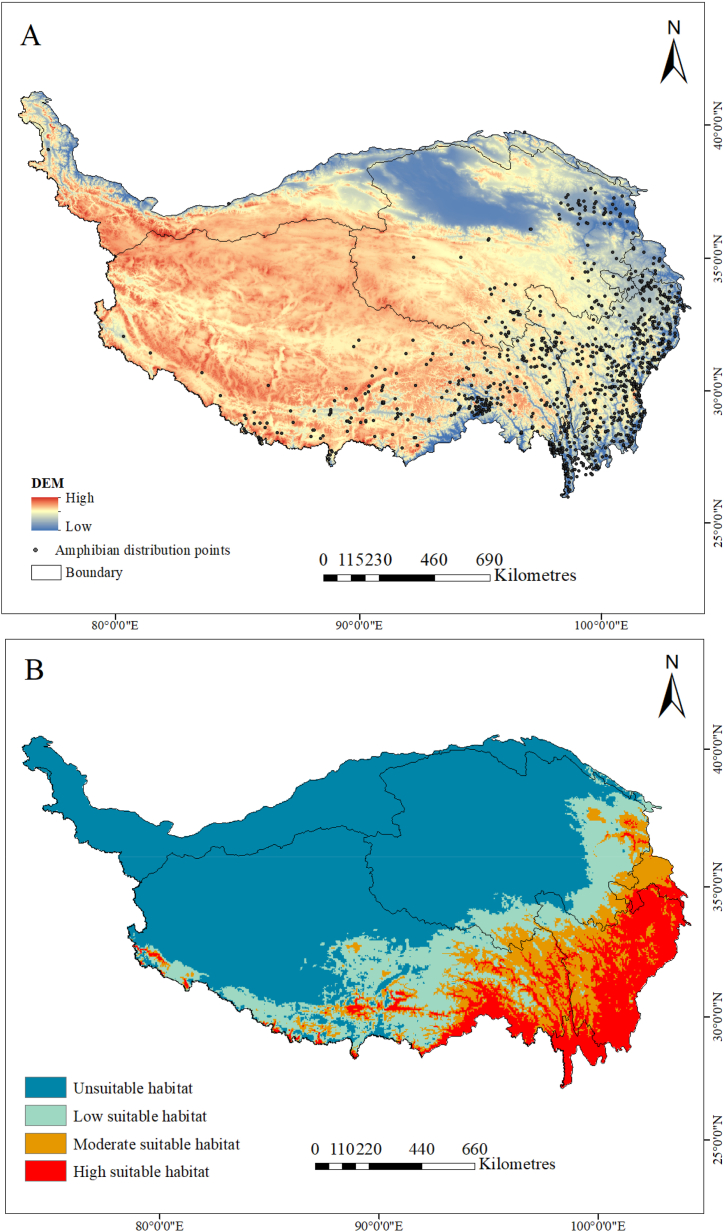
The data collection method was multifaceted and included field surveys, the use of publicly available databases and references to authoritative literature. Data on amphibian distribution on the QTP were collected from four main sources: records from our field investigation (including wild amphibian species, quantities, and distribution coordinates acquired by documenting field surveys on the QTP from 2019), the Global Biodiversity Information Platform (https://www.gbif.org/) to supplement field data (Appendix B), the Chinese amphibian database (https://www.amphibiachina.org/), and inferred from the data were ascertained using Google Maps. In total, 1134 distribution points were recorded. To avoid overfitting the distribution data, ENMtools (Version1.0.4) was employed to remove duplicate, intensive, and invalid data, and 956 effective records were obtained (Fig. 1A) [33].
Fig. 1.
Amphibian distribution points and current potential suitable habitat of whole amphibians on the QTP. Colored Atlas of Chinese Amphibians and Their Distributions. Initially, latitude and longitude data were recorded directly. Subsequently, precise distribution locations.
2.2. Present and future climate
Nineteen bioclimatic factors for the present (1970–2000) and three future scenarios (2041–2060, 2061–2080, 2081–2100) were acquired from the WorldClim Database with a resolution of 2.5° (https://www.worldclim.org/). Table 1 provides a comprehensive overview of the bioclimatic factors used in this study. For future climate change assessments, we employed general circulation model projections of general socioeconomic scenarios produced by CMIP6, which is the sixth phase of The Intergovernmental Panel on Climate Change Model Intercomparison Project [34]. The BCC-CSM2-MR climate system model developed by the National Climate Centre [35] was selected as the general circulation model for this study, containing three future scenarios that represent socioeconomic paths consistent with various models of economic progress and CO2 emissions patterns: SSP 1–2.6, SSP 3–7.0, and SSP 5–8.5 [36]. To prevent potential correlations between climate factors that could affect the predictive accuracy [37], we screened environmental variables with Pearson correlation coefficients less than 0.8 and high contribution values. All climate variables were transformed into ". asc” format and analysed using MaxEnt (V3.4.1) for subsequent analysis.
Table 1.
Climate variables used to model potentially suitable habitat for amphibians on the QTP.
| Code | Environment variable description |
|---|---|
| Bio1 | Annual mean temperature/°C |
| Bio2 | Monthly mean temperature/°C |
| Bio3 | Isothermality |
| Bio4 | Seasonal variation of temperature |
| Bio5 | Maximum temperature in warmest month/°C |
| Bio6 | Minimum temperature in coldest month/°C |
| Bio7 | Annual temperature range/°C |
| Bio8 | Average wettest season temperature/°C |
| Bio9 | Driest quarterly mean temperature/°C |
| Bio10 | Average temperature of the warmest quarter/°C |
| Bio11 | Average temperature of coldest quarter/°C |
| Bio12 | Average annual precipitation/mm |
| Bio13 | Wettest monthly precipitation/mm |
| Bio14 | The driest monthly precipitation/mm |
| Bio15 | Seasonal variation of precipitation |
| Bio16 | Wettest quarterly precipitation/mm |
| Bio17 | Driest quarterly precipitation/mm |
| Bio18 | Precipitation in the warmest quarter/mm |
| Bio19 | Coldest seasonal precipitation/mm |
The National Earth System Science Data Center (https://www.geodata.cn/) provided the QTP boundary, and the QTP climate data were obtained by mass clipping with SDMTools in ArcGIS 10.8 [38].
2.3. Model prediction and evaluation
The potential habitat regions of amphibians in the QTP were predicted using MaxEnt. Occurrence data and climate variables were input into MaxEnt. Additionally, the model predictions were trained using 75 % of the occurrences. Records with a 25 % occurrence were used to test the validity of the model. The cross-validation process was used to verify the duplicates. Furthermore, the background points with maximum count were set to 10,000 by default. The output format was logical [39]. Predictions for each species were duplicated in ten repetitions using MaxEnt. Subsequently, ArcGIS 10.8 was employed to reclassify the data and the Jenks' natural breaks technique was applied for the classification of predicted results of potential habitats using MaxEnt with four levels: 0–0.1 (unsuitable habitat), 0.1–0.3 (low suitable habitat), 0.3–0.5 (moderately suitable habitat), and 0.5–1 (high suitable habitat) [40]. The simulation results were verified mainly using the area under the curve (AUC) values for the receiver operating characteristic curve and true skill statistic (TSS) to evaluate the prediction accuracy of MaxEnt. AUC ranges from 0 to 1 and is classified as bad (0.6–0.7), average (0.7–0.8), well (0.8–0.9), and best (0.9–1), and the higher the value is, the better the prediction effect of the model [41]. TSS ranges from -1–1, with values closer to 1 indicating better prediction performance. Specifically, 0.2–0.5 is bad, 0.6–0.8 is well, and over 0.8 is excellent [42].
2.4. Distribution barycenter migration of amphibians
The research utilised latitude and longitude grid cells to investigate the distribution patterns of barycenter migration of QTP amphibians. Assuming that the study area consisted of n grid cells, the suitable habitat area was calculated using a grid calculator. The SDM toolbox was then employed to simulate the geometric centre position changes of potentially suitable habitats. By comparing the overall migration trends of suitable habitats for amphibians under different scenarios, we revealed the impact of climate change on their distribution. We extracted each centroid's elevation using ArcGIS 10.8 to study the elevation changes.
3. Results
3.1. Accuracy assessment of MaxEnt
The receiver operating characteristic curve provided by MaxEnt revealed an average AUC value of 0.865 in the present and future scenarios (Fig. 2D), with a mean TSS of 0.617. The standard deviation was 0.009 and each family's AUC value was over 0.8 (Fig. 2E), demonstrating a satisfactory prediction result with high reliability.
Fig. 2.
Jackknife tests for influence evaluation of environmental variables on amphibian distribution prediction using training gain (A), test gain (B), and AUC (C). AUC values of MaxEnt for the whole amphibians (D) and AUC bar graph of different families of amphibians on the QTP (E).
3.2. Main environmental factors affecting the distribution
Of the 19 bioclimatic factors, we retained seven environmental variables after screening. The components included temperature factors Bio11(Average temperature of coldest quarter), Bio3(Isothermality), Bio4(Seasonal variation of temperature), Bio6(Minimum temperature in coldest month), and Bio8(Average wettest season temperature) and precipitation factors Bio17(Driest quarterly precipitation) and Bio12(Average annual precipitation). Fig. 2A, B, C and Table 2 demonstrate the results obtained using the jackknife method. It was employed to assess the significance of the chosen bioclimatic variables under current climate conditions to predict the potential distribution areas of the corresponding species. Bio12 manifested the highest quantile in contrast to the remaining variables, followed by Bio11, revealing that the above bioclimatic factors had more significant effects on the distribution of amphibians on the QTP. The variable with the greatest permutation importance was Bio12.
Table 2.
Estimates of relative contributions of the environmental variables to MaxEnt.
| Variable | Percent contribution | Permutation importance |
|---|---|---|
| Bio12 | 63.7 | 57.1 |
| Bio4 | 12.9 | 4.5 |
| Bio11 | 12.7 | 30.9 |
| Bio6 | 5.4 | 1.5 |
| Bio3 | 2.4 | 0.6 |
| Bio17 | 2 | 3.4 |
| Bio8 | 0.9 | 1.9 |
While conducting simulations using a single factor, Bio12 had the highest regularised AUC value, test gain, and training gain, indicating that it possesses the most valuable information. When engaging in simulations with factors other than the specified one, Bio12 exhibited the most substantial reduction in the regularisation of the AUC value, test gain, and training gain. Thus, it may contain information that is absent from the other factors.
3.3. Current suitable habitat prediction
The proportions of the 956 amphibian distribution records in various suitable habitats were as follows: highly suitable habitat (11.13 %), moderately suitable habitat (10.85 %), low-suitability habitat (16.02 %), and unsuitable habitat (62.00 %) (Table 3).
Table 3.
Suitable areas for all amphibians on the QTP in different periods.
| Area ( × 104 km2) | |||||
|---|---|---|---|---|---|
| Time | Scenarios | Highly suitable habitat | Moderately suitable habitat | Low suitable habitat | Unsuitable habitat |
| Current | 27.51042 | 26.80556 | 39.59028 | 153.1858 | |
| 2041–2060 | SSP1-2.6 | 37.43403 | 25.35069 | 39.73959 | 144.5677 |
| SSP3-7.0 | 44.19965 | 25.6441 | 36.51563 | 140.7326 | |
| SSP5-8.5 | 44.2257 | 26.16493 | 35.98264 | 140.7188 | |
| 2061–2080 | SSP1-2.6 | 38.93403 | 25.08333 | 38.22222 | 144.8524 |
| SSP3-7.0 | 50.80382 | 28.07986 | 36.92535 | 131.283 | |
| SSP5-8.5 | 56.89584 | 34.70486 | 44.54514 | 110.9462 | |
| 2081–2100 | SSP1-2.6 | 38.23785 | 24.66667 | 38.5382 | 145.6493 |
| SSP3-7.0 | 58.51563 | 34.69271 | 43.125 | 110.7587 | |
| SSP5-8.5 | 59.11285 | 35.93229 | 43.13715 | 108.9097 | |
The current distribution patterns of potential amphibian habitat areas on the QTP are illustrated in Fig. 1B. The overall suitable habitat covered 93.91 × 104 km2, constituting 38.00 % of the entire study zone. Highly and moderately suitable habitats were mostly located in the eastern and southern regions of the QTP. The regions mentioned were Huangnan and Haidong of Qinghai, Diqing of Yunnan, and the Tibetan regions of Changdu, Shannan, and Linzhi. This included the Qiang Autonomous Prefecture and Aba, along with the Garzê of Sichuan. The southern and central parts of the QTP predominantly hosted unsuitable habitats, encompassing the Haixi Mongolian and Yushu, Golog Tibetan Autonomous Prefecture of Qinghai, and Tibetan regions of Lasa, Naqu, and Shigatse.
The highly suitable habitats of Bufonidae and Salamandridae were located centrally in the southern and eastern regions (Fig. S1). The regions of distribution for the highly suitable habitat of Bufonidae resembled that of Megophryidae but Megophryidae did not inhabit the Hainan Tibetan Autonomous Prefecture or Xining City in Qinghai. Moreover, Bufonidae possessed an obvious expanse of highly and moderately suitable habitats compared with Megophryidae. Notably, among amphibians on the QTP, Salamandridae occupied the largest region, with a highly suitable habitat. The habitats suitable for Ranidae and Dicroglossidae were similar, showing a preference to dwell in the southeastern QTP, including Linzhi of Tibet, Aba, Qiang Autonomous Prefecture and Garzê of Sichuan, Diqing of Yunnan, and Huangnan and Hainan of Qinghai. Highly and moderately suitable habitats of Hynobiidae were uniquely located in the eastern QTP, consisting of Linzhi of Tibet, Diqing of Yunnan, Aba, Qiang Autonomous Prefecture, Garzê, and Liangshan Yi Autonomous Prefecture of Sichuan. Highly and moderately suitable habitats of Rhacophoridae and Ceratobatrachidae were mainly located in the southeastern QTP, including Linzhi in Tibet and Diqing in Yunnan. Ceratobatrachidae had a more suitable habitat than Rhacophoridae. Highly and moderately suitable habitats of Cryptobranchidae were chiefly distributed in the central QTP, including Aba, Qiang Autonomous Prefecture and Garzê of Sichuan, Changdu of Tibet, and Golog and Yushu of Qinghai. Among amphibians, Rhacophoridae possessed the smallest area of Highly and moderately suitable, at 1.33 × 104 and 2 × 104 km2, respectively.
3.4. Future suitable habitat prediction
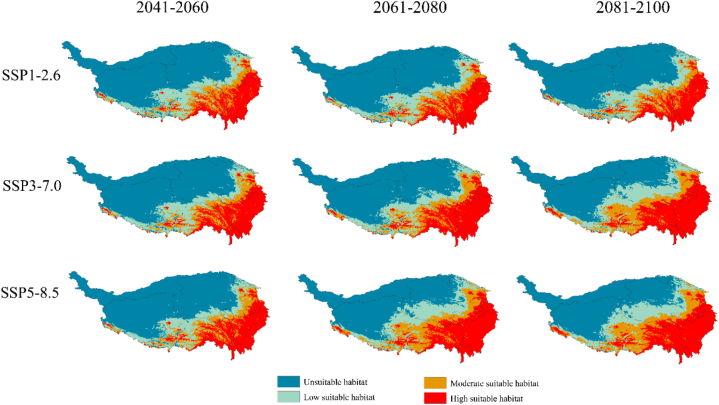
The depiction of potential habitat suitability for amphibians under climate change scenarios SSP 1–2.6, SSP 3–7.0, and SSP 5–8.5 for 2041–2060, 2061–2080, and 2081–2100 are shown in Fig. 3, Fig. 4 and Table 3. For all amphibians, highly suitable habitats would increase in various future scenarios with increased temperatures compared to their current distribution. For 2041–2060, 2061–2080, and 2081–2100, under SSP 1–2.6, the highly suitable habitat increased by 36.07 %, 60.67 %, and 60.76 % compared to the suitable habitat in the current climate scenario, while moderately suitable habitats decreased by 5.43 %, 4.33 %, and 2.39 %, respectively. Under SSP 3–7.0 for 2041–2060, 2061–2080, and 2081–2100, compared to that under the current climate scenario, the highly suitable habitat increased by 41.52 %, 84.67 %, and 106.82 %, respectively, and moderately suitable habitat decreased by 6.43 % in 2041–2060 but increased by 4.75 % and 29.47 % in 2061–2080 and 2081–2100, respectively. Under SSP 5–8.5 for 2041–2060, 2061–2080, and 2081–2100, compared with the suitable habitat for the current climate scenario, the highly suitable habitat increased by 38.99 %, 112.70 %, and 114.87 %, respectively. Meanwhile, the moderately suitable habitat decreased by 7.98 % in 2041–2060, followed by increases of 29.42 % and 34.05 % in 2061–2080 and 2081–2100 respectively.
Fig. 3.
Potential suitable habitat of amphibians on the QTP under future climate scenarios.
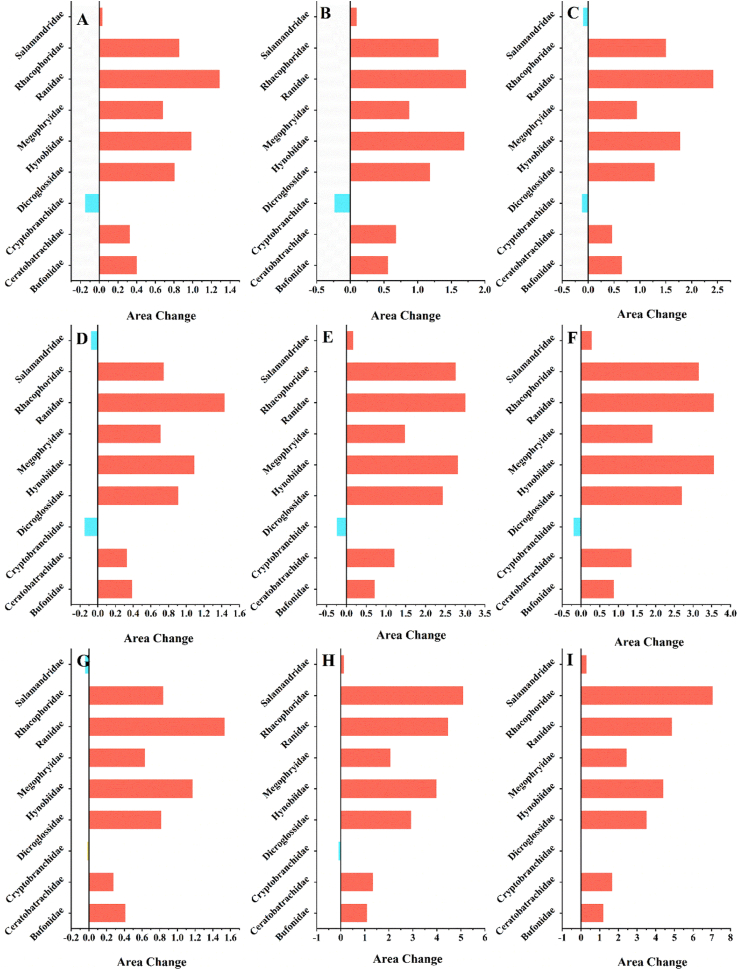
Fig. 4.
High suitable habitat area changes of QTP amphibians under different climate scenarios: (A) SSP 1–2.6 in 2041–2060; (B) SSP3-7.0 in 2041–2060; (C) SSP 5–8.5 in 2041–2060; (D) SSP 1–2.6 in 2061–2080; (E) SSP3-7.0 in 2061–2080; (F) SSP 5–8.5 in 2061–2080; (G) SSP 1–2.6 in 2081–2100; (H) SSP3-7.0 in 2081–2100; (I) SSP 5–8.5 in 2081–2100.
For each amphibian family, the results of future climate model predictions suggested that under various scenarios of global warming, Bufonidae, Ceratobatrachidae, Dicroglossidae, Hynobiidae, Megophryidae, Ranidae, and Rhacophoridae exhibited increasing trends in highly and moderately suitable habitats (Fig. 4A–I, Tables S2, S3, S5–9 and Figs. S3, S4, S6–10). This trend was observed when future climate model predictions were compared with the present results. Among the various amphibian species, Ranidae exhibited the most significant increase in highly and moderately suitable habitats, showing a fluctuation range of 129.11 %–486.73 % and 49.18 %–121.62 %, respectively, and peaked under SSP 5–8.5 for 2081–2100 (Fig. 4I). Furthermore, under SSP 5–8.5 for 2041–2060, habitats suitable for Salamandridae decreased by 9.96 % (Fig. 4C, Fig. S11, Table S10). Under SSP 1–2.6, there was a 7.75 % decrease for 2061–2080 and 4.33 % decrease for 2081–2100 (Fig. 4D–G). However, under other future climate scenarios, these habitats experienced an increase, with the highest rise of 31.30 % for 2081–2100 under SSP 5–8.5 (Fig. 4I). In contrast, habitats for Cryptobranchidae will consistently diminish because of future climate change, with reductions ranging from 1.87 % to 24.53 % (Fig. 4A–I, Fig. S5, Table S4).
3.5. Future migratory trends of amphibians
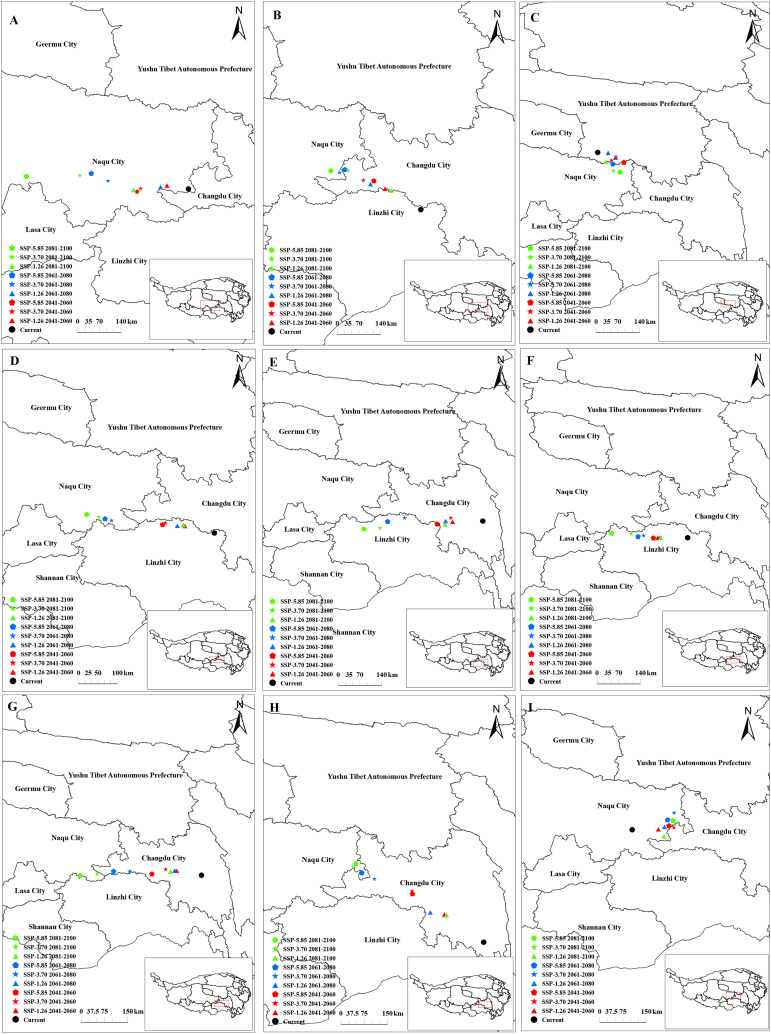
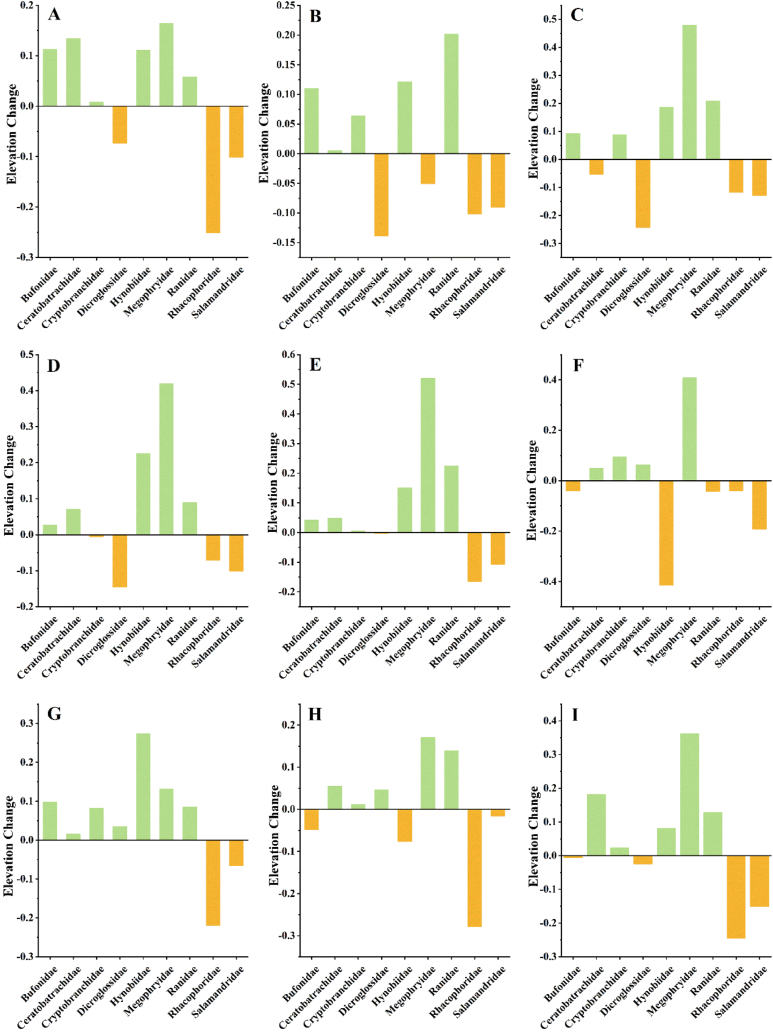
Barycentre findings for amphibians indicated that these species generally tended to migrate northward (Fig. S2). For each amphibian family, the Ceratobatrachidae, Rhacophoridae and Salamandridae tended to move northward in the future climatic scenario (Fig. 5B–H, I). In contrast, Cryptobranchidae mainly migrated southward in future distribution models (Fig. 5C). Furthermore, Bufonidae, Dicroglossidae, and Megophryidae tended to migrate northwest owing to climate change (Fig. 5A–D, F). Hynobiidae and Ranidae tended to migrate west because of climate change (Fig. 5E–G). Moreover, the barycentre elevation for Rhacophoridae and Salamandridae continuously decreased. For the remaining amphibians, the elevation of the barycentres increased or decreased under the future climate scenarios (Fig. 6A–I).
Fig. 5.
Barycenter migration of each family of amphibians in the future climate scenario: (A) Bufonidae; (B) Ceratobatrachidae; (C) Cryptobranchidae; (D) Dicroglossidae; (E) Hynobiidae; (F) Megophryidae; (G) Ranidae; (H) Rhacophoridae; (I) Salamandridae.
Fig. 6.
Elevation change of barycenter for QTP amphibians in the different future climate scenarios: (A) SSP 1–2.6 in 2041–2060; (B) SSP3-7.0 in 2041–2060; (C) SSP 5–8.5 in 2041–2060; (D) SSP 1–2.6 in 2061–2080; I SSP3-7.0 in 2061–2080; (F) SSP 5–8.5 in 2061–2080; (G) SSP 1–2.6 in 2081–2100; (H) SSP3-7.0 in 2081–2100; (I) SSP 5–8.5 in 2081–2100.
4. Discussion
4.1. Model accuracy analysis
MaxEnt predicts the distribution of a species by identifying the distribution based on the maximum entropy model (i.e., it is most geographically uniform) subjected to constraints of environmental conditions at the documented distribution sites. MaxEnt has the advantages of easy operation, high accuracy, and satisfactory results [43]. For all species, the AUC values of the prediction results reached 0.865 (Fig. 2D) and the standard deviation was 0.009; for each family, it was over 0.8 (Fig. 2E) and the mean TSS was 0.617, indicating that the results were satisfactory and had high reliability. Spatial distribution analysis showed that highly and moderately suitable habitats were mainly concentrated in the eastern and southern parts of the QTP, which corresponded well with the known natural distribution points of amphibians in the region. This has important implications for the distribution analysis or prediction of amphibian populations in other regions or globally.
Densely localised distribution points were excluded from the overfitting problem. Although this exclusion method was considered necessary, it may have affected the accuracy of model predictions. To address this limitation, future research efforts should consider expanding detailed field surveys of amphibians. Access to comprehensive natural distribution data can be significantly enhanced by utilising the results of contemporary habitat modelling techniques. Additionally, addressing sampling biases, refining the selection of environmental factors, and optimising MaxEnt are crucial steps for enhancing simulation accuracy. Our study was limited by technical constraints in considering only future climatic factors. Therefore, the accuracy of MaxEnt model predictions requires further validation. Future studies should incorporate a broader range of environmental factors (e.g., water depth, vegetation type and cover, slope, and slope direction), along with biological interactions and the impact of human activities in diverse amphibian habitats. This significantly improved the species prediction model and increased the precision of model predictions.
4.2. Current potential suitable habitat and major climate factors
Under current climatic conditions, highly and moderately suitable habitats for amphibians on the QTP are primarily distributed in the eastern and southern regions. This distribution is influenced by key climatic variables. The seven most critical factors restricting amphibian habitat suitability were Bio11, Bio3, Bio4, Bio8, Bio6, Bio12, and Bio17. These factors align with amphibian preferences for warm, humid climates and their vulnerability to environmental change. However, the current prediction models focus solely on future climatic factors. However, the accuracy of the predictions requires further verification. To enhance the precision of species predictions, it is essential to incorporate additional variables, such as water depth, vegetation type, and cover, and environmental variables, such as slope and aspect, biotic interactions, and human impact. This comprehensive approach can better inform adaptation strategies for climate change conditions.
4.3. Potential distribution change in future climate scenarios
Amphibians are the most endangered vertebrates in the world, with over 30 % of species threatened and the worldwide network of protected regions inadequate for protection [44,45]. Owing to climate change, the distribution areas and patterns of amphibian species on the QTP have changed greatly. Under SSP 1–2.6, SSP 3–7.0, and SSP 5–8.5 in 2041–2060, 2061–2080, and 2081–2100, compared with the current distribution, the increase in highly and moderately suitable habitats for major amphibians in the QTP in various future scenarios suggests that habitats that are currently unsuitable for species distribution would be greatly improved and transformed into suitable habitats. However, this result differed from those of previous studies that found that climate change would lead to habitat loss and population decline [37,46,47], which may be closely related to the increase in annual average precipitation in the future. With increased precipitation under future climates on the QTP, the limitation of water sources for amphibian species is expected to diminish, suggesting that the distribution limitation of amphibian species in unsuitable habitats may decrease.
Our analysis of the current climatic environmental factors affecting amphibians on the QTP revealed that Bio12 (Average annual precipitation) was the most important factor. Consequently, climate change will create more suitable climatic habitats for amphibians living on the QTP and habitats with high species richness will become increasingly available. Notably, suitable habitats for Cryptobranchidae and Salamandridae were reduced. Cryptobranchidae usually inhabit refreshing, swiftly moving, adequately oxygenated, uncontaminated rivers and streams. They require rocks, openings in embankments, crevices in bedrock, or analogous locations for refuge or the construction of their dens. Meanwhile, Salamandridae prefer moist and cool environments to keep their skin moist to facilitate water and air circulation. Liu et al. investigated the effects of elevated water temperatures on the feeding behaviour of A.davidianus [48]. At temperatures of 20 to 22 °C, approximately 35 % of A.davidianus engage in feeding; however, from 26 to 28 °C, the feeding rate decreases significantly to only 10 %. Feeding activity nearly ceases at over 28 °C and death occurs at above 35 °C [49]. Under future global warming scenarios, vulnerability to higher water temperatures may pose a danger to A.davidianus and other Cryptobranchidae species. Similarly, salamanders are also under threat. Another reason is human activity, including reservoir construction, deforestation, mining operations, the use of fertilisers and pesticides, river pollution, and environmental degradation. Cryptobranchidae and Salamandridae habitats have suffered severe degradation. Some species may have already been protected and may have been on the list of endangered species in some areas. In many areas, their resources have been exhausted and they are even on the brink of extinction. However, the exact situation may vary depending on the species and location. Based on further model predictions, it is advisable to consider adjustments to existing nature reserves for species-rich areas. Strategies could include expanding the geographic range or area of current reserves by connecting them with neighbouring reserves, thereby enlarging habitat areas and increasing habitat diversity. Additionally, the establishment of new nature reserves in these regions to protect amphibians should be considered.
4.4. Trend in migration of barycenter
Climate change has altered the habitats of many species, and migration is a natural process of adaptation. The migration trends of the barycentre revealed that in response to climate change, the majority of QTP amphibians tended to migrate towards the northwestern regions. Simultaneously, the majority of QTP amphibians tended to move towards higher elevations to relieve the impacts of climate change. This supports existing research that found that to deal with global warming, various animal species are migrating towards higher elevations or higher latitude regions [50]. In contrast, among amphibians on the QTP, Cryptobranchidae tended to consistently move towards lower latitudes under various climate change scenarios. However, Cryptobranchidae experienced rising barycentre elevations under various climatic conditions. Cryptobranchidae typically inhabit clear streams or caves, where the water temperature must be moderate. To find suitable habitats, they migrate to areas at lower or higher altitudes in the mountains. The warm and humid microclimates of mountain valleys are crucial for the survival of these species. This helps them cope with the threats posed by climate change and reduces the impact of human disturbances. In contrast, Salamandridae and Rhacophoridae tend to migrate to regions with lower elevations but higher latitudes under future climate change. Salamandridae and Rhacophoridae are typically found in temperate climates. With global warming, the migration of these two families to higher or lower latitudes might be advantageous for their survival. Salamandridae and Rhacophoridae require specific environmental conditions for survival and reproduction, including appropriate temperatures, humidity, and ecosystems. Migration to cooler high-latitude areas and warmer low-altitude regions may assist them in finding habitats that are more suitable for the current climatic conditions.
5. Conclusion
The prediction of future climate change impacts on the QTP amphibians using MaxEnt can be applied for their conservation. We revealed that suitable habitats for QTP amphibians were centred in the southern and eastern QTP, including the northwestern areas of the Yunnan and Sichuan Provinces, as well as the eastern regions of Qinghai and Tibet. Bio11, Bio3, Bio4, Bio8, Bio6, Bio12, and Bio17 are crucial factors that shape amphibian habitat suitability. The most important permutation importance was Bio12. Under future scenarios, rising temperatures will generally increase the suitable habitats for amphibians, except for Salamandridae and Cryptobranchidae. Specifically, Dicroglossidae habitats would see a maximum increase of 351.94 % under SSP 5–8.5 for 2081–2100. Although they would increase in other scenarios, peaking at 31.30 % under SSP5-8.5 for 2081–2100, Salamandridae habitats would decrease by 9.96 % under SSP 5–8.5 for 2041–2060 and 7.75 %, and 4.33 % under SSP 1–2.6 scenarios for 2061–2080 and 2081–2100, respectively. In contrast, Cryptobranchidae consistently exhibited reduced habitat suitability owing to climate change. Subsequently, we determined that the suitable habitats for all amphibians, except Cryptobranchidae, Salamandridae, and Rhacophoridae, would migrate to regions with higher elevations and latitudes under future climate change. Our study on the distribution of amphibian habitats is important for biodiversity conservation. This study provides a theoretical reference for understanding the current distribution of this species and lays the foundation for subsequent research on amphibian resources.
Data availability statement
Data included in article/supp. material/referenced in article
Complete ethics statement
Review and/or approval by an ethics committee was not required for this study because it was an amphibian distribution survey instead of an animal experiment.
CRediT authorship contribution statement
Fangfang He: Writing – review & editing, Writing – original draft, Software, Methodology, Investigation, Formal analysis, Conceptualization. Lu Liang: Writing – review & editing, Software, Investigation, Formal analysis. Huichun Wang: Funding acquisition. Aijing Li: Software, Investigation, Data curation. Mencuo La: Writing – review & editing, Methodology, Formal analysis. Yao Wang: Methodology, Investigation. Xiaoting Zhang: Methodology, Investigation. Denglang Zou: Writing – review & editing, Supervision, Project administration, Methodology, Investigation, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This study was financially supported by the National Natural Science Foundation of China (Grants 82304892 and 32260129).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e35860.
Contributor Information
Huichun Wang, Email: whch_66@163.com.
Denglang Zou, Email: dlangzou@foxmail.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Wu X., et al. Impact of climate change on human infectious diseases: empirical evidence and human adaptation. Environ. Int. 2016;86:14–23. doi: 10.1016/j.envint.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 2.Weiskopf S.R., et al. Climate change effects on biodiversity, ecosystems, ecosystem services, and natural resource management in the United States. Sci. Total Environ. 2020;733 doi: 10.1016/j.scitotenv.2020.137782. [DOI] [PubMed] [Google Scholar]
- 3.Pecl G.T., et al. Biodiversity redistribution under climate change: impacts on ecosystems and human well-being. Science. 2017;355(6332):eaai9214. doi: 10.1126/science.aai9214. [DOI] [PubMed] [Google Scholar]
- 4.Peterson A.T., et al. Princeton University Press; Princeton: 2012. Ecological Niches and Geographic Distributions (MPB-49). Ecological Niches and Geographic Distributions (MPB-49) [Google Scholar]
- 5.Bates A.E., et al. Defining and observing stages of climate-mediated range shifts in marine systems. Global Environ. Change. 2014;26:27–38. [Google Scholar]
- 6.Scheffers B.R., et al. The broad footprint of climate change from genes to biomes to people. Science. 2016;354(6313):aaf7671. doi: 10.1126/science.aaf7671. [DOI] [PubMed] [Google Scholar]
- 7.Favre A., et al. The role of the uplift of the Qinghai‐Tibetan Plateau for the evolution of Tibetan biotas. Biol. Rev. 2015;90(1):236–253. doi: 10.1111/brv.12107. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Y., et al. Climate change and human activities altered the diversity and composition of soil microbial community in alpine grasslands of the Qinghai-Tibetan Plateau. Sci. Total Environ. 2016;562:353–363. doi: 10.1016/j.scitotenv.2016.03.221. [DOI] [PubMed] [Google Scholar]
- 9.Sun Y., et al. Effects of the interaction among climate, terrain and human activities on biodiversity on the Qinghai-Tibet Plateau. Sci. Total Environ. 2021;794 doi: 10.1016/j.scitotenv.2021.148497. [DOI] [PubMed] [Google Scholar]
- 10.Li S., et al. Enhancing protected areas for biodiversity and ecosystem services in the Qinghai–Tibet Plateau. Ecosyst. Serv. 2020;43 [Google Scholar]
- 11.Pan T., et al. Contributions of climatic and non-climatic drivers to grassland variations on the Tibetan Plateau. Ecol. Eng. 2017;108:307–317. [Google Scholar]
- 12.Fei S., et al. Impacts of climate on the biodiversity-productivity relationship in natural forests. Nat. Commun. 2018;9(1):5436. doi: 10.1038/s41467-018-07880-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wei Y., et al. Responses of vegetation zones, in the Qinghai-Tibetan Plateau, to climate change and anthropogenic influences over the last 35 years. Pratacult. Sci. 2019;36:1163–1176. [Google Scholar]
- 14.Li H., et al. Greening implication inferred from vegetation dynamics interacted with climate change and human activities over the Southeast Qinghai–Tibet Plateau. Rem. Sens. 2019;11(20):2421. [Google Scholar]
- 15.Liang F., Changyuan Y., Jianping J. Progress and prospects for studies on Chinese amphibians. Asian Herpetol Res. 2010;1:64–85. [Google Scholar]
- 16.Yang C., Anping C., Jingyun F. Geographical distribution patterns of endangered fishes, amphibians, reptiles and mammals and their hotspots in China: a study based on “China Red Data Book of Endangered Animals”. Biodivers. Sci. 2002;10(4):359. [Google Scholar]
- 17.Jiang J., et al. Assessing the threat status of amphibians in China. Biodivers. Sci. 2016;24(5):588. [Google Scholar]
- 18.Jing C., Kai W. AmphibiaChina: an online database of Chinese Amphibians. Zool. Res. 2016;37(1):57. doi: 10.13918/j.issn.2095-8137.2016.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li C., et al. Monitoring and research of amphibians and reptiles diversity in key areas of China. Biodivers. Sci. 2017;25(3):246. [Google Scholar]
- 20.Kai W., et al. The updated checklists of amphibians and reptiles of China. Biodivers. Sci. 2020;28(2):189. [Google Scholar]
- 21.Mi C., et al. Effects of climate and human activity on the current distribution of amphibians in China. Conserv. Biol. 2022;36(6) doi: 10.1111/cobi.13964. [DOI] [PubMed] [Google Scholar]
- 22.Chen Y., et al. Assessing the effectiveness of China's protected areas to conserve current and future amphibian diversity. Divers. Distrib. 2017;23(2):146–157. [Google Scholar]
- 23.Otto M., et al. Assessing vegetation response to precipitation in northwest Morocco during the last decade: an application of MODIS NDVI and high resolution reanalysis data. Theor. Appl. Climatol. 2016;123(1):23–41. [Google Scholar]
- 24.Converse S.J., Grant E.H.C. A three-pipe problem: dealing with complexity to halt amphibian declines. Biol. Conserv. 2019;236:107–114. [Google Scholar]
- 25.Rollins-Smith L.A. Global amphibian declines, disease, and the ongoing battle between Batrachochytrium fungi and the immune system. Herpetologica. 2020;76(2):178–188. [Google Scholar]
- 26.Douglas A.J., Todd L.A., Katzenback B.A. The amphibian invitrome: past, present, and future contributions to our understanding of amphibian immunity. Dev. Comp. Immunol. 2023 doi: 10.1016/j.dci.2023.104644. [DOI] [PubMed] [Google Scholar]
- 27.Bradie J., Leung B. A quantitative synthesis of the importance of variables used in MaxEnt species distribution models. J. Biogeogr. 2016;44(6):1344–1361. [Google Scholar]
- 28.Guo Y., et al. Challenges and development trend of species distribution model. Adv. Earth Sci. 2020;35(12):1292–1305. [Google Scholar]
- 29.Moreno-Amat E., et al. Impact of model complexity on cross-temporal transferability in Maxent species distribution models: an assessment using paleobotanical data. Ecol. Model. 2015;312:308–317. [Google Scholar]
- 30.Yuan H.-S., Wei Y.-L., Wang X.-G. Maxent modeling for predicting the potential distribution of Sanghuang, an important group of medicinal fungi in China. Fungal Ecology. 2015;17:140–145. [Google Scholar]
- 31.Zhang K., et al. Maxent modeling for predicting the potential geographical distribution of two peony species under climate change. Sci. Total Environ. 2018;634:1326–1334. doi: 10.1016/j.scitotenv.2018.04.112. [DOI] [PubMed] [Google Scholar]
- 32.Byeon D.-h., Jung S., Lee W.-H. Review of CLIMEX and MaxEnt for studying species distribution in South Korea. J. Asia Pac. Bus. 2018;11(3):325–333. [Google Scholar]
- 33.Warren D.L., Glor R.E., Turelli M. ENMTools: a toolbox for comparative studies of environmental niche models. Ecography. 2010;33(3):607–611. [Google Scholar]
- 34.Popp A., et al. Land-use futures in the shared socio-economic pathways. Global Environ. Change. 2017;42:331–345. [Google Scholar]
- 35.Xin X. Performance of BCC-CSM2-MR in simulating summer climate changes in East Asia. Geophys. Res. Abstr. 2019;21:4711. [Google Scholar]
- 36.Li B., et al. Threatened birds face new distribution under future climate change on the Qinghai-Tibet Plateau (QTP) Ecol. Indicat. 2023;150 [Google Scholar]
- 37.Pearson R.G., et al. Predicting species distributions from small numbers of occurrence records: a test case using cryptic geckos in Madagascar. J. Biogeogr. 2007;34(1):102–117. [Google Scholar]
- 38.Johnston K., et al. vol. 380. 2001. (Using ArcGIS Geostatistical Analyst). Esri Redlands. [Google Scholar]
- 39.Radosavljevic A., Anderson R.P. Making better Maxent models of species distributions: complexity, overfitting and evaluation. J. Biogeogr. 2014;41(4):629–643. [Google Scholar]
- 40.Ma B., Sun J. Predicting the distribution of stipa purpurea across the Tibetan plateau via the MaxEnt model. BMC Ecol. 2018;18:1–12. doi: 10.1186/s12898-018-0165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao Y., et al. Predicting potential suitable habitats of Chinese fir under current and future climatic scenarios based on Maxent model. Ecol. Inf. 2021;64 [Google Scholar]
- 42.Li S., et al. Predicting the potential suitable distribution area of Emeia pseudosauteri in Zhejiang Province based on the MaxEnt model. Sci. Rep. 2023;13(1):1806. doi: 10.1038/s41598-023-29009-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Phillips S.J., et al. Opening the black box: an open‐source release of Maxent. Ecography. 2017;40(7):887–893. [Google Scholar]
- 44.Ficetola G.F., et al. Habitat availability for amphibians and extinction threat: a global analysis. Divers. Distrib. 2015;21(3):302–311. [Google Scholar]
- 45.Nori J., Villalobos F., Loyola R. Global priority areas for amphibian research. J. Biogeogr. 2018;45(11):2588–2594. [Google Scholar]
- 46.Parmesan C., et al. Poleward shifts in geographical ranges of butterfly species associated with regional warming. Nature. 1999;399(6736):579–583. [Google Scholar]
- 47.Wilson R.J., et al. Changes to the elevational limits and extent of species ranges associated with climate change. Ecol. Lett. 2005;8(11):1138–1146. doi: 10.1111/j.1461-0248.2005.00824.x. [DOI] [PubMed] [Google Scholar]
- 48.Liu J., et al. Research on Chinese giant salamander F2 adaptability and growth advantages. Sichuan J. Zool. 2006;25(2):387–390. [Google Scholar]
- 49.Browne R.K., et al. The giant salamanders (Cryptobranchidae): Part B. Biogeography, ecology and reproduction. Amphib. Reptile Conserv. 2014;5(4):30–50. [Google Scholar]
- 50.Liang Q., et al. Shifts in plant distributions in response to climate warming in a biodiversity hotspot, the Hengduan Mountains. J. Biogeogr. 2018;45(6):1334–1344. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data included in article/supp. material/referenced in article