Abstract
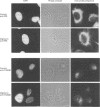
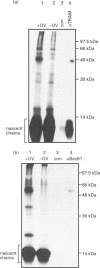
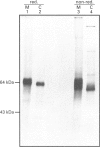
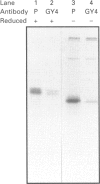
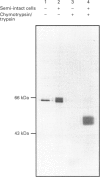
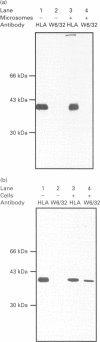
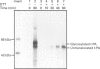
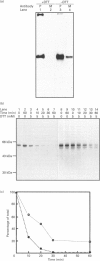
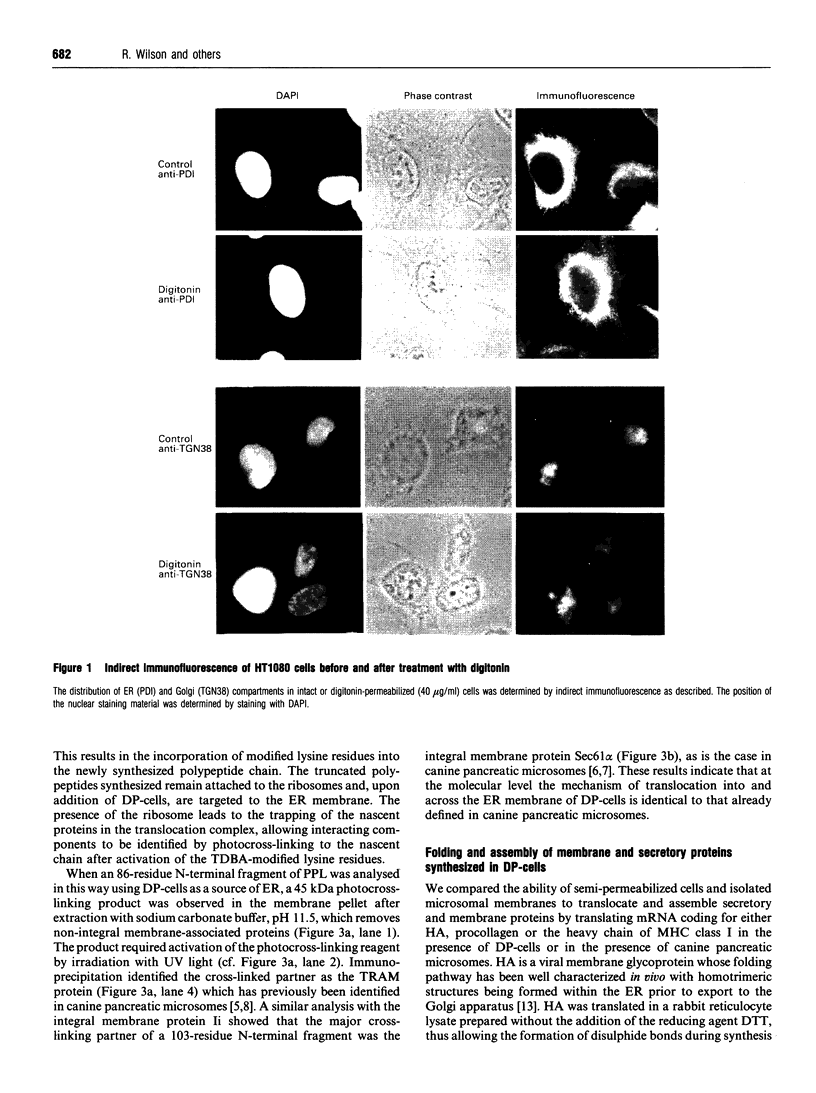
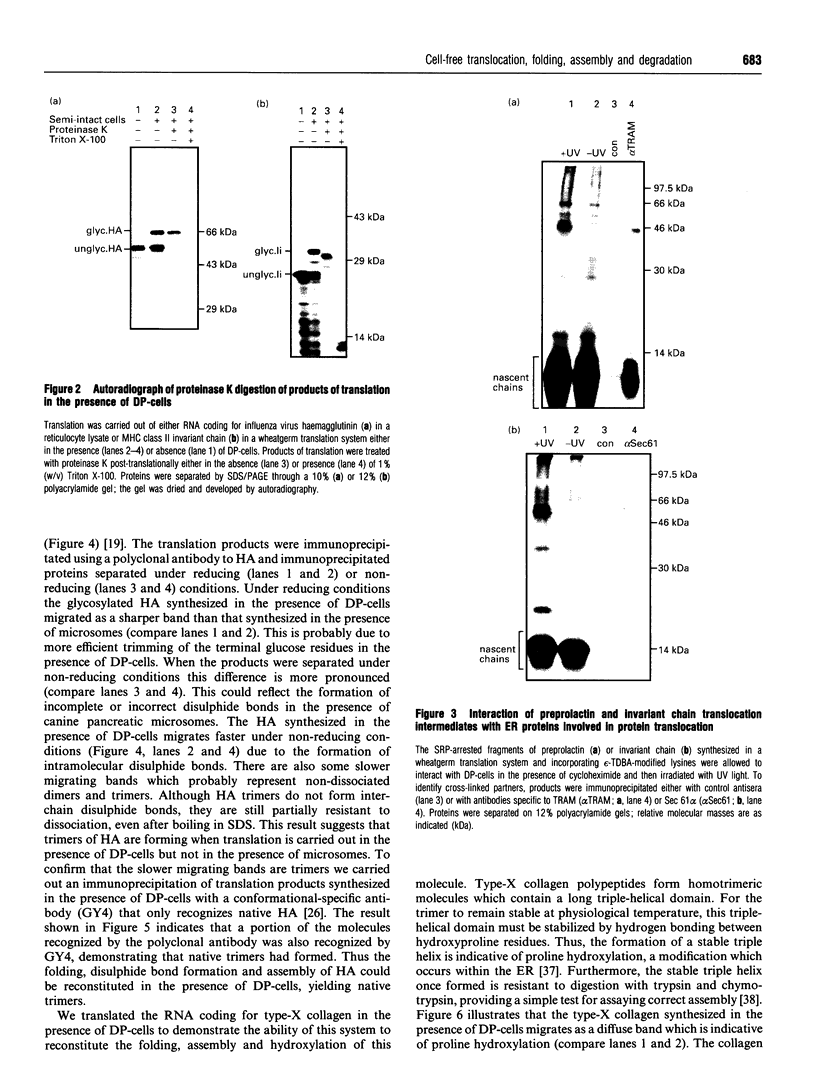
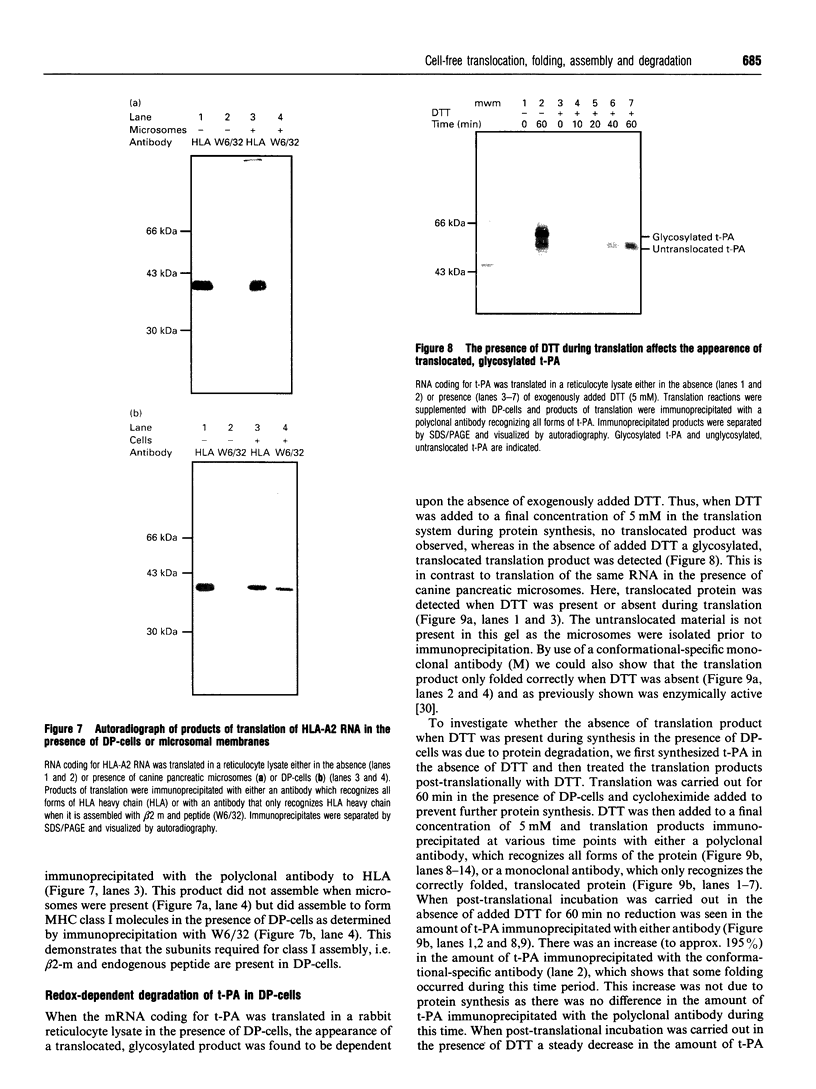
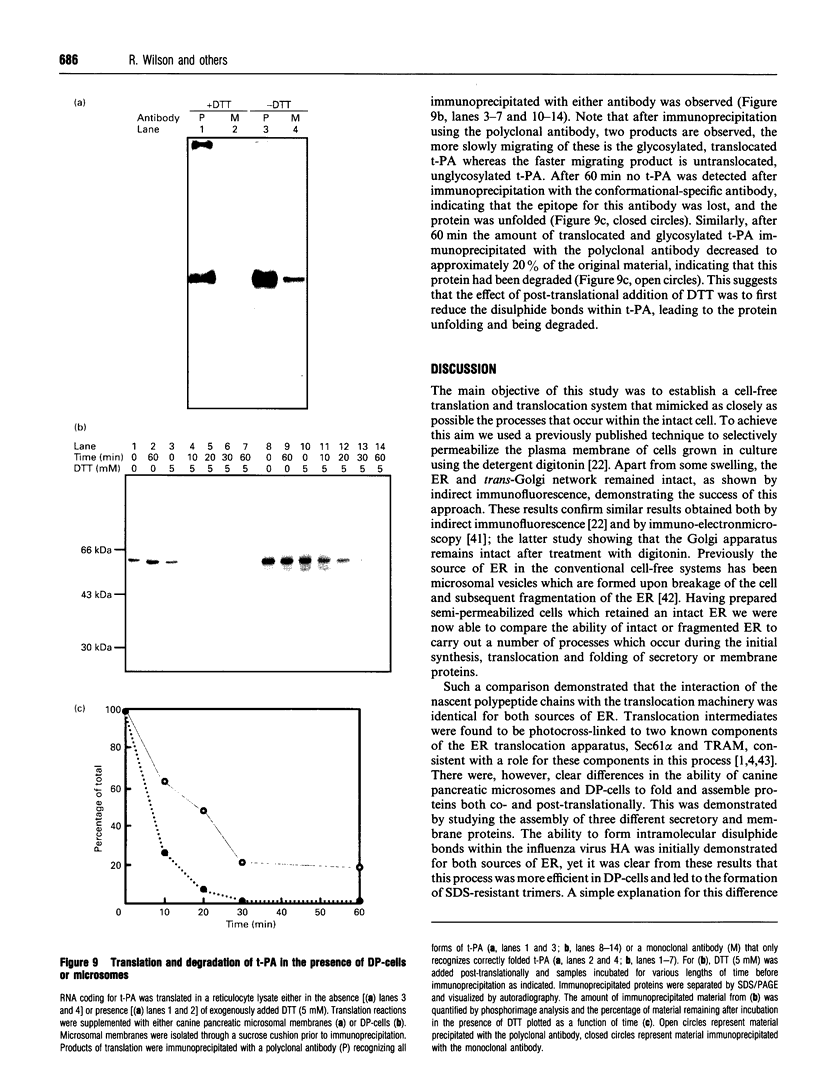
We describe here a semi-permeabilized cell-system which reconstitutes the efficient synthesis, translocation, folding, assembly and degradation of membrane and secretory proteins. Cells grown in culture were treated with the detergent digitonin which selectively permeabilized the plasma membrane leaving the cellular organelles, such as the endoplasmic reticulum (ER) and trans-Golgi network intact. These permeabilized cells were added to an in vitro translation system, either wheatgerm or reticulocyte lysate, supplemented with RNA coding for either membrane or secretory proteins. Efficient translocation and modification of proteins by these cells was demonstrated by protease protection, photocross-linking of nascent chains to components of the translocation apparatus and by post-translational modifications such as glycosylation or hydroxylation. A comparison was made between the ability of semi-permeabilized cells and microsomal vesicles to fold and assemble proteins. The results show that the intact ER within these cells can assemble proteins much more efficiently than vesicularized ER. Furthermore, the semi-permeabilized cells carried out the redox-dependent degradation of tissue-type plasminogen activator. This system has all the advantages of conventional cell-free systems, including speed and, importantly, the ability to manipulate the components of the assay, while retaining intracellular organelles and, therefore, allowing cellular processes to occur as they would in the intact cell.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Austen B. M., Hermon-Taylor J., Kaderbhai M. A., Ridd D. H. Design and synthesis of a consensus signal sequence that inhibits protein translocation into rough microsomal vesicles. Biochem J. 1984 Nov 15;224(1):317–325. doi: 10.1042/bj2240317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balch W. E., McCaffery J. M., Plutner H., Farquhar M. G. Vesicular stomatitis virus glycoprotein is sorted and concentrated during export from the endoplasmic reticulum. Cell. 1994 Mar 11;76(5):841–852. doi: 10.1016/0092-8674(94)90359-x. [DOI] [PubMed] [Google Scholar]
- Barnstable C. J., Bodmer W. F., Brown G., Galfre G., Milstein C., Williams A. F., Ziegler A. Production of monoclonal antibodies to group A erythrocytes, HLA and other human cell surface antigens-new tools for genetic analysis. Cell. 1978 May;14(1):9–20. doi: 10.1016/0092-8674(78)90296-9. [DOI] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braakman I., Helenius J., Helenius A. Manipulating disulfide bond formation and protein folding in the endoplasmic reticulum. EMBO J. 1992 May;11(5):1717–1722. doi: 10.1002/j.1460-2075.1992.tb05223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruckner P., Prockop D. J. Proteolytic enzymes as probes for the triple-helical conformation of procollagen. Anal Biochem. 1981 Jan 15;110(2):360–368. doi: 10.1016/0003-2697(81)90204-9. [DOI] [PubMed] [Google Scholar]
- Bulleid N. J., Bassel-Duby R. S., Freedman R. B., Sambrook J. F., Gething M. J. Cell-free synthesis of enzymically active tissue-type plasminogen activator. Protein folding determines the extent of N-linked glycosylation. Biochem J. 1992 Aug 15;286(Pt 1):275–280. doi: 10.1042/bj2860275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulleid N. J., Freedman R. B. Defective co-translational formation of disulphide bonds in protein disulphide-isomerase-deficient microsomes. Nature. 1988 Oct 13;335(6191):649–651. doi: 10.1038/335649a0. [DOI] [PubMed] [Google Scholar]
- Dottavio-Martin D., Ravel J. M. Radiolabeling of proteins by reductive alkylation with [14C]formaldehyde and sodium cyanoborohydride. Anal Biochem. 1978 Jul 1;87(2):562–565. doi: 10.1016/0003-2697(78)90706-6. [DOI] [PubMed] [Google Scholar]
- Freedman R. B., Bulleid N. J., Hawkins H. C., Paver J. L. Role of protein disulphide-isomerase in the expression of native proteins. Biochem Soc Symp. 1989;55:167–192. [PubMed] [Google Scholar]
- Gething M. J., McCammon K., Sambrook J. Expression of wild-type and mutant forms of influenza hemagglutinin: the role of folding in intracellular transport. Cell. 1986 Sep 12;46(6):939–950. doi: 10.1016/0092-8674(86)90076-0. [DOI] [PubMed] [Google Scholar]
- Gilmore R. Protein translocation across the endoplasmic reticulum: a tunnel with toll booths at entry and exit. Cell. 1993 Nov 19;75(4):589–592. doi: 10.1016/0092-8674(93)90476-7. [DOI] [PubMed] [Google Scholar]
- Gurevich V. V., Pokrovskaya I. D., Obukhova T. A., Zozulya S. A. Preparative in vitro mRNA synthesis using SP6 and T7 RNA polymerases. Anal Biochem. 1991 Jun;195(2):207–213. doi: 10.1016/0003-2697(91)90318-n. [DOI] [PubMed] [Google Scholar]
- Görlich D., Hartmann E., Prehn S., Rapoport T. A. A protein of the endoplasmic reticulum involved early in polypeptide translocation. Nature. 1992 May 7;357(6373):47–52. doi: 10.1038/357047a0. [DOI] [PubMed] [Google Scholar]
- Görlich D., Kurzchalia T. V., Wiedmann M., Rapoport T. A. Probing the molecular environment of translocating polypeptide chains by cross-linking. Methods Cell Biol. 1991;34:241–262. doi: 10.1016/s0091-679x(08)61684-2. [DOI] [PubMed] [Google Scholar]
- Görlich D., Prehn S., Hartmann E., Kalies K. U., Rapoport T. A. A mammalian homolog of SEC61p and SECYp is associated with ribosomes and nascent polypeptides during translocation. Cell. 1992 Oct 30;71(3):489–503. doi: 10.1016/0092-8674(92)90517-g. [DOI] [PubMed] [Google Scholar]
- Görlich D., Rapoport T. A. Protein translocation into proteoliposomes reconstituted from purified components of the endoplasmic reticulum membrane. Cell. 1993 Nov 19;75(4):615–630. doi: 10.1016/0092-8674(93)90483-7. [DOI] [PubMed] [Google Scholar]
- Hartmann E., Sommer T., Prehn S., Görlich D., Jentsch S., Rapoport T. A. Evolutionary conservation of components of the protein translocation complex. Nature. 1994 Feb 17;367(6464):654–657. doi: 10.1038/367654a0. [DOI] [PubMed] [Google Scholar]
- Helenius A., Marquardt T., Braakman I. The endoplasmic reticulum as a protein-folding compartment. Trends Cell Biol. 1992 Aug;2(8):227–231. doi: 10.1016/0962-8924(92)90309-b. [DOI] [PubMed] [Google Scholar]
- High S., Andersen S. S., Görlich D., Hartmann E., Prehn S., Rapoport T. A., Dobberstein B. Sec61p is adjacent to nascent type I and type II signal-anchor proteins during their membrane insertion. J Cell Biol. 1993 May;121(4):743–750. doi: 10.1083/jcb.121.4.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- High S., Görlich D., Wiedmann M., Rapoport T. A., Dobberstein B. The identification of proteins in the proximity of signal-anchor sequences during their targeting to and insertion into the membrane of the ER. J Cell Biol. 1991 Apr;113(1):35–44. doi: 10.1083/jcb.113.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- High S., Martoglio B., Görlich D., Andersen S. S., Ashford A. J., Giner A., Hartmann E., Prehn S., Rapoport T. A., Dobberstein B. Site-specific photocross-linking reveals that Sec61p and TRAM contact different regions of a membrane-inserted signal sequence. J Biol Chem. 1993 Dec 15;268(35):26745–26751. [PubMed] [Google Scholar]
- High S., Stirling C. J. Protein translocation across membranes: common themes in divergent organisms. Trends Cell Biol. 1993 Oct;3(10):335–339. doi: 10.1016/0962-8924(93)90103-8. [DOI] [PubMed] [Google Scholar]
- John D. C., Grant M. E., Bulleid N. J. Cell-free synthesis and assembly of prolyl 4-hydroxylase: the role of the beta-subunit (PDI) in preventing misfolding and aggregation of the alpha-subunit. EMBO J. 1993 Apr;12(4):1587–1595. doi: 10.1002/j.1460-2075.1993.tb05803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungnickel B., Rapoport T. A., Hartmann E. Protein translocation: common themes from bacteria to man. FEBS Lett. 1994 Jun 6;346(1):73–77. doi: 10.1016/0014-5793(94)00367-x. [DOI] [PubMed] [Google Scholar]
- Kivirikko K. I., Myllylä R., Pihlajaniemi T. Protein hydroxylation: prolyl 4-hydroxylase, an enzyme with four cosubstrates and a multifunctional subunit. FASEB J. 1989 Mar;3(5):1609–1617. [PubMed] [Google Scholar]
- Klausner R. D., Sitia R. Protein degradation in the endoplasmic reticulum. Cell. 1990 Aug 24;62(4):611–614. doi: 10.1016/0092-8674(90)90104-m. [DOI] [PubMed] [Google Scholar]
- Li Z., Srivastava P. K. Tumor rejection antigen gp96/grp94 is an ATPase: implications for protein folding and antigen presentation. EMBO J. 1993 Aug;12(8):3143–3151. doi: 10.1002/j.1460-2075.1993.tb05983.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton R. B., Bulleid N. J. Reconstitution of the folding pathway of collagen in a cell-free system: formation of correctly aligned and hydroxylated triple helices. Biochem J. 1993 Dec 1;296(Pt 2):511–517. doi: 10.1042/bj2960511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neefjes J. J., Schumacher T. N., Ploegh H. L. Assembly and intracellular transport of major histocompatibility complex molecules. Curr Opin Cell Biol. 1991 Aug;3(4):601–609. doi: 10.1016/0955-0674(91)90029-x. [DOI] [PubMed] [Google Scholar]
- Ou W. J., Cameron P. H., Thomas D. Y., Bergeron J. J. Association of folding intermediates of glycoproteins with calnexin during protein maturation. Nature. 1993 Aug 26;364(6440):771–776. doi: 10.1038/364771a0. [DOI] [PubMed] [Google Scholar]
- Plutner H., Davidson H. W., Saraste J., Balch W. E. Morphological analysis of protein transport from the ER to Golgi membranes in digitonin-permeabilized cells: role of the P58 containing compartment. J Cell Biol. 1992 Dec;119(5):1097–1116. doi: 10.1083/jcb.119.5.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport T. A. Transport of proteins across the endoplasmic reticulum membrane. Science. 1992 Nov 6;258(5084):931–936. doi: 10.1126/science.1332192. [DOI] [PubMed] [Google Scholar]
- Scheele G., Jacoby R. Conformational changes associated with proteolytic processing of presecretory proteins allow glutathione-catalyzed formation of native disulfide bonds. J Biol Chem. 1982 Oct 25;257(20):12277–12282. [PubMed] [Google Scholar]
- Schleef R. R., Sinha M., Loskutoff D. J. Characterization of two monoclonal antibodies against human tissue-type plasminogen activator. Thromb Haemost. 1985 Apr 22;53(2):170–175. [PubMed] [Google Scholar]
- Schleef R. R., Wagner N. V., Sinha M., Loskutoff D. J. A monoclonal antibody that does not recognize tissue-type plasminogen activator bound to its naturally occurring inhibitor. Thromb Haemost. 1986 Dec 15;56(3):328–332. [PubMed] [Google Scholar]
- Schwaninger R., Plutner H., Bokoch G. M., Balch W. E. Multiple GTP-binding proteins regulate vesicular transport from the ER to Golgi membranes. J Cell Biol. 1992 Dec;119(5):1077–1096. doi: 10.1083/jcb.119.5.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonderfeld-Fresko S., Proia R. L. Synthesis and assembly of a catalytically active lysosomal enzyme, beta-hexosaminidase B, in a cell-free system. J Biol Chem. 1988 Sep 15;263(26):13463–13469. [PubMed] [Google Scholar]
- Stueber D., Ibrahimi I., Cutler D., Dobberstein B., Bujard H. A novel in vitro transcription-translation system: accurate and efficient synthesis of single proteins from cloned DNA sequences. EMBO J. 1984 Dec 20;3(13):3143–3148. doi: 10.1002/j.1460-2075.1984.tb02271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuckwell D. S., Ayad S., Grant M. E., Takigawa M., Humphries M. J. Conformation dependence of integrin-type II collagen binding. Inability of collagen peptides to support alpha 2 beta 1 binding, and mediation of adhesion to denatured collagen by a novel alpha 5 beta 1-fibronectin bridge. J Cell Sci. 1994 Apr;107(Pt 4):993–1005. doi: 10.1242/jcs.107.4.993. [DOI] [PubMed] [Google Scholar]
- Walter P., Blobel G. Signal recognition particle: a ribonucleoprotein required for cotranslational translocation of proteins, isolation and properties. Methods Enzymol. 1983;96:682–691. doi: 10.1016/s0076-6879(83)96057-3. [DOI] [PubMed] [Google Scholar]
- Wilde A., Reaves B., Banting G. Epitope mapping of two isoforms of a trans Golgi network specific integral membrane protein TGN38/41. FEBS Lett. 1992 Nov 30;313(3):235–238. doi: 10.1016/0014-5793(92)81199-v. [DOI] [PubMed] [Google Scholar]
- Yamada A., Brown L. E., Webster R. G. Characterization of H2 influenza virus hemagglutinin with monoclonal antibodies: influence of receptor specificity. Virology. 1984 Oct 30;138(2):276–286. doi: 10.1016/0042-6822(84)90351-9. [DOI] [PubMed] [Google Scholar]
- Young J., Kane L. P., Exley M., Wileman T. Regulation of selective protein degradation in the endoplasmic reticulum by redox potential. J Biol Chem. 1993 Sep 15;268(26):19810–19818. [PubMed] [Google Scholar]
- de Silva A. M., Balch W. E., Helenius A. Quality control in the endoplasmic reticulum: folding and misfolding of vesicular stomatitis virus G protein in cells and in vitro. J Cell Biol. 1990 Sep;111(3):857–866. doi: 10.1083/jcb.111.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]