Abstract
Purpose
To report acute and late bowel, urinary, and sexual dysfunction patient-reported outcome measures, among patients with localized prostate cancer who underwent stereotactic magnetic resonance–guided daily adaptive radiation therapy (SMART).
Methods and Materials
All patients who completed a baseline 12-item Patient-Reported Outcomes Version of the Common Terminology Criteria for Adverse Events questionnaire, before undergoing SMART with 36.25 Gy in 5 fractions, were subsequently followed up with the same graded questionnaire at set time points. Latest prostate-specific antigen levels were recorded. The percentage of patients who reported no change from their baseline adverse event (AE) or reported a new ≥ “frequent or almost constant” or “severe grade or higher” AE grade during follow-up was calculated. The maximum 12-item Patient-Reported Outcomes Version of the Common Terminology Criteria for Adverse Events grade for each item was recorded for each patient. The percentage of toxicity levels for each separate AE item at set time points was calculated.
Results
The total number of patients was 69 with a median follow-up of 27 months. Median age of the cohort was 73 years (range, 54-85 years). The median pretreatment prostate-specific antigen level, T stage, and Gleason score were 7.5 mmol/L (range, 4.5-32 mmol/L), T2b (range, T2-T3b), and 7 (3 + 4; range, 6-9), respectively. No patient had biochemical failure during follow-up. Regarding bowel symptoms, >80% of men reported no change from baseline toxicity during follow-up. New ≥ frequent or almost constant diarrhea was reported in 9% of patients. “Almost constant” diarrhea peaked at 1 month but was absent at >33 months. Regarding urinary symptoms, increased urinary urgency was the most common complaint (39%). Twenty percent of men reported new ≥ frequent or almost constant urinary urgency incidence peaking at 1 month but absent at >33 months. New “severe” sexual dysfunction was seen in 26% of patients and was persistent at >33 months.
Conclusions
Our study is one the largest patient-reported outcomes study after prostate SMART. It shows acceptable levels of toxicity even up to 2 years after treatment.
Introduction
There are approximately 52,300 new prostate cancer cases in the United Kingdom each year,1 accounting for 27% of all UK cancer cases in male patients annually.1 Most patients (78%) with a diagnosis of prostate cancer survive for 10 or more years.1 Against this background, optimizing curative treatments while reducing the burden of long-term treatment-related side effects and loss of function will have an important impact on the quality of life (QoL) of prostate cancer survivors. Over the past decade, stereotactic body radiation therapy (SBRT), a form of high-precision radiation therapy where large daily doses of radiation are delivered in generally 5 or fewer treatments, has emerged as an effective curative treatment option for localized prostate cancer.2, 3, 4, 5, 6 Historically, most prostate SBRT has been planned using computed tomography (CT)–based planning techniques and delivered on linear accelerators (LINACs) reliant on cone beam CT images to guide radiation therapy. Although safe and well tolerated, CT-based prostate SBRT is not without its treatment-related burden with acute and long-term bowel, urinary, and sexual dysfunction reported.5,7, 8, 9
Stereotactic magnetic resonance (MR)–guided daily adaptive radiation therapy (SMART) is a relatively new technique for the delivery of curative treatment for localized prostate cancer.10,11 It brings several advancements to the delivery of ablative radiation therapy. First, by using the enhanced soft tissue definition from high-quality MR imaging (MRI), each individual fraction can be adapted to the patient's presenting onset daily anatomy. Uniquely with the MRIdian MRI LINAC (Viewray), the prostate position is tracked in real-time while coupled with beam gating that prevents treatment delivery if the prostate is outside the treatment boundary,12 negating the need for invasive fiducial markers. Whether this improved accuracy in treatment delivery, which allows smaller margins13 and increased normal tissue sparing of adjacent organs, translates into a meaningful reduction in both acute and late treatment-related symptoms14, 15, 16 is of particular interest to patients and clinicians.
Already there have been results from both prospective databases17 and comparative randomized trials13 that suggest a reduction in acute toxicity with the use of SMART. However, there are limited data available on acute and late patient-reported outcome measures (PROMs) following SMART. PROMs have been shown to integrate the patient perspective into adverse event (AE) reporting and capture QoL outcomes after treatment.18 Several studies have focused on PROMs after CT-based SBRT.19, 20, 21 Further knowledge related specifically to SMART treatment and its relationship to potential QoL improvements will inform patients’ understanding of the treatment side-effect profile and help guide their choice of intervention.
The objective of this study is to report PROMs for gastrointestinal (GI), genitourinary (GU), and sexual function in the acute and late setting among patients who underwent SMART for localized prostate cancer.
Methods and Materials
Patients who underwent SMART for localized prostate cancer at our center between December 2019 to March 2022 and consented to prospective follow-up of their clinical outcomes by means of PROMs were included. All patients gave written consent to participate in the study and for their clinical outcome data to be collected and reported externally.
Inclusion criteria for prostate SBRT were age 18 years or older, World Health Organization performance status 0 to 2, biopsy-proven adenocarcinoma of the prostate, Gleason score 6 or above, prostate volume <90 cc on Transrectal Ultrasound, T stage cT1c to cT3b (on MRI and/or endorectal ultrasound), no evidence of lymph node or distant metastases on radiological staging, International Prostate Symptoms Score <19, minimum 8-week interval from a Transurethral resection of prostate. Exclusion criteria were previous pelvic radiation therapy or any contraindication to having an MRI (presence of non–MRI-compatible implanted cardiac devices, claustrophobia, psychiatric disorders, and metal objects).
Radiation therapy procedures
MR-LINAC simulation and image import/registration
A noncontrast CT and MRI simulation was performed for each patient. Patients were encouraged but not mandated to have a half-full bladder; because it was mandated that all patients undergo daily adaption, bowel preparation was omitted. For each patient, a true fast imaging with steady-state free precession MRI sequence was acquired for planning. The electron density CT scan and MR simulation scan were imported to MIM Maestro planning software (MIM Software Inc) where the subsequent MR-CT fusion was carried out.
Target volume delineation
All contours were peer-reviewed by another site specialist. For low-risk patients, the clinical tumour volume included the prostate only, whereas for intermediate- and high-risk patients (as classified by National Comprehensive Cancer Network),22 the clinical target volume included the prostate plus the base of seminal vesicles (up to 2 cm) plus any visible tumor extension seen on MRI. Planning target volume (PTV) margin was 3 mm in all directions.
Treatment planning
Radiation therapy prescription was 36.25 Gy in 5 fractions on alternative days. Dose was prescribed such that 95% of the volume would receive 100% of the dose, that is, PTV (V95%) = 36.25 Gy. Further detailed overview of the radiation therapy treatment planning process (including dose constraints used), the treatment delivery, and daily adaptive workflow are contained within Appendix E1.
Data collection and analysis (PROMs)
The validated National Cancer Institute's Patient-Reported Outcomes Version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE)18,23 was selected to measure adverse outcomes after SBRT treatment. All patients completed a baseline 12-item PRO-CTCAE questionnaire relating to bowel, urinary, and sexual function (see Appendix E2 for questionnaire) before undergoing SBRT. All were subsequently followed up with the same patient-reported questionnaire at set time points (1, 3, 6, and 12 months after treatment and at the time of study censor—October 2022). Patients had access to the questionnaires via a software application (PROMinet App, GenesisCare UK) that they could download to their phone or could fill it via email or telemedicine. Patients graded their toxicity with the appropriate scales none/never, mild/rarely, moderate/occasionally, severe/frequently, or very severe/almost constantly. Latest prostate-specific antigen levels were recorded. The percentage of patients who reported no change from their baseline symptoms or reported a new ≥ “frequent or almost constant” or “severe grade or higher” AE grade during follow-up was calculated. For individual patients, the maximum AE grade for each PRO-CTCAE item was recorded. The percentage of toxicity levels for each separate AE item was calculated at prespecified timeframes.
Results
Sixty-nine patients, who underwent SMART for localized prostate cancer between December 2019 and March 2022 each completed their PRO-CTCAE questionnaires at baseline; at 1, 3, 6, and 12 months after treatment; and at the time of study censor—October 2022. The median age of patients was 73 years (range, 54-85 years). The median follow-up was 27 months (range, 24-34 months). Sixty-nine patients had a complete PRO-CTCAE data set at 20 to 26, 48 patients at 27 to 32 months, and 31 patients at 33 to 34 months had completed after SBRT. The median T stage was T2 (range, T2-T3b), and median Gleason score was 3 + 4 = 7 (range, 6-8). Pretreatment median prostate-specific antigen level was 7.5 mmol/L (range, 3-22.17 mmol/L). Twenty-nine percent of patients were categorized to be in the high-risk group. All patients underwent 36.25 Gy in 5 fractions with 70% of patients also undergoing a period of androgen deprivation treatment. Table 1 outlines the patient, tumor, and treatment characteristics among the patients included in the study.
Table 1.
Patient, tumor, and treatment characteristics among men who underwent prostate stereotactic magnetic resonance–guided daily adaptive radiation therapy
| Variable | Values |
|---|---|
| Patient characteristics | |
| Age at diagnosis, n (%) | |
| <40 y | 0 (0) |
| 40-49 y | 5 (7) |
| 50-59 y | 5 (7) |
| 60-69 y | 17 (25) |
| 70-79 y | 38 (55) |
| 80+ y | 4 (6) |
| Tumor characteristics | |
| Gleason score, n (%) | |
| 3 + 3 = 6 | 1 (1) |
| 3 + 4 = 7 | 39 (57) |
| 4 + 3 = 7 | 9 (13) |
| 4 + 4 = 8 | 20 (29) |
| Tumor stage, n (%) | |
| T1 | 0 (0) |
| T2 | 56 (81) |
| T3a | 12 (17) |
| T3b | 1 (1) |
| Pretreatment PSA | |
| Median PSA (mmol/L) | 7.5 |
| Treatment characteristics | |
| Radiation therapy, n (%) | |
| SBRT dose | 69 (100) |
| Androgen deprivation therapy, n (%) | |
| Yes | 48 (70) |
| No | 21 (30) |
| Median duration (mo) | 6 |
| All men, n (%) | 69 (100) |
Abbreviations: PSA = prostate-specific antigen; SBRT =stereotactic body radiation therapy.
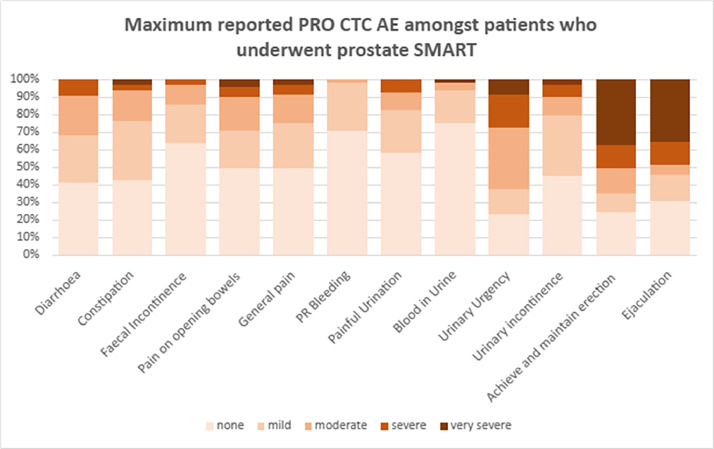
There was no biochemical failure during the follow-up period. Figure 1 displays the proportion of maximum reported PRO-CTCAE grades among the patient cohort during the study time period. Regarding bowel PROMs, >80% of patients reported no increase in the PRO-CTCAE GI categories at any time point after treatment: diarrhea, 84%; constipation, 84%; fecal incontinence, 81%; pain on opening bowels, 84%; general pain, 83%; and GI bleeding, 88%. New patient-reported ≥ frequent or almost constant/severe grade or higher GI AEs were 9% for diarrhea, 3% for constipation, 1% for fecal incontinence, and 6% for pain on opening bowels. Regarding urinary symptoms, urinary urgency was the most common with 39% of patients reporting worse symptoms from baseline during the follow-up period. Nineteen percent of patients reported a change from baseline in painful urination and urinary incontinence. New patient-reported ≥ frequent or almost constant urinary urgency, painful urination, and urinary incontinence were reported at 20%, 3%, and 7%, respectively.
Figure 1.
Maximum Patient-Reported Outcomes Version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE) grades among patients who underwent prostate stereotactic magnetic resonance–guided daily adaptive radiation therapy (SMART).
The peak of new ≥ frequent or almost constant urinary urgency was at 1 month which subsequently settled with no constant urinary urgency reported after 12 months. After 27 months there was a slight flare of urinary urgency with 8% of patients reporting frequent urinary urgency but this flare was absent at >33 months (Fig. 2A). “Almost constant” diarrhea peaked at 1 month but was absent at 3 months, reappearing at 27 months along with a second flare of frequent diarrhea at 20 to 26 months, but both grades were no longer present at >33 months (Fig. 2B). Patient reporting of almost constant incontinence was 3% between 3 and 12 months, but it was no longer reported at >13 months (Fig. 3A). New “severe” sexual dysfunction in the form of erectile dysfunction or problems with ejaculation was 25% and 26%, respectively (Table 2), and remained at >33 months (Fig. 3B).
Figure 2.
(A) Longitudinal display of percentage Patient-Reported Outcomes Version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE) grade levels reported for urinary urgency at different time points after prostate stereotactic magnetic resonance–guided daily adaptive radiation therapy (SMART). (B) Longitudinal display of percentage PRO-CTCAE grade levels reported for diarrhea at different time points after prostate SMART.
Figure 3.
(A) Longitudinal display of percentage Patient-Reported Outcomes v) of the Common Terminology Criteria for Adverse Events (PRO-CTCAE) grade levels reported for urinary incontinence at different time points after prostate stereotactic magnetic resonance–guided daily adaptive radiation therapy (SMART). (B) Longitudinal display of percentage PRO-CTCAE grade levels reported for ejaculation at different time points after prostate SMART.
Table 2.
Percentage of patients who had no change from baseline or a new ≥ frequent or almost constant/severe grade or higher toxicity at any time point
| No increase in AE grade from baseline |
New ≥ frequent or almost constant/severe grade or higher AE reported at any time point |
|||
|---|---|---|---|---|
| PRO-CTCAE | No. of patients | % | No. of patients | % |
| Diarrhea | 58 | 84% | 6 | 9% |
| Constipation | 58 | 84% | 2 | 3% |
| Fecal incontinence | 56 | 81% | 1 | 1% |
| Pain on opening bowels | 58 | 84% | 4 | 6% |
| General pain | 57 | 83% | 4 | 6% |
| GI bleeding | 61 | 88% | 0 | 0% |
| Painful urination | 56 | 81% | 2 | 3% |
| Blood in urine | 66 | 96% | 0 | 0% |
| Urinary urgency | 42 | 61% | 14 | 20% |
| Urinary incontinence | 56 | 81% | 5 | 7% |
| Achieve and maintain erection | 47 | 68% | 17 | 25% |
| Ejaculation | 46 | 67% | 18 | 26% |
Abbreviations: AE = adverse event; GI = gastrointestinal; PRO-CTCAE = Patient-Reported Outcomes Version of the Common Terminology Criteria for Adverse Events.
Table 3 compares PRO-CTCAE GU and sexual function PROMs in patients who underwent adjuvant 6 months of androgen deprivation therapy (ADT). The percentage of patients not on ADT who reported no change from their baseline erectile dysfunction at any time point was 76% compared with 65% on ADT. New ≥ severe grade or higher change in erectile function was 31% in patients on ADT compared with 10% in patients not on ADT.
Table 3.
Comparison of percentage of patients on androgen deprivation therapy versus no androgen deprivation therapy who had no change from baseline or a new ≥ frequent or almost constant/severe grade or higher genitourinary toxicity at any time point
| Patients on androgen deprivation therapy (n = 48) |
Patients not on androgen deprivation therapy (n = 21) |
|||||||
|---|---|---|---|---|---|---|---|---|
| No increase in AE grade from baseline |
New ≥ frequent or almost constant/severe grade or higher AE reported at any time point |
No increase in AE grade from baseline |
New ≥ frequent or almost constant/severe grade or higher AE reported at any time point |
|||||
| PRO-CTCAE | No. of patients | % | No. of patients | % | No. patients | % | No. of patients | % |
| Painful urination | 39 | 81% | 2 | 4% | 17 | 81% | 0 | 0% |
| Blood in urine | 47 | 97% | 0 | 0% | 19 | 90% | 0 | 0% |
| Urinary urgency | 30 | 63% | 10 | 21% | 12 | 57% | 4 | 19% |
| Urinary incontinence | 38 | 79% | 4 | 8% | 18 | 85% | 1 | 5% |
| Achieve and maintain erection | 31 | 65% | 15 | 31% | 16 | 76% | 2 | 10% |
| Ejaculation | 31 | 65% | 14 | 29% | 15 | 71% | 4 | 19% |
Abbreviations: AE = adverse event; PRO-CTCAE = Patient-Reported Outcomes Version of the Common Terminology Criteria for Adverse Events.
Discussion
The radiation oncology community is constantly striving to improve the precision and accuracy of radiation therapy. Prostate cancer accounts for 25% of all new cancer cases in male patients each year in the United Kingdom,1 making it an important target for technological innovation and advances that will reduce treatment-related toxicity, improve overall QoL, and facilitate curative treatment.
Over the past decade, clinical trial data have emerged demonstrating SBRT to be a curative option for localized prostate cancer.2, 3, 4, 5 Traditionally, prostate SBRT is delivered using LINACs dependent on cone beam CT–guided radiation therapy with the invasive insertion of prostate fiducial markers. With CT-based SBRT, both biochemical control3,20 and toxicity are comparable to conventional fractionated radiation therapy.20,24, 25, 26 For example, the Prostate Advances in Comparitive Evidence (PACE-B) trial, which compared intensity modulated fractionated radiation therapy with 62 Gy in 20 fractions (n = 441) with prostate SBRT with 36.25 Gy in 5 fractions (n = 433), showed a grade >2 GI toxicity of 12% in the conventional fractionation arm versus 10% in the SBRT arm.3 However, other trials have suggested that CT-based SBRT is not without its toxicity. In particular, the Hypofractionated radiotherapy of intermediate risk localised prostate cancer (HYPO-RT-PC) trial demonstrated a more pronounced early side-effect profile with SBRT compared with conventional fractionation.2 Indeed, although generally well tolerated, both the acute and late toxic effects of SBRT, namely, bowel, urinary, and sexual dysfunction, can contribute to the treatment-related burden and reduced QoL.6,9 These toxicities and their impact on posttreatment QoL are important considerations for patients when selecting an intervention. The relationship between these toxicities and high-dose radiation to the surrounding bladder, rectum, and surrounding structures has been linked in several studies.14, 15, 16 There is therefore considerable scope for precision radiation therapy to improve accuracy, increase normal tissue sparing, and ultimately improve QoL outcomes of patients with prostate cancer.
SMART has emerged as a promising advancement in precision radiation therapy and is now commercially available.10,11 In this study, we have reported the first 1-month, 3-month, 6-month, 12-month, and most recent follow-up (median follow-up, 27 months) PROM results for patients treated with prostate SMART dose fractionation of 7.25 Gy × 5 fractions on a 0.3 T MRIdian. At our center, it was mandated that all patients undergo daily adaption for each fraction, and of note, bowel preparation was not part of our simulation or treatment protocol. These results add to the reassurance that SMART prostate SBRT remains very tolerable with a low burden of treatment-related side effects even in the late setting. The PROM data we present here are also concordant with the literature. The Multi-outcome evaluation of radiation therapy using MR-Linac (MOMENTUM) study reported GI and GU PROM outcomes from an international registry following prostate patients treated on a 1.5 T MR-LINAC.17 In their study, the results of the Quality of life Prostate Related (QLQ-PR) GI and GU domains showed that there was only a small change in the effect size from baseline and at 3, 6, and 12 months after treatment. This is also consistent with the PROM results from the study by Bruynzeel et al27 in which there was minimal change in QLQ-PR GU and GI domains in a 101-patient cohort followed up for 1 year after treatment. The MOMENTUM study did report a significant increase in the cumulative GI and GU toxicity at 3 months. However, they recorded the highest CTCAE grade of toxicity between 0 and 3 months at the 3-month mark only. Our data suggest that the peak incidence of severe GI and GU toxicity is at 1 month with subsequent recovery of the more burdensome toxicity grades with time. This peak at 1 month was also seen in the overall urinary bother and bowel domain PROM reporting in the PACE-B trial and by another retrospective PROM study after SBRT by Bhattasali et al.8 This knowledge should inform patients’ expectations of the timeframe of treatment bother after completion of prostate SBRT.
Overall, our PROM toxicity levels were low when compared to toxicity reported from other alternative radiation modalities. Eighty percent of patients reported no change in bowel symptoms from baseline at any stage during follow-up. However, in the domain of urinary urgency, 39% of patients did report some worsening of symptoms from baseline with frequent to almost constant new urinary urgency reported at a 20% incidence. This compares favorably to the 30% to 39% incidence of moderate-to-severe urinary toxicity reported after external beam radiation therapy and brachytherapy.28 In comparison with brachytherapy, we did not find a secondary urinary bother flare at 12 months after SMART SBRT,29, 30, 31 but found a slight flare of frequent urinary urgency at 24 months. However, unlike brachytherapy, urinary toxicity was transient and did not persist as a late sequelae of treatment.29, 30, 31 Our reported bowel toxicity was similar to our urinary toxicity patterns. New “frequent” to “almost constant” diarrhea incidence was reported at 9%; fecal incontinence at 1%; pain on opening bowels at 6%; and constipation at 3%, which also compares favorably with the rates of 15% to 16% incidence of moderate-to-severe bowel toxicity reported with other radiation therapy modalities.28 Similar to Bhattasali et al,8 we too saw a slight increase in bowel bother in the late stage, with frequent diarrhea increasing to 14% at 20 to 26 months compared with 6% at baseline. This flare was transient with diarrhea toxicity returning to similar baseline rates after the 27-month follow-up. We report a low incidence of urinary incontinence with patient reporting of new ≥ frequent or almost constant urinary incontinence at 7% which was transient and no longer reported after 13 months. This dip in urinary functional control seems to correspond to the time period when urinary urgency too was an issue for patients. Patient reporting of severe sexual dysfunction was 26% with reporting of dysfunction being persistent at the year mark. Unlike the PACE-B trial where ADT was not permitted, our study's patient cohort had ADT use of >70%, which almost certainly contributed to PROM reporting of sexual dysfunction.
Whether MRI LINAC prostate SBRT is clinically superior to conventional-based CT SBRT has now been formally tested in the context of a recently randomized clinical trial. The Magnetic Resonance Image-Guided Stereotactic Body Radiotherapy for Prostate Cancer (MIRAGE) phase 3 trial has compared 40 Gy in 5 fractions delivered in a 1:1 randomized CT- or MR-guided SBRT (nonadaptive) with acute GU, GI clinician-reported, and Expanded Prostate Cancer Index Composite-26 (EPIC-26) PROMs as endpoints.13 Similar to our patient cohort, their study included 25% of patients in the high-risk prostate cancer category in the MRI arm with 62% of patients also undergoing ADT. This trial also used the opportunity to test smaller PTV margins with MRI-guided technology, namely, 2 mm for patients in the MRI arm and 4 mm in the CT arm. They found that the incidence of acute grade 2 or greater GU toxicity was significantly lower with MRI versus CT guidance (24.4% vs 43.4%; P = .01), as was the incidence of acute grade 2 or greater GI toxicity (0% vs 10.5%). PROM assessments also favored MRI guidance, with there being a significantly reduced percentage of patients with a ≥12-point decrease in EPIC-26 bowel scores at 1 month. Interestingly, in the context of this publication, there was a slight discordance between physician-scored toxicity and the PROM assessments. Clinician assessment reported 0% grade ≥2 GI toxicity, whereas in GI domains of EPIC-26, 30.6% of patients reported a clinically relevant decrement in the bowel domain in the MRI group. This does suggest a potential physician bias in toxicity reporting not seen with PROM techniques of toxic effects assessment.
Our study has several strengths. With SMART being a relatively new technique in the delivery of ablative radiation therapy for localized prostate cancer, most treatment-related toxicity reporting has been in the acute setting. At present, there are limited mature data on late effects after SMART in addition to limited data on PROMs after this relatively new treatment modality. Our study is one of the largest PROM studies to date after prostate SMART and has late effects reporting of up to 2 years after treatment. Our choice of using PROMs over physician reporting offers the benefit of integrating the patient's perspective into AE reporting. Our study provides further knowledge on both the acute and late QoL outcomes after prostate SMART that could facilitate better communication between patients and physicians when deciding the best choice of intervention for localized prostate cancer. Sexual dysfunction specifically is known to be a self-reported major concern for both patients32 and their partners.33, 34, 35 Collection of real-world data on sexual dysfunction can be challenging, with compliance with sexual function questionnaires being a barrier to outcome reporting. For example, in the study conducted by Bruynzeel et al,27 only 33% of patients completed the questions related to sexual function in contrast to our study where all 69 patients had completed a most recent sexual dysfunction questionnaire at the time of the study censor. Compliance with sexual function patient reporting may have been increased with the use of multiple options for patient engagement—mobile application, email, and telemedicine. Our study should help inform a better understanding of risk of sexual dysfunction prior to choosing treatment.
There are some limitations to our study. Our data are from a single center with modest numbers; however, this is in the context of SMART being a novel treatment with limited availability. Although the benefits of using patient-reported outcomes and PROMs to guide real-time patient care are well established,18,36,37 they have not been adopted as the gold standard primary endpoint for most clinical trials. We used the validated National Cancer Institute's PRO-CTCAE questionnaire18 for our study, but at present, there is no consensus on the optimal PROM questionnaire, with the EPIC-26 and Quality of life questionaire prostate related (25 questions) (QLQ-PR25) questionnaires also commonly used. This additional heterogeneity with PROMs brings challenges to the direct comparison of PROM results across different studies. Unlike the PACE-B study, our patient cohort included 29% of patients with high-risk prostate cancer with 70% of patients also using ADT during the follow-up period. With the known detrimental effects of ADT on QoL,38 its high levels of use may have added to the treatment-related burden and potentially masked some of the toxicity effect benefit of MRI guidance. Of note, our center, unlike the MIRAGE trialist, mandated the use of daily adaption for every fraction of SMART, with an optimized plan delivered for each fraction. The quantitative dosimetric benefit of this has not been included in the scope of this study. However, beyond the guiding principle of “as low as reasonably achievable.” the quantitative dosimetric and clinical benefits of resource-heavy daily adaption is an important future question.
As MRI LINAC technology continues to evolve, it will become increasingly important for the oncology community to place a value on its benefit. This will be aided by data maturing from the MIRAGE trial as well as further studies such as ours reporting on clinical outcomes. In addition, further improvements in cost efficiencies will make implementation of this technology more attractive. Currently, the dosimetric advantages of the technology are being used to investigate whether further hypofractionation is feasible—the Randomized trial of five or two MRI-Guided Adaptive Radiotherapy Treatments for Prostate Cancer (FORT) trialists are investigating the safety SMART SBRT treatment with 25 Gy in 2 fractions for localized prostate cancer with PROMs being their primary endpoint.39 Such short curative treatment courses could in the future represent a paradigm shift in the management of localized prostate cancer.
Conclusions
Our study is one of the largest patient-reported outcome studies after stereotactic MR-guided adaptive radiation therapy for localized prostate cancer. At up to 2 years after follow-up, the treatment is well tolerated with treatment-related burden remaining low. Future clinical trials focusing on PROM outcomes from SMART prostate radiation therapy will be important to confirm our findings.
Disclosures
We declare that none of the authors have competing interests.
Acknowledgments
We thank our patients who took part in this study. We also thank the radiographers and research support staff at GenesisCare UK.
Footnotes
Sources of support: This work had no specific funding.
Research data are stored in an institutional repository and will be shared upon request to the corresponding author.
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.adro.2024.101574.
Appendix. Supplementary materials
References
- 1.Cancer Research UK. Prostate cancer statistics. Accessed June 14, 2023.https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/prostate-cancer
- 2.Widmark A, Gunnlaugsson A, Beckman L, et al. Ultra-hypofractionated versus conventionally fractionated radiotherapy for prostate cancer: 5-year outcomes of the HYPO-RT-PC randomised, non-inferiority, phase 3 trial. Lancet. 2019;394:385–395. doi: 10.1016/S0140-6736(19)31131-6. [DOI] [PubMed] [Google Scholar]
- 3.Brand DH, Tree AC, Ostler P, et al. Intensity-modulated fractionated radiotherapy versus stereotactic body radiotherapy for prostate cancer (PACE-B): Acute toxicity findings from an international, randomised, open-label, phase 3, non-inferiority trial. Lancet Oncol. 2019;20:1531–1543. doi: 10.1016/S1470-2045(19)30569-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Dams R, Jiang NY, Fuller DB, et al. Stereotactic body radiotherapy for high-risk localized carcinoma of the prostate (SHARP) consortium: Analysis of 344 prospectively treated patients. Int J Radiat Oncol Biol Phys. 2021;110:731–737. doi: 10.1016/j.ijrobp.2021.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kishan AU, Dang A, Katz AJ, et al. Long-term outcomes of stereotactic body radiotherapy for low-risk and intermediate-risk prostate cancer. JAMA Netw Open. 2019;2 doi: 10.1001/jamanetworkopen.2018.8006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schaeffer E, Srinivas S, Antonarakis ES, et al. NCCN guidelines insights: Prostate Cancer, version 1.2021. J Natl Compr Canc Netw. 2021;19:134–143. doi: 10.6004/jnccn.2021.0008. [DOI] [PubMed] [Google Scholar]
- 7.Van As NJ, Tree A, Ostler PJ, et al. PACE-A: An international phase 3 randomised controlled trial (RCT) comparing stereotactic body radiotherapy (SBRT) to surgery for localised prostate cancer (LPCa)—Primary endpoint analysis. JCO. 2023;41 298-298. [Google Scholar]
- 8.Bhattasali O, Chen LN, Woo J, et al. Patient-reported outcomes following stereotactic body radiation therapy for clinically localized prostate cancer. Radiat Oncol. 2014;9:52. doi: 10.1186/1748-717X-9-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Donovan JL, Hamdy FC, Lane JA, et al. Patient-reported outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N Engl J Med. 2016;375:1425–1437. doi: 10.1056/NEJMoa1606221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pathmanathan AU, van As NJ, Kerkmeijer LGW, et al. Magnetic resonance imaging-guided adaptive radiation therapy: A “game changer” for prostate treatment? Int J Radiat Oncol Biol Phys. 2018;100:361–373. doi: 10.1016/j.ijrobp.2017.10.020. [DOI] [PubMed] [Google Scholar]
- 11.Hall WA, Paulson E, Li XA, et al. Magnetic resonance linear accelerator technology and adaptive radiation therapy: An overview for clinicians. CA Cancer J Clin. 2022;72:34–56. doi: 10.3322/caac.21707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sritharan K, Tree A. MR-guided radiotherapy for prostate cancer: State of the art and future perspectives. Br J Radiol. 2022;95 doi: 10.1259/bjr.20210800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kishan AU, Ma TM, Lamb JM, et al. Magnetic resonance imaging-guided vs computed tomography-guided stereotactic body radiotherapy for prostate cancer: The MIRAGE randomized clinical trial. JAMA Oncol. 2023;9:365–373. doi: 10.1001/jamaoncol.2022.6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Willigenburg T, van der Velden JM, Zachiu C, et al. Accumulated bladder wall dose is correlated with patient-reported acute urinary toxicity in prostate cancer patients treated with stereotactic, daily adaptive MR-guided radiotherapy. Radiother Oncol. 2022;171:182–188. doi: 10.1016/j.radonc.2022.04.022. [DOI] [PubMed] [Google Scholar]
- 15.Mylona E, Acosta O, Lizee T, et al. Voxel-based analysis for identification of urethrovesical subregions predicting urinary toxicity after prostate cancer radiation therapy. Int J Radiat Oncol Biol Phys. 2019;104:343–354. doi: 10.1016/j.ijrobp.2019.01.088. [DOI] [PubMed] [Google Scholar]
- 16.Alayed Y, Davidson M, Quon H, et al. Dosimetric predictors of toxicity and quality of life following prostate stereotactic ablative radiotherapy. Radiother Oncol. 2020;144:135–140. doi: 10.1016/j.radonc.2019.11.017. [DOI] [PubMed] [Google Scholar]
- 17.Teunissen FR, Willigenburg T, Tree AC, et al. Magnetic resonance-guided adaptive radiation therapy for prostate cancer: The first results from the MOMENTUM study—An international registry for the evidence-based introduction of magnetic resonance-guided adaptive radiation therapy. Pract Radiat Oncol. 2023;13:e261–e269. doi: 10.1016/j.prro.2022.09.007. [DOI] [PubMed] [Google Scholar]
- 18.Dueck AC, Mendoza TR, Mitchell SA, et al. Validity and reliability of the US National Cancer Institute's Patient-Reported Outcomes Version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE) JAMA Oncol. 2015;1:1051–1059. doi: 10.1001/jamaoncol.2015.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.King CR, Collins S, Fuller D, et al. Health-related quality of life after stereotactic body radiation therapy for localized prostate cancer: Results from a multi-institutional consortium of prospective trials. Int J Radiat Oncol Biol Phys. 2013;87:939–945. doi: 10.1016/j.ijrobp.2013.08.019. [DOI] [PubMed] [Google Scholar]
- 20.Katz AJ, Santoro M, Diblasio F, Ashley R. Stereotactic body radiotherapy for localized prostate cancer: Disease control and quality of life at 6 years. Radiat Oncol. 2013;8:118. doi: 10.1186/1748-717X-8-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wiegner EA, King CR. Sexual function after stereotactic body radiotherapy for prostate cancer: Results of a prospective clinical trial. Int J Radiat Oncol Biol Phys. 2010;78:442–448. doi: 10.1016/j.ijrobp.2009.07.1748. [DOI] [PubMed] [Google Scholar]
- 22.Mohler JL, Antonarakis ES, Armstrong AJ, et al. Prostate Cancer, version 2.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2019;17:479–505. doi: 10.6004/jnccn.2019.0023. [DOI] [PubMed] [Google Scholar]
- 23.Kluetz PG, Chingos DT, Basch EM, Mitchell SA. Patient-reported outcomes in cancer clinical trials: Measuring symptomatic adverse events with the National Cancer Institute's Patient-Reported Outcomes Version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE) Am Soc Clin Oncol Educ Book. 2016;35:67–73. doi: 10.1200/EDBK_159514. [DOI] [PubMed] [Google Scholar]
- 24.Freeman DE, King CR. Stereotactic body radiotherapy for low-risk prostate cancer: Five-year outcomes. Radiat Oncol. 2011;6:3. doi: 10.1186/1748-717X-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen LN, Suy S, Uhm S, et al. Stereotactic body radiation therapy (SBRT) for clinically localized prostate cancer: The Georgetown University experience. Radiat Oncol. 2013;8:58. doi: 10.1186/1748-717X-8-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McBride SM, Wong DS, Dombrowski JJ, et al. Hypofractionated stereotactic body radiotherapy in low-risk prostate adenocarcinoma: Preliminary results of a multi-institutional phase 1 feasibility trial. Cancer. 2012;118:3681–3690. doi: 10.1002/cncr.26699. [DOI] [PubMed] [Google Scholar]
- 27.Bruynzeel AME, Tetar SU, Oei SS, et al. A prospective single-arm Phase 2 study of stereotactic magnetic resonance guided adaptive radiation therapy for prostate cancer: Early toxicity results. Int J Radiat Oncol Biol Phys. 2019;105:1086–1094. doi: 10.1016/j.ijrobp.2019.08.007. [DOI] [PubMed] [Google Scholar]
- 28.Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med. 2008;358:1250–1261. doi: 10.1056/NEJMoa074311. [DOI] [PubMed] [Google Scholar]
- 29.Crook J, Fleshner N, Roberts C, Pond G. Long-term urinary sequelae following 125iodine prostate brachytherapy. J Urol. 2008;179:141–145. doi: 10.1016/j.juro.2007.08.136. discussion 146. [DOI] [PubMed] [Google Scholar]
- 30.Keyes M, Miller S, Moravan V, et al. Urinary symptom flare in 712 125I prostate brachytherapy patients: long-term follow-up. Int J Radiat Oncol Biol Phys. 2009;75:649–655. doi: 10.1016/j.ijrobp.2008.11.043. [DOI] [PubMed] [Google Scholar]
- 31.Cesaretti JA, Stone NN, Stock RG. Urinary symptom flare following I-125 prostate brachytherapy. Int J Radiat Oncol Biol Phys. 2003;56:1085–1092. doi: 10.1016/s0360-3016(03)00210-4. [DOI] [PubMed] [Google Scholar]
- 32.Watson E, Shinkins B, Frith E, et al. Symptoms, unmet needs, psychological well-being and health status in survivors of prostate cancer: Implications for redesigning follow-up. BJU Int. 2016;117:E10–E19. doi: 10.1111/bju.13122. [DOI] [PubMed] [Google Scholar]
- 33.Bobridge A, Bond MJ, Marshall V, Paterson J. An investigation of the support needs of men and partners throughout the prostate cancer journey. Psychooncology. 2015;24:341–347. doi: 10.1002/pon.3655. [DOI] [PubMed] [Google Scholar]
- 34.Albaugh JA, Sufrin N, Lapin BR, Petkewicz J, Tenfelde S. Life after prostate cancer treatment: A mixed methods study of the experiences of men with sexual dysfunction and their partners. BMC Urol. 2017;17:45. doi: 10.1186/s12894-017-0231-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mehta A, Pollack CE, Gillespie TW, et al. What patients and partners want in interventions that support sexual recovery after prostate cancer treatment: An exploratory convergent mixed methods study. Sex Med. 2019;7:184–191. doi: 10.1016/j.esxm.2019.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Basch E, Snyder C, McNiff K, et al. Patient-reported outcome performance measures in oncology. J Oncol Pract. 2014;10:209–211. doi: 10.1200/JOP.2014.001423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Silveira A, Sequeira T, Gonçalves J. Lopes Ferreira P. Patient reported outcomes in oncology: Changing perspectives-a systematic review. Health Qual Life Outcomes. 2022;20:82. doi: 10.1186/s12955-022-01987-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dacal K, Sereika SM, Greenspan SL. Quality of life in prostate cancer patients taking androgen deprivation therapy. J Am Geriatr Soc. 2006;54:85–90. doi: 10.1111/j.1532-5415.2005.00567.x. [DOI] [PubMed] [Google Scholar]
- 39.ClinicalTrials.gov. Randomized phase II trial of five or two MRI-guided adaptive radiotherapy treatments for prostate cancer. ClinicalTrials.gov identifier: NCT04984343. Accessed June 17, 2023. https://clinicaltrials.gov/ct2/show/NCT04984343
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.