Abstract
Zhoupigan (Citrus reticulata cv. Manau Gan) is a local citrus variety in China. Its peel, known as Zangju peel (ZJP). The metabolic profile and bioactivity of ZJP have not been adequately studied, resulting in underutilization of ZJP and a serious waste of resources. In this study, GC–MS identified 46 components in ZJP, which defined ZJP's distinct aroma. Furthermore, UPLC-ESI-MS/MS detected 1506 metabolites in ZJP, and the differential metabolites were primarily involved in the biosynthesis of flavonoids and phenylacetone. Additionally, 56 key differential metabolites with metabolic pathways were identified. ZJP had significant antioxidant activity and the enzyme inhibitory activity ranking as pancreatic lipase (IC50 = 3.71 mg/mL) > α-glucosidase (IC50 = 6.28 mg/mL) > α-amylase (IC50 = 8.02 mg/mL). This study aimed to evaluate the potential of ZJP as natural antioxidant and functional food source and to serve as foundation for the further development of ZJP products with specific functional attributes.
Keywords: Zhoupigan, Volatile metabolites, Widely targeted metabolomics, Antioxidant, Hypolipidemic, Hypoglycemic
Graphical abstract
Highlights
-
•
The metabolic profile of Zhoupigan (Citrus reticulata cv. Manau Gan) was resolved.
-
•
A total of 1535 metabolites were identified, more than previously reported.
-
•
Metabolite profiles and bioactivities of different citrus peels were compared.
-
•
Zhoupigan peel has excellent antioxidant and enzyme-inhibitory activities.
-
•
Zhoupigan peel is a potential source of natural antioxidants and a functional food.
1. Introduction
Citrus is among the most consumed and extensively distributed fruits globally, renowned for its distinctive flavor and high nutritional value (Wang, Wang, Xiao, et al., 2024). Annually, over 130 million tons of citrus are produced, predominantly for pulp consumption, juice processing, and culinary flavoring. However, the utilization of citrus peels remains limited, leading to the generation of >15 million tons of citrus waste each year (Leporini et al., 2020), which represents a significant resource waste and environmental pollutant. Citrus peels, a primary source of essential oils (EO) stored in citrus oil glands, emit a pleasant aromatic scent (Wang, Ren, Zhou, et al., 2024). Additionally, these peels are abundant in phenolic acids, flavonoids, alkaloids, limonin, and numerous other bioactive compounds that exhibit antioxidant, anti-inflammatory, and lipid-lowering properties (Liu et al., 2021). Consequently, citrus peels can serve as a source of functional ingredients and preservatives. They are often processed into health food products such as candied fruit, jiushichenpi, Ganpu tea, or utilized as food flavoring agents. The dried ripe peel of Citrus reticulata and its cultivated variants, known as “chenpi” (Citri reticulatae pericarpium, CP), has been used for millennia in China for its extensive bioactivities, often as herbal medicine, flavoring, and health food. Despite numerous studies on locally cultivated citrus variants (Costanzo et al., 2022; Tao et al., 2014; Zhang et al., 2014), little research has focused on the peel of Zhoupigan, hindering the optimal utilization of Zhoupigan resources.
Zhoupigan, also known as Zangju or Shitougan, is distinguished by its wrinkled rind, succulent flesh, and a distinctively acidic, slightly bitter taste, which is highly esteemed for its unique flavor (Chen et al., 2021). Cultivated predominantly in the agricultural and pastoral regions along the middle and lower reaches of the Sanjiang River Valley, particularly in Derong and Muli counties (Sichuan Province, China), Zhoupigan covers over 11,600 ha with an annual yield of approximately 130,000 tons (https://www.nongjixie.org/Library/Nmap/view?nmap_id=1516). It is also cultivated in Ankang (Shanxi Province, China) and Yueyang (Hunan Province, China). In the Tibetan region, Zhoupigan is referred to as “Jia Xu,” meaning “longevity of the fruit.” Unlike ordinary citrus trees that typically survive for 40 to 50 years, Zhoupigan trees can flourish for nearly a century, remaining verdant and` robust. The Tibetan area is characterized by high altitude, dry climate with little rain, abundant light, and large temperature differences between day and night. As a result of this unique environment and location, Tibetans have developed distinctive dietary habits. The Tibetan diet primarily consists of high-calorie foods such as Zanba (a flour made from hulless barley), Tibetan milk tea (made from milk and sugar), and buttered tea (a drink made from salt, tea, and milk-derived oil) (Wang, Wang, Shi, et al., 2023; Xiao et al., 2021). However, the consumption of fruits and vegetables is inadequate. In this case, as a fruit cultivated on a large scale in Tibetan areas, Zhoupigan plays an important role in protecting the health of Tibetans. Local residents also incorporate ZJP into meat stews to reduce greasiness and immerse ZJP in water to relieve headaches and coughs. Due to the lack of vegetables and fruits in Tibetan areas, Tibetans generally store Zhoupigan after harvesting for approximately two months to prolong consumption time. In addition, Tibetans believe that storage helps to increase the flavor of Zhoupigan. Therefore, the limited research on Zhoupigan, a widely cultivated citrus variety with a distinct flavor and diverse uses grown in a unique environment, has obstructed its full utilization. It further emphasizes the significance of conducting a comprehensive assessment of its metabolite profile and bioactivity. In our previous research, utilizing HS-GC-IMS analyzed the volatile components of ZJP at different maturity stages, identifying some components not reported in other citrus varieties (Wang, Wang, Zou, et al., 2023).
Hyperglycemia and hyperlipidemia commonly coexist in patients with diabetes (Wang, Ren, et al., 2022). Pancreatic lipase (PL) is essential for the hydrolysis of dietary triglycerides, and lipase inhibitors can produce a hypolipidemic effect, aiding in the management of metabolic disorders (Zeng et al., 2018). α-amylase and α-glucosidase are essential enzymes in starch digestion. α-amylase catalyzes the hydrolysis of starch by breaking the α-1,4-glucosidic bond, followed by α-glucosidase further degrading hydrolysis products into glucose, thus increasing glucose levels. Inhibiting these enzymes can help control blood glucose levels (Wang, Hu, Li, et al., 2024). Currently, Orlistat (ORL) is the only approved PL inhibitor, while hypoglycemic drugs include acarbose (ACA), miglitol, and voglibose; however, these enzyme inhibitors often have side effects (Wu et al., 2013; Zhang et al., 2017). Natural plants have long been a source of medicines; phytotherapy, which uses plant-derived medicines, is often safer and more effective with fewer side effects than chemical medicines. Plant by-products are an important source of phenolic compounds. Phenol extraction from by-products (stems, leaves, and stem-leaf mixtures) of safflower (Carthamus tinctorius L.) and analysis of their composition by UPLC-DAD-MS, followed by assessment of their antioxidant and erythrocyte protective properties, revealed that that they are an ideal source of phenolics that can be used in the food and pharmaceutical sectors (Del-Toro-Sánchez et al., 2021). Plants such as Allium cepa L. and Panax ginseng C. A. Mey. have been utilized in diabetes treatment (Governa et al., 2018). Citrus peels, rich in various bioactive components, are an ideal source of dietary supplements. Functional foods prepared by incorporating citrus extracts or utilizing citrus by-products were shown to exhibit more potent antioxidant capacity (Gómez-Mejía et al., 2023; Laganà et al., 2022; Peng et al., 2022). Research by Huang, Zhang, et al. (2020) identified hesperidin as the primary PL inhibitor, while Huang, Zhu, et al. (2020) suggested that the PL inhibitory activity of citrus peels might be due to their polymethoxyflavonoids (PMFs) content. Phenols extracted from citrus peels exhibit strong antioxidant, antidiabetic, and antihypertensive properties (Alu'datt et al., 2017; Qurtam et al., 2021), and citrus EOs significantly inhibit α-Glucosidase and α-Amylase, demonstrating potential hypoglycemic and antidiabetic effects (Benayad et al., 2021). Currently, studies on the in vitro antidiabetic activity of citrus extracts have mainly focused on either hypolipidemic or hypoglycemic properties individually, while reports on their combined properties are rare and mostly evaluate the activity of a certain class of compounds, such as flavonoids, phenols, and PMFs (Alu'datt et al., 2017; Kong et al., 2020; Loizzo et al., 2019; Zeng et al., 2018). This can hinder our ability to thoroughly assess their hypolipidemic and hypoglycemic activities. However, while the inhibitory activity of citrus extracts on pancreatic lipase, α-glucosidase, and α-amylase has been confirmed by numerous studies, it has not been adequately demonstrated how these extracts or individual compounds inhibit these enzymes. We previously evaluated ZJP's antioxidant and hypolipidemic activities across different maturation stages and revealed excellent antioxidant capacity, lipase inhibition, the ability to inhibit lipid differentiation of 3 T3-L1 cells, and promotion of lipid metabolism. (Wang, Wang, Xiao, et al., 2024). In conclusion, citrus peels exhibit strong hypolipidemic and hypoglycemic activities, and while ZJP also shows excellent antioxidant and hypolipidemic activities, its hypoglycemic potential remains unexplored, and comparisons with other citrus peels' bioactivities are yet to be reported.
Metabolomics is an effective technology for detecting compounds, yet the diversity of secondary metabolites in plants presents significant identification challenges, thereby impeding plant metabolome detection. UPLC-ESI-MS/MS-based widely targeted metabolomics is an emerging analytical technology, combining the “versatility” of untargeted metabolomics with the “accuracy” of targeted metabolomics. Widely targeted metabolomics utilizes QTRAP mass spectrometry in multiple reaction monitoring (MRM) mode, enabling the qualitative and quantitative detection of over 1800 primary and 28,000 secondary metabolites in a single run. This method has gained widespread application in food and drug research due to its high sensitivity, specificity, and throughput (Jia et al., 2023; Qian et al., 2023; Yang et al., 2023; Zheng et al., 2023). Recently, widely targeted metabolomics has been extensively employed to study specificities among different varieties, sites, maturity stages, and colors (Dadwal et al., 2022; Li, Wen, et al., 2021; Shen et al., 2023; Wang, Wang, Xiao, et al., 2024). Consequently, comparing ZJP with other citrus peel metabolites using widely targeted metabolomics can elucidate the similarities and differences among the metabolites more effectively.
Although we have previously conducted preliminary studies on ZJP composition and activity, we have not analyzed the composition of ZJP's EO or compared its composition and activity with other citrus peels, which limits the use of ZJP. Therefore, GC–MS and UPLC-ESI-MS/MS-based widely targeted metabolomics were initially implemented to analyze the composition of ZJP. Its composition was then compared with that of C. reticulata ‘Unshiu’ peel (WZP) and C. reticulata ‘Chachi’ peel (XHP), which are included in the Chinese Pharmacopoeia, to assess their metabolite profile differences. Subsequently, multivariate analyses such as principal component analysis (PCA) and partial least squares discriminant analysis (OPLS-DA) were conducted to extract valuable insights from the metabolomics data. In addition, differences in PL, α-glucosidase, α-amylase inhibitory activity and antioxidant activity between ZJP and XHP and WZP were compared, and these differences were correlated with key differential metabolites (DMs). In addition, we compared the hypolipidemic and hypoglycemic-related enzyme inhibitory activities in the peels from the different citrus genotypes. This study aimed to assess ZJP's potential and feasibility as a natural antioxidant and functional food source and to compare the metabolic profiles and in vitro bioactivities between ZJP, XHP and WZP.
2. Materials and methods
2.1. Materials and reagents
2.1.1. Plant material
Zhoupigan was sourced from Derong County, Ganzi Tibetan Autonomous Region, Sichuan Province, China (99°16′37′′ E; 28°32′32′′ N; 2225 m). C. reticulata ‘Unshiu’ was obtained from Danling County, Meishan City, Sichuan Province, China (103°30′57.3″ E; 30°0′54.0″ N; 509 m). Upon collection, the samples were transported to the laboratory, where the peels were manually washed, separated, and dried using hot air at a constant temperature of 50 °C (101–3 A, Beijing Zhongxingweiye Century Instrument Co., Ltd., Beijing, China) for 18 h to produce the Zhoupigan peel (ZJP) and C. reticulata ‘Unshiu’ peel (WZP). These peels were then stored in a dry place at room temperature (25–28 °C). C. reticulata ‘Chachi’ peel (XHP) was procured in 2023 from Xinhui District, Jiangmen County, Guangdong Province, China (113°01′52″ E; 22°31′41″ N) as a one-year storage sample. The samples are illustrated in Fig. S1.
2.1.2. Chemicals
Methanol, acetonitrile, and formic acid were purchased from Merck Drugs & Biotechnology, USA, and were chromatographically pure. 1,1-diphenyl-2-trinitrophenylhydrazine (DPPH), 2,2′-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS), 4-nitrophenyl laurate, p-nitrophenyl-α-D-glucopyranoside (PNPG), and α-glucosidase (α-glu) were purchased from Shanghai Macklin Biochemical Technology Co., Ltd., (Shanghai, China). Total antioxidant capacity (T-AOC) assay kit (ferric ion reducing antioxidant capacity, FRAP) was purchased from Nanjing Jiancheng Biotechnology Research Institute Co., Ltd., (Nanjing, Jiangsu Province, China). α-amylase was purchased from Sigma Aldrich (Shanghai) Trading Co., Ltd., (Shanghai, China). Porcine PL was purchased from Shanghai yuanye Bio-Technology Co., Ltd., (Shanghai, China). Soluble starch was provided by China National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). All the above reagents were analytically pure.
2.2. Sample preparation and extraction for GC–MS
For essential oil extraction, 100 g of dried sample powder was soaked in 10 times the volume of water for 2 h. The essential oil was then extracted using a volatile oil extractor until no further increase in oil quantity was observed. The upper essential oil was carefully collected and dried with about 0.5 g of anhydrous sodium sulfate at 4 °C for 24 h. The essential oil was diluted 100-fold with n-hexane, filtered through a microporous filter membrane (pore size 0.22 μm), and prepared for GC–MS analysis.
2.3. GC–MS conditions
Gas chromatography-mass spectrometry (GC–MS) was conducted using an Agilent 7890 A-5975C gas chromatograph equipped with an HP-5 MS capillary column (0.25 mm × 30 m, 0.25 μm, Agilent J&W Scientific, Folsom, CA, USA). The carrier gas was high-purity He (99.999%), with an injection volume of 1 μL and a split ratio of 10:1, at a flow rate of 1.0 mL/min. The heating program started at an initial temperature of 40 °C, maintained for 3.5 min, followed by an increase of 10 °C/min to 100 °C, then 25 °C/min to 260 °C, held for 5 min. The EI ion source operated at 70 eV, with an ion source temperature of 230 °C, a quadrupole temperature of 150 °C, an interface temperature of 250 °C, a solvent delay of 3.0 min, and in full scan mass scan mode. The NIST 11 (2011), NIST 14 (2014), and RTLPEST 3 (2011) standard spectral libraries were searched, and the relative percentage content of each component was calculated using the peak area normalization method.
2.4. Sample preparation and extraction for widely targeted metabolomic analysis
Using a vacuum freeze-drying technique, samples were placed in a lyophilizer (Scientz-100F, Ningbo Xinzhi Bio-technology Co., Ltd., Ningbo, Zhejiang Province, China) and subsequently ground into a fine powder using a grinder (MM 400, Verder Shanghai Instruments and Equipment Co., Ltd., Shanghai, China) at 30 Hz for 1.5 min. Precisely 50 mg of the powdered sample was weighed using an electronic balance (MS105DΜ, Mettler Toledo Group, Zurich, Switzerland) and combined with 1200 μL of −20 °C pre-cooled 70% methanol aqueous solution. The mixture was vortexed every 30 min for 30 s, repeated six times. After centrifugation at 12,000 rpm for 3 min, the supernatant was aspirated, filtered through a microporous filter membrane (pore size 0.22 μm), and stored in an injection vial for UPLC-MS/MS analysis.
2.5. UPLC–MS/MS conditions
The sample extracts were analyzed using an UPLC-ESI-MS/MS system (UPLC, ExionLC™ ADˈ https: //sciex.com.cn/; MS, Applied Biosystems 6500 Q TRAP, https://sciex.com.cn/). The analytical conditions were as follows, UPLC: column, Agilent SB-C18 (1.8 μm, 2.1 mm * 100 mm); The mobile phase was consisted of solvent A, pure water with 0.1% formic acid, and solvent B, acetonitrile with 0.1% formic acid. Sample measurements were performed with a gradient program that employed the starting conditions of 95% A, 5% B. Within 9 min, a linear gradient to 5% A, 95% B was programmed, and a composition of 5% A, 95% B was kept for 1 min. Subsequently, a composition of 95% A, 5.0% B was adjusted within 1.1 min and kept for 2.9 min. The flow velocity was set as 0.35 mL per minute; The column oven was set to 40 °C; The injection volume was 2 μL. The effluent was alternatively connected to an ESI-triple quadrupole-linear ion trap (QTRAP)-MS.
The ESI source operation parameters were as follows: source temperature 500 °C; ion spray voltage (IS) 5500 V (positive ion mode)/−4500 V (negative ion mode); ion source gas I (GSI), gas II(GSII), curtain gas (CUR) were set at 50, 60, and 25 psi, respectively; the collision-activated dissociation(CAD) was high. QQQ scans were acquired as MRM experiments with collision gas (nitrogen) set to medium. DP(declustering potential) and CE(collision energy) for individual MRM transitions was done with further DP and CE optimization. A specific set of MRM transitions were monitored for each period according to the metabolites eluted within this period.
2.6. Identification and quantification of metabolites
Mass spectrometry data were processed using Analyst 1.6.3 software for qualitative and quantitative analysis of metabolites. Total ion chromatography (TIC) illustrated chromatographic differences (Fig. S2), while the MRM Metabolite Assay Multi-Peak Chart depicted detectable metabolites, with each color representing a distinct metabolite (Fig. S3). Qualitative analysis was conducted by comparing accurate precursor ion (Q1) and product ion (Q3) values and retention times (RT), matching these with the self-built database MWDB (MetWare Biological Co., Ltd., Wuhan, Hebei Province, China). Quantitative analysis involved MRM analysis using a mass spectrometer to acquire metabolite data from different samples. Integration and calibration of chromatographic peaks were performed using MultiQuant (AB SCIEX, Framingham, MA, USA) software to calculate the relative concentration of each substance in the peak area (Fig. S4).
2.7. Biological activity evaluation
2.7.1. Sample extraction
For further analysis, 0.2 g of ground sample powder was mixed with 10 mL of 70% methanol and extracted by ultrasonication (SB25-12D, Ningbo Xinyi Ultrasonic Equipment Co., Ltd., Ningbo, Zhejiang Province, China) for 1 h at 100 W and 50 Hz. The mixture was then centrifuged at 4000 rpm for 20 min using a centrifuge (TDZS-WS, Hunan Xiangyi Laboratory Instrument Development Co., Ltd., Changsha, Hunan Province, China). The supernatant was collected, filtered through a microporous membrane, diluted as needed, and subjected to antioxidant activity, PL, α-glucosidase, and α-amylase inhibitory activities within 2 weeks.
2.7.2. Evaluation of antioxidant activity
The DPPH and ABTS free radical scavenging capacities, along with the FRAP total antioxidant capacity, of various concentrations of citrus peel extracts (0.1–5 mg/mL) were assessed following the methodology of Wang, Wang, Xiao, et al. (2024). The DPPH and ABTS free radical scavenging capacities were quantified as the semi-inhibitory concentration (IC50), while the total antioxidant capacity was represented in milligrams of FeSO4 per gram of dry weight sample (mg FeSO4 g−1 DW). The standard curve was defined as y = 3.1387× + 0.0217 (R2 = 0.9999).
2.7.3. Evaluation of inhibition capacity of enzyme activity
The PL inhibitory capacity of citrus peel extracts (1–20 mg/mL) was measured following the method outlined by Wang, Wang, Xiao, et al. (2024), with ORL serving as a positive control. Similarly, the α-glucosidase and α-amylase inhibitory capacities of the citrus peel extracts (1–20 mg/mL) were determined using the method described by Wang, Li, et al. (2022), with ACA as a positive control. Results were reported as IC50 values.
2.8. Data analysis
PCA, Venn diagrams, Metabolite Classification Charts, and Correlation analyses were conducted using the Metware Cloud, a complimentary online platform for data analysis (https://cloud.metware.cn). The pheatmap package in R software was utilized for clustering heatmap plotting, while the MetaboAnalystR package in R software was employed to compute VIP values, rankings, and rating plots in OPLS-DA. DMs were screened using the criteria of FC ≥ 2 or FC ≤ 0.5 and VIP > 1. Statistical analyses, including bar graphs, line plots, and ANOVA, were performed using GraphPad Prism 8.4.3 (GraphPad Software, USA), with a P-value of <0.05 indicating statistical significance. Metabolites were annotated through the KEGG database (compounds: https://www.kegg.jp/kegg/compound/;Pathways,http://www.kegg.jp/kegg/pathway.html).
3. Results and discussion
3.1. GC–MS analysis of ZJP
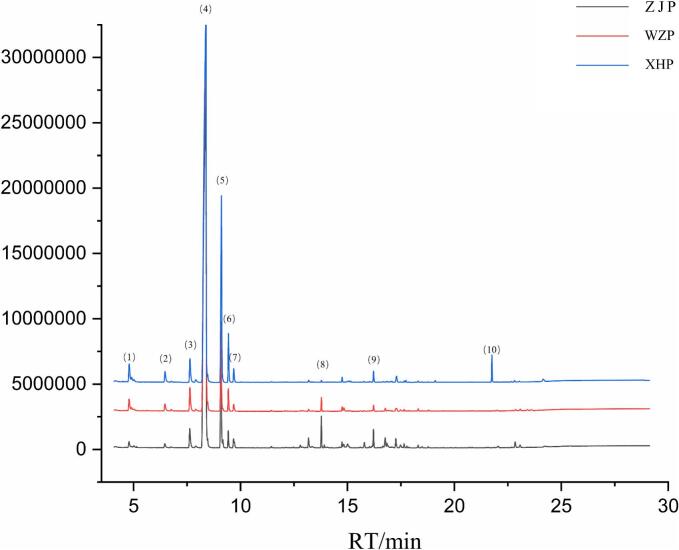
Citrus peel is a major source of EOs, containing numerous volatile organic compounds (VOCs) that determine the unique aroma and flavor of citrus. In this study, the VOCs of ZJP EO were analyzed via GC–MS and compared with those of WZP EO and XHP EO. A total of 67 VOCs, including 32 hydrocarbons, 20 alcohols, 5 aldehydes, 4 phenols, 2 esters, 2 ketones, 1 ether, and 1 pyran, were identified across the three EOs (Table 1 and Fig. S5A). PCA revealed significant separation among the three citrus peels, with ZJP distinctly diverging from WZP and XHP, which was further corroborated by clustered heatmaps (Fig. S5C-D). d-Limonene, γ-Terpinene, β-Myrcene, and linalool were the predominant compounds found in all three citrus peel EOs. Notably, ZJP contained 46 VOCs, 18 of which, such as (+)-2-Carene, Caryophyllene, and carvacrol, were unique to ZJP and exhibited woody, herbal, and waxy odors (Fig. 1, Fig. S5B). To explore the differences between ZJP and the other citrus peel VOCs, differentially abundant VOCs were identified using criteria of FC ≥ 2 or FC ≤ 0.5 and VIP > 1. A total of 28 differential VOCs were identified between ZJP and WZP, with 18 significantly upregulated in ZJP (P < 0.05); similarly, 28 differential VOCs were found between ZJP and XHP, with 22 significantly upregulated in ZJP (P < 0.05). Decanal, 2,6-Octadiene, 2,6-dimethyl-, (+)-Δ-Cadinene, dl-Perillaldehyde, Carveol, Carvone, (R)-(+)-citronellal, and Nerol were consistently upregulated in ZJP in both comparisons. These eight components, along with the 18 unique to ZJP, contribute to its distinctive flavor profile. Liu et al. (2024) characterized the flavor metabolic profiles of XHP tea liquor with varying aging times and identified d-limonene, α-phellandrene, γ-terpinene, and dimethyl anthranilate as characteristic aroma compounds of XHP.
Table 1.
GC–MS analysis of volatile components in the essential oil of ZJP, WZP, and XHP.
| No. | Compounds | RT(min) | CAS | Formula | Molecular weight | Major ions | Relative amount (%) |
Class | ||
|---|---|---|---|---|---|---|---|---|---|---|
| WZP | ZJP | XHP | ||||||||
| 1 | L-(−)-α-pinene | 4.801 | 7785-26-4 | C10H16O | 152.233 | 93, 91, 92, 77 | 1.24 ± 0.01 | 0.58 ± 0.01 | 1.81 ± 0.03 | Hydrocarbons |
| 2 | α-thujene | 4.911 | 2867-05-2 | C10H16 | 136.234 | 93, 91, 77, 92 | 0.24 ± 0.21 | ND | 0.51 ± 0.03 | Hydrocarbons |
| 3 | Camphene | 5.644 | 79–92-5 | C10H16 | 136.234 | 93, 121, 79, 91 | 0.06 ± 0.01 | ND | ND | Hydrocarbons |
| 4 | β-Pinene | 6.474 | 127–91-3 | C10H16 | 136.234 | 93, 69, 91, 41 | 0.77 ± 0.01 | 0.42 ± 0.00 | 1.13 ± 0.02 | Hydrocarbons |
| 5 | β-Phellandrene | 6.761 | 555–10-2 | C10H16 | 136.234 | 93, 91, 77, 79 | 0.16 ± 0.01 | 0.10 ± 0.01 | 0.09 ± 0.01 | Hydrocarbons |
| 6 | β-Myrcene | 7.641 | 123–35-3 | C10H16 | 136.234 | 93, 69, 41, 91 | 2.60 ± 0.17 | 1.87 ± 0.04 | 2.37 ± 0.08 | Hydrocarbons |
| 7 | α-terpinene | 7.909 | 99–86-5 | C10H16 | 136.234 | 121, 93, 136, 91, 77 | 0.28 ± 0.02 | ND | ND | Hydrocarbons |
| 8 | 4-Carene | 7.909 | 29,050–33-7 | C10H16 | 136.234 | 121, 93, 136, 91, 76 | ND | 0.27 ± 0.02 | 0.38 ± 0.03 | Hydrocarbons |
| 9 | d-Limonene | 8.379 | 5989-27-5 | C10H16 | 136.234 | 93, 68, 67, 79 | 77.34 ± 1.34 | 70.92 ± 1.30 | 66.79 ± 0.68 | Hydrocarbons |
| 10 | γ-Terpinene | 9.088 | 99–85-4 | C10H16 | 136.234 | 93, 91, 136, 77 | 7.25 ± 0.32 | 9.61 ± 0.45 | 15.09 ± 0.31 | Hydrocarbons |
| 11 | o-Cymene | 9.436 | 527–84-4 | C10H14 | 134.218 | 119, 134, 91, 117 | 1.54 ± 0.08 | 1.04 ± 0.07 | 3.01 ± 0.03 | Hydrocarbons |
| 12 | Terpinolene | 9.68 | 586–62-9 | C10H16 | 136.234 | 121, 93, 136, 91, 79 | 0.71 ± 0.06 | 0.91 ± 0.08 | 1.00 ± 0.06 | Hydrocarbons |
| 13 | 3-Methyl-2-buten-1-ol | 10.016 | 556–82-1 | C5H10O | 86.132 | 71, 41, 43, 53 | ND | ND | 0.02 ± 0.03 | Alcohols |
| 14 | p-1,3,8-menthatriene | 11.444 | 18,368–95-1 | C10H14 | 134.218 | 57, 41, 119, 91 | ND | 0.04 ± 0.06 | ND | Hydrocarbons |
| 15 | p-Mentha-1,5,8-triene | 11.963 | 21,195–59-5 | C10H14 | 134.218 | 91, 134, 119, 117 | ND | 0.01 ± 0.01 | ND | Hydrocarbons |
| 16 | 2-p-Tolyl-1-propene | 12.098 | 1195-32-0 | C10H12 | 132.202 | 132, 117, 115, 91 | 0.05 ± 0.01 | 0.04 ± 0.01 | 0.01 ± 0.01 | Hydrocarbons |
| 17 | 1,2-diethylbenzene | 12.177 | 135–01-3 | C10H14 | 134.218 | 91, 119, 134, 105 | ND | 0.01 ± 0.02 | ND | Hydrocarbons |
| 18 | trans-limonene oxide | 12.47 | 4959-35-7 | C10H16O | 152.233 | 43, 67, 94, 108 | ND | 0.07 ± 0.02 | ND | pyrans |
| 19 | (R)-(+)-citronellal | 12.812 | 2385-77-5 | C10H18O | 154.249 | 69, 41, 95, 55 | 0.08 ± 0.01 | 0.24 ± 0.02 | ND | aldehydes |
| 20 | (+)-2-Carene | 12.91 | 4497-92-1 | C10H16 | 136.234 | 121, 93, 136, 161, 91 | ND | 0.09 ± 0.01 | ND | Hydrocarbons |
| 21 | Δ-elemene | 12.916 | 20,307–84-0 | C15H24 | 204.35 | 121, 93, 136, 91, 161 | 0.09 ± 0.01 | ND | ND | Hydrocarbons |
| 22 | Decanal | 13.185 | 112–31-2 | C10H20O | 156.265 | 57, 41, 43, 55 | 0.16 ± 0.02 | 0.66 ± 0.06 | 0.14 ± 0.02 | aldehydes |
| 23 | α-Cubebene | 13.356 | 17,699–14-8 | C15H24 | 204.351 | 119, 105, 161, 93 | ND | 0.20 ± 0.02 | ND | Hydrocarbons |
| 24 | Linalool | 13.783 | 78–70-6 | C10H18O | 154.249 | 71, 93, 43, 55 | 0.79 ± 0.03 | 1.43 ± 0.11 | 0.10 ± 0.00 | Alcohols |
| 25 | 1-Octanol | 13.923 | 111–87-5 | C8H18O | 130.228 | 56, 55, 41, 69 | ND | 0.15 ± 0.01 | ND | Alcohols |
| 26 | Germacrene D | 14.039 | 23,986–74-5 | C15H24 | 204.35 | 161, 105, 91, 119 | ND | 0.07 ± 0.07 | ND | Hydrocarbons |
| 27 | β-ylangene | 14.638 | 20,479–06-5 | C15H24 | 204.35 | 161, 120, 91, 149 | ND | 0.09 ± 0.01 | ND | Hydrocarbons |
| 28 | 2-Isopropyl-5-methylanisole | 14.644 | 1076-56-8 | C11H16O | 164.244 | 149, 164, 91, 117 | ND | ND | 0.02 ± 0.02 | Phenols |
| 29 | 4-terpineol | 14.748 | 562–74-3 | C10H18O | 154.249 | 71, 111, 93, 43 | 0.29 ± 0.02 | 0.33 ± 0.03 | 0.29 ± 0.03 | Alcohols |
| 30 | β-elemene | 14.833 | 515–13-9 | C15H24 | 204.351 | 81, 93, 67, 107 | 0.40 ± 0.03 | 0.33 ± 0.03 | ND | Hydrocarbons |
| 31 | Caryophyllene | 15.01 | 87–44-5 | C15H24 | 204.351 | 93, 133, 91, 79 | ND | 0.55 ± 0.08 | ND | Hydrocarbons |
| 32 | isocaryophyllene | 15.016 | 118–65-0 | C15H24 | 204.351 | 93, 91, 133, 69 | ND | ND | 0.09 ± 0.08 | Hydrocarbons |
| 33 | β-terpineol | 15.163 | 138–87-4 | C10H18O | 154.249 | 71, 93, 43, 107 | ND | ND | 0.08 ± 0.01 | Alcohols |
| 34 | γ-Elemene | 15.597 | 29,873–99-2 | C15H24 | 204.351 | 121, 93, 107, 91 | 0.06 ± 0.01 | ND | ND | Hydrocarbons |
| 35 | (E)-p-Menth-2,8-dien-1-ol; | 15.756 | 7212-40-0 | C10H16O | 152.233 | 109, 134, 79, 137 | ND | ND | 0.05 ± 0.04 | Alcohols |
| 36 | 2,6-Octadiene, 2,6-dimethyl- | 15.798 | 2792-39-4 | C10H18 | 138.25 | 81, 69, 43, 95 | 0.07 ± 0.01 | 0.38 ± 0.04 | ND | Hydrocarbons |
| 37 | Humulene | 16.152 | 6753-98-6 | C15H24 | 204.351 | 93, 121, 80, 41 | 0.02 ± 0.04 | ND | ND | Hydrocarbons |
| 38 | α-Terpineol | 16.226 | 98–55-5 | C10H18O | 154.249 | 59, 93, 121,136 | 0.45 ± 0.06 | 1.13 ± 0.11 | 0.60 ± 0.04 | Alcohols |
| 39 | Dodecanal | 16.659 | 112–54-9 | C12H24O | 184.318 | 57, 43, 41, 82 | ND | 0.10 ± 0.09 | 0.05 ± 0.04 | aldehydes |
| 40 | β-copaene | 16.769 | 18,252–44-3 | C15H24 | 204.351 | 69, 161, 93, 41 | ND | 0.81 ± 0.08 | ND | Hydrocarbons |
| 41 | neryl propionate | 16.775 | 105–91-9 | C13H22O2 | 210.313 | 69, 93, 41, 43 | 0.26 ± 0.02 | ND | ND | Esters |
| 42 | Carvone | 16.854 | 99–49-0 | C10H14O | 150.218 | 82, 108, 54, 93 | ND | 0.47 ± 0.05 | 0.07 ± 0.01 | ketones |
| 43 | Valencene | 16.916 | 4630-07-3 | C15H24 | 204.351 | 161, 91, 79, 119 | 0.18 ± 0.02 | ND | ND | Hydrocarbons |
| 44 | (−)-cis-Isopiperitenol | 17.08 | 96,555–02-1 | C10H16O | 152.233 | 84, 83, 41, 69 | 0.02 ± 0.03 | 0.08 ± 0.07 | ND | Alcohols |
| 45 | Citronellol | 17.27 | 106–22-9 | C10H20O | 156.265 | 69, 41, 67, 55 | ND | 0.66 ± 0.06 | ND | Alcohols |
| 46 | α-Farnesene | 17.288 | 502–61-4 | C15H24 | 204.351 | 93, 69, 41, 107 | 0.29 ± 0.25 | ND | 0.37 ± 0.32 | Hydrocarbons |
| 47 | (+)-Δ-Cadinene | 17.496 | 483–76-1 | C15H24 | 204.351 | 161, 119, 134, 105 | 0.16 ± 0.02 | 0.31 ± 0.03 | 0.05 ± 0.01 | Hydrocarbons |
| 48 | dl-Perillaldehyde | 17.654 | 2111-75-3 | C10H14O | 150.218 | 79, 68, 67, 107 | 0.12 ± 0.02 | 0.24 ± 0.02 | 0.08 ± 0.01 | aldehydes |
| 49 | trans-p-mentha-1(7),8-dien-2-ol | 17.734 | 21,391–84-4 | C10H16O | 152.233 | 109, 134, 91, 119 | ND | ND | 0.12 ± 0.01 | Alcohols |
| 50 | Nerol | 17.783 | 106–25-2 | C10H18O | 154.249 | 69, 41, 93, 68 | 0.01 ± 0.02 | 0.12 ± 0.01 | ND | Alcohols |
| 51 | t-Butylbenzene | 17.874 | 98–06-6 | C10H14 | 134.218 | 119, 43, 59, 134 | 0.03 ± 0.03 | ND | ND | Hydrocarbons |
| 52 | cis-carveol | 18.314 | 1197-06-4 | C10H16O | 152.233 | 109, 84, 83, 55 | 0.16 ± 0.02 | 0.19 ± 0.02 | 0.05 ± 0.05 | Alcohols |
| 53 | Geraniol | 18.497 | 106–24-1 | C10H18O | 154.249 | 69, 43, 135, 41 | 0.02 ± 0.02 | 0.03 ± 0.06 | ND | Alcohols |
| 54 | cherry propanol | 18.503 | 1197-01-9 | C10H14O | 150.218 | 135, 43, 91, 150 | ND | ND | 0.02 ± 0.02 | Alcohols |
| 55 | Carveol | 18.772 | 99–48-9 | C10H16O | 152.233 | 84, 109, 134, 69 | ND | 0.12 ± 0.05 | 0.01 ± 0.02 | Alcohols |
| 56 | cis-isocarveol | 19.108 | 22,626–43-3 | C10H16O | 152.233 | 109, 55, 67, 41 | ND | ND | 0.07 ± 0.06 | Alcohols |
| 57 | Menth-1-en-9-ol | 19.846 | 18,479–68-0 | C10H18O | 154.249 | 45, 94, 121, 67 | ND | 0.03 ± 0.02 | ND | Alcohols |
| 58 | perill alcohol | 20.835 | 536–59-4 | C10H16O | 152.233 | 79, 67, 121, 91 | ND | 0.01 ± 0.02 | ND | Alcohols |
| 59 | Nerolidol, cis-(+) | 21.574 | 142–50-7 | C15H26O | 222.366 | 69, 93, 107, 41 | ND | 0.06 ± 0.01 | ND | Alcohols |
| 60 | methyl n-methylanthranilate | 21.758 | 85–91-6 | C9H11NO2 | 165.189 | 165, 105, 104, 132 | ND | ND | 1.36 ± 0.01 | Esters |
| 61 | α-elemol | 22.002 | 639–99-6 | C15H26O | 222.366 | 59, 93, 161, 81 | 0.08 ± 0.01 | ND | ND | Alcohols |
| 62 | Thymol | 22.704 | 89–83-8 | C10H14O | 150.218 | 135, 150, 91, 115 | 0.05 ± 0.00 | 0.06 ± 0.00 | 0.05 ± 0.01 | Phenols |
| 63 | o-Acetyl-p-cresol | 22.857 | 1450–72-2 | C9H10O2 | 150.174 | 135, 150, 106, 77 | 0.14 ± 0.01 | ND | ND | ketones |
| 64 | carvacrol | 22.985 | 499–75-2 | C10H14O | 150.218 | 135, 150, 91, 77 | ND | 0.03 ± 0.03 | ND | Phenols |
| 65 | o-Isopropylanisole | 22.991 | 2944-47-0 | C10H14O | 150.218 | 135, 150, 91, 136, 133 | ND | 0.01 ± 0.02 | ND | Ether |
| 66 | o-cymen-5-ol | 23.07 | 3228-02-2 | C10H14O | 150.218 | 135, 150, 91, 136, 107 | ND | 0.16 ± 0.14 | ND | Phenols |
| 67 | α-sinensal | 24.243 | 17,909–77-2 | C15H22O | 114.189 | 93, 45, 55, 134 | ND | 0.15 ± 0.27 | 0.46 ± 0.05 | aldehydes |
Note: Data are presented as mean ± standard deviation of three replicate measurements; RT indicates retention time; ND represents not detected.
Fig. 1.
GC chromatograms of the VOCs of ZJP, WZP, and XHP. (1) L-(−)-alpha-pinene; (2) β-Pinene; (3) β-Myrcene; (4) d-Limonene; (5) γ-Terpinene; (6) o-Cymene; (7) Terpinolene; (8) Linalool; (9) α-Terpineol; (10) methyl n-methylanthranilate.
3.2. Metabolomic analysis of ZJP
3.2.1. Metabolite profiles
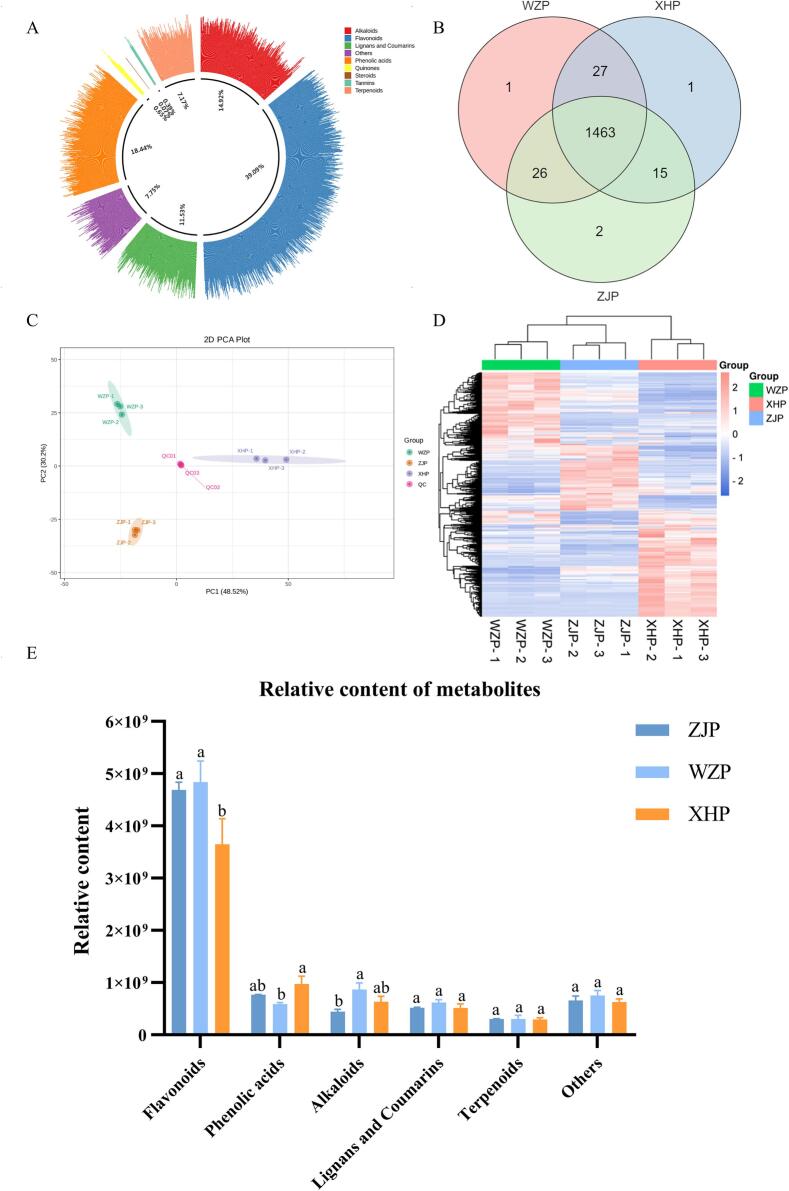
WZP and XHP are included in the Chinese Pharmacopoeia and are utilized as herbal medicines, benefiting from extensive and standardized cultivation bases across China. Consequently, they have been extensively studied and well-researched, unlike ZJP, which remains underexplored. To comprehensively resolve the metabolic information of ZJP and facilitate a detailed comparison of its metabolic profile with WZP and XHP, widely targeted metabolomics based on UPLC-ESI-MS/MS was employed to analyze citrus peel metabolites. A total of 1535 metabolites were detected in the three citrus peel species, classified into nine groups (Fig. 2A; Table S1): 600 flavonoids (39.09%), 283 phenolic acids (18.44%), 229 alkaloids (14.92%), 177 lignans and coumarins (11.53%), 110 terpenoids (7.17%), 10 quinones (0.65%), 6 tannins (0.39%), 1 steroid (< 0.1%), and 117 other species (7.75%). The metabolite count identified in this study surpasses that of previous studies (Wang, Chen, et al., 2022; Wang, Peng, Gao, et al., 2024; Yuan et al., 2024), highlighting the efficacy of UPLC-ESI-MS/MS-based widely targeted metabolomics in metabolite identification. The primary components of citrus peels—flavonoids, phenolic acids, and alkaloids—endow them with a broad spectrum of bioactivities, facilitating their use in functional food production. ZJP revealed 1506 metabolites, including Bergaptol and dimethoxylamine, absent in WZP and XHP (Fig. 2B). Bergaptol, a furanocoumarin commonly found in Citrus spp., is non-phototoxic and non-photomutagenic. Modern pharmacological studies have demonstrated Bergaptol's anti-inflammatory, antioxidant, anticancer, anti-osteoporosis, antibacterial, and antilipidemic effects (Phucharoenrak & Trachootham, 2024), along with robust antioxidant activity and free radical scavenging abilities compared to ascorbic acid (Girennavar et al., 2007).
Fig. 2.
Differences and metabolite profiles between ZJP, WZP, and XHP. (A) Pie chart categorizing identified metabolites. (B) Metabolite distribution. (C) PCA analysis. (D) Heatmap of metabolite clustering. (E) Relative content of metabolites. Each peak representing a metabolite and peak height indicating relative content. Different letters in the graphs indicate significant differences in the same index (P < 0.05).
PCA score plot extracted two principal components (PC1 and PC2), accounting for 48.52% and 30.2% of the variance, respectively. The tight clustering of the three replicate QC samples confirmed the reproducibility and reliability of the experiment, with ZJP distinctly separated from WZP and XHP (Fig. 2C), indicating significant differences among the three citrus peels. Additionally, heatmap categorization clearly distinguished the three classes (Fig. 2D), signifying substantial differences in the metabolite content of ZJP compared to WZP and XHP. The relative contents of various metabolites are depicted in Fig. 2E, showing higher relative contents of flavonoids, alkaloids, coumarins, and lignans in WZP, while phenolic acids were more abundant in XHP. Interestingly, ZJP did not exhibit significant enrichment in a major class of compounds but displayed higher relative contents of flavones, isoflavones, flavanonols, and phenolamines compared to WZP and XHP (Fig. S6).
3.2.2. Key differential metabolites
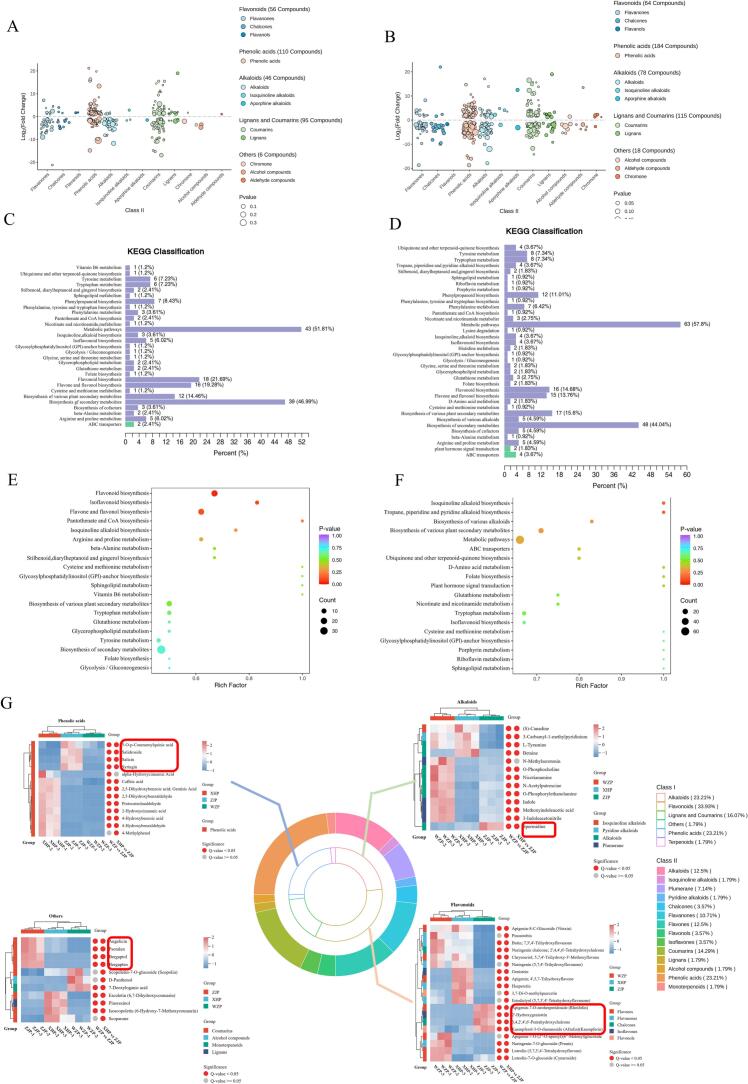
Comparative metabolomics comprehensively characterizes the chemical compositions across different samples, facilitating the identification of key active metabolites (Wang, Chen, et al., 2022; Wang, Hu, Chen, et al., 2023). In this study, ZJP served as the control group, with WZP and XHP as experimental groups, to analyze metabolic differences. OPLS-DA pairwise comparisons between WZP and ZJP (R2X = 0.778, R2Y = 1, Q2 = 0.987) and between XHP and ZJP (R2X = 0.801, R2Y = 1, Q2 = 0.993) assessed these differences. The Q2 values for all control groups exceeded 0.9, indicating model stability (Fig. S7). The distinct separation in these pairwise comparisons confirmed significant differences in their metabolic profiles (Fig. S8). DMs were screened using the criteria of FC ≥ 2 or FC ≤ 0.5 and VIP > 1, with results visualized in volcano plots (Fig. S9). Between WZP and ZJP, 731 DMs were identified, focusing on phenolic acids, lignans, coumarins, flavonoids, and alkaloids (Fig. 3A). Among these, 17 DMs, such as Psoralen and Angelicin, were unique to ZJP. Although isoflavones and phenolic acids were most abundant among the common compounds, the top five upregulated metabolites in ZJP included four lignans and coumarins—Aurapten (2706.73-fold), Bergapten (1712.67-fold), Isobergapten (1703.61-fold), and Sphondin (700.82-fold)—and the phenolic acid Protocatechuic acid glucosyl xyloside (351.56-fold). In comparisons between XHP and ZJP, 955 DMs were identified, also focusing on flavonoids, phenolic acids, lignans, coumarins, and alkaloids (Fig. 3B). Of these, 25 DMs were unique to ZJP. The top five upregulated metabolites in ZJP were Kaempferol-3-O-rutinoside-7-O-rhamnoside (2385.37-fold), Zanthoxyloside (2147.56-fold), Neoeriocitrin (1902.24-fold), Sibiricin (1814.66-fold), and Hydrangeifolin I (1598.11-fold). Additionally, PMFs were notably downregulated in ZJP, including 2’-Hydroxy-3′,4′,6′,3,4-pentamethoxychalcone (0.0010-fold), 5,6,7,8,3′,4’-Hexamethoxyflavanone (0.0012-fold), 6’-Hydroxy-2,4,2′,3′,4′,5’-Hexamethoxychalcone (0.0014-fold), and 6’-Hydroxy-4,2′,3′,4′,5’-Pentamethoxychalcone (0.0034-fold). Interestingly, while glycosides did not exhibit the most significant differences, they were consistently more upregulated in ZJP in both comparison groups.
Fig. 3.
DMs analysis between ZJP vs. WZP and XHP. (A) WZP vs. ZJP DMs bubble plots. (B) XHP vs. ZJP DMs bubble plots. (C) WZP vs. ZJP KEGG pathway annotation. (D) XHP vs. ZJP KEGG pathway annotations. (E) WZP vs. ZJP KEGG enrichment analysis. (F) XHP vs. ZJP KEGG enrichment analysis. (G) Key DMs with clustering analysis.
Bubble plots were created by selecting the top 5 Class I categories, sorted by the number of substances in Class I. For each Class I category, the top 3 Class II categories were plotted, sorted by the number of substances in Class II. The size of the points represents the P-value. In the KEGG enrichment plots, the horizontal coordinate indicates the Rich Factor of each pathway, the vertical coordinate lists the pathway names (sorted by P-value), the color of the dots reflects the P-value size (with red indicating more significant enrichment), and the size of the dots represents the number of enriched DMs. Metabolites circled in the clustering heatmap represent significant up-regulation in ZJP (P < 0.05). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
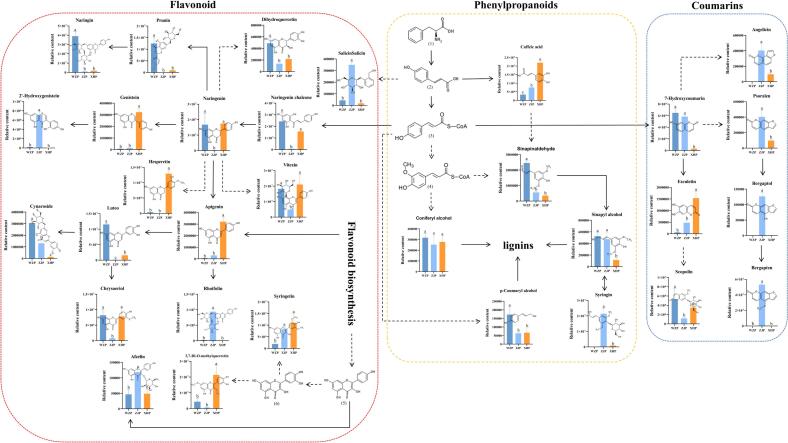
KEGG pathway annotation revealed that most DMs were primarily involved in the biosynthesis of flavonoids, phenylpropanoids, and alkaloids, as well as amino acid biosynthesis (Fig. 3C-D). The phenylpropanoid metabolic pathway enhances plant resistance and participates in defense mechanisms through the synthesis of phenolic compounds, flavonoids, lignans, and alkaloids (Jin et al., 2009). Activation of the phenylpropanoid pathway increases the activity of related enzymes and the content of phenylpropanoids in fruits, strengthening the cell wall and preventing pathogenic bacterial invasion. This has significant implications for the preservation and antisepsis of fruits and vegetables (Wang et al., 2019). KEGG enrichment analysis demonstrated that the DMs between WZP and ZJP were mainly enriched in the biosynthesis of flavonoids, pantothenic acid, and coenzyme A (Fig. 3E). Additionally, the DMs between XHP and ZJP were involved in the biosynthesis of amino acids and alkaloids (Fig. 3F). To provide a clearer understanding of DM metabolism, the metabolic pathways of flavonoids, lignans, and coumarins were mapped based on KEGG annotation results (Fig. 4). In the flavonoid synthesis pathway, DMs were more prevalent in WZP and XHP, whereas lignans and coumarins were predominantly produced in ZJP. p-Coumaroyl-CoA is ortho-hydroxylated by cinnamoyl-CoA 2′-hydroxylase and subsequently forms 7-Hydroxycoumarin (Umbelliferone) through lactone ring closure (Phucharoenrak & Trachootham, 2024). Umbelliferone, a precursor of several coumarins, is catalyzed by marmesin synthase and psoralen synthase to form Bergaptol, a unique component of ZJP, and ultimately Bergapten, catalyzed by bergaptol 5-O-methyltransferase (Hehmann et al., 2004).
Fig. 4.
Diagram of synthetic pathways of DMs: flavonoids, lignans, and coumarins components. Different letters indicate significant differences (P < 0.05) for the same metabolite, with the absence of a letter indicating the component was not detected in the group. Solid lines indicate direct synthesis pathways, while dashed lines indicate synthesis through multiple steps. (1) L-Phenylalanine; (2) p-Coumaric acid; (3) p-Coumaroyl-CoA; (4) Feruloyl-CoA; (5) Kaempferol; (6) Quercetin.
A Venn diagram of the DMs revealed 509 common DMs in both groups (Fig. S10), with 166 DMs significantly upregulated in both groups, indicating significant biological activity in ZJP. KEGG annotated 56 key DMs with metabolic pathways (Table S2), encompassing 19 pathways such as flavonoid biosynthesis, amino acid metabolism, and glycolysis (Fig. S11A), which were primarily enriched in secondary metabolite synthesis and phenylpropanoid biosynthesis (Fig. S11B). These included 19 flavonoids, 13 phenolic acids, 13 alkaloids, and 9 coumarins and lignans, along with 1 terpenoid and 1 other. 13 metabolites were significantly upregulated in ZJP (Fig. 3G), including Rhoifolin, Salidroside, Salicin, and Angelicin, which exhibited potent anti-inflammatory, antioxidant, and anti-osteoporotic activities.
3.3. Biological activity evaluation
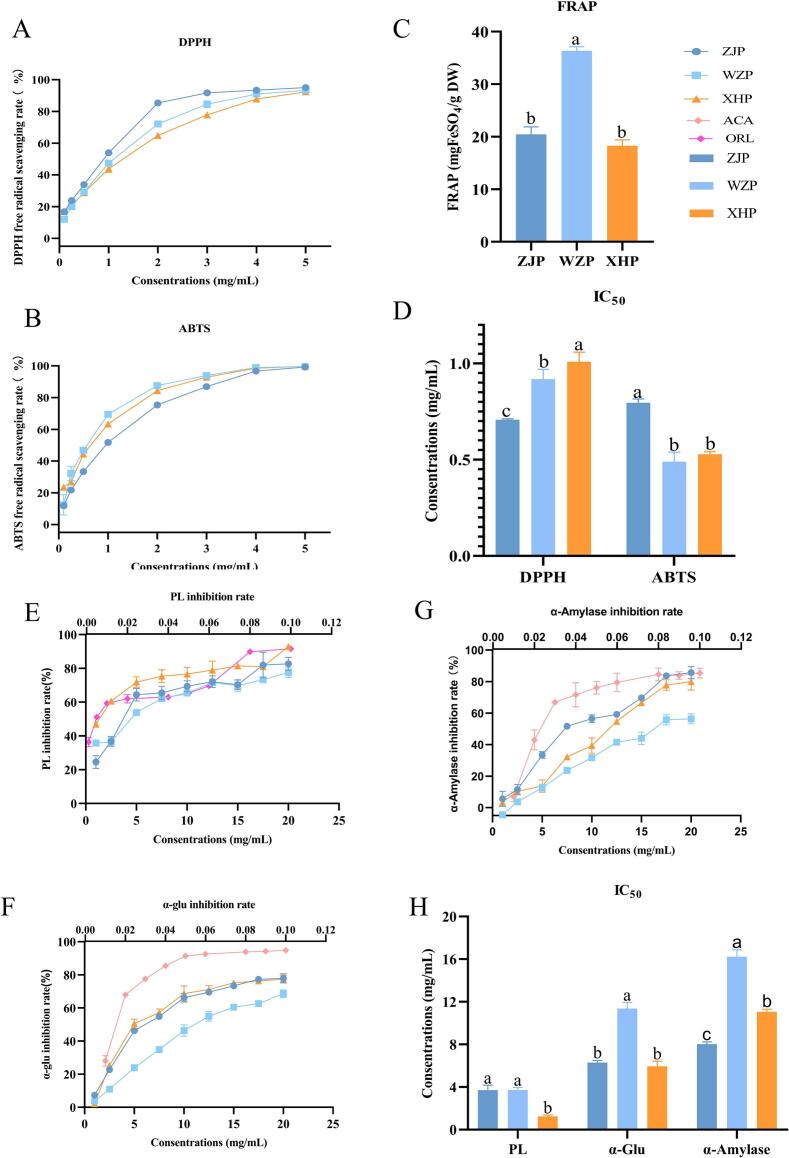
3.3.1. Antioxidant activities
Oxidative stress is linked to various chronic diseases, and natural antioxidants can effectively mitigate the damage caused by oxidative stress. Citrus peels are rich in secondary metabolites such as flavonoids and phenolic acids, which significantly contribute to human health, particularly through their antioxidant activity, making them an ideal source of natural antioxidants (Peng et al., 2022). This study evaluated the antioxidant activity of three citrus peel extracts using DPPH, ABTS, and FRAP methods. All three citrus peel extracts exhibited strong DPPH and ABTS free radical scavenging activities at various concentrations, which increased with concentration (Fig. 5A-B). The highest total antioxidant capacity, as measured by FRAP, was observed in WZP (36.33 mg FeSO4/g DW), significantly surpassing that of ZJP and XHP (P < 0.05), although no significant difference was noted between ZJP and XHP (P > 0.05) (Fig. 5C). The DPPH radical scavenging capacity (IC50) of the three citrus peel extracts ranged from 0.71 to 1.01 mg/mL, with ZJP exhibiting the strongest DPPH radical scavenging rate (IC50 = 0.71 mg/mL), significantly higher than WZP and XHP (P < 0.05). The ABTS radical scavenging capacity (IC50) ranged from 0.49 to 0.80 mg/mL (Fig. 5D), with WZP showing the strongest ABTS radical scavenging capacity (IC50 = 0.49 mg/mL), not significantly different from XHP (P > 0.05), but both were significantly higher than ZJP (P < 0.05). Interestingly, ZJP demonstrated the strongest DPPH radical scavenging capacity but the lowest ABTS scavenging capacity. The mechanisms of different antioxidant capacity assays vary, potentially leading to inconsistent results for the same sample across different methods (Chen et al., 2020).
Fig. 5.
Comparison of bioactivity of three citrus peels. (A) DPPH radical scavenging capacity. (B) ABTS free radical scavenging capacity. (C) FRAP total antioxidant capacity. (D) IC50 value of DPPH and ABTS scavenging rates. (E) PL inhibitory activity. (F) α-glucosidase inhibitory activity. (G) α-amylase inhibitory activity. (H) IC50 value of enzyme activity inhibitory capacities. Different letters in the graphs indicate significant differences in the same index (P < 0.05).
The primary methods that are commonly employed for determining the in vitro antioxidant capacity include inhibition of lipid peroxidation, free radical scavenging capacity, ferric ion reducing capacity, and copper ion reducing capacity (Gulcin, 2020). In addition, the antioxidant capacity determination methods can be divided into those based on electron transfer and hydrogen ion transfer. However, these two mechanisms cannot distinguish between ABTS and DPPH (Apak et al., 2016). Nonetheless, the principle of the assay remains the same: measuring the scavenging capacity against colored free radicals or the ability to inhibit redox-active compounds (Floegel et al., 2011). The assessment of antioxidant capacity involves measuring changes in the color of the colored ABTS and DPPH radicals, or the colorless FRAP, following their reaction with antioxidants (Gulcin, 2020). Factors such as concentration, pH, polarity, and color of the sample can influence the results (Munteanu & Apetrei, 2021), indicating that a single model should not be used to evaluate antioxidant capacity comprehensively. Polyphenols comprise a wide range of compounds that have a benzene ring and multiple hydroxyl groups in their chemical structure. They mainly include flavonoids, phenolic acids, and other compounds. The antioxidant activity of polyphenols lies in their ability to provide hydrogen or electrons and to delocalize unpaired electrons within aromatic structures (Fernandez-Panchon et al., 2008). Polyphenol polarity has an important effect on its biological activity, with a higher number of phenolic hydroxyl groups and a higher polarity associated with a stronger antioxidant activity (Xing et al., 2020). Hagerman et al. (1998) also demonstrated that the potency of antioxidants is associated with the number of phenolic hydroxyl groups they contain. Moreover, Feng and Liu (2009) further found that their antioxidant capacity is determined by the substituted phenolic hydroxyl groups rather than enol hydroxyl groups. Interestingly, a similar discovery was made in lignans (Nsimba et al., 2012). ZJP and WZP have relatively high flavonoid content, which explains their higher antioxidant capacity. The notable antioxidant activity of citrus peels may be attributed to their rich content of phenolic acids, flavonoids, carotenoids, and other compounds (Singh et al., 2020). Specifically, the number and position of hydroxyl groups bound to the aromatic ring significantly affect the antioxidant capacity of phenolic acids (Gulcin, 2020). In conclusion, this study demonstrated that ZJP possesses substantial antioxidant activity, consistent with previous findings (Cao et al., 2023; Wang, Wang, Wang, et al., 2024; Wang, Wang, Xiao, et al., 2024), confirming it as an excellent source of natural antioxidants.
3.3.2. PL inhibitory activities
PL is an essential enzyme in the lipid metabolic pathway, facilitating the conversion of accumulated lipids into fatty acids and monoacylglycerols, which are then absorbed by small intestinal epithelial cells (Li, Xue, et al., 2021). Thus, the application of natural PL inhibitors is crucial in reducing lipid accumulation and promoting lipid catabolism and absorption. This study demonstrated that all three citrus peel extracts inhibited PL activity, with the inhibitory effects intensifying as concentration increased (Fig. 5E). XHP exhibited the most potent PL inhibitory activity (IC50 = 1.24 mg/mL, Fig. 5H), significantly surpassing ZJP (IC50 = 3.71 mg/mL) and WZP (IC50 = 3.70 mg/mL), with no significant difference between the latter two. The robust PL inhibitory capacity of citrus peels may be attributed to the adsorption of insoluble dietary fiber to PL (Yu et al., 2023). Previous studies by Zeng et al. (2018) observed that PL inhibition in XHP progressively increased from August to December, while our earlier research noted a rise and subsequent decline in ZJP PL inhibition from October to December (Wang, Wang, Xiao, et al., 2024).
3.3.3. α-glucosidase and α-amylase inhibitory activities
Hyperglycemia and hyperlipidemia often coexist in patients with diabetes, posing major risk factors for atherosclerotic cardiovascular disease. α-amylase and α-glucosidase catalyze the hydrolysis of starch into products such as maltose, maltotriose, and dextrin, leading to elevated blood glucose levels and hyperglycemia. The α-glucosidase and α-amylase inhibitory activities of ZJP, WZP, and XHP increased with concentration (Fig. 5F-G), with α-glucosidase inhibition stronger than α-amylase inhibition across all three citrus peels, though all were weaker than PL inhibition (Fig. 5H). XHP exhibited slightly stronger α-glucosidase inhibitory activity (IC50 = 5.92 mg/mL) compared to ZJP (IC50 = 6.28 mg/mL), with both significantly higher than WZP (IC50 = 11.35 mg/mL), reflecting an inverse trend to the FRAP total antioxidant capacity. Significant differences in α-amylase inhibitory activities were noted, with ZJP demonstrating the strongest inhibition (IC50 = 8.02 mg/mL), followed by XHP (IC50 = 11.06 mg/mL), and WZP exhibiting the weakest (IC50 = 16.19 mg/mL). Citrus flavonoids significantly inhibit starch digestion, with naringin and neohesperidin primarily inhibiting amylose digestion, while hesperidin and nobiletin inhibit both amylose and amylopectin digestion (Shen et al., 2012).
The dried ripe peels of C. reticulata ‘Chachi’ and C. reticulata ‘Unshiu’, known as “Chenpi” in China, are widely used as herbal medicine and food flavoring agents, and are processed into functional foods such as ‘Jiuzhichenpi’, ‘Chenpi Candy’, and ‘Ganpu Tea’. The aging process of Chenpi is a natural fermentation process (Yang et al., 2022), and “Ganpu tea” is a novel tea beverage made by co-fermenting Puer tea leaves wrapped in intact citrus peels (Deng et al., 2022). Modern pharmacological studies have demonstrated that both “Chenpi” and “Ganpu Tea” possess potent hypolipidemic and hypoglycemic activities (Huang, Zhang, et al., 2020; Wang, Gmitter Jr, et al., 2022; Zeng et al., 2018), corroborated by this study. This research further demonstrated that ZJP exhibits strong PL, α-glucosidase, and α-amylase inhibitory activities, highlighting its potential as a functional product for lowering blood lipids and glucose levels.
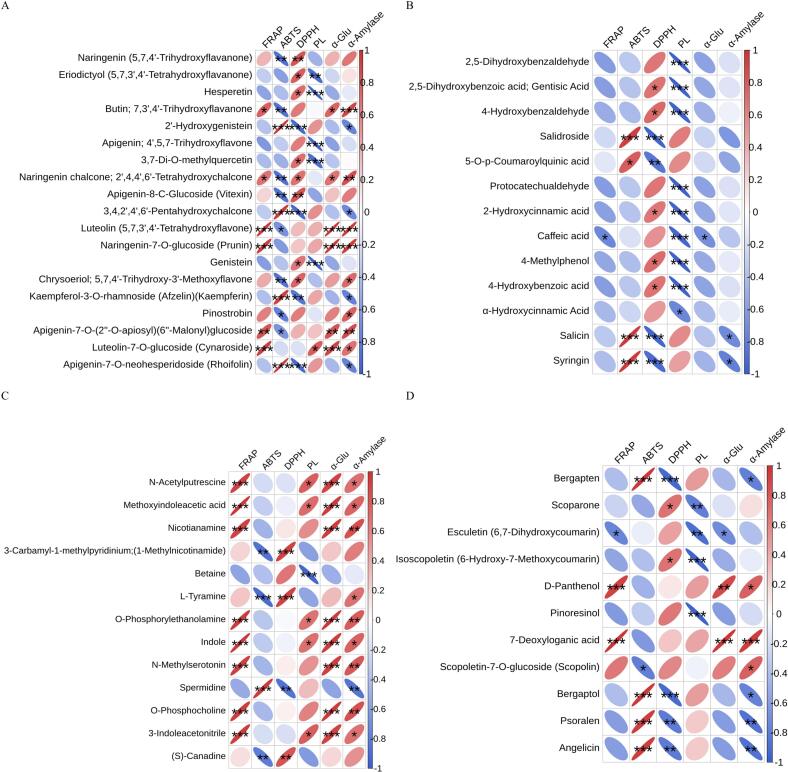
3.4. Correlation analysis
To further analyze activity and key DM correlations, Pearson correlation analyses were conducted for ABTS, DPPH, FRAP, PL, α-glucosidase, and α-amylase inhibitory activities (all expressed as IC50 except FRAP), along with 56 key DMs (Fig. 6A-D). Luteolin, Luteolin-7-O-glucoside, Prunin, Nicotianamine, Indole, and 3-Indoleacetonitrile exhibited significant positive correlations with the total antioxidant capacity measured by FRAP. Conversely, L-Tyramine, Naringenin, Naringenin chalcone, Butin, Vitexin, Chrysoeriol, (S)-Canadine, and Scopolin showed significant negative correlations with ABTS. Metabolites such as 2’-Hydroxygenistein, 3,4,2′,4′,6’-Pentahydroxychalcone, Rhoifolin, Salidroside, Salicin, Syringin, Bergapten, Bergaptol, Angelicin, and Psoralen, which were significantly up-regulated in ZJP, demonstrated significant negative correlations with DPPH. Hesperetin, Apigenin, 3,7-Di-O-methylquercetin, Genistein, Protocatechualdehyde, Caffeic acid, 4-Hydroxybenzoic acid, α-Hydroxycinnamic acid, 2-Hydroxycinnamic acid, Betaine, and Pinoresinol exhibited significant negative correlations with PL inhibitory activity. Spermidine, Bergapten, Bergaptol, Psoralen, and Angelicin showed significant negative correlations with α-amylase inhibitory activity. Interestingly, only Caffeic acid and Esculetin were significantly negatively correlated with α-glucosidase inhibitory activity, while most key DMs were significantly positively correlated with α-glucosidase inhibitory activity. The correlation analysis, combined with experimental data, indicates that the activity of the samples was not solely determined by the key DMs identified. Metabolites such as Gallic acid, Rutin, Nobiletin, Quercetin, Limonin, Ferulate, Chlorogenic acid, and Naringin, although not identified as key DMs in this study, have been extensively researched for their roles in maintaining human health (Singh et al., 2020; Zhao et al., 2020). Additionally, anthocyanins and carotenoids, which were not examined in this study, also play significant roles in plant growth and human health (Rapisarda et al., 2022). In summary, this study revealed that ZJP, WZP, and XHP, along with their metabolites such as Bergapten, Hesperetin, Luteolin, Luteolin-7-O-glucoside, Naringenin, Naringenin chalcone, Chrysoeriol, 2’-Hydroxygenistein, Rhoifolin, and Cynaroside, possess potential antioxidant, hypolipidemic, and hypoglycemic activities. Furthermore, ABTS showed a significant negative correlation with DPPH, and the different mechanisms of ABTS, DPPH, and FRAP assays resulted in varying antioxidant capacities for the same sample (Chen et al., 2020). α-glucosidase and α-amylase, two key enzymes in glucose regulation, exhibited a significant positive correlation with each other and with the FRAP total antioxidant capacity.
Fig. 6.
Correlation analysis of key DMs with antioxidant and enzyme activity inhibition capacity. (A) Flavonoids. (B) Phenolic acids. (C) Alkaloids. (D) Other classes. In the figure, * indicates P < 0.05; ** indicates P < 0.01; *** indicates P < 0.001.
4. Conclusion
This study utilized GC–MS to analyze the volatile components of ZJP, identifying 46 species, predominantly d-Limonene, γ-Terpinene, and β-Myrcene. Additionally, 18 unique components, such as Caryophyllene, Citronellol, and carvacrol, were detected exclusively in ZJP, contributing to its distinctive herbal, woody, and waxy aroma. Among the three citrus peels analyzed, a total of 1535 metabolites were detected, with 1506 found in ZJP. The DMs were concentrated in phenolic acids, lignans, coumarins, flavonoids, and alkaloids. KEGG pathway annotation indicated that most DMs were involved in the biosynthesis of flavonoids, phenylpropanoids, alkaloids, and amino acids, a finding corroborated by enrichment analysis. From the three citrus peels, 509 DMs were screened, and KEGG annotation identified 56 key DMs involved in 19 metabolic pathways, including flavonoid biosynthesis, amino acid metabolism, and glycolysis. These pathways were primarily enriched in the synthesis of secondary metabolites and phenylpropanoid biosynthesis, with 13 metabolites, such as Psoralen and Salicin, significantly up-regulated in ZJP. In vitro activity assays demonstrated that ZJP exhibited the strongest DPPH radical scavenging (IC50 = 0.71 mg/mL) and α-amylase inhibitory activity (IC50 = 8.02 mg/mL). WZP showed the highest ABTS radical scavenging (IC50 = 0.49 mg/mL) and FRAP total antioxidant capacity (36.33 mg FeSO4/g DW), while XHP had the strongest PL and α-glucosidase inhibitory activities (IC50 = 1.24 mg/mL and 5.92 mg/mL, respectively). The study highlighted ZJP's potential antioxidant, hypolipidemic, and hypoglycemic activities. Pearson correlation analysis revealed significant correlations between metabolites such as Bergaptol, Vitexin, and Luteolin with antioxidant activity, and Apigenin, Caffeic acid, Esculetin, Butin, and Rhoifolin with PL, α-glucosidase, and α-amylase inhibitory activities.
This study provided the first comprehensive metabolite profile of ZJP and evaluated its antioxidant, lipid-lowering, and blood sugar-lowering capacities compared to WZP and XHP. The results showed that ZJP was generally similar to WZP and XHP in terms of metabolite composition but differed in their content, with key differential metabolites annotated to pathways such as glycolysis. Meanwhile, the in vitro activity study found that ZJP had greater hypoglycemia capacity compared to hypolipidemic capacity. However, there is still uncertainty regarding the absorption and transformation of metabolites and the mechanism of action of ZJP in glycolipid metabolism, and we will further study the mechanisms underlying the hypolipidemic and hypoglycemia effects of ZJP through in vivo experiments in the future. Besides, we did not compare the composition and activity of ZJP to more citrus peels. In conclusion, our study illustrated that ZJP is an ideal source of natural antioxidants and functional ingredients. Subsequent research should be conducted to develop ZJP into functional health-promoting food products to fully utilize Zhoupigan resources.
CRediT authorship contribution statement
Jialiang Zou: Writing – original draft, Visualization, Methodology, Investigation, Formal analysis, Conceptualization. Peng Wang: Writing – review & editing, Data curation. Huanhuan Xu: Writing – review & editing, Methodology, Data curation. Xuelian Gan: Writing – review & editing, Methodology, Data curation. Huangsheng Zhang: Writing – review & editing, Methodology, Data curation. Lin Chen: Writing – review & editing, Methodology, Data curation. Hongping Chen: Writing – review & editing, Methodology, Data curation. Fu Wang: Writing – review & editing, Methodology, Data curation. Yuan Hu: Validation, Formal analysis. Youping Liu: Writing – review & editing, Supervision, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This study was supported by the National Science Foundation of China (NO. 81973436 and 82104340). In addition, The authors would like to thank all those who contributed directly or indirectly to the project.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2024.101719.
Appendix A. Supplementary data
Supplementary material 1
Supplementary material 2
Supplementary material 3
Data availability
Data will be made available on request.
References
- Alu’datt M.H., Rababah T., Alhamad M.N., Al-Mahasneh M.A., Ereifej K., Al-Karaki G.…Ghozlan K.A. Profiles of free and bound phenolics extracted from Citrus fruits and their roles in biological systems: Content, and antioxidant, anti-diabetic and anti-hypertensive properties. Food & Function. 2017;8(9):3187–3197. doi: 10.1039/c7fo00212b. [DOI] [PubMed] [Google Scholar]
- Apak R., Özyürek M., Güçlü K., Çapanoğlu E. Antioxidant activity/capacity measurement. 1. Classification, physicochemical principles, mechanisms, and electron transfer (ET)-based assays. Journal of Agricultural and Food Chemistry. 2016;64(5):997–1027. doi: 10.1021/acs.jafc.5b04739. [DOI] [PubMed] [Google Scholar]
- Benayad O., Bouhrim M., Tiji S., Kharchoufa L., Addi M., Drouet S.…Mimouni M. Phytochemical profile, α-glucosidase, and α-amylase inhibition potential and toxicity evaluation of extracts from Citrus aurantium (L) Peel, a valuable by-product from northeastern Morocco. Biomolecules. 2021;11(11):1555. doi: 10.3390/biom11111555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X., Shi K., Xu Y., Zhang P., Zhang H., Pan S. Integrated metabolomics and network pharmacology to reveal antioxidant mechanisms and potential pharmacological ingredients of citrus herbs. Food Research International (Ottawa, Ont.) 2023;174(Pt 1) doi: 10.1016/j.foodres.2023.113514. [DOI] [PubMed] [Google Scholar]
- Chen P., Liu J., Wang L., Liu X., Guo L., Li F.…Deng Z. Genetic background of citrus landrace ‘Huarongdao Zhoupigan’revealed by molecular marker and genomic analysis. The 8th International Horticulture Research Conference. 2021;289 doi: 10.1016/j.scienta.2021.110456. [DOI] [Google Scholar]
- Chen Q., Wang D., Tan C., Hu Y., Sundararajan B., Zhou Z. Profiling of flavonoid and antioxidant activity of fruit tissues from 27 Chinese local Citrus cultivars. Plants (Basel, Switzerland) 2020;9(2):196. doi: 10.3390/plants9020196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costanzo G., Vitale E., Iesce M.R., Naviglio D., Amoresano A., Fontanarosa C.…Arena C. Antioxidant properties of pulp, peel and seeds of phlegrean mandarin (Citrus reticulata Blanco) at different stages of fruit ripening. Antioxidants (Basel, Switzerland) 2022;11(2):187. doi: 10.3390/antiox11020187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadwal V., Joshi R., Gupta M. A comparative metabolomic investigation in fruit sections of Citrus medica L. and Citrus maxima L. detecting potential bioactive metabolites using UHPLC-QTOF-IMS. Food Research International (Ottawa, Ont.) 2022;157 doi: 10.1016/j.foodres.2022.111486. [DOI] [PubMed] [Google Scholar]
- Del-Toro-Sánchez C.L., Rodríguez-Félix F., Cinco-Moroyoqui F.J., Juárez J., Ruiz-Cruz S., Wong-Corral F.J.…Tapia-Hernández J.A. Recovery of phytochemical from three safflower (Carthamus tinctorius L.) by-products: Antioxidant properties, protective effect of human erythrocytes and profile by UPLC-DAD-MS. Journal of Food Processing and Preservation. 2021;45(9) doi: 10.1111/jfpp.15765. [DOI] [Google Scholar]
- Deng M., Dong L., Jia X., Huang F., Chi J., Muhammad Z.…Zhang R. The flavonoid profiles in the pulp of different pomelo (Citrus grandis L. Osbeck) and grapefruit (Citrus paradisi Mcfad) cultivars and their in vitro bioactivity. Food Chemistry: X. 2022;15 doi: 10.1016/j.fochx.2022.100368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J.Y., Liu Z.Q. Phenolic and enolic hydroxyl groups in curcumin: Which plays the major role in scavenging radicals? Journal of Agricultural and Food Chemistry. 2009;57(22):11041–11046. doi: 10.1021/jf902244g. [DOI] [PubMed] [Google Scholar]
- Fernandez-Panchon M.S., Villano D., Troncoso A.M., Garcia-Parrilla M.C. Antioxidant activity of phenolic compounds: From in vitro results to in vivo evidence. Critical Reviews in Food Science and Nutrition. 2008;48(7):649–671. doi: 10.1080/10408390701761845. [DOI] [PubMed] [Google Scholar]
- Floegel A., Kim D.O., Chung S.J., Koo S.I., Chun O.K. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. Journal of Food Composition and Analysis. 2011;24(7):1043–1048. doi: 10.1016/j.jfca.2011.01.008. [DOI] [Google Scholar]
- Girennavar B., Jayaprakasha G.K., Jadegoud Y., Nagana Gowda G.A., Patil B.S. Radical scavenging and cytochrome P450 3A4 inhibitory activity of bergaptol and geranylcoumarin from grapefruit. Bioorganic & Medicinal Chemistry. 2007;15(11):3684–3691. doi: 10.1016/j.bmc.2007.03.047. [DOI] [PubMed] [Google Scholar]
- Gómez-Mejía E., Sacristán I., Rosales-Conrado N., León-González M.E., Madrid Y. Valorization of Citrus reticulata Blanco peels to produce enriched wheat bread: Phenolic bioaccessibility and antioxidant potential. Antioxidants (Basel, Switzerland) 2023;12(9):1742. doi: 10.3390/antiox12091742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Governa P., Baini G., Borgonetti V., Cettolin G., Giachetti D., Magnano A.R.…Biagi M. Phytotherapy in the management of diabetes: A review. Molecules (Basel, Switzerland) 2018;23(1):105. doi: 10.3390/molecules23010105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulcin İ. Antioxidants and antioxidant methods: An updated overview. Archives of Toxicology. 2020;94(3):651–715. doi: 10.1007/s00204-020-02689-3. [DOI] [PubMed] [Google Scholar]
- Hagerman A.E., Riedl K.M., Jones G.A., Sovik K.N., Ritchard N.T., Hartzfeld P.W., Riechel T.L. High molecular weight plant polyphenolics (tannins) as biological antioxidants. Journal of Agricultural and Food Chemistry. 1998;46(5):1887–1892. doi: 10.1021/jf970975b. [DOI] [PubMed] [Google Scholar]
- Hehmann M., Lukačin R., Ekiert H., Matern U. Furanocoumarin biosynthesis in Ammi majus L. cloning of bergaptol O-methyltransferase. European Journal of Biochemistry. 2004;271(5):932–940. doi: 10.1111/j.1432-1033.2004.03995.x. [DOI] [PubMed] [Google Scholar]
- Huang R., Zhang Y., Shen S., Zhi Z., Cheng H., Chen S., Ye X. Antioxidant and PL inhibitory effects of flavonoids from different citrus peel extracts: An in vitro study. Food Chemistry. 2020;326 doi: 10.1016/j.foodchem.2020.126785. [DOI] [PubMed] [Google Scholar]
- Huang X., Zhu J., Wang L., Jing H., Ma C., Kou X., Wang H. Inhibitory mechanisms and interaction of tangeretin, 5-demethyltangeretin, nobiletin, and 5-demethylnobiletin from citrus peels on PL: Kinetics, spectroscopies, and molecular dynamics simulation. International Journal of Biological Macromolecules. 2020;164:1927–1938. doi: 10.1016/j.ijbiomac.2020.07.305. [DOI] [PubMed] [Google Scholar]
- Jia X., Wu F., Lu A., Tan D., Zhang Q., He Y., Qin L. Widely targeted metabolomics analysis of Dendrobium officinale at different altitudes. Chemistry & Biodiversity. 2023;20(4) doi: 10.1002/cbdv.202201082. [DOI] [PubMed] [Google Scholar]
- Jin P., Zheng Y., Tang S., Rui H., Wang C.Y. Enhancing disease resistance in peach fruit with methyl jasmonate. Journal of the Science of Food and Agriculture. 2009;89(5):802–808. doi: 10.1002/jsfa.3516. [DOI] [Google Scholar]
- Kong F., Ding Z., Zhang K., Duan W., Qin Y., Su Z., Bi Y. Optimization of extraction flavonoids from Exocarpium Citri Grandis and evaluation its hypoglycemic and hypolipidemic activities. Journal of Ethnopharmacology. 2020;262 doi: 10.1016/j.jep.2020.113178. [DOI] [PubMed] [Google Scholar]
- Laganà V., Giuffrè A.M., De Bruno A., Poiana M. Formulation of biscuits fortified with a flour obtained from bergamot by-products (Citrus bergamia, Risso) Foods (Basel, Switzerland) 2022;11(8):1137. doi: 10.3390/foods11081137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leporini M., Loizzo M.R., Sicari V., Pellicanò T.M., Reitano A., Dugay A.…Tundis R. Citrus × Clementina Hort. Juice enriched with its by-products (peels and leaves): Chemical composition, in vitro bioactivity, and impact of processing. Antioxidants (Basel, Switzerland) 2020;9(4):298. doi: 10.3390/antiox9040298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R., Xue Z., Jia Y., Wang Y., Li S., Zhou J.…Chen H. Polysaccharides from mulberry (Morus alba L.) leaf prevents obesity by inhibiting PL in high-fat diet induced mice. International Journal of Biological Macromolecules. 2021;192:452–460. doi: 10.1016/j.ijbiomac.2021.10.010. [DOI] [PubMed] [Google Scholar]
- Li W., Wen L., Chen Z., Zhang Z., Pang X., Deng Z.…Guo Y. Study on metabolic variation in whole grains of four proso millet varieties reveals metabolites important for antioxidant properties and quality traits. Food Chemistry. 2021;357 doi: 10.1016/j.foodchem.2021.129791. [DOI] [PubMed] [Google Scholar]
- Liu N., Li X., Zhao P., Zhang X., Qiao O., Huang L.…Gao W. A review of chemical constituents and health-promoting effects of citrus peels. Food Chemistry. 2021;365 doi: 10.1016/j.foodchem.2021.130585. [DOI] [PubMed] [Google Scholar]
- Liu Y., Wen H., Kong J., Hu Z., Hu Y., Zeng J.…Xu J. Flavor characterization of Citri Reticulatae Pericarpium (Citrus reticulata “Chachiensis”) with different aging years via sensory and metabolomic approaches. Food Chemistry. 2024;443 doi: 10.1016/j.foodchem.2024.138616. [DOI] [PubMed] [Google Scholar]
- Loizzo M.R., Sicari V., Tundis R., Leporini M., Falco T., Calabrò V. The influence of ultrafiltration of Citrus limon L. Burm. cv Femminello Comune juice on its chemical composition and antioxidant and hypoglycemic properties. Antioxidants (Basel, Switzerland) 2019;8(1):23. doi: 10.3390/antiox8010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munteanu I.G., Apetrei C. Analytical methods used in determining antioxidant activity: A review. International Journal of Molecular Sciences. 2021;22(7):3380. doi: 10.3390/ijms22073380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nsimba R.Y., West N., Boateng A.A. Structure and radical scavenging activity relationships of pyrolytic lignins. Journal of Agricultural and Food Chemistry. 2012;60(51):12525–12530. doi: 10.1021/jf3037787. [DOI] [PubMed] [Google Scholar]
- Peng M., Gao Z., Liao Y., Guo J., Shan Y. Development of citrus-based functional jelly and an investigation of its anti-obesity and antioxidant properties. Antioxidants (Basel, Switzerland) 2022;11(12):2418. doi: 10.3390/antiox11122418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phucharoenrak P., Trachootham D. Bergaptol, a major furocoumarin in citrus: Pharmacological properties and toxicity. Molecules (Basel, Switzerland) 2024;29(3):713. doi: 10.3390/molecules29030713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian G., Li X., Zhang H., Zhang H., Zhou J., Ma X.…Li L. Metabolomics analysis reveals the accumulation patterns of flavonoids and phenolic acids in quinoa (Chenopodium quinoa Willd.) grains of different colors. Food Chemistry: X. 2023;17 doi: 10.1016/j.fochx.2023.100594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qurtam A.A., Mechchate H., Es-Safi I., Al-Zharani M., Nasr F.A., Noman O.M.…Alqahtani A.S. Citrus flavanone narirutin, in vitro and in silico mechanistic antidiabetic potential. Pharmaceutics. 2021;13(11):1818. doi: 10.3390/pharmaceutics13111818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapisarda P., Amenta M., Ballistreri G., Fabroni S., Timpanaro N. Distribution, antioxidant capacity, bioavailability and biological properties of anthocyanin pigments in blood oranges and other Citrus species. Molecules (Basel, Switzerland) 2022;27(24):8675. doi: 10.3390/molecules27248675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J., Wang H., Sun L., Fan K., Zhang X., Huang Q.…Ding Z. Metabolic variations among three new tea varieties cultivated in Shandong, China. Foods (Basel, Switzerland) 2023;12(6):1299. doi: 10.3390/foods12061299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W., Xu Y., Lu Y.H. Inhibitory effects of Citrus flavonoids on starch digestion and antihyperglycemic effects in HepG2 cells. Journal of Agricultural and Food Chemistry. 2012;60(38):9609–9619. doi: 10.1021/jf3032556. [DOI] [PubMed] [Google Scholar]
- Singh B., Singh J.P., Kaur A., Singh N. Phenolic composition, antioxidant potential and health benefits of citrus peel. Food Research International (Ottawa, Ont.) 2020;132 doi: 10.1016/j.foodres.2020.109114. [DOI] [PubMed] [Google Scholar]
- Tao N., Jia L., Zhou H. Anti-fungal activity of Citrus reticulata Blanco essential oil against Penicillium italicum and Penicillium digitatum. Food Chemistry. 2014;153:265–271. doi: 10.1016/j.foodchem.2013.12.070. [DOI] [PubMed] [Google Scholar]
- Wang C., Peng M., Gao Z., Han Q., Fu F., Li G.…Shan Y. Untargeted metabolomic analyses and antilipidemic effects of citrus physiological premature fruit drop. International Journal of Molecular Sciences. 2024;25(3):1876. doi: 10.3390/ijms25031876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F., Chen L., Chen H., Yan Z., Liu Y. Discovery of the key active compounds in Citri Reticulatae Pericarpium (Citrus reticulata “Chachi”) and their therapeutic potential for the treatment of COVID-19 based on comparative metabolomics and network pharmacology. Frontiers in Pharmacology. 2022;13:1048926. doi: 10.3389/fphar.2022.1048926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F., Hu H., Li Y., Zhong J., Pan H., Sheng Y., Bi Y., Kong F. Characterization, antioxidant and α-amylase inhibition of polysaccharides and phosphorylated derivatives from finger citron (Citrus medica L. var. sarcodactylis Swingle) Food Bioscience. 2024;59 doi: 10.1016/j.fbio.2024.103985. [DOI] [Google Scholar]
- Wang F., Hu Y., Chen H., Chen L., Liu Y. Exploring the roles of microorganisms and metabolites in the 30-year aging process of the dried pericarps of Citrus reticulata 'Chachi' based on high-throughput sequencing and comparative metabolomics. Food Research International (Ottawa, Ont.) 2023;172 doi: 10.1016/j.foodres.2023.113117. [DOI] [PubMed] [Google Scholar]
- Wang H., Ren J., Zhou S., Duan Y., Zhu C., Chen C.…Zhang F. Molecular regulation of oil gland development and biosynthesis of essential oils in Citrus spp. Science (New York, N.Y.) 2024;383(6683):659–666. doi: 10.1126/science.adl2953. [DOI] [PubMed] [Google Scholar]
- Wang H., Wang P., Wang F., Chen H., Chen L., Hu Y., Liu Y. Integrated HS-GC-IMS and UPLC-Q-Orbitrap HRMS-based metabolomics revealed the characteristics and differential volatile and nonvolatile metabolites of different citrus peels. Current Research in Food Science. 2024;8 doi: 10.1016/j.crfs.2024.100755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Wang Y., Shi Z., Zhao L., Jian W., Li K.…Peng W. Association between dietary patterns and metabolic syndrome and modification effect of altitude: A cohort study of Tibetan adults in China. Nutrients. 2023;15(9):2226. doi: 10.3390/nu15092226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Wu Y., Yu R., Wu C., Fan G., Li T. Effects of postharvest application of methyl jasmonate on physicochemical characteristics and antioxidant system of the blueberry fruit. Scientia Horticulturae. 2019;258 doi: 10.1016/j.scienta.2019.108785. [DOI] [Google Scholar]
- Wang P., Wang H., Xiao Y., Zou J., Chen H., Chen L.…Liu Y. Insights into metabolic characteristics and biological activity changes in Zangju (Citrus reticulata cv. Manau Gan) peel at different maturity stages through UPLC-MS/MS-based metabolomics. Food Chemistry. 2024;X, 21 doi: 10.1016/j.fochx.2024.101197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P., Wang H., Zou J., Chen L., Chen H., Hu Y.…Liu Y. Electronic nose and head space GC-IMS provide insights into the dynamic changes and regularity of volatile compounds in Zangju (Citrus reticulata cv. Manau Gan) Peel at different maturation stages. Molecules (Basel, Switzerland) 2023;28(14):5326. doi: 10.3390/molecules28145326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Li Y., Huang D., Chen S., Xia Y., Zhu S. The inhibitory mechanism of chlorogenic acid and its acylated derivatives on α-amylase and α-glucosidase. Food Chemistry. 2022;372 doi: 10.1016/j.foodchem.2021.131334. [DOI] [PubMed] [Google Scholar]
- Wang S., Ren H., Zhong H., Zhao X., Li C., Ma J.…Wang W. Combined berberine and probiotic treatment as an effective regimen for improving postprandial hyperlipidemia in type 2 diabetes patients: A double blinded placebo controlled randomized study. Gut Microbes. 2022;14(1):2003176. doi: 10.1080/19490976.2021.2003176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Gmitter F.G., Jr., Grosser J.W., Wang Y. Natural sweeteners and sweetness-enhancing compounds identified in Citrus using an efficient metabolomics-based screening strategy. Journal of Agricultural and Food Chemistry. 2022;70(34):10593–10603. doi: 10.1021/acs.jafc.2c03515. [DOI] [PubMed] [Google Scholar]
- Wu X., He W., Yao L., Zhang H., Liu Z., Wang W.…Cao J. Characterization of binding interactions of (−)-epigallocatechin-3-gallate from green tea and lipase. Journal of Agricultural and Food Chemistry. 2013;61(37):8829–8835. doi: 10.1021/jf401779z. [DOI] [PubMed] [Google Scholar]
- Xiao Z., Sun X., Zhaxi D., Zhang F., Ji Y., Cheng T.…Xu X. Distinct nutrient intake style in inhabitants of ultra-high-altitude areas in north of Tibet, China: A cross-sectional study based on newly developed Tibetan food frequency questionnaires. Frontiers in Nutrition. 2021;8 doi: 10.3389/fnut.2021.743896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing H., Huang L., Ding B., Zhao W., Zhao J., Zhang X., Wang X. Effects of in vitro digestion on antioxidant activity and bioavailability of plant polyphenols with different polarities. Food and Fermentation Industries. 2020;16:70–77. doi: 10.13995/j.cnki.11-1802/ts.023627. [DOI] [Google Scholar]
- Yang J., Qiu M., Lu T., Yang S., Yu J., Lin J.…Zhang D. Discovery and verification of bitter components in Panax notoginseng based on the integrated strategy of pharmacophore model, system separation and bitter tracing technology. Food Chemistry. 2023;428 doi: 10.1016/j.foodchem.2023.136716. [DOI] [PubMed] [Google Scholar]
- Yang M., Jiang Z., Wen M., Wu Z., Zha M., Xu W., Zhang L. Chemical variation of Chenpi (Citrus peels) and corresponding correlated bioactive compounds by LC-MS metabolomics and multibioassay analysis. Frontiers in Nutrition. 2022;9 doi: 10.3389/fnut.2022.825381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu B., Tang Q., Fu C., Regenstein J., Huang J., Wang L. Effects of different particle-sized insoluble dietary fibre from citrus peel on adsorption and activity inhibition of PL. Food Chemistry. 2023;398 doi: 10.1016/j.foodchem.2022.133834. [DOI] [PubMed] [Google Scholar]
- Yuan Y., Duan Y., Zhang Q., Hou J., Xu C., Zhao J.…Wang Y. Untargeted metabolomics analysis of Gannan navel orange at different storage periods under room temperature using HS-SPME-GC-MS and UPLC-Q-TOF/MS. Food Chemistry. 2024;440 doi: 10.1016/j.foodchem.2023.138186. [DOI] [PubMed] [Google Scholar]
- Zeng S.L., Li S.Z., Lai C.J., Wei M.Y., Chen B.Z., Li P.…Liu E.H. Evaluation of anti-lipase activity and bioactive flavonoids in the Citri Reticulatae Pericarpium from different harvest time. Phytomedicine :Iinternational Journal of Phytotherapy and Phytopharmacology. 2018;43:103–109. doi: 10.1016/j.phymed.2018.04.008. [DOI] [PubMed] [Google Scholar]
- Zhang B.W., Li X., Sun W.L., Xing Y., Xiu Z.L., Zhuang C.L., Dong Y.S. Dietary flavonoids and acarbose synergistically inhibit α-glucosidase and lower postprandial blood glucose. Journal of Agricultural and Food Chemistry. 2017;65(38):8319–8330. doi: 10.1021/acs.jafc.7b02531. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Sun Y., Xi W., Shen Y., Qiao L., Zhong L.…Zhou Z. Phenolic compositions and antioxidant capacities of Chinese wild mandarin (Citrus reticulata Blanco) fruits. Food Chemistry. 2014;145:674–680. doi: 10.1016/j.foodchem.2013.08.012. [DOI] [PubMed] [Google Scholar]
- Zhao C., Wang F., Lian Y., Xiao H., Zheng J. Biosynthesis of citrus flavonoids and their health effects. Critical Reviews in Food Science and Nutrition. 2020;60(4):566–583. doi: 10.1080/10408398.2018.1544885. [DOI] [PubMed] [Google Scholar]
- Zheng W., Zhang W., Liu D., Yin M., Wang X., Wang S.…Ma Z. Evolution-guided multiomics provide insights into the strengthening of bioactive flavone biosynthesis in medicinal pummelo. Plant Biotechnology Journal. 2023;21(8):1577–1589. doi: 10.1111/pbi.14058. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1
Supplementary material 2
Supplementary material 3
Data Availability Statement
Data will be made available on request.