Abstract
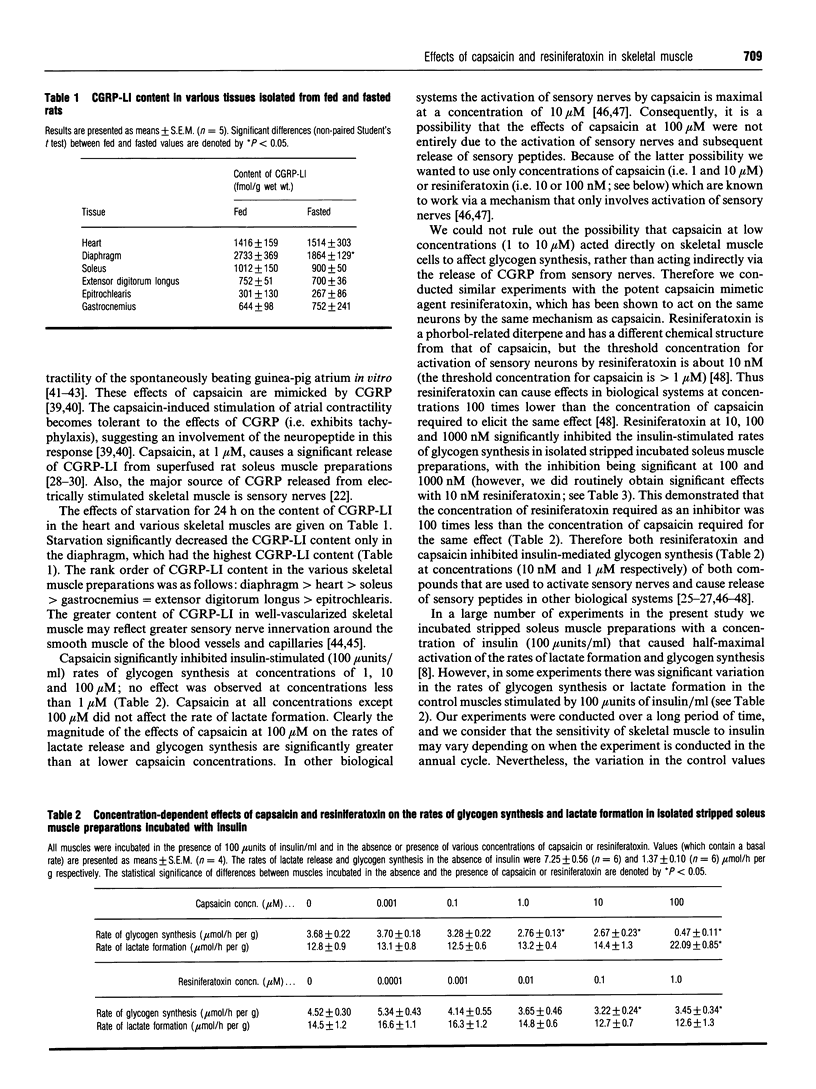
1. The content of calcitonin-gene-related-peptide-like immunoreactivity (CGRP-LI) in various rat muscles was measured. Starvation for 24 h did not affect the content of CGRP-LI in these muscles, except for a decreased level in the starved-rat diaphragm. Higher contents of CGRP-LI were observed in well-vascularized muscles. 2. Capsaicin (at 1, 10 and 100 microM) inhibited insulin-stimulated rates of glycogen synthesis in isolated stripped incubated soleus muscle preparations by a mechanism independent of catecholamine release, since the effects of capsaicin were not altered by the beta-adrenoreceptor antagonist DL-propranolol. 3. Resiniferatoxin (10 nM), which is a potent capsaicin agonist, also significantly inhibited the insulin-stimulated rate of glycogen synthesis. Furthermore, the concentration of resiniferatoxin required to inhibit glycogen synthesis was 100 times less than the concentration of capsaicin needed for the same effect. 4. Capsaicin (10 microM) decreased the content of CGRP-LI in isolated stripped incubated soleus muscle preparations by about 40%. 5. Neonatal treatment of rats with capsaicin, which causes de-afferentation of some sensory nerves such, we hypothesize, that CGRP can no longer be released to counteract the effects of insulin in vivo, caused increased rates of glycogen synthesis and increased glycogen content in stripped soleus muscle preparations in vitro when muscles were isolated from the adult rats. 6. These findings support the hypothesis that capsaicin and resiniferatoxin elicit an excitatory response on sensory nerves in skeletal muscle in vitro to cause the efferent release of CGRP. Consequently, CGRP is delivered to skeletal muscle fibres to inhibit insulin-stimulated glycogen synthesis. The role of CGRP in recovery of blood glucose levels during hypoglycaemia is discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amara S. G., Jonas V., Rosenfeld M. G., Ong E. S., Evans R. M. Alternative RNA processing in calcitonin gene expression generates mRNAs encoding different polypeptide products. Nature. 1982 Jul 15;298(5871):240–244. doi: 10.1038/298240a0. [DOI] [PubMed] [Google Scholar]
- Challiss R. A., Espinal J., Newsholme E. A. Insulin sensitivity of rates of glycolysis and glycogen synthesis in soleus, stripped soleus, epitrochlearis, and hemi-diaphragm muscles isolated from sedentary rats. Biosci Rep. 1983 Jul;3(7):675–679. doi: 10.1007/BF01172878. [DOI] [PubMed] [Google Scholar]
- Chantry A., Leighton B., Day A. J. Cross-reactivity of amylin with calcitonin-gene-related peptide binding sites in rat liver and skeletal muscle membranes. Biochem J. 1991 Jul 1;277(Pt 1):139–143. doi: 10.1042/bj2770139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba T., Yamaguchi A., Yamatani T., Nakamura A., Morishita T., Inui T., Fukase M., Noda T., Fujita T. Calcitonin gene-related peptide receptor antagonist human CGRP-(8-37). Am J Physiol. 1989 Feb;256(2 Pt 1):E331–E335. doi: 10.1152/ajpendo.1989.256.2.E331. [DOI] [PubMed] [Google Scholar]
- Choi S. B., Frontoni S., Rossetti L. Mechanism by which calcitonin gene-related peptide antagonizes insulin action in vivo. Am J Physiol. 1991 Feb;260(2 Pt 1):E321–E325. doi: 10.1152/ajpendo.1991.260.2.E321. [DOI] [PubMed] [Google Scholar]
- Cooper G. J., Day A. J., Willis A. C., Roberts A. N., Reid K. B., Leighton B. Amylin and the amylin gene: structure, function and relationship to islet amyloid and to diabetes mellitus. Biochim Biophys Acta. 1989 Dec 14;1014(3):247–258. doi: 10.1016/0167-4889(89)90220-6. [DOI] [PubMed] [Google Scholar]
- Cooper G. J., Willis A. C., Clark A., Turner R. C., Sim R. B., Reid K. B. Purification and characterization of a peptide from amyloid-rich pancreases of type 2 diabetic patients. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8628–8632. doi: 10.1073/pnas.84.23.8628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel P. C., Jones J. B. Causes and elimination of erratic blanks in enzymatic metabolite assays involving the use of NAD+ in alkaline hydrazine buffers: improved conditions for the assay of L-glutamate, L-lactate, and other metabolites. Anal Biochem. 1978 Aug 1;88(2):475–484. doi: 10.1016/0003-2697(78)90447-5. [DOI] [PubMed] [Google Scholar]
- Fleming B. P., Gibbins I. L., Morris J. L., Gannon B. J. Noradrenergic and peptidergic innervation of the extrinsic vessels and microcirculation of the rat cremaster muscle. Microvasc Res. 1989 Nov;38(3):255–268. doi: 10.1016/0026-2862(89)90004-6. [DOI] [PubMed] [Google Scholar]
- Forsgren S., Bergh A., Carlsson E., Thornell L. E. Calcitonin gene-related peptide expression at endplates of different fibre types in muscles in rat hind limbs. Cell Tissue Res. 1993 Dec;274(3):439–446. doi: 10.1007/BF00314540. [DOI] [PubMed] [Google Scholar]
- Franco-Cereceda A., Henke H., Lundberg J. M., Petermann J. B., Hökfelt T., Fischer J. A. Calcitonin gene-related peptide (CGRP) in capsaicin-sensitive substance P-immunoreactive sensory neurons in animals and man: distribution and release by capsaicin. Peptides. 1987 Mar-Apr;8(2):399–410. doi: 10.1016/0196-9781(87)90117-3. [DOI] [PubMed] [Google Scholar]
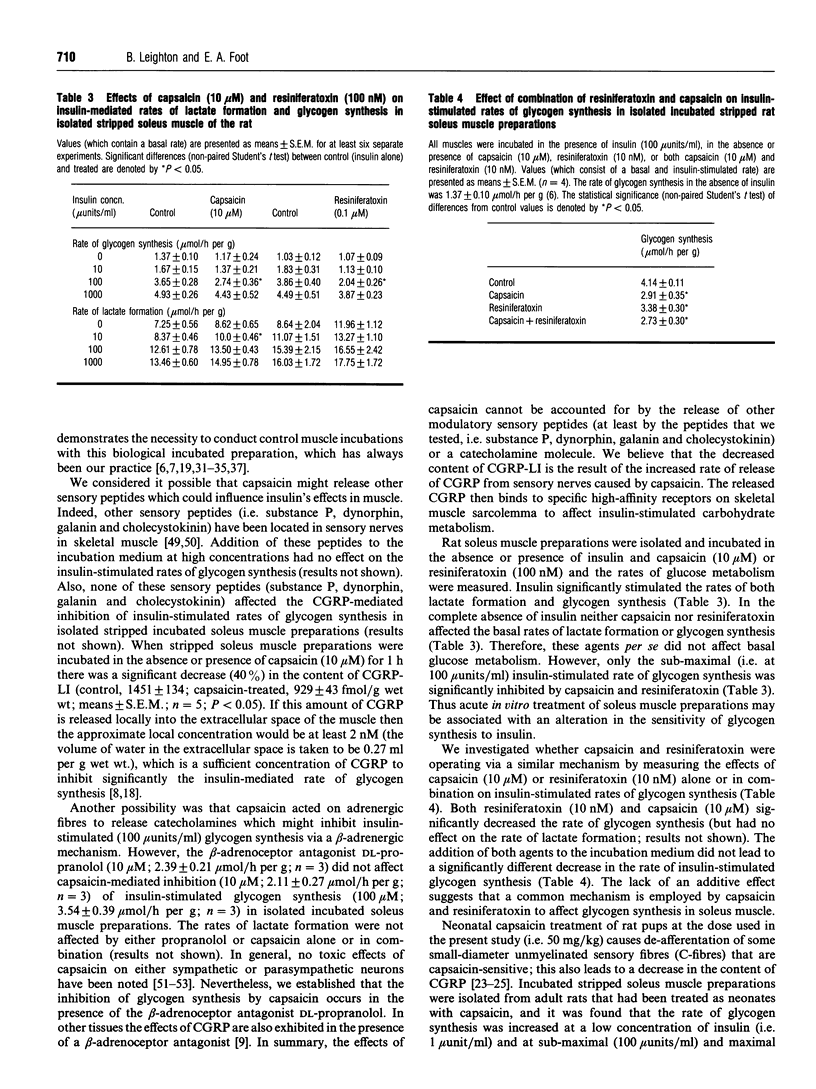
- Franco-Cereceda A., Lundberg J. M. Actions of calcitonin gene-related peptide and tachykinins in relation to the contractile effects of capsaicin in the guinea-pig and rat heart in vitro. Naunyn Schmiedebergs Arch Pharmacol. 1988 Jun;337(6):649–655. doi: 10.1007/BF00175791. [DOI] [PubMed] [Google Scholar]
- Franco-Cereceda A., Lundberg J. M. Calcitonin gene-related peptide (CGRP) and capsaicin-induced stimulation of heart contractile rate and force. Naunyn Schmiedebergs Arch Pharmacol. 1985 Nov;331(2-3):146–151. doi: 10.1007/BF00634231. [DOI] [PubMed] [Google Scholar]
- Frontoni S., Choi S. B., Banduch D., Rossetti L. In vivo insulin resistance induced by amylin primarily through inhibition of insulin-stimulated glycogen synthesis in skeletal muscle. Diabetes. 1991 May;40(5):568–573. doi: 10.2337/diab.40.5.568. [DOI] [PubMed] [Google Scholar]
- Fukuda N., Fujiwara M. Effect of capsaicin on the guinea-pig isolated atrium. J Pharm Pharmacol. 1969 Sep;21(9):622–624. doi: 10.1111/j.2042-7158.1969.tb08333.x. [DOI] [PubMed] [Google Scholar]
- Gamse R., Molnar A., Lembeck F. Substance P release from spinal cord slices by capsaicin. Life Sci. 1979 Aug 13;25(7):629–636. doi: 10.1016/0024-3205(79)90558-7. [DOI] [PubMed] [Google Scholar]
- Geppetti P., Del Bianco E., Patacchini R., Santicioli P., Maggi C. A., Tramontana M. Low pH-induced release of calcitonin gene-related peptide from capsaicin-sensitive sensory nerves: mechanism of action and biological response. Neuroscience. 1991;41(1):295–301. doi: 10.1016/0306-4522(91)90218-d. [DOI] [PubMed] [Google Scholar]
- Gibbins I. L., Furness J. B., Costa M. Pathway-specific patterns of the co-existence of substance P, calcitonin gene-related peptide, cholecystokinin and dynorphin in neurons of the dorsal root ganglia of the guinea-pig. Cell Tissue Res. 1987 May;248(2):417–437. doi: 10.1007/BF00218210. [DOI] [PubMed] [Google Scholar]
- Gibbins I. L., Wattchow D., Coventry B. Two immunohistochemically identified populations of calcitonin gene-related peptide (CGRP)-immunoreactive axons in human skin. Brain Res. 1987 Jun 23;414(1):143–148. doi: 10.1016/0006-8993(87)91335-7. [DOI] [PubMed] [Google Scholar]
- Holzer P. Local effector functions of capsaicin-sensitive sensory nerve endings: involvement of tachykinins, calcitonin gene-related peptide and other neuropeptides. Neuroscience. 1988 Mar;24(3):739–768. doi: 10.1016/0306-4522(88)90064-4. [DOI] [PubMed] [Google Scholar]
- Jancsó G., Kiraly E., Jancsó-Gábor A. Pharmacologically induced selective degeneration of chemosensitive primary sensory neurones. Nature. 1977 Dec 22;270(5639):741–743. doi: 10.1038/270741a0. [DOI] [PubMed] [Google Scholar]
- Ju G., Hökfelt T., Brodin E., Fahrenkrug J., Fischer J. A., Frey P., Elde R. P., Brown J. C. Primary sensory neurons of the rat showing calcitonin gene-related peptide immunoreactivity and their relation to substance P-, somatostatin-, galanin-, vasoactive intestinal polypeptide- and cholecystokinin-immunoreactive ganglion cells. Cell Tissue Res. 1987 Feb;247(2):417–431. doi: 10.1007/BF00218323. [DOI] [PubMed] [Google Scholar]
- Kitamura K., Kangawa K., Kawamoto M., Ichiki Y., Nakamura S., Matsuo H., Eto T. Adrenomedullin: a novel hypotensive peptide isolated from human pheochromocytoma. Biochem Biophys Res Commun. 1993 Apr 30;192(2):553–560. doi: 10.1006/bbrc.1993.1451. [DOI] [PubMed] [Google Scholar]
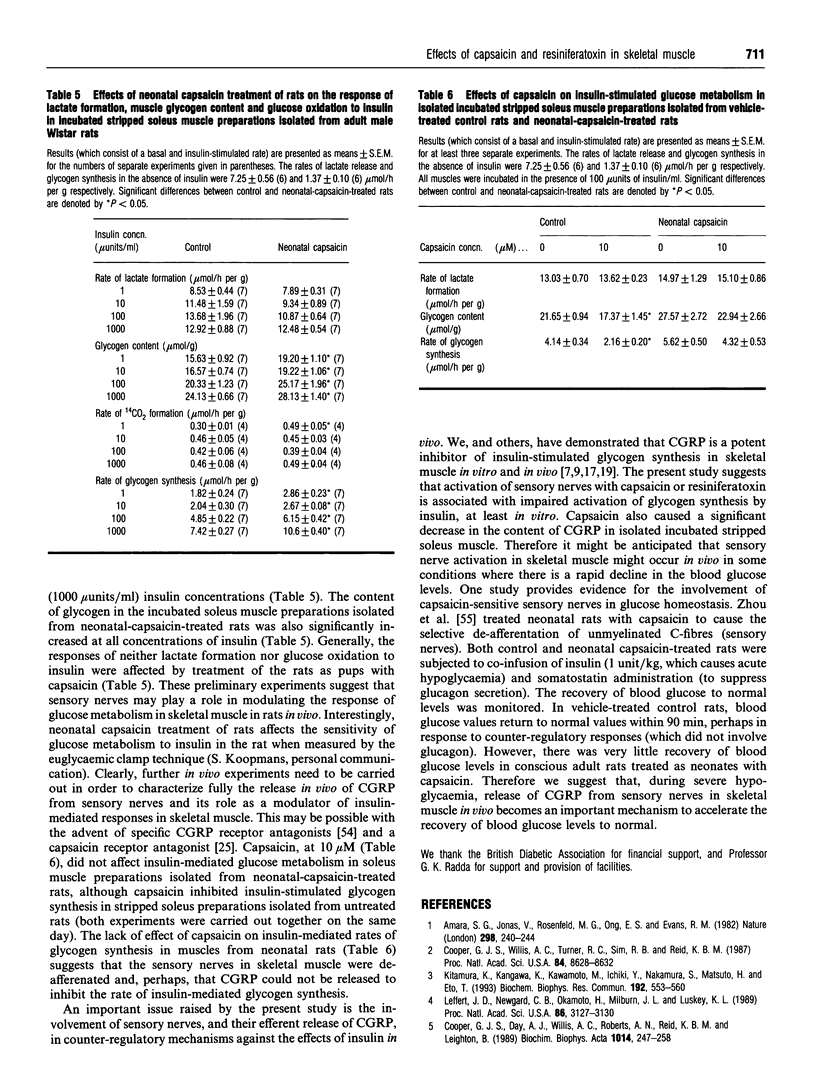
- Koopmans S. J., van Mansfeld A. D., Jansz H. S., Krans H. M., Radder J. K., Frölich M., de Boer S. F., Kreutter D. K., Andrews G. C., Maassen J. A. Amylin-induced in vivo insulin resistance in conscious rats: the liver is more sensitive to amylin than peripheral tissues. Diabetologia. 1991 Apr;34(4):218–224. doi: 10.1007/BF00405079. [DOI] [PubMed] [Google Scholar]
- Leffert J. D., Newgard C. B., Okamoto H., Milburn J. L., Luskey K. L. Rat amylin: cloning and tissue-specific expression in pancreatic islets. Proc Natl Acad Sci U S A. 1989 May;86(9):3127–3130. doi: 10.1073/pnas.86.9.3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leighton B., Cooper G. J. Pancreatic amylin and calcitonin gene-related peptide cause resistance to insulin in skeletal muscle in vitro. Nature. 1988 Oct 13;335(6191):632–635. doi: 10.1038/335632a0. [DOI] [PubMed] [Google Scholar]
- Leighton B., Cooper G. J. The role of amylin in the insulin resistance of non-insulin-dependent diabetes mellitus. Trends Biochem Sci. 1990 Aug;15(8):295–299. doi: 10.1016/0968-0004(90)90015-4. [DOI] [PubMed] [Google Scholar]
- Leighton B., Dimitriadis G. D., Parry-Billings M., Lozeman F. J., Newsholme E. A. Effects of aging on the responsiveness and sensitivity of glucose metabolism to insulin in the incubated soleus muscle isolated from Sprague-Dawley and Wistar rats. Biochem J. 1989 Jul 15;261(2):383–387. doi: 10.1042/bj2610383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leighton B., Foot E. A., Cooper G. G., King J. M. Calcitonin gene-related peptide-1 (CGRP-1) is a potent regulator of glycogen metabolism in rat skeletal muscle. FEBS Lett. 1989 Jun 5;249(2):357–361. doi: 10.1016/0014-5793(89)80658-1. [DOI] [PubMed] [Google Scholar]
- Leighton B., Foot E. Effects of capsaicin on glucose metabolism in isolated incubated skeletal muscle in vitro. Biochem Soc Trans. 1990 Jun;18(3):476–477. doi: 10.1042/bst0180476. [DOI] [PubMed] [Google Scholar]
- Leighton B., Foot E. Inhibition of insulin-stimulated glycogen synthesis in skeletal muscle by resiniferatoxin is probably due to a mechanism involving CGRP. Ann N Y Acad Sci. 1992 Jun 30;657:549–551. doi: 10.1111/j.1749-6632.1992.tb22831.x. [DOI] [PubMed] [Google Scholar]
- Leighton B., Foot E. The effects of amylin on carbohydrate metabolism in skeletal muscle in vitro and in vivo. Biochem J. 1990 Jul 1;269(1):19–23. doi: 10.1042/bj2690019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leighton B., Kowalchuk J. M., Challiss R. A., Newsholme E. A. Circadian rhythm in sensitivity of glucose metabolism to insulin in rat soleus muscle. Am J Physiol. 1988 Jul;255(1 Pt 1):E41–E45. doi: 10.1152/ajpendo.1988.255.1.E41. [DOI] [PubMed] [Google Scholar]
- Lundberg J. M., Hua Y., Fredholm B. B. Capsaicin-induced stimulation of the guinea-pig atrium. Involvement of a novel sensory transmitter or a direct action on myocytes? Naunyn Schmiedebergs Arch Pharmacol. 1984 Feb;325(2):176–182. doi: 10.1007/BF00506198. [DOI] [PubMed] [Google Scholar]
- Miyauchi T., Ishikawa T., Sugishita Y., Saito A., Goto K. Effects of capsaicin on nonadrenergic noncholinergic nerves in the guinea pig atria: role of calcitonin gene-related peptide as cardiac neurotransmitter. J Cardiovasc Pharmacol. 1987 Dec;10(6):675–682. doi: 10.1097/00005344-198712000-00011. [DOI] [PubMed] [Google Scholar]
- Molina J. M., Cooper G. J., Leighton B., Olefsky J. M. Induction of insulin resistance in vivo by amylin and calcitonin gene-related peptide. Diabetes. 1990 Feb;39(2):260–265. doi: 10.2337/diab.39.2.260. [DOI] [PubMed] [Google Scholar]
- Molnár J., György L., Unyi G., Kenyeres J. Effect of capsaicin on the isolated ileum and auricle of the guinea pig. Acta Physiol Acad Sci Hung. 1969;35(3):369–374. [PubMed] [Google Scholar]
- Ohlén A., Lindbom L., Staines W., Hökfelt T., Cuello A. C., Fischer J. A., Hedqvist P. Substance P and calcitonin gene-related peptide: immunohistochemical localisation and microvascular effects in rabbit skeletal muscle. Naunyn Schmiedebergs Arch Pharmacol. 1987 Jul;336(1):87–93. doi: 10.1007/BF00177756. [DOI] [PubMed] [Google Scholar]
- Ohlén A., Thureson-Klein A., Raud J., Hökfelt T., Hedqvist P. Calcitonin gene-related peptide in nerves of the hamster cheek pouch. Acta Physiol Scand. 1988 Dec;134(4):579–580. doi: 10.1111/j.1748-1716.1998.tb08541.x. [DOI] [PubMed] [Google Scholar]
- Piehl F., Arvidsson U., Hökfelt T., Cullheim S. Calcitonin gene-related peptide-like immunoreactivity in motoneuron pools innervating different hind limb muscles in the rat. Exp Brain Res. 1993;96(2):291–303. doi: 10.1007/BF00227109. [DOI] [PubMed] [Google Scholar]
- Popper P., Micevych P. E. Localization of calcitonin gene-related peptide and its receptors in a striated muscle. Brain Res. 1989 Sep 4;496(1-2):180–186. doi: 10.1016/0006-8993(89)91064-0. [DOI] [PubMed] [Google Scholar]
- Renzi D., Santicioli P., Maggi C. A., Surrenti C., Pradelles P., Meli A. Capsaicin-induced release of substance P-like immunoreactivity from the guinea pig stomach in vitro and in vivo. Neurosci Lett. 1988 Oct 17;92(3):254–258. doi: 10.1016/0304-3940(88)90598-8. [DOI] [PubMed] [Google Scholar]
- Rodrigo J., Polak J. M., Fernandez L., Ghatei M. A., Mulderry P., Bloom S. R. Calcitonin gene-related peptide immunoreactive sensory and motor nerves of the rat, cat, and monkey esophagus. Gastroenterology. 1985 Feb;88(2):444–451. doi: 10.1016/0016-5085(85)90505-0. [DOI] [PubMed] [Google Scholar]
- Sakaguchi M., Inaishi Y., Kashihara Y., Kuno M. Release of calcitonin gene-related peptide from nerve terminals in rat skeletal muscle. J Physiol. 1991 Mar;434:257–270. doi: 10.1113/jphysiol.1991.sp018468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santicioli P., Del Bianco E., Figini M., Bevan S., Maggi C. A. Effect of capsazepine on the release of calcitonin gene-related peptide-like immunoreactivity (CGRP-LI) induced by low pH, capsaicin and potassium in rat soleus muscle. Br J Pharmacol. 1993 Oct;110(2):609–612. doi: 10.1111/j.1476-5381.1993.tb13854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santicioli P., Del Bianco E., Geppetti P., Maggi C. A. Release of calcitonin gene-related peptide-like (CGRP-LI) immunoreactivity from rat isolated soleus muscle by low pH, capsaicin and potassium. Neurosci Lett. 1992 Aug 31;143(1-2):19–22. doi: 10.1016/0304-3940(92)90223-t. [DOI] [PubMed] [Google Scholar]
- Sternini C., Brecha N. Immunocytochemical identification of islet cells and nerve fibers containing calcitonin gene-related peptide-like immunoreactivity in the rat pancreas. Gastroenterology. 1986 May;90(5 Pt 1):1155–1163. doi: 10.1016/0016-5085(86)90380-x. [DOI] [PubMed] [Google Scholar]
- Szallasi A., Blumberg P. M. Resiniferatoxin, a phorbol-related diterpene, acts as an ultrapotent analog of capsaicin, the irritant constituent in red pepper. Neuroscience. 1989;30(2):515–520. doi: 10.1016/0306-4522(89)90269-8. [DOI] [PubMed] [Google Scholar]
- Winter J., Dray A., Wood J. N., Yeats J. C., Bevan S. Cellular mechanism of action of resiniferatoxin: a potent sensory neuron excitotoxin. Brain Res. 1990 Jun 18;520(1-2):131–140. doi: 10.1016/0006-8993(90)91698-g. [DOI] [PubMed] [Google Scholar]
- Yamaguchi A., Chiba T., Morishita T., Nakamura A., Inui T., Yamatani T., Kadowaki S., Chihara K., Fukase M., Fujita T. Calcitonin gene-related peptide and induction of hyperglycemia in conscious rats in vivo. Diabetes. 1990 Feb;39(2):168–174. doi: 10.2337/diab.39.2.168. [DOI] [PubMed] [Google Scholar]
- Young D. A., Deems R. O., Deacon R. W., McIntosh R. H., Foley J. E. Effects of amylin on glucose metabolism and glycogenolysis in vivo and in vitro. Am J Physiol. 1990 Sep;259(3 Pt 1):E457–E461. doi: 10.1152/ajpendo.1990.259.3.E457. [DOI] [PubMed] [Google Scholar]


