Abstract
Objective:
To conduct an economic cost analysis and budget impact assessment (BIA) of implementing the Frailty Care Bundle (FCB) intervention nationally over five-years for hip fracture patients. The FCB was designed to reduce hospital associated decline in older hospitalised patients.
Methods:
The FCB was delivered in two Irish hospitals on two wards per hospital. A micro level cost analysis, from the Irish health service perspective was undertaken. Direct costs of the FCB were considered (personnel, training, resources), expressed in Euros (2020). For the BIA national population estimates for hip fracture and costs avoided were based on 18% difference in patients returning to their baseline capability in the post compared to pre-intervention group, valued using cost estimates of functional decline.
Results:
We estimated total intervention costs at €53,619 (89% for personnel) and the average cost per patient was €156.03. The expected costs of implementing the FCB nationally over 12-months was €57,274 per hospital (€72.92 per patient). The BIA for an expected targeted population (16,000 over 5 years), estimated that the cost of implementing the FCB (€1.2m) was less than the expected value of functional decline avoided owing to the intervention (€3.6m), suggesting a positive net effect (€2.4m).
Conclusion:
Investment in the FCB can be offset with more rapid patient return to baseline functional capability, reducing health care costs. Trial and Protocol Registration (retrospective): BMC ISRCTN 15145850, (https://doi.org/10.1186/ISRCTN15145850).
Keywords: Economic analysis, Fundamental care, Hip fracture, Nursing
Introduction
Older patients are more susceptible to functional decline and decondition during hospitalisation, which is termed hospital associated decline (HAD). HAD is defined as the onset of a new or a deterioration in an existing disability during hospitalisation not present at hospital admission[1]. International research suggests approximately a third of older patients hospitalised for acute care, experience functional decline[2,3]. Older patients who experience a sudden loss in functional capability due to trauma related fractures are particularly vulnerable to HAD; while the prevalence of falls related fractures such as hip fractures increase with age[4]. For patients who sustain a fracture, the initial functional loss due to injury can be compounded by delayed mobilisation, prolonged bed rest and sedentary behaviour, especially for more complex patients with underlying medical conditions[5]. Early mobilisation combined with good nutrition and cognitive engagement activities are protective against HAD[1,6]. There is growing evidence on interventions to improve consistency in delivering and prioritising these aspects of fundamental care above competing demands[7,8], but there is very limited information on the costs and possible cost-off sets of delivering such interventions.
Multicomponent interventions that target the major modifiable elements of HAD (mobilisation, nutrition and cognition) may be more effective than single strand interventions in promoting functional recovery to baseline capability of older people admitted to hospital after major trauma[8-11]. One such intervention the Frailty Care Bundle (FCB), developed by the authors in this paper, was designed to improve consistency in mobilisation, enhanced nutrition and increased cognitive engagement to prevent HAD in older patients following orthopaedic surgery[12]. The FCB was delivered to orthopaedic trauma patients across two Irish hospitals in addition to usual care between November 2019 and November 2021. The intervention was delivered on two acute trauma wards (62 beds in total) in the acute trauma care centre (site 2) and two orthopaedic rehabilitation wards in the elective and rehabilitation hospital (33 beds in total) (site 1)[13].
The intervention primarily focused on the nursing teams with wider multidisciplinary involvement. Among the main changes to routine clinical practice on the participating wards were making visible daily mobilisation goals to both patients and the nursing team in order to increase nurse assisted and supervised mobilisation (in addition to scheduled physiotherapy); offering a wider range of protein-based snacks combined with assisted mealtimes to increase nutritional intake; and to provide distraction activities and environmental changes to help with orientation[13]. The intervention used a clinical facilitation model, whereby by an experienced orthopaedic trauma nurse worked with ward teams (education, coaching, role modelling, audit and feedback) to implement changes to routine ward practices[12]. In addition, expertise on implementation science was provided by the principal investigator (PI) and other members of the research team. The intervention implementation was guided by the integrated-Promoting Action on Research Implementation in Health Services, (i-PARIHS), framework which emphasises the interaction between evidence, context and facilitation in implementing evidence-based practice[14].
In the original study protocol, we planned to recruit a sample size of 180 patients (pre n=90 and post n=90), in the final sample 120 patients consented to participate (pre n=60, post n=60), at 6–8-week follow-up after hospital discharge 79 participants remained in the study (pre n=43, post n=36)[12]. The study was thus under-powered to demonstrate statistical significance, but there was a positive trend toward an 11% increase in average daily patient step count (odds ratio (OR) 1.11 95% confidence Interval (CI) 0.72-1.7) in the post intervention compared to the pre-intervention group. At 6-8 week post discharge follow-up, 18% more patient participants in the post intervention group reported a higher likelihood of returning to pre-injury physical activity as measured by the modified Barthel Index (mBI) (OR 2.29 (95% CI 0.98-5.36) (p=0.056) compared to the pre-intervention group[13].
An important dimension in evaluating intervention outcomes is the cost of implementation to the service[15]. In the nursing literature on fundamental care, there is a dearth of evidence on how to cost interventions that are delivered at ward level and primarily reliant on the nursing team (nurses and health care assistants) time[16,17]. Time spent delivering the FCB activities meant the nursing teams were not free to undertake other care activities. Related literature on hospital falls prevention or early mobilisation interventions was used to inform this economic analysis.
While interventions to prevent falls in hospital settings and to optimise functional recovery to baseline capability are widely discussed in the literature, very few provide economic analyses[18-20]. Of the relevant studies, three provide full economic evaluations estimating cost effectiveness. Galbraith et al.[21], and Haines et al[22], focused on falls prevention programmes, Milte et al.[23], reported on a nutrition and exercise intervention for rehabilitation following a hip fracture, while Lieten et al.[24], undertook a pilot study of an orthogeriatric co-management model. With regards to perspective and range of costs included, the studies adopted a health care provider perspective, only Lieten et al.[24] included patient out of pocket expenses. More specifically, Galbraith et al.[21] included staff costs (opportunity cost of attending training and audit meetings) and infrastructure costs (provision of new equipment, repairs, and labour cost). Haines et al.[22] included costs associated with acute hospitalisation (directly and not directly related to falls), and inpatient rehabilitation in Australia. Milte et al.[23] included costs of healthcare resources (e.g. allied health professional (AHP) community visits, travel costs, equipment, nutrition supplements) and cost offsets (e.g., hospitalisation, transitional care programme, residential care). While Lieten et al.[24] included total hospital costs, costs sustained by the Belgian healthcare system, private hospitalisation insurance and patient out of pocket expenses.
The aim of this paper is to describe a micro level cost analysis of implementing the Frailty Care Bundle (FCB) intervention following national guidelines from the Health Information and Quality Authority (HIQA)[25]. Scenario analyses are also conducted to consider the implications of implementing the FCB in a non-trial setting. The results of which are used to inform a budget impact assessment (BIA) of implementing the FCB nationally over a five-year horizon for hip fracture patients aged 65 years or older.
Materials and Methods
Costs Analysis
Costs associated with the implementation of the FCB intervention were estimated at a micro level, following national guidelines[25]. Only direct costs of resources involved in the programme from the health care provider and payer (i.e. Health Service Executive) were included and these were identified, measured and valued as per Drummond et al.[26]. Resources consist of personnel costs and FCB materials. Personnel costs accounted for the clinical facilitator’s remuneration for the ward situational analysis and implementation of the intervention, and for staff training (considered as an opportunity cost of attending educational sessions). It was assumed no additional costs were incurred for staff mobilising patients or assisting with nutrition as this was considered part of usual fundamental care activity. Total personnel costs (including clinical facilitator cost) were derived as shown in Table 1, using national salary scales per staff grade level and accounting for PRSI (pay related social insurance, 11.05%), pension costs (4%) and overheads (25%), as per national guidelines[25,27]. Hourly salaries were calculated according to Regulatory Impact Analysis Guidelines[28], whereby annual salary was calculated as per national guidelines[25], divided by minimum of net days worked per annum (that is, 249 days minus annual leave) expressed as working hours (i.e. net days times 6.95)[29]. Annual and hourly salary of the Implementation Science/Quality Improvement Expert – involved in Scenario Analysis programme implementation – has been derived from the average national Senior Lecturers salary scales across five Irish universities.
Table 1.
Cost Analysis.
| Baseline | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| €/unit | Site 1(2 wards) | Site 2 (2 wards) | Total Costs (€) | Scenario Analysis 1 hospital (4 wards) | |||||
| #Units | Duration | Cost (€) | # Units | Duration | Cost (€) | Total Cost (€) | |||
| Clinical Facilitator | Annual | Months | Months | ||||||
| CNM 1 (General) | 68,568.481 | 1 | 3.5 | 19,999.14 | 1 | 4.5 | 25,713.18 | 45,712.32 | - |
| Staff Nurse | 55,063.461 | 27,531.738 | |||||||
| Implementation Science / Quality Improvement Expert | 120,100.161 | 13,317.169 | |||||||
| Training | Hourly | Hours | Hours | ||||||
| CNM 1 (General) | 46.961 | 2 | 1.50 | 140.87 | 2 | 0.75 | 70.43 | 211.30 | 211.30 |
| CNM 2 (General) | 50.771 | 1 | 1.50 | 76.15 | 1 | 0.75 | 38.07 | 114.22 | 114.22 |
| Staff Nurse | 35.691 | 33 | 1.50 | 1,766.57 | 49 | 0.75 | 1,311.54 | 3,078.11 | 3,292.24 |
| Health Care Assistant | 30.111 | 4 | 3.00 | 361.29 | 10 | 0.75 | 225.81 | 587.10 | 790.33 |
| FCB Materials2 | |||||||||
| Whiteboards | 30.58 | 0 | - | 0.00 | 8 | - | 244.64 | 244.64 | 489.28 |
| Patient information leaflets | 1.39 | 5605 | - | 778.40 | 5605 | - | 778.40 | 1,556.80 | 1,563.75 |
| Floor distance markers | 92.47 | 2 | - | 184.94 | 2 | - | 184.94 | 369.88 | 369.88 |
| Patient mobility sheets | 1.30 | 30 | - | 39.00 | 0 | - | 0.00 | 39.00 | 78.00 |
| Orientation clocks | 191.75 | 4 | - | 767.00 | 0 | - | 0.00 | 767.00 | 1,534.00 |
| Distraction resources | 2.00 | 10 | - | 20.00 | 0 | - | 0.00 | 20.00 | 174.55 |
| Freezer depreciation (intervention period) | 4.75 | 0 | - | 0.00 | 1 | - | 4.75 | 4.75 | 19.00 |
| High protein ice-creams | 0.90 | 0 | - | 0.00 | 150 | - | 135.00 | 135.00 | 1,080.00 |
| Fruit pots | 0.90 | 600 | - | 539.10 | 0 | - | 0.00 | 539.10 | 6,469.20 |
| Radios6 | 60.00 | 2 | - | 120 | 2 | - | 120 | 240.00 | 240.00 |
| Total | 24,792.46 | 28,826.77 | 53,619.23 | 57,274.64 | |||||
| Total/patient | 275.473 | 113.653 | 156.03 | 72.927 | |||||
| Total/month | 12,396.234 | 9,608.924 | 10,723.85 | 4,772.898 | |||||
See Supplemental Table 1 for staff cost calculations.
FCB Study Records.
Number of patients: 254 at site 2, 90 at site 1.
Intervention length: 3 months at site 2, 2 months at site 1 plus 1.5 months per site for ward situational analysis undertaken by clinical facilitator.
1,500 leaflets purchased and 75% of them were used (equally split across hospitals).
For the trial radios were borrowed in two hospitals. The opportunity cost of purchasing them is included here for completeness.
Number of patients: 785. CNM: Clinical Nurse Manager.
FCB materials refer to the mobility, nutrition and cognition materials needed for the implementation of the intervention (note variations between hospitals owing to prior practices). These included whiteboards, patient mobility sheets, orientation clocks, distraction resources for patients with dementia, freezer to store high-protein ice-creams, and fruit pots. Quantities of each resource were identified and measured using project documentation and based on typical ward occupancy (patient numbers) and were valued using market prices (Table 1).
The FCB project collected outcomes data on a sample of patients (n=120) who provided informed consent to participate in the evaluation[13]. In contrast, the intervention was delivered at ward level thus all patients were eligible to be involved in the FCB during the intervention period. The total number of patients was estimated based on average length of stay for the study cohort (22 days), across participating wards, assuming 100% occupancy for the duration of the intervention. Therefore, the intervention was delivered to 344 patients, across two hospitals and four wards (two wards per site). Of which, 254 patients were in site 2, and 90 in site 1. Only the intervention delivery time was included, data collection time for the study evaluation was excluded. Difference in intervention time between the sites (3.5 months at site 1 and 4.5 months at site 2) was due to smaller ward size and thus nursing teams on Site 1 (33 beds) compared to Site 2 (62 beds). All costs were expressed in 2020 Euros and calculated at hospital level. As the total duration of the intervention was shorter than one year, discounting was not required. Depreciation was applied on relevant assets (i.e., the freezer) as per national guidelines[25].
Scenario Analysis
The FCB intervention was based on a clinical facilitation model, whereby the intervention implementation was supported by a clinical facilitator who was an experienced orthopaedic nurse[30]. In this study, the clinical facilitator was costed at a Clinical Nurse Manager 1 (General) grade operating for 4.5 months at site 2 (two wards) and 3.5 months at site 1 (two wards). Of which 1.5 months was dedicated to ward situational analysis in each hospital (to inform implementation design) and the remaining time was spent on intervention implementation. In the scenario analysis we considered the impact on costs if the clinical facilitator role was undertaken by a staff nurse (reducing the hourly cost) and delivering the FCB programme for 12 months in a single hospital to simulate national implementation. A clinical facilitator at staff nurse level would be employed for 12 months part-time, along with support from an implementation science expert 3.5 hours per week (salary equivalent to Academic Senior Lecturer in Health Science Research), to implement the intervention with all FCB elements across four wards.
Budget Impact Assessment
A budget impact assessment (BIA) estimated the resource impact of the FCB intervention, over a five-year horizon using cost estimates from the scenario analysis (Table 1)[31]. Central Statistics Office (CSO) projections for over 65s population[32] and estimates of hip fractures amongst over 60s aged population from the Irish Hip Fracture database[4,33-35] were used to estimate a sample population cohort. Results from the FCB study were used to estimate the proportion of this cohort in which FCB could reduce functional decline[13]. Best available estimates on the cost of functional decline were applied to this cohort to estimate expected costs avoided. The net cost were also estimated wherein cost avoided were compared to costs of implementing the FCB nationally.
The CSO provides population projections for 2021-2051 (5-year intervals)[32] across six different population growth scenarios, characterised by combination of different fertility rates (constant and decreasing) and different levels of net migration increases (high, moderate, and low). For the scope of the current time horizon analysed (i.e., 2023-2027), variations in fertility rate do not affect population projections and the moderate migration scenario was considered. To estimate annual population for the intermediate years (not detailed in CSO projections (2023, 2024, 2025, and 2027) a constant growth rate was assumed. The constant growth annual rate (expressed as %) equates to the population at the end over the population at the start of the census period, raised to the power of 1 over the number of years in the census period, all minus one.
In the Irish health care system interventions like FCB are often rolled out by clinical diagnosis via National Clinical Guidelines. To reflect this the i.e. BIA was performed on a sub-group of potential patients, hip fracture patients, rather than the entire population. Estimates of hip fractures for the cohort were derived from the National Office of Clinical Audit’s (NOCA) Irish Hip Fracture Database National Reports of 2018, 2019, and 2020[4,33-35]. Data on hip fractures amongst adults aged over 60 years were extracted from the latter and the three-year average probability of a hip fracture amongst the population was estimated (0.391%). This was applied to CSO population estimates.
Results
Cost Analysis: Baseline Total Costs
The total cost of the intervention at site 1 and 2 was €53,619.23; an accumulation of €28,826.77 at site 2 and €24,792.46 at site 1 (Table 1). The distribution of costs across categories, at site 2, showed that 89% of total costs were attributable to the clinical facilitator’s remuneration (€25,713.18); 6% were for staff training (€1,645.86) and the remaining 5% were for FCB materials (€1,467.73). At site 1, 81% of total costs were attributable to the clinical facilitator’s remuneration (€19,999.14); 10% were for FCB materials (€2,448.44); the remaining 9% were for staff training (€2,344.88). As detailed on Table 1, staff training time was slightly longer on site 1 as there was additional training on dementia communication (staff request) and more FCB materials were adopted at site 1 only (i.e., the fruit pots and the orientation clocks). Disaggregating total costs to patient level, average cost per patient was €156.03 across the two hospitals. At individual hospital level, the intervention was over twice as expensive at site 1 (€275.47 per patient) compared to site 2 (€113.65 per patient). In site 1 average fixed costs were higher owing to fewer patient numbers and shorter intervention duration.
Scenario Analyses
In a Scenario Analysis we considered a single hypothetical hospital consisting of four wards with a staff nurse undertaking the clinical facilitator role, to simulate national implementation of the FCB over a 12-month period. In addition to the clinical facilitator, an Implementation Science/Quality Improvement Expert would contribute to implementing the FCB for 3.5 hours per week for the duration of the intervention, totalling 168 hours. We estimated (Table 1) total annual costs at €57,274.64 or €4,772.89 per month, which was €3,655.41 (7%) more than the baseline model. Average patient costs were estimated to be €72.92; €83.12 (53%) lower than the baseline. The clinical facilitator’s remuneration costs remained the largest proportion of costs (48%); followed by the implementation scientist’s / quality improvement expert’s remuneration (23%), FCB material costs (21%) and staff training at 8%.
Budget Impact Assessment
A budget impact assessment (BIA) estimated the resource impact of the FCB intervention, over a five-year horizon (as advocated by HIQA[29]) using cost estimates from the scenario analysis (Table 1) for a targeted population – hip fracture patients. Wherein, total cost of implementing the FCB intervention and estimated costs avoided were estimated for hip fracture patients over 65 years nationally. We acknowledge the real-world population is higher as there are other types of traumatic fractures however, data on their national prevalence is less accurate, so only a subset of the whole population is considered here. Furthermore, it’s likely that such an intervention would be implemented as a phased roll out based on clinical diagnosis (e.g. incorporated as part of the National Hip Fracture Standards)[33].
The targeted population for FCB in the BIA was individuals with hip fractures aged 65 years and older. Population estimates from the CSO[32] predicted the population of people aged 65 years and older to increase from 782,326 in 2023 to 887,896 by 2027 (Table 2). Using data from the Irish Hip Fracture database[4,33-35] we estimated 0.391% of Irish adults aged over 60s are diagnosed with hip fracture annually. Applying this to the national population aged over 65 years, we estimated the expected population for FCB to be approximately 16,000.
Table 2.
Budget Impact Analysis FCB.
| 2023 | 2024 | 2025 | 2026 | 2027 | TOTAL | |
|---|---|---|---|---|---|---|
| Population age 65+1 | 782,326 | 802,709 | 823,623 | 867,100 | 887,896 | 4,163,654 |
| Projected hip fractures2 | 3,056 | 3,135 | 3,217 | 3,387 | 3,468 | 16,262 |
| € intervention3 | 222,808 | 228,613 | 234,570 | 246,952 | 252,875 | 1,185,819 |
| Functional Decline: Projected hip fractures NOT returned to baseline functionality | ||||||
| # Pre FCB4 | 1,803 | 1,850 | 1,898 | 1,998 | 2,046 | 9,595 |
| # Post FCB5 | 1,253 | 1,285 | 1,319 | 1,389 | 1,422 | 6,667 |
| # avoided | 550 | 564 | 579 | 610 | 624 | 2,927 |
| € Avoided 6 | 676,857 | 694,492 | 712,587 | 750,202 | 768,195 | 3,602,333 |
| Net Effect (€)7 | 454,049 | 465,879 | 478,017 | 503,250 | 515,320 | 2,416,515 |
Scenario with moderate net migration increase[32].
Cost per patient estimated in Scenario analysis 2 (€72.61) applied to hip fractures estimates.
Share of hip fractures returning (41%) and not returning (59%) to baseline functionality without FCB[12].
Share of hip fractures returning (59%) and not returning (41%) to baseline functionality with FCB[12].
Annual cost of functional decline per patient equal to €1,230.65 (calculated from Bash & Kerr[36].
Derived as cost avoided minus cost of intervention.
Estimating Intervention Cost
Applying the cost per patient estimate from the Scenario Analysis (€72.92) to the estimated population above revealed the estimated cost of implementing the FCB nationally to be approximately €1.2m for 5 years, or €237,164 on average per year.
Estimating Costs Avoided
The FCB study demonstrated that in the pre-intervention population (63% hip fracture, 37% other fractures) 41% had returned to baseline (pre-injury) physical functioning level. In the post intervention group (51% hip fractures, 49% other fractures) the return to baseline function increased to 59%. These proportions were applied to the cohort estimates above. As no national estimates for the cost of functional decline were available, UK costs were used as a proxy as they represented the best available estimates[36]. Bash & Kerr[36] calculations were used to compute the average cost of functional decline (€1,230.65) and these estimates were adjusted for exchange and inflation rates and applied to the population under consideration. Total costs of functional decline avoided were approximately €3.6m.
Net Effect
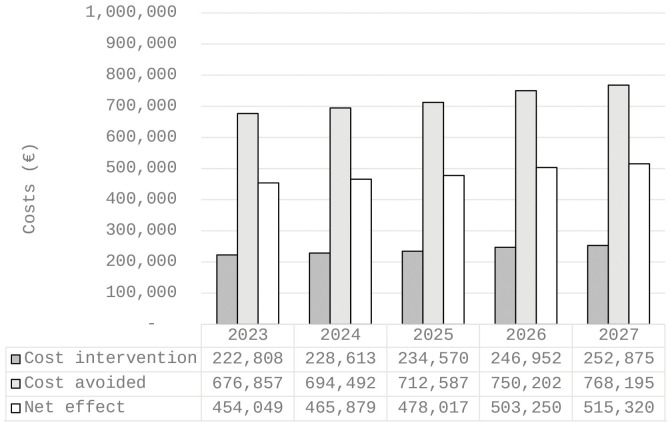
Comparing the intervention costs (€1.2m) to expected costs avoided (€3.6m), the net effect was approximately €2.4m (Figure 1). Suggesting the investment in the FCB intervention can be off set with patients’ more rapid returns to baseline functional capability.
Figure 1.
Estimated costs with moderate net migration increase, 2023-2027.
Discussion
The purpose of this economic analysis was to examine the cost of the FCB intervention and identify possible cost offsets. The cost analysis outlines the cost of implementing the FCB at the local hospital level and extrapolate the cost at a national level using a hip fracture population (€237,164 on average per year). We have presented a model to consider costs offset based on a more rapid return to baseline functional capability and reduced risk of hospital associated decline, our model suggests a cost saving of 2.4 million over a five-year period. There is a lack of high-quality data in this area especially on the cost of functional decline and we have outlined these limitations below.
Economic analyses of interventions like FCB are sparse and the existing literature covers a diverse range of interventions so direct comparisons between our results and the literature discussed above would be inappropriate. Nevertheless, there is a common theme in the economic analysis of this type of intervention, targeted investment focusing on care processes and supporting front-line teams to reduce risks for functional decline are likely to be cost saving when considered at the health system level[21-24]. There has always been a strong moral argument for preventing functional decline during hospitalisation. The findings in this paper suggests that there may be a strong economic argument to invest in the organisational and health system capacity to enable nursing and multidisciplinary teams to prioritise early, consistent mobilisation, enhanced nutrition and cognitive engagement for older patients.
As health care environments become busier due to increases in population ageing, prevalence of chronic disease and injuries including hip fractures combined with prioritisation of technological care and growing shortages of health care workers, especially nurses[37,38] the delivery of fundamental care becomes more important but also more precarious and strained[17,39]. Interventions to improve ward culture, leadership and multidisciplinary collaboration around fundamental care requires sustained implementation resources to support ward teams to prioritise fundamental care and prevent functional decline[7,11]. Welch et al. in a systematic review of interventions to reduce hospital associated decline in older patients suggested that walking alone may be insufficient to prevent or treat acute sarcopenia (loss of muscle strength) but commented that it ‘is safe to do when possible and should be commended’[8]. Our study shows functional decline may not be inevitable and for a modest investment in health care provider time and access to implementation/quality improvement expertise we can improve outcomes for older people.
In resource constrained health systems, there must be optimal use of the existing health workforce to deliver high quality fundamental care and that such care has both a moral and financial value. This paper provides a model to cost strategies to prioritise fundamental care and to consider cost offsets. We accept there are limitations to some of the assumptions we have used in this analysis, nonetheless, our approach can inform cost analysis for nursing care interventions.
Limitations
There are several limitations in the economic analysis, the FCB study limitations are discussed elsewhere[13]. The BIA considers only one group of patients who could benefit from FCB, others may too. With regards to estimation of the cohort size, a constant growth rate was assumed for population growth within year classifications to estimate annual population. The Irish Hip Fracture database reports data in absolute numbers, the annual probability is estimated by considering these cases as a proportion of the total population in that age cohort per annum. In addition, the database reports data for the 60+ age group, it is not disaggregated to 5-year intervals to enable estimates for the 65+ age group. So, the probability of hip fracture employed here is for the 60+ age group and was assumed to be constant over the 5 years. In the absence of Irish estimates for costs of functional decline, UK estimates are used as a proxy. Some simplifying assumptions were made in estimating this cost, including computing an average for one stage functional decline only. So, in applying the cost to those who do not return to original functional status we apply this average cost and do not model for different stages of decline. We acknowledge there may be other cost offsets for the health service in a hospital setting and community setting (including publicly provided home care etc.) not accounted for in the cost of functional decline employed, additional follow-up data collection would be required to estimate these. Furthermore, the perspective of this analysis was the health care provider only, it is likely the FCB programme and resultant avoidance of functional decline would have positive impacts for informal and privately arranged care too. Finally, this study presents a partial economic evaluation wherein only costs are considered. As the review of the literature reveals economic evaluations are sparse in the area to examine the cost effectiveness of FCB a comparator and effectiveness data for both the intervention and comparator would be required.
Conclusion
This economic cost analysis valued the resource use associated with the implementation of the FCB intervention initially as per the trial and used a scenario to consider costs under ideal national implementation circumstances. The average baseline cost per patient was €156.03. As demonstrated in the scenarios, scaling the FCB to hospital level with dedicated personal overseeing implementation reduced per patient costs (€72.92). The budget impact analysis considered the cohort of expected hip fracture patients in the next five years. Applying the average implementation cost estimates and comparing them to potential functional decline avoided, indicated the potential for a positive net economic effect (€2.4m) from a health care provider perspective.
Ethical approval
The Frailty Care Bundle study and the associated economic costing received ethical approval from the Clinical Research Ethics Committee of the Cork Teaching Hospitals (Ref ECM 3 (d) 12/11/2019).
Funding
The research project was funded by the Health Research Board (HRB) of Ireland (Ref APA-2019-009). Funding was also provided by the HSE South South West Hospital Group as part of the HRB Applied Partnership funding award. The funder monitored the project’s progress but did not have any role in intervention implementation, data collection, analyzing or interpretation.
Authors’ contributions
Dr Aileen Murphy, Federica de Blasio, Dr Ann Kirby undertook the cost analysis calculations, literature review, and drafting this article. Professor Corina Naughton was the project PI and along with Marguerite de Foubert collected the cost data, reviewed and contributed to the drafting of the current article. All authors read and approved the final version of the article.
Acknowledgements
The research team would like to acknowledge the time, commitment and engagement of patients and staff, especially ward managers for their participation in the study. We would also like to acknowledge members of the research steering group and Directors of Nursing for their time and advice.
Supplemental Table 1.
Staff costs.
| Basic Pay1 (€) | Employers PRSI2 | Pension Costs3 | Overhead3 | Total Costs3 (€) | Annual Leave4 | € per Hour5 | |
|---|---|---|---|---|---|---|---|
| Clinical Facilitator | 48,9606 | 11.05% | 4% | 25% | 68,568.48 | 31 | 45.26 |
| Clinical Nurse Manager 1 (General) | 50,798 | 11.05% | 4% | 25% | 71,142.60 | 31 | 46.96 |
| Clinical Nurse Manager 2 (General) | 54,920 | 11.05% | 4% | 25% | 76,915.46 | 31 | 50.77 |
| Staff Nurse | 39,317 | 11.05% | 4% | 25% | 55,063.46 | 27 | 35.69 |
| Health Care Assistant | 33,169 | 11.05% | 4% | 25% | 46,453.18 | 27 | 30.11 |
| Implementation Science/Quality Improvement Expert7 | 85,755 | 11.05% | 4% | 25% | 120,100.16 | 31 | 79.27 |
See national salary scales for mid-point salary (values from October 1, 2020) (HSE, 2021).
See national employers PRSI (Grant Thornton Ireland, 2020).
See national guidelines for total costs calculation (HIQA, 2020).
Annual leave days for health sector staff (HSE, 2019).
Based on 249 working days per year, and 6.95 working hours per day as per RIA (2009).
6 Third point on Clinical Nurse Manager 1 (General) salary scale (values from October 1, 2020) (HSE, 2021).
Average national salary of an Academic Senior Lecturer in Health Services Research.
Footnotes
Edited by: Jagadish K. Chhetri
References
- 1.Zisberg A, Shadmi E, Gur-Yaish N, Tonkikh O, Sinoff G. Hospital-associated functional decline:the role of hospitalization processes beyond individual risk factors. J Am Geriatr Soc. 2015;63(1):55–62. doi: 10.1111/jgs.13193. [DOI] [PubMed] [Google Scholar]
- 2.Lafrenière S, Folch N, Dubois S, Bédard L, Ducharme F. Strategies Used by Older Patients to Prevent Functional Decline During Hospitalization. Clin Nurs Res. 2017;26(1):6–26. doi: 10.1177/1054773815601392. [DOI] [PubMed] [Google Scholar]
- 3.de Vos AJ, Asmus-Szepesi KJ, Bakker TJ, de Vreede PL, van Wijngaarden JD, Steyerberg EW, Mackenbach JP, Nieboer AP. Integrated approach to prevent functional decline in hospitalized elderly:the Prevention and Reactivation Care Program (PReCaP) BMC Geriatr. 2012;12:7. doi: 10.1186/1471-2318-12-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.NOCA. (National Office of Clinical Audit) Irish Hip Fracture Database National Report. Dublin: National Office of Clinical Audit; 2020. [Accessed on 06/07/2022]. https://d7g406zpx7bgk.cloudfront.net/cb1eb5610b/irish_hip_fracture_database_national_report_2020_design_final.pdf. [Google Scholar]
- 5.Sheehan KJ, Goubar A, Martin FC, Potter C, Jones GD, Sackley C, Ayis S. Discharge after hip fracture surgery in relation to mobilisation timing by patient characteristics:linked secondary analysis of the UK National Hip Fracture Database. BMC Geriatr. 2021;21(1):694. doi: 10.1186/s12877-021-02624-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parr JM, Bell J, Koziol-McLain J. Evaluating fundamentals of care:The development of a unit-level quality measurement and improvement programme. J Clin Nurs. 2018;27(11-12):2360–2372. doi: 10.1111/jocn.14250. [DOI] [PubMed] [Google Scholar]
- 7.de Foubert M, Cummins H, McCullagh R, Brueton V, Naughton C. Systematic review of interventions targeting fundamental care to reduce hospital-associated decline in older patients. J Adv Nurs. 2021;77(12):4661–4678. doi: 10.1111/jan.14954. [DOI] [PubMed] [Google Scholar]
- 8.Welch C, Majid Z, Greig C, Gladman J, Masud T, Jackson T. Interventions to ameliorate reductions in muscle quantity and function in hospitalised older adults:a systematic review towards acute sarcopenia treatment. Age Ageing. 2021;50(2):394–404. doi: 10.1093/ageing/afaa209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCullagh R, O'Connell E, O'Meara S, Dahly D, O'Reilly E, O'Connor K, Horgan NF, Timmons S. Augmented exercise in hospital improves physical performance and reduces negative post hospitalization events:a randomized controlled trial. BMC Geriatr. 2020;20(1):46. doi: 10.1186/s12877-020-1436-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu SY, Li C, Zhang PX. Enhanced recovery after surgery for hip fractures:a systematic review and meta-analysis. Perioper Med (Lond) 2021;10(1):31. doi: 10.1186/s13741-021-00201-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mudge AM, McRae P, Banks M, Blackberry I, Barrimore S, Endacott J, Graves N, Green T, Harvey G, Hubbard R, Kurrle S, Lim WK, Lee-Steere K, Masel P, Pandy S, Young A, Barnett A, Inouye SK. Effect of a Ward-Based Program on Hospital-Associated Complications and Length of Stay for Older Inpatients:The Cluster Randomized CHERISH Trial. JAMA Intern Med. 2022;182(3):274–282. doi: 10.1001/jamainternmed.2021.7556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Naughton C, Cummins H, de Foubert M, Barry F, McCullagh R, Wills T, et al. Implementation of the Frailty Care Bundle (FCB) to promote mobilisation, nutrition and cognitive engagement in older people in acute care settings:protocol for an implementation science study. HRB Open Res. 2022;5:3. [Google Scholar]
- 13.Naughton C, de Foubert M, Cummins H, McCullagh R, Wills T, Skelton DA, Dahly D, O'Mahony D, Ahern E, Tedesco S, Sullivan BO. Implementation of a Frailty Care Bundle (FCB) Targeting Mobilisation, Nutrition and Cognitive Engagement to Reduce Hospital Associated Decline in Older Orthopaedic Trauma Patients:Pretest-Posttest Intervention Study. J Frailty Sarcopenia Falls. 2024;9(1):32–50. doi: 10.22540/JFSF-09-032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harvey G, Kitson A. PARIHS revisited:from heuristic to integrated framework for the successful implementation of knowledge into practice. Implement Sci. 2016;11:33. doi: 10.1186/s13012-016-0398-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Proctor E, Silmere H, Raghavan R, Hovmand P, Aarons G, Bunger A, Griffey R, Hensley M. Outcomes for implementation research:conceptual distinctions, measurement challenges, and research agenda. Adm Policy Ment Health. 2011;38(2):65–76. doi: 10.1007/s10488-010-0319-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chiang B. Estimating nursing costs--a methodological review. Int J Nurs Stud. 2009;46(5):716–22. doi: 10.1016/j.ijnurstu.2008.10.015. [DOI] [PubMed] [Google Scholar]
- 17.Kitson A, Carr D, Conroy T, Feo R, Grønkjær M, Huisman-de Waal G, et al. Speaking Up for Fundamental Care:the ILC Aalborg Statement. BMJ Open. 2019;9(12):e033077. doi: 10.1136/bmjopen-2019-033077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cameron ID, Dyer SM, Panagoda CE, Murray GR, Hill KD, Cumming RG, Kerse N. Interventions for preventing falls in older people in care facilities and hospitals. Cochrane Database Syst Rev. 2018;9(9):CD005465. doi: 10.1002/14651858.CD005465.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han CY, Miller M, Yaxley A, Baldwin C, Woodman R, Sharma Y. Effectiveness of combined exercise and nutrition interventions in prefrail or frail older hospitalised patients:a systematic review and meta-analysis. BMJ Open. 2020;10(12):e040146. doi: 10.1136/bmjopen-2020-040146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spetz J, Brown DS, Aydin C. The economics of preventing hospital falls:demonstrating ROI through a simple model. J Nurs Adm. 2015;45(1):50–7. doi: 10.1097/NNA.0000000000000154. [DOI] [PubMed] [Google Scholar]
- 21.Galbraith JG, Butler JS, Memon AR, Dolan MA, Harty JA. Cost analysis of a falls-prevention program in an orthopaedic setting. Clin Orthop Relat Res. 2011;469(12):3462–8. doi: 10.1007/s11999-011-1932-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haines TP, Hill AM, Hill KD, Brauer SG, Hoffmann T, Etherton-Beer C, McPhail SM. Cost effectiveness of patient education for the prevention of falls in hospital:economic evaluation from a randomized controlled trial. BMC Med. 2013;11:135. doi: 10.1186/1741-7015-11-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Milte R, Miller MD, Crotty M, Mackintosh S, Thomas S, Cameron ID, Whitehead C, Kurrle S, Ratcliffe J. Cost-effectiveness of individualized nutrition and exercise therapy for rehabilitation following hip fracture. J Rehabil Med. 2016;48(4):378–85. doi: 10.2340/16501977-2070. [DOI] [PubMed] [Google Scholar]
- 24.Lieten S, Pien K, Van Laere S, Bravenboer B, Scheerlinck T. Introduction of the orthogeriatric co-management model increases the quality of care:a pilot study. Acta Orthop Belg. 2020;86(4):580–587. [PubMed] [Google Scholar]
- 25.HIQA (Health Information and Quality Authority) (2020) Guidelines for the economic evaluation of health technologies in Ireland. [Accessed on 09/06/2022]. https://www.hiqa.ie/reports-and-publications/health-technology-assessment/guidelines-economic-evaluation-health.
- 26.Drummond M, Sculpher MJ, Claxton K, Stoddart GL, Torrance G.W. Methods for the economic evaluation of health care programmes. (4th ed) Oxford: Oxford University Press; 2015. [Google Scholar]
- 27.HSE. Health Sector Consolidated Salary Scales. 2021. [Accessed on 09/06/2022]. from https://www.hse.ie/eng/staff/resources/hr-circulars/1-october-2021-consolidated-salary-scales.pdf .
- 28.RIA. Best practice principles for regulatory impact analysis. 2020. [Accessed on 09/06/2022]. https://www.oecd-ilibrary.org/docserver/663f08d9-en.pdf?expires=1709003120&id=id&accname=guest&checksum=F01A1105C72C8E4C4999C0DFDD7396E4.
- 29.HSE. (Health Service Executive) HR Circular 034/2019:Standardisation of Annual Leave Health and Social Care Professionals. 2019. [Accessed on 02/05/2022]. https://www.hse.ie/eng/staff/resources/hr-circulars/hr-circular-034-2019-re-annual-leave-for-health-social-care-professionals.pdf.
- 30.Kilbourne AM, Geng E, Eshun-Wilson I, et al. How does facilitation in healthcare work?Using mechanism mapping to illuminate the black box of a meta-implementation strategy. Implement Sci Commun. 2023;4(1):53. doi: 10.1186/s43058-023-00435-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.HIQA. (Health Information and Quality Authority) (2018) Guidelines for the budget impact analysis of health technologies in Ireland. [Accessed on 02/05/2022]. https://www.hiqa.ie/sites/default/files/2018-01/HIQA_BIA_Guidelines_2018_0.pdf.
- 32.CSO. (Central Statistics Office) (2018) Population Projections Results. Population and Labour Force Projections 2017. 2051. [Accessed on 20/06/2022]. https://www.cso.ie/en/releasesandpublications/ep/p-plfp/populationandlabourforceprojections2017-2051/populationprojectionsresults/
- 33.NOCA (2020) Irish Hip Fracture Database National Report. Dublin: National Office of Clinical Audit; 2019. [Accessed on 02/06/2022]. https://www.noca.ie/audits/irish-hip-fracture-database/ [Google Scholar]
- 34.NOCA (|y2020) Major Trauma Audit National Report. Dublin: National Office of Clinical Audit; 2018. [Accessed on 02/06/2022]. https://www.noca.ie/audits/irish-hip-fracture-database/ [Google Scholar]
- 35.NOCA (|y2019) Irish Hip Fracture Database National Report. Dublin: National Office of Clinical Audit; 2018. [Accessed on 02/06/2022]. https://www.noca.ie/audits/irish-hip-fracture-database/ [Google Scholar]
- 36.Bash K, Kerr M. Current &Future Cost of Frailty to Health and Care, Third National Frailty Conference, British Geriatrics Society. 2017. [Accessed on 06/07/2022]. https://www.bgs.org.uk/sites/default/files/content/attachment/2018-05-02/Bash_Current_and_future_cost_of_frailty.pdf.
- 37.Feo R, Frensham LJ, Conroy T, Kitson A. “It's just common sense”:Preconceptions and myths regarding fundamental care. Nurse Educ Pract. 2019;36:82–84. doi: 10.1016/j.nepr.2019.03.006. [DOI] [PubMed] [Google Scholar]
- 38.WHO (|y2020) The state of the Worlds Nursing. [Accessed on 26/07/2022]. https://www.who.int/news/item/07-04-|y2020-who-and-partners-call-for-urgent-investment-in-nurses.
- 39.Richards DA, Hilli A, Pentecost C, Goodwin VA, Frost J. Fundamental nursing care:A systematic review of the evidence on the effect of nursing care interventions for nutrition, elimination, mobility and hygiene. J Clin Nurs. 2018;27(11-12):2179–2188. doi: 10.1111/jocn.14150. [DOI] [PMC free article] [PubMed] [Google Scholar]