Abstract
Objectives:
Sarcopenia is a skeletal muscle mass (SMM) disease characterized by loss of strength with generalized loss of SMM. The aim of this study is to evaluate the effects of a 12-week intervention on SMM, strength, and functionally in older adults.
Methods:
This is a retrospective analysis of an intervention protocol with older adults at risk of sarcopenia who performed a daily intake of oral nutritional supplements (ONS) and resistance training exercise (RET), 3 times a week. Calf circumference (CC), bioelectrical impedance analysis (BIA), handgrip strength (HGS) and Timed Up and Go (TUG) were performed at baseline and at 12 weeks.
Results:
Fifty-one older adults were included. The mean age was 76.3 ± 8.3 years and 68.6% were women. After 12 weeks, the study showed an increase of CC in cm (1.9 ± 2.5, p < 0.001), increase of strength in kg (5.4 ± 2.1, p < 0.001), reduction of TUG in seconds (-2.4 ± 4.8, p = 0.001), increase of free-fat mass in kg (1.0 ± 1.3, p < 0.001) and SMM in kg (0.9 ± 0.5, p < 0.001).
Conclusions:
Nutritional intervention with ONS associated with RET, can increase muscle strength, SMM and functionality among older adults at risk for sarcopenia.
Keywords: Nutritional supplementation, Older adults, Rehabilitation, Resistance exercise, Sarcopenia
Introduction
Sarcopenia is considered a skeletal muscle mass (SMM) disease associated with aging, and it is characterized as out of proportion loss of muscle strength combined with cumulative and generalized loss of muscle mass and functional ability[1]. As populations grow older exponentially, sarcopenia is considered an important public health problem because it is associated with adverse results such as functional decline, fragility, admission to hospital, low quality of life and increased risk of mortality[2-4]. In terms of health economics, sarcopenia is costly to healthcare systems directly and indirectly due to the increased risk of hospitalization and the high costs of medical care: increased occurrence of falls, fractures, dysphagia, pressure ulcers and need for post-discharge rehabilitation[5,6].
The European Work Group of Sarcopenia in Older People (EWGSOP) published a screening algorithm for sarcopenia risk assessment. The SARC-F is a pioneer developed screening tool for sarcopenia and the questions cover about five domains: strength, assistance with walking, rising from a chair, climbing stairs, and falls[7,8]. As SARC-F has been reported to have low sensitivity, despite high specificity, modifications of this sarcopenia screening tool have been proposed such as the SARC-CalF[9]. The incorporation of muscle mass measurement such as calf circumference (CC), proposed by the SARC-CalF, increased the sensitivity of the screening tool when compared to the SARC-F, without modifying the specificity[9,10].
Older people with positive sarcopenia screening tool need to be referred for assessment of the sarcopenia diagnosis proposed by the EWGSOP2[1]. These patients can be classified into probable sarcopenia (only loss of muscle strength), confirmed sarcopenia (loss of strength associated with loss of quantity and/or quality of SMM) or severe sarcopenia (loss of functionality and physical performance)[1].
Nutritional care interventions for older people with sarcopenia comprises different approaches, including, dietary advice, enrichment of meals, provision of protein snacks, supply of oral nutritional supplements (ONS), as well as calcium, vitamin D, and protein, which can complement each other regarding effects on food intake and nutritional status[11]. The current recommendations for caloric and protein intake for older adults with sarcopenia are 30 kcal/kg/day and at least 1.2 g/kg/day, respectively, depending on nutritional status, physical activity, presence of comorbidities and disease-related catabolism[12-14].
Physical activity is safe and recommended to target strength and endurance, flexibility, elasticity, and stability. In addition, to alleviate SMM loss and increase strength, resistance exercises are strongly recommended for 3 or more non-consecutive days per week[13]. Immediately after resistance physical training (RET), an intake of at least 20 g of protein is recommended, as sensitivity to amino acids can be increased due to the “anabolic window” and stimulation of muscle protein synthesis (MPS)[13]. The quality of the protein source is also of great importance in stimulating MPS. Proteins rich in leucine, at a dose of 2.0 to 2.5 g per intake, generate greater muscle anabolic stimulus[15,16].
The purpose of treating sarcopenia in the older people is, first and foremost, to supply adequate amounts of energy, protein, micronutrients, and fluids to reach out nutritional needs and thereby preserve or improve nutritional status and quality of life[14,17]. Therefore, the objective of this study is to evaluate the effects of a 12-week intervention with nutritional supplementation and resistance training on SMM, strength, and functionality in older adults at risk for sarcopenia in a geriatric rehabilitation center.
Materials and Methods
Study design and setting
This is a retrospective analysis of an intervention protocol with older adults referred by clinical physicians or geriatricians to a single geriatric rehabilitation center (Prevent Senior, Brazil) from March to June 2019. We included data from older patients with positive screening for sarcopenia (SARC-CalF > 10) who started the nutritional and physical rehabilitation protocol with 12-week intervention.
The inclusion criteria were as follows: age ≥ 60 years old; SARC-CalF > 10 (positive risk for sarcopenia); patients with preserved mobility and who maintained autonomy in activities of daily living; patients with complete data on assessment of muscle strength, muscle mass, anthropometric measures and functional tests in medical records; patients free of acute disorders, decompensated chronic diseases, acute cardiovascular or cerebrovascular events, bleeding and disorders that cause acute pain; older adults with adequate cognitive level to perform resistance training and functional tests.
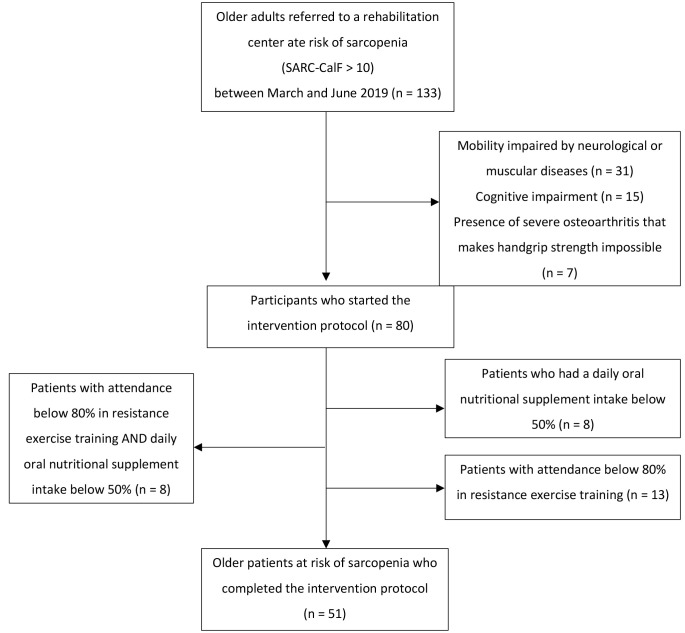
The exclusion criteria were patients unable to perform grip strength measurement due to specific comorbidities, including preceding stroke or osteoarthritis, and older people with severe cognitive impairment. Patients with cancer, palliative care, neurodegenerative diseases, or disabling orthopedic diseases were also excluded. The selection of participants as well as the inclusion and exclusion criteria flow chart are shown in Figure 1.
Figure 1.
Flow chart describing the selection, inclusion, and exclusion criteria of the participants.
Procedure
After informed consent was obtained, we searched the characteristics of the patients in the medical providing information on age, medical records, comorbidities, self-reported history of unbalance, type of assistive device used for locomotion, and use of prescription medications. This information was used to describe the demographics and health status of subjects participating in the study.
Anthropometric Measurement
Anthropometric data were achieved by a single nutritionist on all occasion: baseline and 12-week intervention. The body weight was assessed using a scale electronic model (Seca®, Germany) and measured in kilograms (kg). The patient was oriented to remain standing, in the center of the weighted platform, without shoes or sandals, free from any objects.
Body mass index (BMI) was measured as weight in kg divided by height in meters squared (kg/m2). The Mini Nutritional Assessment – Short Form (MNA-SF) was performed and the subjects were classified as: normally nourished, at risk for malnutrition or malnourished[18].
The mid upper arm circumference (MUAC) was recorded using a measuring tape. The right upper arm was assessed at the midpoint between the top of the shoulder and the top of the elbow. Another anthropometric measurement such as CC was evaluated on the right leg, using an inelastic measuring tape, in its most prominent part, with the patient having the leg inclined at an angle 90° with the knee[19]. CC value was considered the average of two measurements. In obese and overweight subjects (BMI ≥ 25 kg/m2), the correction factor for adjustment was performed, as previously reported[20]. We considered as normal values > 34 cm for men and > 33 cm for women, as validated for the Brazilian population[9].
Muscle strength
The muscle strength was evaluated by the handgrip strength (HGS), a usual indicator of strength and power of muscle groups[21,22], assessed through manual dynamometry (Jamar®, Canada), performed by a physical therapist.
Patients were oriented to sit with the shoulder adducted and in neutral rotation, the elbow in 90° flexion, forearm in neutral position and wrist between 0 and 30° of extension and between 0 and 15° of adduction. The contraction was measured three times, and for each contraction lasting 5 seconds there was a rest period of 30 seconds between each test. The maximum value of the three measurements was used for muscle strength interpretation.
The cutoff values for HGS were obtained according to the EWGSOP[1], with low muscle strength being estimated for values in women < 16 kg and in men < 27 kg.
Physical Performance
To evaluate functionality, we executed the Timed Up and Go (TUG). The patients were oriented to perform the TUG, and they received verbal commands to get up from a chair, walk 3 meters as quickly and as safely as possible, cross a line marked on the floor, turn around, go back, and sit down. The time to perform the TUG test ≥ 20 seconds demonstrated low physical performance[1].
Bioelectrical Impedance Analysis
The body composition evaluated by this method was performed by multifrequency bioelectrical impedance analysis (BIA) (Seca®, Germany). BIA was performed before the rehabilitation session because patients were instructed to remain fasting for the 4 hours prior to the exam, in addition to being informed in avoid the consumption of coffee and other stimulating drinks in the previous 48 hours. After BIA, they were taken to eat a snack, and only after that, they started the resistance training.
To perform the BIA, the patients were in dorsal decubitus, free of any metallic materials, keeping the upper and lower limbs away from the trunk at a 45° angle. The electrodes were positioned according to the manufacturer’s recommendation.
The data collected by the BIA were the body fat mass, free fat mass (FFM), the amount of SMM, and phase angle (AF). Cutoff values for low SMM assessed by BIA were obtained according to the criteria established by the EWGSOP[1].
Intervention Protocol
All patients included were prescribed ONS enriched with proteins, calcium, and vitamin D (Nutren Senior®, Nestlé Health Science) which provides 117 kcal of energy, 10g of proteins, 219mg of calcium and 100U of vitamin D in 3 tablespoons of powdered supplement.
Patients were instructed to dilute 3 tablespoons of powdered supplement in 180mL of skimmed milk and ingest it twice a day for 12 weeks. The ONS diluted in milk provided 34g of protein, 1014mg of calcium and 400U of vitamin D per day. A nutritionist made calls three times a week to intensify the intervention protocol and check the intake percentage of the proteinrich ONS.
The physical activity was performed by RET applied with 36 sessions, 3 times a week with a duration of 50 minutes each session, for 12 consecutive weeks, in the same geriatric rehabilitation center. Tests were preceded by a regular warm up (10 min at a easeful speed on a treadmill) and a specific warm up of 3 repetitions with a weight they could normally lift 10 times. The weight was progressively increased until the subject could only execute one repetition. All training sessions were conducted by an exercise specialist to guarantee safety and adherence to the protocol, and minimum training attendance was set at 80%. All patients underwent a cardiological assessment before starting the physical training.
Statistical analysis
Continuous data were showed as mean and standard deviation and categorical data were expressed as N and percentage. Data comparison for anthropometric measurements, muscle strength, physical function, body composition measurements by BIA was performed by paired t test. The Shapiro Wilk test was performed to test data normality. The effect size of the mean difference was calculated using the Glass’s delta. Comparison of proportions was performed using the chisquare test. We use SPSS version 25.0 (SPSS, Inc. Chicago IL, USA) for data analysis. Statistical significance will be associate with to an alpha error of 5% (p < 0.05).
Results
Baseline
A total of 133 older adults were referred to the geriatric rehabilitation center at risk for sarcopenia during the analysis period. However, only fifty-one older adults who completed the 12 weeks of nutritional supplementation and physical exercise intervention were included. The mean age was 76.3 ± 8.3 years and 68.6% were women. Polypharmacy has presented in 84.3% of all included patients. All patients had CC measurements below the cutoff values at baseline.
Regarding muscle strength and functionality, 70.6% had low muscle strength and 35.3% had compromised functionality, at the beginning of the intervention protocol. The baseline characteristics of subjects are demonstrated in Table 1. According to the sarcopenia diagnosis flowchart proposed by the EWGSOP2, 19.6% had confirmed sarcopenia and 13.7% had severe sarcopenia at baseline.
Table 1.
Baseline characteristics of older adults at risk for sarcopenia.
| Variables | Older patients at risk for sarcopenia (n = 51) |
|---|---|
| Age (years) | 76.3 ± 8.3 |
| Sex (n, %) | |
| Female | 35 (68.6) |
| Male | 16 (31.4) |
| Comorbidities (n, %) | |
| Hypertension | 29 (56.9) |
| Diabetes mellitus | 18 (35.3) |
| COPD | 12 (23.5) |
| Cardiovascular disease | 10 (19.6) |
| Polypharmacy (n, %) | |
| No | 8 (15.7) |
| Yes (≥ 5 daily medications) | 43 (84.3) |
| Weight (kg) | 58.9 ± 17.4 |
| BMI (kg/m2) | 22.3 ± 5.2 |
| MNA-SF (n, %) | |
| Malnourished | 39 (76.4) |
| At risk for malnutrition | 6 (11.8) |
| Normally nourished | 6 (11.8) |
| SARC-CalF | 13.2 ± 1.7 |
| Low CC (n, %) ≤ 33 cm (female); ≤ 34 cm (male) | 51 (100) |
| Anthropometric measurements | |
| CC (cm) | 30.7 ± 2.2 |
| MUAC (cm) | 26.1 ± 4.6 |
| HGS (kg) | 16.4 ± 5.9 |
| Low HGS (n, %) < 16 kg (female); < 27 kg (male) | 36 (70.6) |
| Time Up and Go (s) | 18.9 ± 9.6 |
| Low Physical Performance (n, %) ≥ 20 seconds | 18 (35.3) |
| Bioelectrical Impedance Analysis | |
| Fat Mass (kg) | 15.0 ± 7.8 |
| Free Fat Mass (kg) | 43.9 ± 11.6 |
| Skeletal Muscle Mass (kg) | 22.4 ± 6.2 |
| Phase Angle (°) | 4.2 ± 0.5 |
COPD: chronic obstructive pulmonary disease; BMI: body mass index; MNA-SF: Mini Nutrition Assessment – Short Form; SARC-CalF: strength, assistance with walking, rising from a chair, climbing stairs, falls, and calf circumference; CC: calf circumference; MUAC: mid upper arm circumference; HGS: handgrip strength.
12-week intervention
After 12-week intervention protocol, the comparison of the older adults in relation to the baseline are demonstrated in Table 2. The comparison showed an increase in mean difference of anthropometric measurements: body weight in kg (1.1 ± 1.5, p < 0.001) and CC in cm (1.9 ± 2.5, p < 0.001). Also, it was demonstrated an increase in muscle strength by HGS in kg (5.4 ± 2.1, p < 0.001) and a reduction in the time to perform the TUG in seconds (-2.4 ± 4.8, p = 0.001). The largest effect size was seen in increasing CC and HGS after 12-week intervention.
Table 2.
Comparison of variables after nutritional and physical intervention in geriatric rehabilitation center.
| Variables | Baseline | 12-week intervention | Mean Difference | Effect Size | 95% CI Lower | 95% CI Upper | p |
|---|---|---|---|---|---|---|---|
| Weight (kg) | 58.9 ± 17.4 | 60.0 ± 16.6 | 1.1 ± 1.5 | 0.06 | -0.33 | 0.45 | < 0.001* |
| BMI (kg/m2) | 22.3 ± 5.2 | 22.9 ± 4.8 | 0.5 ± 0.6 | 0.12 | -0.27 | 0.51 | < 0.001* |
| SARC-CalF | 13.2 ± 1.7 | 7.9 ± 4.1 | -5.2 ± 4.4 | -3.12 | -3.70 | -2.48 | < 0.001* |
| Anthropometric measures | |||||||
| CC (cm) | 30.7 ± 2.2 | 32.6 ± 2.5 | 1.9 ± 2.5 | 0.84 | 0.44 | 1.23 | < 0.001* |
| MUAC (cm) | 26.1 ± 4.6 | 26.2 ± 4.5 | 0.1 ± 0.5 | 0.02 | -0.37 | 0.41 | 0.262 |
| HGS (kg) | 16.4 ± 5.9 | 21.8 ± 5.6 | 5.4 ± 2.1 | 0.92 | 0.51 | 1.31 | < 0.001* |
| TUG (s) | 18.9 ± 9.6 | 16.6 ± 7.8 | -2.4 ± 4.8 | -0.24 | -0.63 | -0.16 | 0.001* |
| BIA | |||||||
| FM (kg) | 15.0 ± 7.8 | 15.1 ± 7.5 | 0.1 ± 1.2 | 0.01 | -0.38 | 0.40 | 0.486 |
| FFM (kg) | 43.9 ± 11.6 | 44.9 ± 11.2 | 1.0 ± 1.3 | 0.09 | -0.30 | 0.48 | < 0.001* |
| SMM (kg) | 22.4 ± 6.2 | 23.3 ± 6.1 | 0.9 ± 0.5 | 0.15 | -0.24 | 0.53 | < 0.001* |
| PA (°) | 4.2 ± 0.5 | 4.3 ± 0.5 | 0.1 ± 0.2 | 0.20 | -0.19 | 0.59 | 0.002* |
BMI: body mass index; SARC-CalF: strength, assistance with walking, rising from a chair, climbing stairs, falls, and calf circumference; CC: calf circumference; MUAC: mid upper arm circumference; HGS: handgrip strength; TUG: Timed Up and Go; BIA: bioelectrical impedance analysis; FM: fat mass; FFM: free fat mass; SMM: skeletal muscle mass; PA: phase angle.
Paired t test.
In addition, there was a significant increase in the mean difference in body composition measures: FFM in kg (1.0 ± 1.3, p < 0.001), SMM in kg (0.9 ± 0.5, p < 0.001) and PA in degrees (0.1 ± 0.2, p = 0.002), assessed by BIA when data were compared with baseline.
The comparison of the proportion of sarcopenia diagnosis between baseline and 12-week intervention is shown in Table 3. Sarcopenia severity reduced from 13.7% to 5.9% (p < 0.001) in relation to baseline.
Table 3.
Comparison of the diagnosis of sarcopenia between baseline and 12-week intervention among older adults in a geriatric rehabilitation center.
| Baseline | 12-week intervention | p-value | |
|---|---|---|---|
| No sarcopenia (n, %) Normal HGS | 15 (29.4) | 32 (62.7) | < 0.001* |
| Probable sarcopenia (n, %) Low HGS and normal SMM | 19 (37.3) | 10 (19.6) | 0.002* |
| Confirmed sarcopenia (n, %) Low HGS and low SMM | 10 (19.6) | 6 (11.8) | 0.002* |
| Severe sarcopenia (n, %) Low HGS, low SMM and low functionality | 7 (13.7) | 3 (5.9) | < 0.001* |
HGS: handgrip strength; SMM: skeletal muscle mass.
Chi square test.
Discussion
In our study about older adults referred to a geriatric rehabilitation center due to a positive screening tool test for sarcopenia, there was an increase in SMM and muscle strength, and an improvement in physical performance after a nutritional intervention with ONS and physical activity by RET for 12 consecutive weeks. In addition, the percentage of diagnosed sarcopenia confirmed, and severe sarcopenia reduced at the end of the intervention.
Aging is related with a progressive and cumulative loss of SMM and muscle strength. Loss of muscle mass is valued at approximately 35–40% between the ages of 20 and 80 years[23]. The difference in muscle strength between young people and healthy older adults ages 60 to 80 years is 20–40%, and this difference increases to ≥ 50% when compared with those older than 80 years[3,24].
Sufficient dietary protein intake in addition with adequate vitamin D and calcium status are proposed to attenuate age-related SMM and bone loss[25,26]. This is particularly important for frail and sarcopenic older people who are at greater risk of osteoporosis, falls, fractures and sequential institutionalization[13].
Recent studies propound that the older individuals demonstrated a blunted MPS response to protein administration and physical exercise when compared with that in the young people[27,28]. An important factor is that older adults are anabolically resistant and request higher doses of protein to reach similar rates of MPS, the main variable that regulates changes in SMM[27]. Some factors impair protein digestion and amino acid absorption, insulin-mediated muscle tissue perfusion, absorption of amino acids into SMM, or a reduced amount or activation status of key signaling proteins that may contribute to this suggested anabolic resistance in aging[13,27,28].
Vitamin D deficiency is a common condition among older adults and has a negative impact and contributes to greater loss of muscle mass[29]. Worldwide data show that 5% to 25% of the independent people over 65 years are deficient or insufficient in vitamin D[30]. Several factors contribute to a higher prevalence of hypovitaminosis D as a reduce in cutaneous vitamin D synthesis after sun exposure due to atrophic changes of the skin itself[31]. Adequate levels of vitamin D intake are already well established as aiding post-exercise recovery due to its positive effects on SMM regeneration through promoting muscle cell development and immune response[32].
Previous studies demonstrated that protein enriched ONS with calcium and vitamin D in combination with RET have been identified as an efficient intervention for increase muscle mass and strength gain in older adults[33-35]. Resistance exercise is the very potent physical stimulus for hypertrophy of SMM, improving strength and physical performance and, therefore, is the most effective non-pharmacological treatment for age-related sarcopenia[35].
Following a RET session performed, the MPS is essentially raised; although, muscle protein breakdown (MPB) is also increased, and muscle protein balance remains negative[27]. Therefore, ingestion of protein, primarily essential amino acids, in temporal vicinity after RET, leads to a synergistic increase in MPS along with a mitigation of the exercise-induced increase in MPB, which provides a positive protein balance[29].
In a systematic review by Liao et al, it was demonstrated that an increase of >2.0% to 3.0% in SMM predicts a positive effect of protein enriched ONS plus RET on leg strength and walking ability[36]. Regardless of the apparent benefit of resistance exercise in combatting sarcopenia, adherence to the RET guidelines (≥2/week, >80% of 1-repetition maximum - RM) remains low, especially in older adults[29].
Morton et al[34] demonstrated benefits similar to our study in one of the largest meta-analysis of interventions including dietary protein supplementation and assessments of SMM and strength during prolonged RET. Their findings were that dietary protein supplementation augmented RET-induced increases in 1RM strength and FFM. They assumed that dietary protein supplementation is both sufficient and necessary to improve RET adaptations in muscle mass and strength in older adults[34]. However, further analysis of trends observed in this study regarding effective nutritional interventions and the result to wider understanding is needed.
Previous reviews[24,33,37,38] and following the current recommendations of the European Society for Clinical Nutrition and Metabolism Expert Group[39], the results of the current meta-analysis support the urgent need for older adults at risk for sarcopenia to combine protein-based nutrition intervention and RET to avoid functional disabilities, especially frail older patients who are at high risk of insufficient protein intake and physical inactivity.
Another highly prevalent factor in our study population was polypharmacy (intake of 5 or more daily medications). Polypharmacy is well-known with its association with falls in older adults and this might be described by its association with poor physical performance[40]. Epidemiological data suggest that polypharmacy is present in more than a third of the elderly worldwide, being a factor of great relevance and impact in older adults[41]. It is widely known that polypharmacy in older populations has been associated with many negative health care outcomes, including increased loss of SMM, physical disability, and loss of equilibrium[41].
Several limitations to our findings should be elucidated. Firstly, this study was retrospective, and, since it was not a double-blind study, it could grant to some form of bias. Therefore, the entirety of the information in medical records was limited. Secondly, based on the variation among protein supplement intake (protein source, supplied amounts, timing of ingestion) and different exercise schemes (training volume), endorsing a definite conclusion for the effect of specific type of physical exercise and nutritional supplementation on muscle mass or strength gains was difficult. Third, the study has no control group, all patients were part of an institutional protocol being attended by the same geriatric rehabilitation center, therefore, all patients received the ONS intervention plus RET. Fourth, we only control the protein intake from the ONS, but we do not control the overall intake from the diet.
Conclusion
Nutritional intervention with frequent ingestion of ONS enriched with protein, calcium and vitamin D associated with supervised physical activity, especially with resistance training, can increase muscle strength, SMM and functionality among older adults at risk for sarcopenia. Other studies with a control group and robust scientific methodology should be performed to confirm these findings.
Ethics approval
The study was reviewed and approved by the local Research Ethics Committee (CAAE 63001521.5.0000.8114, Prevent Senior Institute, Brazil). All procedures were performed following the Declaration of Helsinki.
Authors’ contributions
Thiago J.M. Gonçalves contributed to the conception of the research and writing the manuscript; Bruna T Carlos contributed to the acquisition of the data; Mayara S. de Souza contributed to the analysis and interpretation of the data; Valeria C. Jorge contributed to the metodology and validation of the research. Sandra E.A.B. Gonçalves contributed to writing the manuscript. Rafaela A. Campos and Valeria A.S. Rosenfeld equally contributed to the conception and design of the research. All authors drafted the manuscript, critically revised the manuscript, agree to be fully accountable for ensuring the integrity and accuracy of the work, and read and approved the final manuscript.
Acknowledgments
The authors wish to thank the volunteers for their participation.
Footnotes
Edited by: Yannis Dionyssiotis
References
- 1.Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyere O, Cederholm T, et al. Sarcopenia:revised European consensus on definition and diagnosis. Age Ageing. 2019;48(4):16–31. doi: 10.1093/ageing/afy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia:European consensus on definition and diagnosis:Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39(4):412–23. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gonçalves TJM, Horie LM, Gonçalves SEAB, Bacchi MK, Bailer MC, Barbosa-Silva TG, et al. Diretriz BRASPEN de Terapia Nutricional no Envelhecimento. BRASPEN J. 2019;34(3):2–58. [Google Scholar]
- 4.Bachettini NP, Bielemann RM, Barbosa-Silva TG, Menezes AMB, Tomasi E, Gonzalez MC. Sarcopenia as a mortality predictor in community-dwelling older adults:a comparison of the diagnostic criteria of the European Working Group on Sarcopenia in Older People. Eur J Clin Nutr. 2020;74(4):573–80. doi: 10.1038/s41430-019-0508-8. [DOI] [PubMed] [Google Scholar]
- 5.Cawthon PM, Lui LY, Taylor BC, McCulloch CE, Cauley JA, Lapidus J, et al. Clinical Definitions of Sarcopenia and Risk of Hospitalization in Community-Dwelling Older Men:The Osteoporotic Fractures in Men Study. J Gerontol A Biol Sci Med Sci. 2017;72(10):1383–9. doi: 10.1093/gerona/glw327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mijnarends DM, Luiking YC, Halfens RJG, Evers SMAA, Lenaerts ELA, Verlaan S, et al. Muscle, Health and Costs:A Glance at their Relationship. J Nutr Health Aging. 2018;22(7):766–73. doi: 10.1007/s12603-018-1058-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Malmstrom TK, Miller DK, Simonsick EM, Ferrucci L, Morley JE. SARC-F:a symptom score to predict persons with sarcopenia at risk for poor functional outcomes. J Cachexia Sarcopenia Muscle. 2016;7(1):28–36. doi: 10.1002/jcsm.12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vidal-Cuellar CL, Mas G, Ayamamani-Torres P, Yazawa T, Rosas-Carrasco O, Tello T. Identification of Probable sarcopenia based on SARC-F and SARC-CalF in older adults from a low-resource setting. J Frailty Sarcopenia Falls. 2022;7(4):222–30. doi: 10.22540/JFSF-07-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barbosa-Silva TG, Menezes AM, Bielemann RM, Malmstrom TK, Gonzalez MC. Enhancing SARC-F:Improving Sarcopenia Screening in the Clinical Practice. J Am Med Dir Assoc. 2016;17(12):1136–41. doi: 10.1016/j.jamda.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 10.Bahat G, Oren MM, Yilmaz O, Kılıç C, Aydin K, Karan MA. Comparing SARC-F with SARC-CalF to Screen Sarcopenia in Community Living Older Adults. J Nutr Health Aging. 2018;22(9):1034–8. doi: 10.1007/s12603-018-1072-y. [DOI] [PubMed] [Google Scholar]
- 11.Tessier AJ, Chevalier S. An Update on Protein, Leucine, Omega-3 Fatty Acids, and Vitamin D in the Prevention and Treatment of Sarcopenia and Functional Decline. Nutrients. 2018;10(8):1099. doi: 10.3390/nu10081099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bauer J, Morley JE, Schols A, Ferrucci L, Cruz-Jentoft AJ, Dent E, et al. Sarcopenia:A Time for Action. An SCWD Position Paper. J Cachexia Sarcopenia Muscle. 2019;10(5):956–961. doi: 10.1002/jcsm.12483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bauer J, Biolo G, Cederholm T, Cesari M, Cruz-Jentoft AJ, Morley JE, et al. Evidence-based recommendations for optimal dietary protein intake in older people:a position paper from the PROT-AGE Study Group. J Am Med Dir Assoc. 2013;14(8):542–59. doi: 10.1016/j.jamda.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 14.Volkert D, Beck AM, Cederholm T, Cruz-Jentoft A, Goisser S, Hooper L, et al. ESPEN guideline on clinical nutrition and hydration in geriatrics. Clin Nutr. 2019;38(1):10–47. doi: 10.1016/j.clnu.2018.05.024. [DOI] [PubMed] [Google Scholar]
- 15.Kim HK, Suzuki T, Saito K, Yoshida H, Kobayashi H, Kato H, et al. Effects of exercise and amino acid supplementation on body composition and physical function in community-dwelling elderly Japanese sarcopenic women:a randomized controlled trial. J Am Geriatr Soc. 2012;60(1):16–23. doi: 10.1111/j.1532-5415.2011.03776.x. [DOI] [PubMed] [Google Scholar]
- 16.Casperson SL, Sheffield-Moore M, Hewlings SJ, Paddon-Jones D. Leucine supplementation chronically improves muscle protein synthesis in older adults consuming the RDA for protein. Clin Nutr. 2012;31(4):512–9. doi: 10.1016/j.clnu.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Woo J, Leung J, Morley JE. Defining sarcopenia in terms of incident adverse outcomes. J Am Med Dir Assoc. 2015;16(3):247–52. doi: 10.1016/j.jamda.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 18.Rubenstein LZ, Harker JO, Salvà A, Guigoz Y, Vellas B. Screening for undernutrition in geriatric practice:developing the short-form mini-nutritional assessment (MNA-SF) J Gerontol A Biol Sci Med Sci. 2001;56(6):M366–72. doi: 10.1093/gerona/56.6.m366. [DOI] [PubMed] [Google Scholar]
- 19.Kawakami R, Murakami H, Sanada K, Tanaka N, Sawada SS, Tabata I, et al. Calf circumference as a surrogate marker of muscle mass for diagnosing sarcopenia in Japanese men and women. Geriatrics &gerontology international. 2015;15(8):969–76. doi: 10.1111/ggi.12377. [DOI] [PubMed] [Google Scholar]
- 20.Gonzalez MC, Mehrnezhad A, Razaviarab N, Barbosa-Silva TG, Heymsfield SB. Calf circumference:cutoff values from the NHANES 1999-2006. The American journal of clinical nutrition. 2021;113(6):1679–87. doi: 10.1093/ajcn/nqab029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bruyère O, Beaudart C, Reginster JY, Buckinx F, Schoene D, Hirani V, et al. Assessment of muscle mass, muscle strength and physical performance in clinical practice:an international survey. Eur Geriatr Med. 2016;7:243–46. [Google Scholar]
- 22.Beaudart C, Rolland Y, Cruz-Jentoft AJ, Bauer JM, Sieber C, Cooper C, et al. Assessment of Muscle Function and Physical Performance in Daily Clinical Practice :A position paper endorsed by the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO) Calcif Tissue Int. 2019;105(1):1–14. doi: 10.1007/s00223-019-00545-w. [DOI] [PubMed] [Google Scholar]
- 23.Cruz-Jentoft AJ, Sayer AA. Sarcopenia. Lancet. 2019;393(10191):2636–46. doi: 10.1016/S0140-6736(19)31138-9. [DOI] [PubMed] [Google Scholar]
- 24.Gielen E, Beckwée D, Delaere A, De Breucker S, Vandewoude M, Bautmans I, et al. Nutritional interventions to improve muscle mass, muscle strength, and physical performance in older people:an umbrella review of systematic reviews and meta-analyses. Nutr Rev. 2021;79(2):121–47. doi: 10.1093/nutrit/nuaa011. [DOI] [PubMed] [Google Scholar]
- 25.Rizzoli R, Stevenson JC, Bauer JM, van Loon LJ, Walrand S, Kanis JA, et al. The role of dietary protein and vitamin D in maintaining musculoskeletal health in postmenopausal women:a consensus statement from the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) Maturitas. 2014;79(1):122–32. doi: 10.1016/j.maturitas.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 26.Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine:what clinicians need to know. J Clin Endocrinol Metab. 2011;96(1):53–8. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burd NA, Gorissen SH, van Loon LJ. Anabolic resistance of muscle protein synthesis with aging. Exerc Sport Sci Rev. 2013;41(3):169–73. doi: 10.1097/JES.0b013e318292f3d5. [DOI] [PubMed] [Google Scholar]
- 28.Moore DR, Churchward-Venne TA, Witard O, Breen L, Burd NA, Tipton KD, et al. Protein ingestion to stimulate myofibrillar protein synthesis requires greater relative protein intakes in healthy older versus younger men. J Gerontol A Biol Sci Med Sci. 2015;70(1):57–62. doi: 10.1093/gerona/glu103. [DOI] [PubMed] [Google Scholar]
- 29.McKendry J, Currier BS, Lim C, Mcleod JC, Thomas ACQ, Phillips SM. Nutritional Supplements to Support Resistance Exercise in Countering the Sarcopenia of Aging. Nutrients. 2020;12(7):2057. doi: 10.3390/nu12072057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lips P, Duong T, Oleksik A, Black D, Cummings S, Cox D, et al. A global study of vitamin D status and parathyroid function in postmenopausal women with osteoporosis:baseline data from the multiple outcomes of raloxifene evaluation clinical trial. J Clin Endocrinol Metab. 2001;86(3):1212–21. doi: 10.1210/jcem.86.3.7327. [DOI] [PubMed] [Google Scholar]
- 31.Remelli F, Vitali A, Zurlo A, Volpato S. Vitamin D Deficiency and Sarcopenia in Older Persons. Nutrients. 2019;11(12):2861. doi: 10.3390/nu11122861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thomas DR. Vitamins in aging, health, and longevity. Clin Interv Aging. 2006;1(1):81–91. doi: 10.2147/ciia.2006.1.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liao CD, Lee PH, Hsiao DJ, Huang SW, Tsauo JY, Chen HC, et al. Effects of Protein Supplementation Combined with Exercise Intervention on Frailty Indices, Body Composition, and Physical Function in Frail Older Adults. Nutrients. 2018;10(12):1916. doi: 10.3390/nu10121916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morton RW, Murphy KT, McKellar SR, Schoenfeld BJ, Henselmans M, Helms E, et al. A systematic review, meta-analysis and meta-regression of the effect of protein supplementation on resistance training-induced gains in muscle mass and strength in healthy adults. Br J Sports Med. 2018;52(6):376–84. doi: 10.1136/bjsports-2017-097608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Phillips SM. Nutritional supplements in support of resistance exercise to counter age-related sarcopenia. Adv Nutr. 2015;6(4):452–60. doi: 10.3945/an.115.008367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liao CD, Chen HC, Huang SW, Liou TH. The Role of Muscle Mass Gain Following Protein Supplementation Plus Exercise Therapy in Older Adults with Sarcopenia and Frailty Risks:A Systematic Review and Meta-Regression Analysis of Randomized Trials. Nutrients. 2019;11(8):1713. doi: 10.3390/nu11081713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gupta P, Kumar S. Sarcopenia and Endocrine Ageing:Are They Related?Cureus. 2022;14(9):e28787. doi: 10.7759/cureus.28787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ladang A, Beaudart C, Reginster JY, Al-Daghri N, Bruyère O, Burlet N, et al. Biochemical Markers of Musculoskeletal Health and Aging to be Assessed in Clinical Trials of Drugs Aiming at the Treatment of Sarcopenia:Consensus Paper from an Expert Group Meeting Organized by the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO) and the Centre Académique de Recherche et d'Expérimentation en Santé(CARES SPRL), Under the Auspices of the World Health Organization Collaborating Center for the Epidemiology of Musculoskeletal Conditions and Aging. Calcif Tissue Int. 2023;112(2):197–217. doi: 10.1007/s00223-022-01054-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Deutz NE, Bauer JM, Barazzoni R, Biolo G, Boirie Y, Bosy-Westphal A, et al. Protein intake and exercise for optimal muscle function with aging:recommendations from the ESPEN Expert Group. Clin Nutr. 2014;33(6):929–36. doi: 10.1016/j.clnu.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ozkok S, Aydin CO, Sacar DE, Catikkas NM, Erdogan T, Kilic C, et al. Associations between polypharmacy and physical performance measures in older adults. Arch Gerontol Geriatr. 2022;98:104553. doi: 10.1016/j.archger.2021.104553. [DOI] [PubMed] [Google Scholar]
- 41.Prokopidis K, Giannos P, Reginster JY, Bruyere O, Petrovic M, Cherubini A, et al. Sarcopenia is associated with a greater risk of polypharmacy and number of medications:a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. 2023;14(2):671–683. doi: 10.1002/jcsm.13190. [DOI] [PMC free article] [PubMed] [Google Scholar]