Abstract
Introduction
Although musculoskeletal involvement of Neurofibromatosis type 1 (NF1) has been well documented, bone formation, or ossification, within neurofibroma, has been scarcely documented in literature. Here, we report a rare case of ossified neurofibroma in a patient with long history of NF1.
Presentation of case
73-Year-old female with childhood-onset NF1 and surgical history of resection for multiple neurofibromas, presented with right ptosis and eyebrow ptosis. A growing tumor on the right eyebrow was surgically resected. Microscopically, the dermal tumor consists of bland spindle cells with thin, wavy nuclei, without atypia, showing S100 immunoreactivity, consistent with neurofibroma. Multiple metaplastic bone formation composed of mature bone trabeculae surrounding adipose tissue were apparent.
Discussion
Up to date, ossification of neurofibroma has been scarcely reported in literature. The etiology is unclear but might involve the response to chronic stress and tissue damage over the years, and/or might indicate the potential differentiation plasticity of mesenchymal stem cell-like population.
Conclusion
The unusual presentation of ossification provides insights on the pathogenesis and differentiation plasticity of neurofibroma.
Keywords: Ossification, Neurofibroma, Neurofibromatosis type 1, Metaplasia, Mesenchymal stem cell, Case report
Highlights
-
•
Rare presentation of ossification in forehead neurofibroma from a patient with long history of neurofibromatosis type 1.
-
•
Bone formation in neurofibroma has been scarcely reported in literature.
-
•
The etiology of ossification might involve the metaplastic change over the tissue damage, chronic stress and aging.
-
•
Ossification can provide insights on the potential differentiation plasticity of mesenchymal stem cell-like population.
1. Introduction
Neurofibromatosis type 1 (NF1), also called von Recklinghausen disease, is a complex autosomal dominant disorder caused by germline mutations in the NF1 tumor suppressor gene, that affects 1 in 3000 persons worldwide [1,2]. The mutated NF1 results in dysfunction of the guanosine triphosphatase–activating protein (GAP) neurofibromin leads to overactivation of multiple signaling pathways including the RAS pathway, leading to the development of multiple types of benign and malignant tumors, including neurofibromas, malignant peripheral nerve sheath tumors (MPNSTs) and optic pathway gliomas (OPGs) [[3], [4], [5]]. Series of molecular targeted therapies, including selumetinib, selectively inhibiting the RAS pathway with mitogen activated protein kinase (MAPK) kinase (MEK) inhibition, have shown promising results in clinical trials or in preclinical studies [5,6]. However, no effective pharmacological agent has not been yet available for cure of the disease, and clinical management largely relies on surgical resection of tumors [5].
Clinical manifestation of NF1 patients include dermal neurofibromas, pigmented skin lesions, such as café-au-lait macules, and occasional skeletal abnormalities and cognitive disabilities [1]. Although musculoskeletal involvement of NF1, such as scoliosis, tibial pseudarthrosis, have been well documented [1,2], bone formation, or ossification, within neurofibroma, has been scarcely documented in literature. Here, we report a rare case of ossified neurofibroma in a patient with long history of NF1, with literature review and discussions on potential etiologies. The work has been reported in line with the Surgical CAse REport (SCARE) criteria [7].
2. Presentation of case
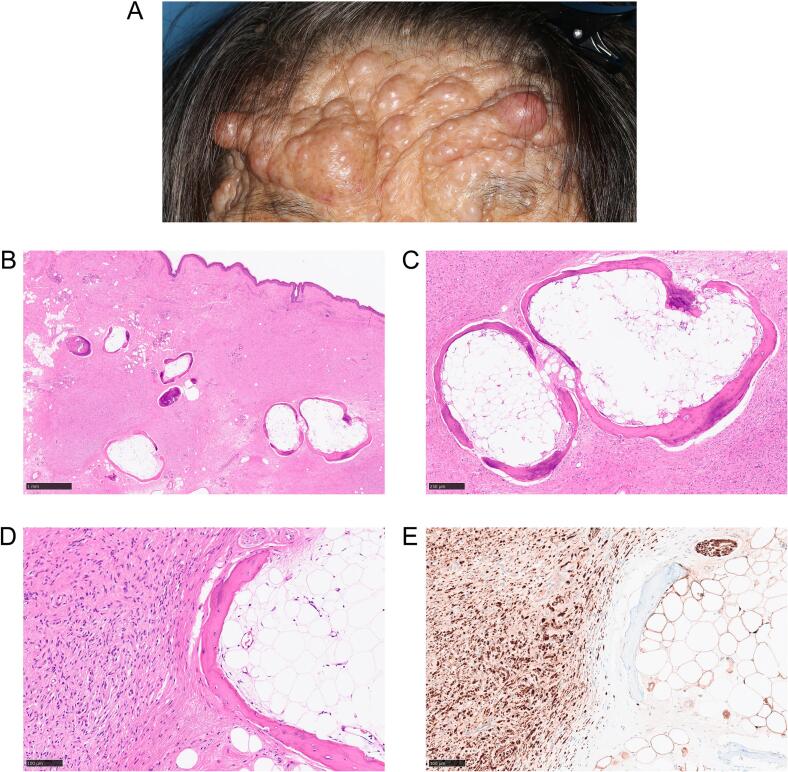
73-year-old female with childhood onset of neurofibromatosis type 1 presented with right ptosis and eyebrow ptosis. She underwent numerous surgeries, including ptosis surgeries, resection of subungual tumors and subcutaneous tumors of eyebrows and the right forearms. Her past medical history includes malignant lymphoma with complete remission, gastric duodenal ulcer, left lung abscess, atrial fibrillation, and about 20 years history of facial paralysis. Physical examination showed widespread nodules of various sizes on her face: on the forehead, eyebrows, eyelids, cheeks, external ears, nose, perioral skin and lips (Fig. 1A). Manual elevation of the right eyebrow still left right ptosis.
Fig. 1.
Ossified neurofibroma in a neurofibromatosis type 1 (NF1) patient.
(A) Preoperative image of the patient's forehead with numerous nodules. (B) Neurofibroma in dermis containing multiple ossified lesions. Scale bar = 1 mm. (C) Ossified lesions consist of mature bone trabeculae with osteoblasts, surrounding adipose tissue. Scale bar = 250 μm. (D) Magnified view of the boundary of ossification and neurofibroma. (E) Immunohistochemistry showed S100 positivity. (D), (E): Scale bar = 100 μm.
Subcutaneous tumors on the right eyebrow, left eyebrow and left external ear were surgically resected. Microscopically, all tumors showed dermal, nonencapsulated proliferation of hypocellular bland spindle cells with thin, wavy nuclei with interspersed collagen bundles (Fig. 1B, C, D). Immunohistochemistry showed the tumor cells were positive for S100 (Fig. 1E). Pathological diagnosis was consistent with neurofibroma. Notably, in the right eyebrow neurofibroma, multiple ossified lesions comprising almost complete set of mature bone structure: bone trabeculae containing osteoblast surrounding adipose tissue, resembling fatty marrow, were observed (Fig. 1B, C, D). Neither massive immune cell infiltration nor foreign body reaction were observed in the tumor and around the ossified lesions. Cartilaginous tissues were not observed either. In addition, ossified lesions did not show atypia nor sign of malignancy. Collectively, ossification was considered metaplastic, and additional therapeutic intervention for the ossification post resection was not performed. The patient was discharged without postsurgical complications.
3. Discussion
Although musculoskeletal involvement of NF1 has been well documented [1,2], ossification of neurofibroma has been scarcely reported; our search hit only 4 cases of ossified neurofibromas in PubMed-indexed English literatures [[8], [9], [10], [11]], other than this case (Table 1). All 4 cases were male [[8], [9], [10], [11]]. 3 cases were solitary neurofibromas [[8], [9], [10]], and 1 case showed diffuse neurofibroma, with family history of skin nodules but the diagnosis for neurofibromatosis was only provisional [11], making our case the first reported female NF1 patient with ossified neurofibroma. In particular, bone formation observed in our case was not simple calcification but rather, almost complete form of the mature bones, comprised of osteoblasts, mature trabeculae, and fatty marrow-like adipose tissue including vascular structures, within the trabeculae.
Table 1.
Reported cases of ossification in neurofibroma.
| Age | Gender | Location | Etiology | Pathological diagnosis | Reference (year) | |
|---|---|---|---|---|---|---|
| 1 | 61 | Male | Right thigh | – | Neurofibroma | Sarma et al. [8] (1983) |
| 2 | 50 | Male | Right foot | – | Solitary neurofibroma | Kapoor et al. [9] (1986) |
| 3 | 41 | Male | Left buccal mucosa | – | Most likely a myxoid variant of neurofibroma | Farthing et al. [10] (1989) |
| 4 | 20 | Male | Right buccal, masticator and parapharyngeal space | Diffuse neurofibroma | Dua et al. [11] (2012) | |
| 5 | 73 | Female | Right eyebrow | Neurofibro-matosis type 1 | Neurofibroma | Present case (2024) |
Etiology of ossification in neurofibroma is still unclear. One possibility is that the ossification might be the response to previous injuries, including surgical history, chronic inflammation and accumulation of stress, and tissue damage and repair over the years [11,12]. Another possibility is that the ossification stems from the differentiation of recently highlighted mesenchymal stem cell-like population, indicating their plasticity [11,[13], [14], [15], [16], [17]]. In heterotopic ossification (HO), bone morphogenetic proteins (BMP), including BMP2, which are members of the transforming growth factor-β (TGF-β) superfamily, have been indicated to promote transformation of stromal mesenchymal cells to osteoblasts [[18], [19], [20]]. Although cell origin of neurofibroma is still controversial, some studies indicate the existence of the multipotent stem cell-like population in neurofibroma [[13], [14], [15]]. A recent study suggests that mesenchymal stem cell-like cells with osteogenic induction potential are more abundant in head and neck neurofibromas compared with those of trunk, perhaps associated with different levels of brain-derived neurotrophic factor (BDNF) across tissue microenvironment [13]. The presented case underwent multiple surgeries for different locations such as subungual tumors and subcutaneous tumors of eyebrows and the right forearms, but the most recently resected one in the right eyebrow that existed for decades, was the only site reported for clear ossification. Thus, it might be consistent with the proposed mesenchymal stem-like cells predilection for head and neck location. The above-described possibilities are not mutually exclusive; indeed, considering that stress, aging, inflammation, and tissue microenvironment collectively impact recruitment and differentiation of mesenchymal stem cells [16,21], the etiologies can be the hybrid of the above-mentioned possibilities with complex interaction. Taken together, the ossification in neurofibroma observed in this case might indicate the differentiation plasticity of potential mesenchymal/multipotent stem cell-like population over the long history of neurofibroma undergoing tissue damage, aging and chronic stress. Further studies are warranted to further dissect the pathogenesis of ossification to identify novel therapeutic targets and develop effective therapeutic strategies to improve the quality of life for patients suffering from neurofibromatosis and ossification.
4. Conclusion
Here, we report a rare case of NF1 patient with ossified neurofibroma. The bone formation over the long clinical course in neurofibroma provide insights on the pathogenesis of metaplastic ossification and potential differentiation plasticity of mesenchymal stem cell-like population.
Ethics statement including patient consent statement
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request. This study was approved by the Tohoku University Hospital Institutional Review Board (Protocol Identification Number 35324).
Funding information
None.
Guarantor
Takashi Suzuki.
CRediT authorship contribution statement
Yuki Muroyama: Conceptualization, Formal analysis, Investigation, Project administration, Validation, Visualization, Writing – original draft, Writing – review & editing. Chieko Miura: Investigation, Project administration, Validation, Writing – review & editing. Yoshimichi Imai: Supervision, Writing – review & editing. Takashi Suzuki: Formal analysis, Investigation, Project administration, Supervision, Validation, Writing – review & editing.
Declaration of competing interest
The authors declare no conflicts of interest associated with this manuscript.
References
- 1.Gutmann David H., Ferner Rosalie E., Listernick Robert H., Korf Bruce R., Wolters Pamela L., Johnson Kimberly J. Neurofibromatosis type 1. Nat. Rev. Dis. Primers. 2017;3(1) doi: 10.1038/nrdp.2017.4. [DOI] [PubMed] [Google Scholar]
- 2.Feldman D.S., Jordan C., Fonseca L. Orthopaedic manifestations of neurofibromatosis type 1. J. Am. Acad. Orthop. Surg. 2010;18(6) doi: 10.5435/00124635-201006000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Baez-Flores J., Rodriguez-Martin M., Lacal J. The therapeutic potential of neurofibromin signaling pathways and binding partners. Commun. Biol. 2023;6(1):436. doi: 10.1038/s42003-023-04815-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ratner N., Miller S.J. A RASopathy gene commonly mutated in cancer: the neurofibromatosis type 1 tumour suppressor. Nat. Rev. Cancer. 2015;15(5):290–301. doi: 10.1038/nrc3911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brosseau J.P., Liao C.P., Le L.Q. Translating current basic research into future therapies for neurofibromatosis type 1. Br. J. Cancer. 2020;123(2):178–186. doi: 10.1038/s41416-020-0903-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gross A.M., Wolters P.L., Dombi E., Baldwin A., Whitcomb P., Fisher M.J., et al. Selumetinib in children with inoperable plexiform neurofibromas. N. Engl. J. Med. 2020;382(15):1430–1442. doi: 10.1056/NEJMoa1912735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sohrabi C., Mathew G., Maria N., Kerwan A., Franchi T., Agha R.A. The SCARE 2023 guideline: updating consensus surgical CAse REport (SCARE) guidelines. Int. J. Surg. Lond. Engl. 2023;109(5):1136. doi: 10.1097/JS9.0000000000000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sarma D.P., Robichaux J., Fondak A. Ossified neurofibroma. J. La State Med. Soc. 1983;135(0024-6921):22–23. [PubMed] [Google Scholar]
- 9.Kapoor R., Mittal K.P., Jayaram G. Solitary neurofibroma of foot — an unusual case with extensive calcification and ossification. Australas. Radiol. 1986;30(2):150–152. doi: 10.1111/j.1440-1673.1986.tb02409.x. [DOI] [PubMed] [Google Scholar]
- 10.Farthing P.M., Malamos D., Williams D.M. Metaplastic bone formation in an unusual neural tumour of the oral cavity. Br. J. Oral Maxillofac. Surg. 1989;27(6):517–519. doi: 10.1016/s0266-4356(89)80012-9. [DOI] [PubMed] [Google Scholar]
- 11.Dua S.G., Kulkarni A.V., Kulkarni S.S., Shetty N.S., Shet T. Hemifacial mass with extensive intralesional ossification and fat. Dentomaxillofac. Radiol. 2012;41(5):436–439. doi: 10.1259/dmfr/28313752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meyers C., Lisiecki J., Miller S., Levin A., Fayad L., Ding C., et al. Heterotopic ossification: a comprehensive review. JBMR Plus. 2019;3(4) doi: 10.1002/jbm4.10172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shi J., Yang Z., Zhang Y., Abdelrehem A., Wu Z., Zhang B., et al. Distinctive mesenchymal-like neurofibroma stem cells shape NF1 clinical phenotypes controlled by BDNF microenvironment. Transl. Oncol. 2024;40 doi: 10.1016/j.tranon.2023.101852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Le L.Q., Shipman T., Burns D.K., Parada L.F. Cell of origin and microenvironment contribution for NF1-associated dermal neurofibromas. Cell Stem Cell. 2009;4(5):453–463. doi: 10.1016/j.stem.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jouhilahti E.M., Peltonen S., Callens T., Jokinen E., Heape A.M., Messiaen L., et al. The development of cutaneous neurofibromas. Am. J. Pathol. 2011;178(2):500–505. doi: 10.1016/j.ajpath.2010.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uccelli A., Moretta L., Pistoia V. Mesenchymal stem cells in health and disease. Nat. Rev. Immunol. 2008;8(9):726–736. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- 17.Takahashi M., Kobayashi H., Mizutani Y., Hara A., Iida T., Miyai Y., et al. Roles of the mesenchymal stromal/stem cell marker Meflin/Islr in cancer fibrosis. Front. Cell Dev. Biol. 2021;9 doi: 10.3389/fcell.2021.749924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Imai N., Iwai A., Hatakeyama S., Matsuzaki K., Kitagawa Y., Kato S., et al. Expression of bone morphogenetic proteins in colon carcinoma with heterotopic ossification. Pathol. Int. 2001;51(8):643–648. doi: 10.1046/j.1440-1827.2001.01243.x. [DOI] [PubMed] [Google Scholar]
- 19.Ramirez D.M., Ramirez M.R., Reginato A.M., Medici D. Molecular and cellular mechanisms of heterotopic ossification. Histol. Histopathol. 2014;29(10):1281–1285. doi: 10.14670/HH-29.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Imaeda Y., Arakawa S., Yasuoka H., Kato H., Nagata H., Asano Y., et al. Heterotopic ossification in primary rectal cancer with squamous cell carcinoma-like differentiation. Fujita Med. J. 2022;8(4):134–138. doi: 10.20407/fmj.2021-013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oreffo R.O.C., Cooper C., Mason C., Clements M. Mesenchymal stem cells. Stem Cell Rev. 2005;1(2):169–178. doi: 10.1385/SCR:1:2:169. [DOI] [PubMed] [Google Scholar]