Highlights
-
•
A 1.5 T MR/HDR suite allows for customized adaptive treatment of cervical cancer.
-
•
Recontouring on repeated MR scans gives a better estimation of the delivered dose.
-
•
Extra rectal degassing leads on average to a decrease of the delivered rectum dose.
Keywords: Cervical cancer, MRI guided brachytherapy, Adaptive workflow, Repeated imaging, OARs
Abstract
Introduction
At our department we have a dedicated 1.5 Tesla MRI/HDR brachytherapy suite, which provides the possibility of repeated MRI scanning before, during and after applicator insertion and before and/or after irradiation for patients with advanced cervical cancer. In this study we analysed the effect of this adaptive workflow. We investigated the number of interventions, their impact on organ doses (OAR) and the respective dose differences between total prescribed and total delivered doses.
Materials and methods
Seventy patients with locally advanced cervical cancer FIGO2009 stages IB-IVA, treated from June 2016 till August 2020, were retrospectively analysed. The standard brachytherapy schedule consisted of two applicator insertions and delivery of three or four HDR fractions.
OARs were recontoured on the repeated MRI scans. The D2cm3 dose difference between total prescribed and total delivered dose for bladder, rectum, sigmoid and bowel were calculated.
Results
In total 153 interventions were performed, 3 replacements of the applicator, 23 adaptations of needle positions, bladder filling was changed 74 times and repeated rectal degassing 53 times. The impact of the rectal interventions was on average −1.2 Gy EQD23. Dose differences between total delivered and total prescribed D2cm3 for bladder, rectum, sigmoid and bowel were −0.6, 0.3, 2.2 and −0.6 Gy EQD23, respectively.
Conclusions
An MRI scanner integrated into the brachytherapy suite enables multiple interventions based on the scans before treatment planning and dose delivery. This allows for customized treatment according to the changing anatomy of the individual patient and a better estimation of the delivered dose.
Introduction
Magnetic resonance imaging guided adaptive brachytherapy (MRI-guided adaptive BT) has become a standard approach for the treatment of advanced cervical cancer with high dose rate (HDR) BT. Given the superior soft-tissue contrast of MRI in comparison with computed tomography (CT), residual gross tumor volume (GTVres), high-risk clinical target volume (CTVHR) and surrounding organs at risk (OARs) are better visualized on MRI [1].
Repeated MR imaging during the course of BT has demonstrated that inter- and intra-fraction movements of the target in relation to the inserted applicator are limited [2], [3], [4]. However, the relation of the applicator and the surrounding OARs is less stable due to OARs movement and changes in filling status [2], [3]. Therefore, the delivered doses to the OARs can differ from the prescribed doses. Nomden et al. [3] reported no significant dose differences overall, however large, individual differences were discovered in certain cases. For the rectum, differences for individual fractions could be up to 6 Gy EQD2, with differences up to 10.2 Gy EQD2 for a single patient. Mazeron et al. [5] also did not detect major movements of the sigmoid and bladder, whereas the rectum got significantly closer to the implant at day 2. The increase of the D2cm3 of the rectum was reported in 17 patients, ranging from 0.4 to 9.4 Gy, leading to a 10.5% overcoming of the dose constraint (75 Gy). Studies have shown a dose volume effect relation between organ dose and treatment related morbidity for the urinary tract [6], [7], gastrointestinal tract [8], [9] and vagina [10]. Since clinical results, in terms of tumor control and overall survival, are improving [11], the reduction of organ dose and treatment related morbidity [12] becomes even more important.
At our department a MR scanner is integrated into the HDR brachy suite and therefore repeated MR imaging during brachytherapy is possible. This allows for an adaptive workflow which has been introduced clinically in 2010 [3], [13]. The workflow includes multiple MR scans before, during or directly after applicator insertion to repeatedly check the applicator position in relation to targets and OARs and to perform various interventions when needed. Prior to irradiation additional MR images are acquired to assess the stability of the implant and possible changes in the surrounding OARs positions or filling status and intervene again, if necessary. Finally, MR images are performed prior to or just after dose delivery which provides information about the delivered dose to the OARs.
In this study, we retrospectively analysed the adaptive MRI guided workflow at our department, and investigated the number of interventions and the impact on OARs dose for patients with cervical cancer. We also determined the dose difference between the total prescribed and the total delivered OARs doses.
Materials and methods
Data of 70 patients with locally advanced cervical cancer FIGO2009 stages 1B-IVA, treated from June 2016 till August 2020, were analysed for this study. All patients were treated according to the EMBRACE II protocol using our routinely applied clinical workflow [14]. Overall treatment consisted of external beam radiotherapy (EBRT) with Volumetric Modulated Arc Therapy (VMAT) to an elective dose of 45 Gy and Simultaneous Integrated Boost (SIB) of 55.0 or 57.5 Gy to pathological lymph nodes in 25 fractions, in combination with weekly chemotherapy with cisplatin 40 mg/m2. BT was usually applied after 22 fractions of EBRT and after the last EBRT fraction. Incidentally, other schedules have been used due to patient related or logistic factors.
The standard BT schedule consisted of two applications with a one-week interval. Each insertion comprised 2 HDR fractions. Eight patients received 3 fractions (in 2 insertions) and 2 patients underwent 3 applicator insertions and got consecutively 2, 1 and 1 fractions. We used the Utrecht tandem/ovoid or Venezia tandem/ring-shaped intracavitary/interstitial (IC/IS) applicator systems (Elekta Brachytherapy, The Netherlands).
Applicator insertion, MR scanning and dose delivery was performed in our MR/HDR treatment suite which contains a 1.5 T MR widebore Ingenia scanner (Philips Medical Systems, The Netherlands) and a HDR Microselectron afterloader (Elekta, The Netherlands) [13]. Applicator insertion was performed under spinal or general anesthesia. For placement of the tandem and rough guidance of needle position abdominal ultrasound was used. A urinary catheter was inserted, which enables adaptive bladder filling or ensuring an empty bladder. Before MR scanning a rectal catheter was inserted for degassing and was immediately removed.
MR imaging
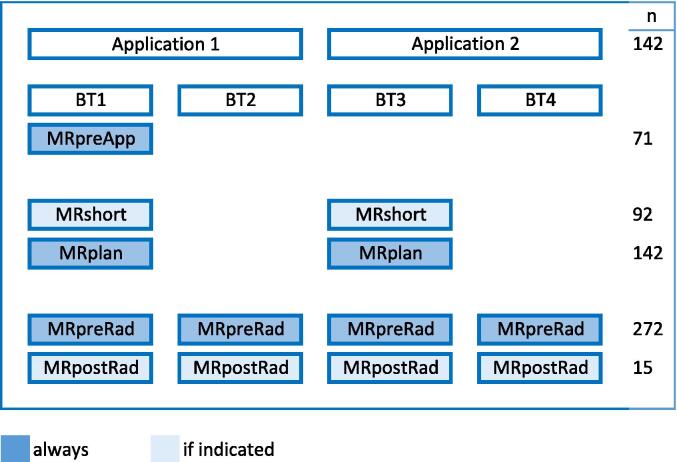
MR imaging was performed for treatment planning, contouring of target and OARs and for position verification. T2 weighted survey, sagittal, transversal, coronal scans and a Diffusion Weighted Image (DWI), all in the same frame of reference, [15] were made. Fig. 1 shows the moments of acquiring the MR images during the workflow. MRpreApp was taken just before the first applicator insertion. MRshort was a quick scan to check the quality of the implant and the anatomical situation. MRplan was the scan used for treatment planning, target and OARs contouring and applicator reconstruction. MRpreRad was acquired before irradiation and MRpostRad was an optional scan after irradiation. In total 70 MRpreApp scans, 92 MRshort scans, 142 MRplan scans, 272 MRpreRad scans and 15 MRpostRad scans were acquired. For these patients all OARs were also contoured on the MRpreRad or the MRpostRad scan.
Fig. 1.
Schematic overview of MR imaging during the brachytherapy cervical treatment procedure. MRpreApp: before the first applicator insertion, MRshort if adaptations are to be expected, MRplan for treatment planning, MRpreRad just before irradiation, MRpostRad after irradiation when interventions are performed based on MRpreRad. Colored in dark blue for every patient, in light blue optional. N is the number of insertions for applications and scans for imaging.
Contouring, dose parameters, treatment planning
On MRplan, GTVres, CTVHR, intermediate-risk CTV (CTVIR) and the OARs (bladder, rectum, sigmoid and bowel) were contoured by the radiation oncologist (RO) according to GEC-ESTRO recommendations [1]. Simultaneously the applicator reconstruction was performed by the Radiation Therapist (RTT) and checked by the Medical Physics Expert (MPE) [16], [17]. After contouring, the structures were exported from an in-house developed software package, Volumetool [18], to the treatment planning system (Oncentra Brachy®, Elekta, The Netherlands) and an optimized plan was generated, based on soft and hard dose constraints according to the EMBRACE II protocol [14]. Physical doses were converted to EQD2 using the linear quadratic model with α/β = 10 Gy for targets and α/β = 3 Gy for OARs. Dose planning and reporting was according to ICRU 89 and the GEC-ESTRO Handbook of Brachytherapy [19], [20]. The planning aim was to achieve a CTVHR D90% > 90 Gy EQD2 while keeping the OARs below the soft constraints, respectively bladder D2cm3 < 80 Gy EQD2, rectum D2cm3 < 65 Gy EQD2, sigmoid and bowel D2cm3 < 70 Gy EQD2. If the planning aim could not be achieved, we attempted for the prescribed dose to stay below the hard constraints for the OARs, respectively bladder D2cm3 < 90 Gy EQD2, rectum, sigmoid and bowel < 75 Gy EQD2. In case OAR dose was a limiting factor for target dose, a minimum of 85 Gy for D90% CTVHR was aimed at, but final decisions based on the individual clinical situation was performed by the responsible RO.
Adaptive workflow
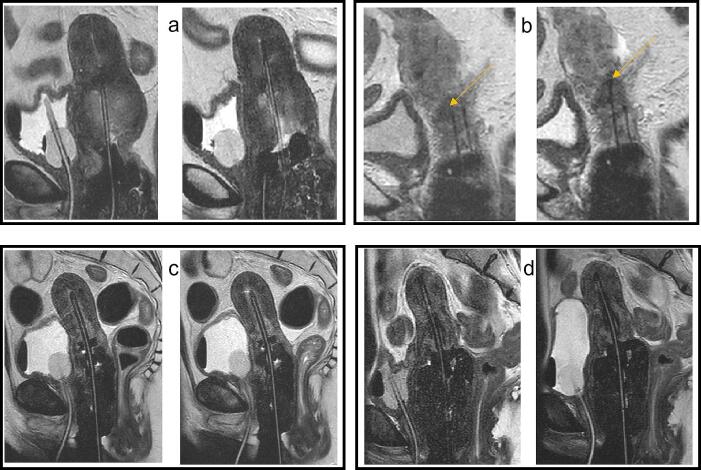
MRpreApp was acquired prior to anesthesia. MRI information together with findings from clinical investigation were used to evaluate tumor regression, to decide on the applicator to be used and possible needle positions. With patient still under anesthesia, MRshort was acquired on indication for applicator position check and/or guidance of needle depth and allowing for additional interventions if needed. Adaptations of bladder or rectal filling, dependent on the specific anatomical situation, were also possible based on information from MRshort. OAR interventions could also be based on MRplan information. After possible adaptations, the final MRplan was made. Fig. 2 shows examples of an applicator replacement intervention, adaptation of needle depth, rectal degassing and change of bladder filling. Every adaptation and possible deviation from the standard workflow were reported.
Fig. 2.
Examples of the effect of different interventions. Sagittal T2 MR images before (left) and after (right) adaptations prior to MRplan or just before irradiation. a) Applicator not in right position followed by replacement, b) needle adaptation; the needle indicated by the yellow arrow was placed deeper, c) extra rectal degassing to decrease the rectal dose, d) filling the bladder to reduce the bowel dose.
Contouring, applicator reconstruction and treatment planning followed. During this process the patient was cared for on recovery or nursing ward. After having finalized the optimized treatment plan with the prescribed doses, the patient was transferred back to the MR/HDR treatment suite. Rectal degassing was done routinely before MR scanning. Based on the treatment planning situation, bladder filling could be added or removed if deemed necessary. On MRpreRad a first check on the T2 weighted survey or sagittal T2 weighted scan allowed to decide if extra degassing was necessary and/or if bladder filling was appropriate. Then the final MRpreRad scan was performed. The MRpreRad tT2 scan was registered to the MRplan tT2 scan in Oncentra Brachy TPS. Image registration was based on the applicator with a box for mutual information, and the planning contours were than projected on MRpreRad. Possible anatomical changes could be assessed. The RO evaluated whether the situation was comparable to the planning situation and acceptable considering the intended dose distribution and if so, the respective HDR fraction was delivered. If not, various interventions could be applied. We could add/remove bladder filling or degas the rectum again. On day 2 the workflow around dose delivery was repeated for the next HDR fraction.
After any intervention for a particular OAR, a new MRpreRad or MRpostRad was made to check the actual anatomical situation again. Recontouring of that OAR was done to assess the delivered dose. For the rectal interventions, all scans before and after extra degassing were contoured to analyse the dose effect of the interventions. If indicated, the RO decided to contour one or more of the OARs before the next BT fraction or the next application. The best estimation of the delivered dose was used as input for planning the next fraction or application. To detect possible developments in the adaptive workflow regarding bladder intervention, we divided the four years studied period into two parts of two years each and analysed on which MR scan (short, plan, preRad) the decision of intervention was made.
Total delivered dose versus total prescribed dose
For the determination of the best estimate of the delivered dose, the OARs were contoured on MRpreRad or MRpostRad, depending on which scan seemed the best representation of the treatment situation. For each patient, we calculated the total delivered OAR dose in D2cm3 over all fractions and compared this with the total prescribed dose as determined by the treatment plan based on the MRplan of both applications. The difference between the total prescribed and total delivered dose for all patients was compared with the results of a previously published analysis, where we performed repeated MR imaging without individualized interventions [3].
Results
Interventions during adaptive workflow
On 3/92 (2%) MRshort scans an incorrect positioning of the applicator was detected, and replacement of the applicator was performed. Needles were inserted in 79 applications (44 patients) and for 77/79 applications an MRshort scan was made. Based on these MRshort scans adaptation of needle depth was performed in 23/77 cases (30%). Bladder filling was adapted 60 times (42% of all 142 applications) before the final MRplan scan was aquired. In the first two years of the studied period, adaptation of bladder filling was mostly based on MRshort scans compared to the MRplan survey scans (27 vs. 3), in the last two years of the studied period, the MRplan survey scans were equally used as the MRshort scans (15 vs. 15). Bladder filling adaptations based on information from MRpreRad scans were done 14 times (5% of all fractions). Adaptations of bladder filling was performed 6 times after recontouring the previous fraction(s). Recontouring before the next application was done once (3% of 36 patients) in the first period and 5 times (14% of 36 patients) in the second period.
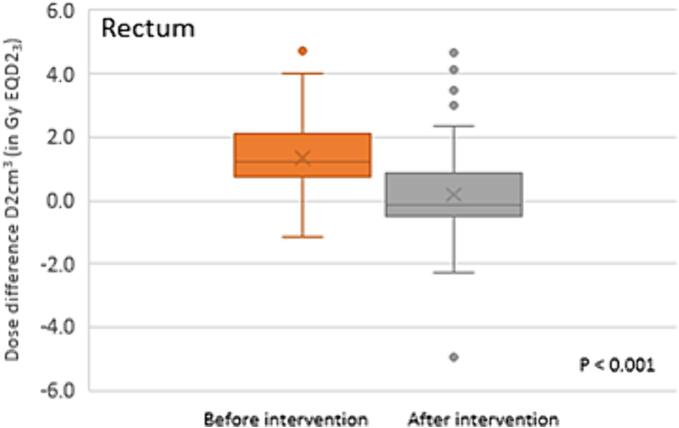
Rectal volume adaptation by additional degassing was done 53 times after MRpreRad accounting for 19% of all MRpreRad scans. Degassing was performed for 17 (12%) fractions on day one (BT1 and BT3) versus 36 (25%) on day two (BT2 and BT4). In 7 of 53 cases the respective MR scans were not saved and not included in the analysis. However, the adaptation was reported on the respective form. The mean D2cm3 rectum dose difference between the 46 MR scans prior to and the 46 MR scans just after extra degassing was −1.2 Gy EQD2 range (−5.0–2.9) (Fig. 3 and Appendix A).
Fig. 3.
Effect in dose difference due to extra rectal degassing. Boxplots of the dose difference of the rectum between the prescribed dose and the dose according to the MRpreRad scan before extra degassing, in orange. And in grey the dose difference between the prescribed dose of the rectum and the dose according to the scan after extra degassing (the delivered dose). The boxplot indicates the 25-75th percentiles, minimum, median (line), mean (X) and outlier points.
Total delivered dose versus total prescribed dose
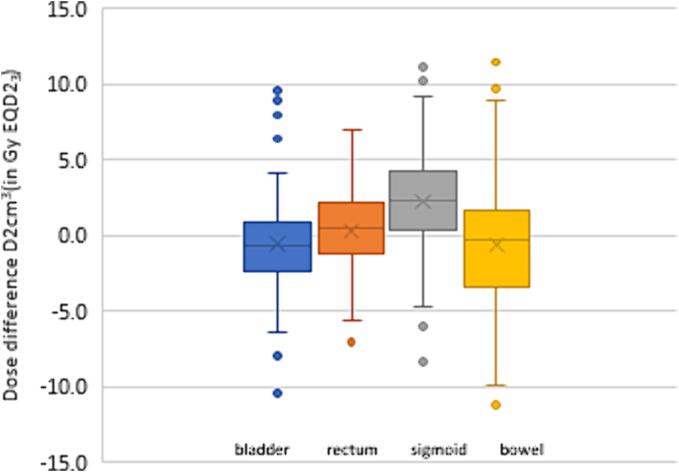
For rectum, bladder, sigmoid and bowel the total delivered dose minus the total prescribed dose were 0.3 (range −7.1–7.0), −0.6 (range −10.4–9.6), 2.2 (range −8.3–11.1), −0.6 (range −11.2–11.5) Gy EQD2, respectively (Fig. 4). For the rectum the delivered dose never violated the hard constraint of 75 Gy EQD2. However, hard constraints were violated four times for bladder and sigmoid, and once for the bowel, (Appendix B). For all cases with hard constraint violations of the delivered dose, the hard constraints of the prescribed dose were not violated during planning.
Fig. 4.
Total delivered dose minus prescribed dose. Boxplots showing the dose differences as total delivered minus total prescribed dose of bladder, rectum, sigmoid and bowel, for 70 patients. The box plot indicates the 25-75th percentiles, minimum, median (line), mean (X) and outlier points.
The mean dose differences are shown in Table 1 for all OARs as determined in this study compared to a previously published study [3].
Table 1.
Total delivered minus total prescribed D2cm3 for bladder, rectum, sigmoid and bowel in Gy EQD23. At the left from this study, compared to a previous study [3] at the right. The values in bold indicates the large difference since our adaptive workflow.
|
N=70 |
N=15 |
|||||||
|---|---|---|---|---|---|---|---|---|
| GγEQD23 | mean | SD | Min | max | mean | SD | Min | max |
| Bladder D2cm3 | −0.6 | 3.6 | −10.4 | 9.6 | −0.3 | 3.8 | −8.5 | 5.4 |
| Recturm D2cm3 | 0.3 | 3.0 | −7.1 | 7.0 | 2.1 | 4.0 | −5.3 | 10.2 |
| Sigmoid D2cm3 | 2.2 | 3.7 | −8.3 | 11.1 | 0.9 | 2.9 | −5.7 | 5.5 |
| Bowel D2cm3 | −0.6 | 4.6 | −11.2 | 11.5 | ||||
Discussion & conclusions
Having a combined 1.5 T MR/HDR treatment suite allows for an adaptive workflow for BT procedures based on information from repeated MR imaging. Having the MRI scanner in the same room where applicator insertion and HDR delivery takes place allows for a highly customized patient centered treatment approach. Multiple interventions before dose planning or dose delivery can be performed at different moments in time according to the needs of the individual and daily patient anatomy. Applicator and needle positions can be adapted if indicated and MR scanning before and/or after dose delivery gives a better estimation of the real delivered dose.
Our adaptive workflow helps to better understand uncertainties in BT dose delivery [2], [3], [4], [5]. Constant evaluation of the dosimetric results of the performed interventions in daily clinical practice was and still is an ongoing process of learning and acquiring knowledge. Since the introduction of the adaptive workflow, we notice a change of practice. The number of interventions has increased and we can better predict the possible dosimetric effects. We nowadays find ongoing evaluation of our daily practice mandatory for monitoring and improving the quality of the treatments and for educational purposes. It is one of the methods of continuous learning, whether on a department level or in cooperation with multiple departments as proposed previously [21], [22], [23].
The EMBRACE II protocol prescribes dose volume constraints for OARs based on clinical evidence from previous clinical studies [14]. Therefore, in clinical routine, we aim to keep not only the prescribed but also the delivered dose below the known OAR constraints without compromising the target dose. By repeatedly imaging, just before or directly after irradiation, better estimates of the total delivered doses to the different OARs can be made. For all patients investigated in this study, the mean difference in D2cm3 between delivered and prescribed doses for rectum, bowel and bladder are close to zero, which suggests that our workflow eliminated earlier described systematic deviations [3]. For the rectum D2cm3, (Fig. 3) the mean difference between the total delivered and the prescribed dose was significantly decreased compared to results acquired before the introduction of the adaptive workflow (Table 1) [3]. However, we observed a large range of dose differences for individual patients, which could have multiple causes. Clinical decisions for an individual patient as well as inter- and intra-observer variations in contouring and reconstruction might result in dose uncertainties of about 5–10% [2], [24]. In some cases a considerable increase of delivered dose relative to the prescribed dose was accepted based on clinical decision making. Additionally, a higher OAR dose could be accepted for a single fraction to achieve a higher dose to the target as the overall hard constraints for the organ [14] was not violated due to respectively lower delivered doses in previous fractions.
Over time, bladder interventions were more often based on information from the survey of MRplan scans instead of MRshort scans. This saves scanning time since less MRshort scans were needed. This also indicates that we went through a learning curve and that decision making for individual interventions can as well be made on a slightly less quality survey scan. Over time we also learned about possible effects of more or less bladder filling. For instance, if bowel dose would be too high while the bladder is relatively empty, bladder filling before MRpreRad could be adjusted within a certain margin. If the bladder was already filled on MRplan, and bladder dose would be too high, we could empty the bladder before MRpreRad and could evaluate the dose after re-contouring.
The sigmoid is highly mobile, regular position changes can be observed by repeated imaging but cannot be controlled. This might be the reason that as of yet, a dose response relation could not be established [25]. Imaging directly before and/or after an intervention and radiation gives a better estimation of the delivered dose. In our cohort, we observed that the mean dose difference for sigmoid is above zero, meaning that on average the delivered dose to the sigmoid is somewhat higher than planned.
Not all interventions had a positive effect on the delivered OARs dose; in some cases, for example the rectum was positioned closer to the target after extra degassing. Moreover, it was not always necessary to deflate, e.g. when the gas bubble was not close to de CTVHR and we learnt that a gas bubble could also have a positive effect by pushing the bowel or sigmoid away from the high dose region.
Over time we started to re-contour OARs that received a high dose per fraction on the MRpreRad scans of the first application (BT1 and BT2) before the second application. In that way the already delivered dose could be taken into account in the treatment planning for BT3 and BT4. In the beginning the RO decided for this procedure in incidental situations, but nowadays we always recontour the OARs before the next application if the dose is close to its constraint.
We realize that having a MRI scanner installed in the HDR brachy suite is an unique situation. Our patients are positioned on the MR table in the same position during pre-treatment imaging, dose delivery and post-treatment imaging if indicated. We use this specific environment to individualize brachytherapy fractions and adapt to the changing anatomical situation of the individual patients Besides being able to apply highly conformal dose distributions we use our facility to increase knowledge regarding anatomical changes during fractionated HDR brachytherapy and for educational purposes.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.tipsro.2024.100262.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Haie-Meder C., Pötter R., Van Limbergen E., Briot E., De Brabandere M., Dimopoulos J., et al. Gynaecological (GYN) GEC-ESTRO Working Group. Recommendations from Gynaecological (GYN) GEC-ESTRO Working Group (I): concepts and terms in 3D image based 3D treatment planning in cervix cancer brachytherapy with emphasis on MRI assessment of GTV and CTV. Radiother Oncol. 2005 Mar;74(3):235–245. doi: 10.1016/j.radonc.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 2.Nesvacil N., Tanderup K., Hellebust T.P., De Leeuw A., Lang S., Mohamed S., et al. A multicentre comparison of the dosimetric impact of inter- and intra-fractional anatomical variations in fractionated cervix cancer brachytherapy. Radiother Oncol. 2013 Apr;107(1):20–25. doi: 10.1016/j.radonc.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nomden C.N., de Leeuw A.A., Roesink J.M., Tersteeg R.J., Westerveld H., Jürgenliemk-Schulz I.M. Intra-fraction uncertainties of MRI guided brachytherapy in patients with cervical cancer. Radiother Oncol. 2014 Aug;112(2):217–220. doi: 10.1016/j.radonc.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 4.Karlsson L., Thunberg P., With A., Mordhorst L.B., Persliden J. J Contemp 3D image-based adapted high-dose-rate brachytherapy in cervical cancer with and without interstitial needles: measurement of applicator shift between imaging and dose delivery. Brachytherapy. 2017 Feb;9(1):52–58. doi: 10.5114/jcb.2017.66110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mazeron R., Champoudry J., Gilmore J., Dumas I., Goulart J., Oberlander A.S., et al. Intrafractional organs movement in three-dimensional image-guided adaptive pulsed-dose-rate cervical cancer brachytherapy: assessment and dosimetric impact. Brachytherapy. 2015;14(2):260–266. doi: 10.1016/j.brachy.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 6.Spampinato S., Fokdal L.U., Pötter R., Haie-Meder C., Lindegaard J.C., Schmid M.P., et al. EMBRACE Collaborative Group. Risk factors and dose-effects for bladder fistula, bleeding and cystitis after radiotherapy with imaged-guided adaptive brachytherapy for cervical cancer: An EMBRACE analysis. Radiother Oncol. 2021 May;158(312–320) doi: 10.1016/j.radonc.2021.01.019. [DOI] [PubMed] [Google Scholar]
- 7.Spampinato S, Fokdal LU, Pötter R, Haie-Meder C, Lindegaard JC, Schmid MP, et al. Importance of the ICRU bladder point dose on incidence and persistence of urinary frequency and incontinence in locally advanced cervical cancer: An EMBRACE analysis. Radiother Oncol. 2021 May;158:300-308. Doi: 10.1016/j.radonc.2020.10.003. [DOI] [PubMed]
- 8.Jensen N.B.K., Pötter R., Kirchheiner K., Fokdal L., Lindegaard J.C., Kirisits C., et al. EMBRACE Collaborative Group. Bowel morbidity following radiochemotherapy and image-guided adaptive brachytherapy for cervical cancer: Physician- and patient reported outcome from the EMBRACE study. Radiother Oncol. 2018 Jun;127(3):431–439. doi: 10.1016/j.radonc.2018.05.016. [DOI] [PubMed] [Google Scholar]
- 9.Mazeron R., Fokdal L.U., Kirchheiner K., Georg P., Jastaniyah N., Šegedin B., et al. EMBRACE collaborative group. Dose-volume effect relationships for late rectal morbidity in patients treated with chemoradiation and MRI-guided adaptive brachytherapy for locally advanced cervical cancer: Results from the prospective multicenter EMBRACE study. Radiother Oncol. 2016 Sep;120(3):412–419. doi: 10.1016/j.radonc.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 10.Westerveld H., Kirchheiner K., Nout R.A., Tanderup K., Lindegaard J.C., Spampinato S., et al. Dose-effect relationship between vaginal dose points and vaginal stenosis in cervical cancer: An EMBRACE-I sub-study. Radiother Oncol. 2022 Mar;168:8–15. doi: 10.1016/j.radonc.2021.12.034. [DOI] [PubMed] [Google Scholar]
- 11.Pötter R., Tanderup K., Schmid M.P., Jürgenliemk-Schulz I., Haie-Meder C., Fokdal L.U., et al. EMBRACE Collaborative Group. MRI-guided adaptive brachytherapy in locally advanced cervical cancer (EMBRACE-I): a multicentre prospective cohort study. LancetOncol. 2021 Apr;22(4):538–547. doi: 10.1016/S1470-2045(20)30753-1. [DOI] [PubMed] [Google Scholar]
- 12.Fokdal L., Pötter R., Kirchheiner K., Lindegaard J.C., Jensen N.B.K., Kirisits C., et al. Physician assessed and patient reported urinary morbidity after radio-chemotherapy and image guided adaptive brachytherapy for locally advanced cervical cancer. Radiother Oncol. 2018 Jun;127(3):423–430. doi: 10.1016/j.radonc.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 13.Virtual tour through the UMC Utrecht 1.5T MRI HDR brachytherapy suite: https://my.matterport.com/show/?m=Vi3chUt4j6F.
- 14.Pötter R., Tanderup K., Kirisits C., de Leeuw A., Kirchheiner K., Nout R., et al. The EMBRACE II study: The outcome and prospect of two decades of evolution within the GEC-ESTRO GYN working group and the EMBRACE studies. EMBRACE Collaborative Group. Clin Transl Radiat Oncol. 2018 Jan;11(9):48–60. doi: 10.1016/j.ctro.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dimopoulos J.C., Petrow P., Tanderup K., Petric P., Berger D., Kirisits C., et al. Recommendations from Gynaecological (GYN) GEC-ESTRO Working Group (IV): Basic principles and parameters for MR imaging within the frame of image based adaptive cervix cancer brachytherapy. Radiother Oncol. 2012 Apr;103(1):113–122. doi: 10.1016/j.radonc.2011.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hellebust T.P., Kirisits C., Berger D., Pérez-Calatayud J., De Brabandere M., De Leeuw A., et al. Recommendations from Gynaecological (GYN) GEC-ESTRO Working Group: considerations and pitfalls in commissioning and applicator reconstruction in 3D image-based treatment planning of cervix cancer brachytherapy. Gynaecological (GYN) GEC-ESTRO Working Group. Radiother Oncol. 2010 Aug;96(2):153–160. doi: 10.1016/j.radonc.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 17.Nomden C.N., de Leeuw A.A., Moerland M.A., Roesink J.M., Tersteeg R.J., Jürgenliemk-Schulz I.M. Clinical use of the Utrecht applicator for combined intracavitary/interstitial brachytherapy treatment in locally advanced cervical cancer. Int J Radiat Oncol Biol Phys. 2012 Mar 15;82(4):1424–1430. doi: 10.1016/j.ijrobp.2011.04.044. [DOI] [PubMed] [Google Scholar]
- 18.Bol G.H., Kotte A.N.T.J., van der Heide U.A., Lagendijk J.J.W. Simultaneous multi-modality ROI delineation in clinical practice. Comput Methods Programs Biomed. 2009;96:133–140. doi: 10.1016/j.cmpb.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 19.International Commission on Radiation Units and Measurements. Prescribing, Recording, and Reporting Brachytherapy for Cancer of the Cervix (ICRU report 89). Bethesda; 2013.
- 20.Tan LT, Tanderup K, Lindegaard J, Serban M, Nout R, Pötter R. The GEC ESTRO Handbook of Brachytherapy, second edition Part II: Clinical practice, chapter 16: Cervical cancer.
- 21.De Leeuw A.A.C., Nout R.A., Van Leeuwen R.G.H., Mans A., Verhoef L.G., Jürgenliemk-Schulz I.M. Implementation of state-of-the-art (chemo)radiation for advanced cervix cancer in the Netherlands: A quality improvement program. Tech Innov Patient Support Radiat Oncol. 2018 Oct;31(9):1–7. doi: 10.1016/j.tipsro.2018.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim H., Houser C.J., Kalash R., Maceil C.A., Palestra B., Malush D., et al. Workflow and efficiency in MRI-based high-dose-rate brachytherapy for cervical cancer in a high-volume brachytherapy center. Brachytherapy. 2018;17(5):753–760. doi: 10.1016/j.brachy.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 23.Coffey M., Leech M. Philip Poortmans on behalf of the ESTRO RTT Committee. Benchmarking Radiation TherapisT (RTT) education for safe practice: The time is now. Radiother Oncol. 2016;119(1):p12–p13. doi: 10.1016/j.radonc.2016.03.008. Published online: March 22. [DOI] [PubMed] [Google Scholar]
- 24.Kari Tanderup, Taran Paulsen Hellebust, Stefan Lang, Jørgen Granfeldt, Richard Pötter, Jacob Christian Lindegaard, et al. Consequences of random and systematic reconstruction uncertainties in 3D image based brachytherapy in cervical cancer. Radiother Oncol 2008; 89(2):156-163. Doi: 10.1016/j.radonc.2008.06.010. [DOI] [PubMed]
- 25.Spampinato S, Jensen NBK, Pötter R, Fokdal LU, Chargari C, Lindegaard JC, et al. Severity and Persistency of Late Gastrointestinal Morbidity in Locally Advanced Cervical Cancer: Lessons Learned From EMBRACE-I and Implications for the Future. Int J Radiat Oncol Biol Phys 2022;112(3):681-693. Epub 2021 Oct 20. PMID: 34678431. Doi: 10.1016/j.ijrobp.2021.09.055. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.