Abstract
Introduction and importance: Large retroperitoneal schwannomas are rare and present significant challenges in surgical management, particularly when located in the pelvic region. Gynecologists can encounter rare problems when a pelvic schwannoma is mistaken for an adnexal pathology. Case Presentation: A 62-year-old woman presented with a giant retroperitoneal mass suspected of a potentially malignant ovarian tumor preoperatively. Computed tomography revealed a large mixed solid-cystic mass near the right adnexa measuring 118 × 100 × 80 mm. The cancer antigen 125 level was 196 U/mL. We performed a diagnostic-operative laparoscopy, which showed a retroperitoneal neoformation below the cava and aortic bifurcation adherent to the sacrum, right pelvic vessels, and hypogastric nerve up to the vagina. We carefully detached the mass from the nearby tissues using the most appropriate laparoscopic devices. The entire neoplasm was removed through the vagina into a surgical bag. The surgery lasted 180 min without complications. Histology revealed a grade I benign schwannoma. At the 12-month follow-up, the patient was asymptomatic without signs of recurrence. Clinical Discussion: Pelvic retroperitoneal schwannomas can mimic ovarian carcinomas; misdiagnosis may occur due to their rarity and the difficulty of interpreting preoperative imaging. In case of unexpected giant presacral schwannomas surgical management is challenging due to their peculiar location. Conclusion: This case underscores the need for a skilled, experienced team of gynecological oncologists to achieve favorable outcomes when performing laparoscopic surgery of giant pelvic retroperitoneal schwannoma. Adequate knowledge of the complex pelvic anatomy, careful surgical planning, and familiarity with the most appropriate surgical tools are critical points.
Keywords: Laparoscopy, Large retroperitoneal mass, Minimally invasive surgery, Schwannoma, Presacral space
Highlights
-
•
Large retroperitoneal schwannomas are exceptionally rare and present significant challenges in surgical management.
-
•
This case highlights the feasibility of laparoscopic surgery for the excision of a giant pelvic retroperitoneal schwannoma.
-
•
The rare giant schwannoma highlights some key points to consider when managing a pelvic retroperitoneal presacral mass.
1. Introduction
Schwannomas are soft tissue tumors derived from Schwann cells in the myelin sheaths of peripheral nerves [1]. They are primarily found in the limbs, head, neck, and mediastinum. However, retroperitoneal and pelvic schwannomas are rare, accounting for only 0.5 %–12 % of retroperitoneal tumors [2] and 1–3 % of all schwannomas [3]. Schwannomas are usually small (size, 5–6 cm) [4]. Large retroperitoneal pelvic schwannomas are particularly challenging to treat because of their complex anatomy, which requires specialized surgical skills and a thorough understanding of the location of organs, vessels, and nerves. Pelvic schwannomas usually arise from the sacral nerves or hypogastric plexus and obturator nerves in a complex region that includes organs, other nerves, and several vascular structures [5]. The preaortic nervous system extends to the pelvic cavity, specifically to an area called the superior hypogastric plexus. This plexus is situated on both sides of the iliac arteries and ureters. It divides into two hypogastric nerves that end on either side of a complex nerve network known as the inferior hypogastric plexus [6].
Dissecting the inferior hypogastric plexus is a complex process because of its location, multiple roots, and complex distribution of terminal branches. The surrounding connective tissue is dense, making recognition of pelvic anatomy challenging. The preservation of these nerves is complex because of the small size and narrowness of the pelvis. The branches of the inferior hypogastric plexus are closely associated with the bladder and uterine vessels. Hence, any surgical intervention in this region must be performed with utmost care to preserve nerve plexus integrity and avoid severe complications in the urogenital and rectal tract [7].
During the resection of large tumors from the presacral space, severe bleeding may occur from the major veins on the surface of the sacrum or from the foramina of the sacral basivertebral veins [8]. The presacral veins' outer layer is closely connected to the sacral periosteum. Damage can cause bleeding from the presacral venous plexus. The pelvic venous system lacks valves, and the presacral space can have up to three times higher hydrostatic pressure than that in the inferior vena cava. Bleeding from 2 to 4 mm presacral veins can be as high as 1000 mL, making it challenging to manage [9].
Gynecologists may occasionally encounter a rare issue in which a schwannoma located in the pelvic region may be mistaken for uterine or adnexal pathology. In this report, we discussed a case in which a giant retroperitoneal pelvic schwannoma was misdiagnosed as a potentially malignant ovarian tumor during preoperative evaluation. We discussed the feasibility and safety of laparoscopic surgery for excision, including intraoperative difficulties, and the need for an experienced surgical team. In a previous paper, we described a case of laparoscopic treatment of a retroperitoneal pelvic schwannoma attached to vital vessels, presenting anatomical and surgical challenges.
2. Presentation of case
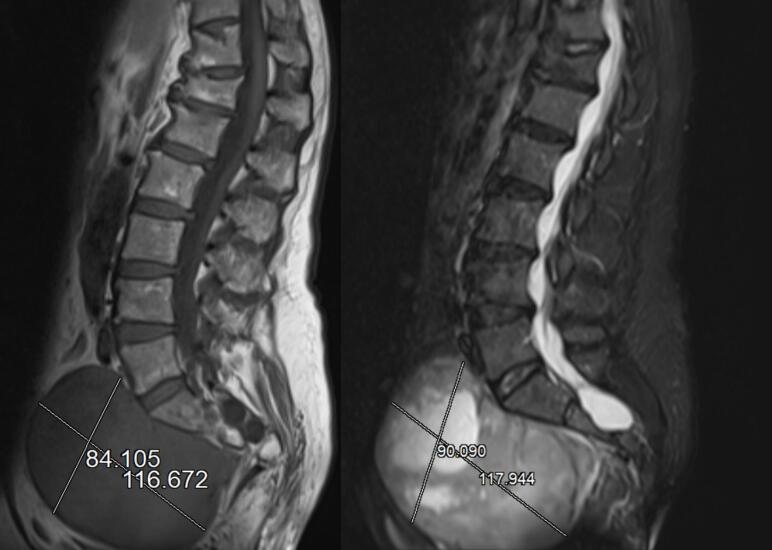
The present work has been reported in line with the SCARE criteria [10]. We presented the case of a 62-year-old patient suspected of having a malignant tumor of the right ovary. Computed tomography of the abdomen revealed a well-defined, mixed solid cystic mass in the pelvis, with a more prominent solid component located near the right adnexa, measuring 118 × 100 × 80 mm (Fig. 1). This mass compressed the iliac arteriovenous vessels, pushing them off their usual paths from the aorta to the cava. The right ureter was also affected. Radiological findings suggested adnexal neoplasia. At our time of observation, the patient was in a fair condition. She experienced abdominopelvic pain, constipation, and an urge to urinate for several months. Abdominal examination revealed no pain or bloating, but new growth extending above the pubis and occupying the pelvis was observed. The lesion was moderately painful and hypomobile upon palpation. During bimanual examination, an abnormal rigid mass completely occupied the pelvis, pushing the uterine cervix forward. However, it was difficult to evaluate the uterus. Laboratory tests, including those for C-reactive protein, fibrinogen, carcinoembryonic antigen, He4, cancer antigen (CA) 19.9, and Ca 15.3, were normal, except for CA 125, which was 196 U/mL. Transvaginal ultrasonography revealed a mixed echogenic mass of approximately 12 cm extending from the right ovary and occupying the entire pelvis. After discussing this with the patient, we decided to perform diagnostic-operative laparoscopy to resect the mass, uterus, and adnexa. The procedure may also require surgery on the large and small intestines and bladder and re-implantation of the ureters. This study was conducted in accordance with the Declaration of Helsinki for studies involving human participants. The patient provided written informed consent for the surgical procedure, publication of this report, and the accompanying images.
Fig. 1.
Computed tomography sagittal scan showing the large mixed solid cystic mass measuring approximately 118 × 100 × 80 mm extending from the right ovary and occupying the entire pelvis.
3. Surgical procedures
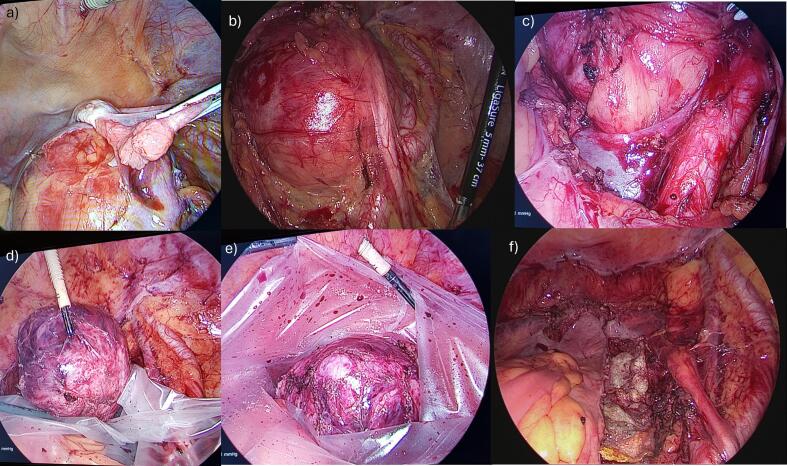
Bowel preparation involved taking oral antibiotics and polyethylene glycol electrolyte powder for 2 days before surgery. Antibiotic prophylaxis was also administered to minimize the risk of postoperative infections, thus allowing safe rectal repair or resection. After surgery, the patients received nutritional support and on-demand pain relief. To minimize the risk of complications, particularly vessel damage, we ensured that all the necessary laparoscopic instruments were available in the operating room. Moreover, we prepared instruments that could be used for open surgery (abdominal laparotomy) in cases of severe injury and uncontrollable bleeding. We also ensured that blood packs for transfusion were available before the surgery began. Surgery was performed under general anesthesia with the patient in the supine position. The patient was then placed in the Trendelenburg position to remove the small bowel and omentum of the upper abdominal cavity. Five trocars were used, based on the patient's body constitution. The first trocar was an optical one measuring 10–12 mm, placed in the periumbilical position. A 10-mm-diameter, 0° laparoscope was introduced through it. A 5-mm trocar was inserted in the left hypochondrium, lateral to the rectus abdominis muscle, another 5-mm trocar in the suprapubic position; a 10–12-mm trocar on the right hypochondrium, lateral to the rectus abdominis muscle; and a 5-mm trocar in the left lower sotto costal space, 5 cm from the umbilicus. We maintained a pneumoperitoneum of 10–12 mmHg throughout surgery. Upon introduction of laparoscopic optic surgery, we observed a large retroperitoneal mass occupying the entire pelvis, mainly originating from the right pelvic wall. The right ovary was situated on top of the mass as if it originated from it (Fig. 2a). The uterus, tubes, and left ovary appeared normal in morphology but were compressed anteriorly by the mass without any signs of infiltration. Initially, we performed a complete hysterectomy along with bilateral adnexectomy as per our standard procedure. This allowed easy visualization of the pelvic organs, ureters, and iliac vessels. Hence, the peritoneal opening extended anteriorly to the aortic bifurcation. This helped us to better isolate the right ureter and identify the vessels and right hypogastric nerve as it entered the pelvis (Fig. 2b). The neoplasm was fixed in the retroperitoneal space below the cava and aortic bifurcation and adherent to the sacrum, right pelvic vessels, and hypogastric nerve up to the vagina (Fig. 2c). After removing the lymphatic tissue, we could accurately visualize the vena cava and its bifurcation, the aortic bifurcation, and the common external iliac artery. We carefully detached the mass using LigaSure Small Jaw Open Sealer/Divider (Covidien, Boulder, CO) for all surgical procedures, LigaSure™ Blunt Tip Laparoscopic Sealer/Divider and LigaSure™ Maryland Tip Laparoscopic Sealer/Divider, Medtronic-Covidien Products (United States), BiClamp® LAP forceps/BiClamp® E LAP forceps (Erbe Italia S.r.l.), Endoscopic 5−/10-mm blunt tip dissectors (Ethicon Endopath, Hamburg, Germany), and gauze for dissection and compression for the control of constant bleeding. We resected the tumor from the nearby tissues, which was challenging because of the dense network of surrounding blood vessels. The mass was detached from the vessels using endoscopic 5-mm blunt cherry dissectors (Ethicon Endopath, Hamburg, Germany) and gauze dissection; bleeding was managed with a BiClamp and compressing gauze. The excision was continued on the right side of the mesorectum toward the retrorectal space. Dissection through the anterior mesorectum and posterior presacral fascia plane was performed to prevent damage to the rectal and iliac vessels and venous plexus. The neoplasm was removed entirely, placed in a surgical bag (Endocatch, Ethicon) (Fig. 2d-e), and extracted through the vagina. A hemostatic suture using Vicryl was placed between the rectum and the internal iliac artery to prevent bleeding. After ensuring hemostasis was controlled, TachoSil® (Human Thrombin, Human Fibrinogen absorbable collagen fibrin sealant patch) was used to aid healing at the implantation bed of the excised neoplasm (Fig. 2f). The laparoscopic procedure is described in Video S1 (https://doi.org/10.5281/zenodo.13286385).
Fig. 2.
The main steps of the surgical procedure: (a) identification of the mass attached to the right ovary; (b) visualization of the mass in the presacral space and identification of the right ureter that is moved up by the mass; (c) identification of the mass in the retroperitoneal presacral space below the cava bifurcation, with the aortic bifurcation clearly visible; (d–e) resection of the mass and placement in a surgical bag; and (f) view of the surgical bed after the resection of the mass.
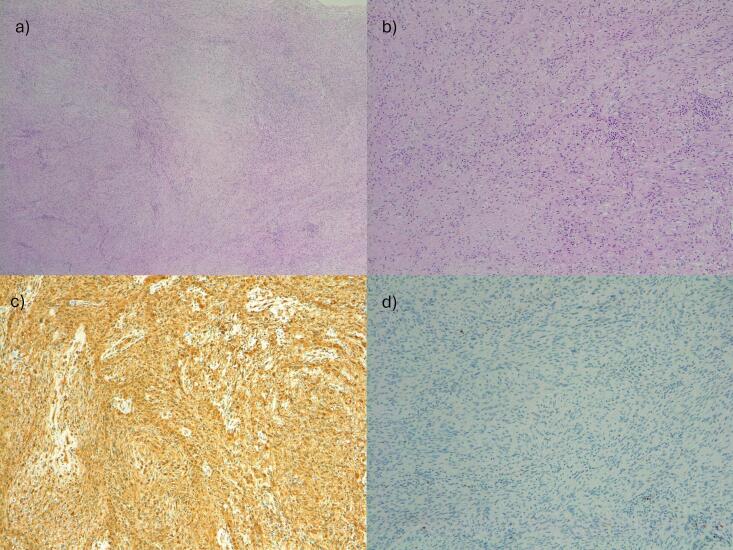
Extemporaneous histological examination revealed a neurinoma without any signs of atypia. The surgery lasted for 3 h, with no complications during the operation. The estimated blood loss volume was 500 mL. The patient's recovery was uneventful, and she was discharged 48 h after the procedure. At the 12-month follow-up, the patient was asymptomatic with no signs of recurrence. The final histological report revealed a benign schwannoma, graded as I according to the World Health Organization classification. The tumor strongly and diffusely expressed the neurogenic marker protein S100 with rare mitoses (Fig. 3).
Fig. 3.
Histological examination of the resected mass. 1a (10×) and 1b (20×): hematoxylin and eosin staining showed two patterns of alternating Antoni A and Antoni B areas; in Antoni A areas, compact spindle cells with indistinct cytoplasmic borders were arranged in short bundles or interlacing fascicles, whirling and sometimes ill-defined fascicles. Antoni B areas are less orderly and cellular with spindle or oval cells arranged haphazardly in the hyalinized matrix, collagen fibers, and inflammatory cells; mitotic figures were occasionally present. 1c (20×): the S100 protein is highly expressed in the schwannoma. 1d (20×): at immunohistochemistry, Ki67 is expressed in scattered tumoral cells.
4. Discussion
Diagnosis of pelvic masses is challenging because of the complex anatomy of the pelvis and adnexa. The positions of the ovaries can vary among and within the same patient at various ages. After menopause, identifying the ovaries may become more difficult because of their small size and lack of follicles. In this context, imaging is crucial for evaluating the mass size, location, relationship with adjacent pelvic structures, and differential diagnoses [1,2]. Differentiating whether a mass is of ovarian or extraovarian origin is a key step in the diagnosis of pelvic masses. However, this is not always clear on imaging, which can lead to misdiagnosis of ovarian malignancy, as was the case in our patient. The pelvic extraperitoneal region consists of pre-vesical, perivesical, perirectal, retrorectal, and presacral spaces. The presacral space is the area between the rectum and sacrum. Tumors affecting this region usually originate from the gastrointestinal or genitourinary system; however, primary neurogenic tumors can also occur, including schwannomas.
Pain represents one of the prevailing symptoms among individuals diagnosed with pelvic tumor. Nonetheless, the opioid crisis has exacerbated the complexities of pain management, shedding light on the necessity for nonpharmacological treatment approaches. Acupuncture and electroacupuncture, which is a modern variation of acupuncture that uses electrical energy, have been proven to reduce nociceptors activation-causing pain: they may represent a feasible and widely available approach for the treatment of pain associated with chronic pelvic tumors [11].
Retroperitoneal schwannomas are primarily found in the pelvis [12]. Surgical treatment for presacral schwannomas typically involves complete excision of the tumor while minimizing damage to surrounding structures and preserving organ function. The approach and technique may vary depending on tumor size, location, and individual patient factors. Surgery in the presacral space is challenging because of the proximity of vital structures. The complexity of surgery depends on various factors such as the specific procedure, the patient's anatomy, and the potential risks associated with accessing and manipulating structures in this area.
Giant presacral schwannomas pose a significant challenge to neurosurgeons. Although these tumors are benign and do not infiltrate the surrounding tissues, their proximity to the pelvic organs and large blood vessels makes it difficult to determine the best surgical approach. Owing to its rarity, there is no universally accepted surgical approach. The literature often recommends an anterior approach via laparotomy, although a dorsal approach involving laminotomy and stabilization has also been described. However, both approaches can be traumatic for patients and carry risks during and after surgery.
Laparoscopic surgery is a minimally invasive option for resecting presacral giant schwannomas using small incisions and cameras, leading to less scarring and faster recovery [13]. It was first described in the 1990s and mainly consists of case reports and rare case series [[14], [15], [16], [17], [18], [19], [20], [21]]. The decision to proceed with laparoscopic surgery depends on several factors, such as the size and location of the tumor and, as well as the surgeon's experience. Given the complexity and specialized location of these tumors, consultation with a vascular surgeon, orthopedic surgeon, urologist, or neurosurgeon may be necessary to ensure patient safety and quality of life Certainly, surgical management of presacral tumors should be performed by an experienced surgeon capable of performing various surgical procedures.
Deng et al. [22] recently reported that 12 patients with presacral tumors underwent laparoscopy without the need for conversion to laparotomy. Eight cases were related to tumors located in the presacral space, whereas the remaining four were found in the lateral wall of the pelvis. The median duration of the operation was 145 min, and the median blood loss was 35 mL. Only three patients experienced minor postoperative complications, and the median postoperative hospital stay was 4 days [22].
It is common for gynecologic oncologists to misdiagnose a pelvic mass as an ovarian tumor when, in reality, it may be a tumor of another origin. Moreover, clinicians should be aware that retroperitoneal schwannomas can closely resemble ovarian carcinomas, even in young women. Because of their infrequency and the fact that preoperative imaging methods may only suggest a diagnosis, misdiagnosis is common in these cases.
Gynecologic oncologists provide a comprehensive approach to the diagnosis and surgical treatment of cancerous and noncancerous (benign) conditions affecting the female pelvis. As previously reported, this area presents unique surgical challenges because of the intricate relationship among multiple organs, vessels, and nerves. Two main types of procedures are performed in the field of gynecologic oncology: (a) standard gynecological procedures (mandatory) and (b) extended-scope surgical procedures (desirable). The latter may include a range of general, plastic, colorectal, urological, and palliative surgeries. These procedures are typically related to the extended cytoreductive procedures used in the surgical management of ovarian cancer. An oncology gynecologist must have the knowledge and expertise to perform these procedures. Therefore, mandatory skills that gynecological oncologists should perform regularly are considered the standard of care. Desirable skills for gynecological oncologists include skills that can be accomplished with or without the assistance of specialist colleagues. These procedures involve allied surgical specialties, such as urological, colorectal, plastic, and upper gastrointestinal surgeries. Some skills desirable for patient care do not yet have level 1 evidence, but they will affect care quality in the future. These skills are research-based practices that are not yet supported by level 1 evidence but are expected to become standard practice. Some procedures in the desirable category may be routine in specific centers based on their resource settings [23].
This case study demonstrates that a team of skilled gynecological oncologists effectively resected a giant presacral pelvic schwannoma. During the presurgical phase, the tumor was initially mistaken for a malignant ovarian neoplasm. This case highlights the importance of gynecological oncologists' knowledge of pelvic anatomy and surgical management skills when dealing with critical anatomical spaces such as the pre-sacral region. This region is rich in venous vascularization and dense nerve lines, making it vital for gynecologic oncologists to successfully resect tumors from essential vessels and nerves.
We want to bring to your attention our previous reports [18,24], which showcased our ability to independently manage the laparoscopic resection of voluminous neoplastic masses from the main arteriovenous vessels. One highlighted case was the successful laparoscopic resection of a giant retroperitoneal schwannoma (45 mm × 32 mm × 39 mm) attached to major vital vessels. We also described the feasibility and safety of laparoscopic surgery for isolated lymph node recurrence up to 8 cm due to gynecological malignancies.
In this clinical case, we wish to confirm that the resection of large pelvic retroperitoneal masses in a minimally invasive way is possible through the expert use of various devices, as showed in the included supplementary video.
We believe that gynecological oncologists who perform laparoscopic surgery for large pelvic retroperitoneal schwannomas may benefit from specific skills. These skills include adequate and rigorous training of surgeons who intend to perform minimally invasive surgical approaches using the most appropriate coagulation and tissue resection devices, using suitable forceps for vascular structures, and having a skilled and expert surgeon to deal with potential vascular damage or injury to adjacent organs.
5. Conclusion
Although prompt recognition of ovarian cancer remains essential, awareness of the processes that mimic ovarian tumors avoids potential misdiagnosis and unnecessary surgery [25]. The complex anatomy and similarity of imaging features of various pelvic pathologies can make accurate radiological interpretation difficult [26]. If gynecological oncologists encounter an unexpected extraovarian retroperitoneal presacral neoplasm that needs to be resected, they can perform the procedure successfully while minimizing complications if they have appropriate skills. This rare case of a giant schwannoma highlights some key points to consider when evaluating and managing a pelvic retroperitoneal presacral mass. It is crucial to have adequate knowledge of the pelvic space, plan the best surgical approach, and familiarize oneself with the most appropriate instrumental tools. Laparoscopy is the surgical technique of choice and has several technical advantages over traditional surgery. In cases with complex pelvic anatomy, laparoscopy can help overcome difficulties by providing a clear view of the dissection plane when entering the presacral area. If organ trauma occurs, laparoscopy can be used to detect and repair the tissue with a minimal incision. Moreover, laparoscopy provides better visualization of the hemostasis of the iliac vessels and venous plexus in the presacral space. By offering a magnified view, laparoscopy allows for more precise preservation of autonomous nerves in the pelvic area, significantly reducing the risk of urinary incontinence due to accidental injury to the autonomic nerves of the pelvis or autonomic neurovascular bundles, even in cases of huge masses.
The following is the supplementary data related to this article.
Video showing radical laparoscopic resection of a large retroperitoneal pelvic schwannoma. The supplementary video can be downloaded from: https://doi.org/10.5281/zenodo.13286385.
Institutional review board (IRB) approval
Ethics approval by the Institutional Local Ethics Committee (Ethics Committee of the Sardinian Region) is not required for case reports. Written informed consent was obtained from the patient for publication and any accompanying images.
Clinical trial registration
NA
Consent
Written informed consent was obtained from the patient for publication and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Ethical approval
Ethics approval by the Institutional Local Ethics Committee (Ethics Committee of the Sardinian Region) is not required for case reports.
Funding
The authors thank the “Associazione Sarda per la Ricerca in Ginecologia Oncologica-ONLUS” for supporting the research. The study sponsor had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to submit the manuscript for publication.
Author contribution
Antonio Macciò and Clelia Madeddu: Conceptualization or design of the work. Antonio Macciò, Paola Abis, Gabriele Sole, Nicola D’Angelo, Sonia Nemolato, and Clelia Madeddu: Acquisition, analysis, collection, and interpretation of data for the work. Antonio Macciò, Paola Abis, and Clelia Madeddu: Drafting of the work or critical review of the work for important intellectual content. All authors reviewed and approved the version to be published.
Guarantor
Antonio Macciò
Declaration of competing interest
The authors declare no conflicts of interest.
Acknowledgments
The authors thank the “Associazione Sarda per la Ricerca in Ginecologia Oncologica-ONLUS” for supporting the research. The study sponsor had no role in the design of the study, in the collection, analysis, or interpretation of data, in the writing of the manuscript, or in the decision to submit the manuscript for publication.
Data availability statement
Data are available from the corresponding author upon reasonable request.
References
- 1.Lamris M.A., El Yamine O., El Jay S.R., et al. Retroperitoneal schwannoma: a case report. Ann. Med. Surg. (Lond.) 2021;70 doi: 10.1016/j.amsu.2021.102785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cury J., Coelho R.F., Srougi M. Retroperitoneal schwannoma: case series and literature review. Clinics (Sao Paulo) 2007;62:359–362. doi: 10.1590/s1807-59322007000300024. [DOI] [PubMed] [Google Scholar]
- 3.Poojari V.G., Pai M.V., Nambiar J., Mathew M. Pelvic schwannoma mimicking as an adnexal mass. Int. J. Reprod. Contracept. Obstet. Gynecol. 2015;4:1206–1208. [Google Scholar]
- 4.Sakalauskaite M., Stanaitis J., Cepkus S., Pleckaitis M., Lunevicius R. Retroperitoneal giant schwannoma eroding lumbal vertebra: a case report with a literature review. Open Med. 2008;3:233–244. [Google Scholar]
- 5.Okuyama T., Tagaya N., Saito K., Takahashi S., Shibusawa H., Oya M. Laparoscopic resection of a retroperitoneal pelvic schwannoma. J. Surg. Case Rep. 2014;2014:rjt122. doi: 10.1093/jscr/rjt122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goidescu O.C., Dogaru I.A., Badea T.G., et al. The distribution of the inferior hypogastric plexus in female pelvis. J. Med. Life. 2022;15:784–791. doi: 10.25122/jml-2022-0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoham AL, Bordoni B. Anatomy, abdomen and pelvis: inferior hypogastric plexus. [Updated 2022 Dec 11]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. (https://www.ncbi.nlm.nih.gov/books/NBK567711/). [PubMed]
- 8.Casal Núñez J.E., Vigorita V., Ruano Poblador A., et al. Presacral venous bleeding during mobilization in rectal cancer. World J. Gastroenterol. 2017;23:1712–1719. doi: 10.3748/wjg.v23.i9.1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Landriel F., Padilla Lichtenberger F., Guiroy A., Soto M., Molina C., Hem S. Minimally invasive approaches for lumbosacral plexus schwannomas. Oper. Neurosurg. (Hagerstown) 2024;26:149–155. doi: 10.1227/ons.0000000000000877. [DOI] [PubMed] [Google Scholar]
- 10.Sohrabi C., Mathew G., Maria N., Kerwan A., Franchi T., Agha R.A. The SCARE 2023 guideline: updating consensus surgical CAse REport (SCARE) guidelines. Int. J. Surg. Lond. Engl. 2023;109(5):1136. doi: 10.1097/JS9.0000000000000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Handayani S., Kartikasari M.N.D., Suparyanti E.L., Moelyo A.G., Kusumawati I., Muhammad F., Kushare V. In: Improving Health for Better Future Life: Strengthening from Basic Science to Clinical Research. Muthmainah M., Hidayati H.B., Yanti B., editors. CRC Press, Taylor & Francis; London: 2023. The effectiveness of electroacupuncture for treating labor pain in primary healthcare: A preliminary study; pp. 114–119. [DOI] [Google Scholar]
- 12.Poudel D., Shrestha S., Poddar E., Pacchai P., Kandel B.P., Lakhey P.J. Retrorectal schwannoma in a middle-aged female: a case report. Int. J. Surg. Case Rep. 2022;96 doi: 10.1016/j.ijscr.2022.107270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Körfer D., Jentschura D. Surgical management of presacral tumors: report of 5 cases with video vignette of laparoscopic removal. Surg. Laparosc. Endosc. Percutan. Tech. 2023;33:198–201. doi: 10.1097/SLE.0000000000001164. [DOI] [PubMed] [Google Scholar]
- 14.Melvin W.S. Laparoscopic resection of a pelvic schwannoma. Surg. Laparosc. Endosc. 1996;6:489–491. [PubMed] [Google Scholar]
- 15.Nishio A., Adachi W., Igarashi J., Koide N., Kajikawa S., Amano J. Laparoscopic resection of a retroperitoneal schwannoma. Surg. Laparosc. Endosc. Percutan. Tech. 1999;9:306–309. [PubMed] [Google Scholar]
- 16.Ohigashi T., Nonaka S., Nakanoma T., Ueno M., Deguchi N. Laparoscopic treatment of retroperitoneal benign schwannoma. Int. J. Urol. 1999;6:100–103. doi: 10.1046/j.1442-2042.1999.06222.x. [DOI] [PubMed] [Google Scholar]
- 17.Ribeiro M.A., Jr., Elias Y.G., Augusto S.S., et al. Laparoscopic resection of primary retroperitoneal schwannoma: a case report. World J. Clin. Cases. 2020;8:4114–4121. doi: 10.12998/wjcc.v8.i18.4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Macciò A., Kotsonis P., Aste L., et al. An interdisciplinary approach for laparoscopic removal of a large retroperitoneal pelvic schwannoma attached to vital vessels: a case report. Medicine (Baltimore) 2019;98 doi: 10.1097/MD.0000000000018149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ohsawa M., Miguchi M., Yoshimitsu M., et al. Laparoscopic excision of a retroperitoneal schwannoma: a case report. Asian J. Endosc. Surg. 2019;12:192–196. doi: 10.1111/ases.12607. [DOI] [PubMed] [Google Scholar]
- 20.Jiang S., Li Q.S., Sheng X.G., Song Q.Q., Lu C.H., Pan C.X. Schwannomas of female genitalia from a gynaecologist’s perspective: report of two cases and review of the literature. Eur. J. Gynaecol. Oncol. 2016;37:254–257. [PubMed] [Google Scholar]
- 21.Dawley B. A retroperitoneal femoral nerve schwannoma as a cause of chronic pelvic pain. J. Minim. Invasive Gynecol. 2008;15:491–493. doi: 10.1016/j.jmig.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 22.Deng C., Wang P., Liu Y., et al. Laparoscopic resection of pelvic schwannomas: a 9-year experience at a single center. World Neurosurg. X. 2022;17 doi: 10.1016/j.wnsx.2022.100150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Minig L., Padilla-Iserte P., Zorrero C. The relevance of gynecologic oncologists to provide high-quality of care to women with gynecological cancer. Front. Oncol. 2016;5:308. doi: 10.3389/fonc.2015.00308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanna E., Madeddu C., Lavra F., et al. Laparoscopic management of isolated nodal recurrence in gynecological malignancies is safe and feasible even for large metastatic nodes up to 8 cm: a prospective case series. Int. J. Surg. 2022;104 doi: 10.1016/j.ijsu.2022.106744. [DOI] [PubMed] [Google Scholar]
- 25.Elsherif S.B., Agely A., Gopireddy D.R., et al. Mimics and pitfalls of primary ovarian malignancy imaging. Tomography. 2022;8:100–119. doi: 10.3390/tomography8010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vilos G.A., Vilos A.G., Hollett-Caines J., Abu-Rafea B., Jacob G.P., Ettler H. Retroperitoneal pelvic tumours in women: diagnostic and therapeutic challenges. Facts Views Vis. Obgyn. 2020;11:299–306. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video showing radical laparoscopic resection of a large retroperitoneal pelvic schwannoma. The supplementary video can be downloaded from: https://doi.org/10.5281/zenodo.13286385.
Data Availability Statement
Data are available from the corresponding author upon reasonable request.