Highlights
-
•
Respiratory-gated volumetric arc therapy with internal markers is clinically feasible.
-
•
Mean treatment time for stereotactic liver irradiation was below 10 min.
-
•
Target conformity and stomach-sparing profit from gated volumetric arc therapy.
Keywords: Motion management, SBRT, VMAT, Internal marker, Respiratory gating
Abstract
Real-time tumor-tracking volumetric modulated arc therapy (RT-VMAT) enabling beam-gating based on continuous X-ray tracking of the three-dimensional position of internal markers is relevant for moving tumors. Dose-volume characteristics and treatment time were evaluated in ten consecutive patients who underwent liver stereotactic body radiation therapy with RT-VMAT. Target dose conformity and sparing of the stomach and the intestine were improved comparing RT-VMAT with RT-3D conformal radiotherapy. The mean treatment time for each fraction was less than 10 min. RT-VMAT could be effective, especially for targets located adjacent to organs at risk.
1. Introduction
Respiratory-gated volumetric modulated arc therapy (VMAT) is promising for achieving better dose distribution than three-dimensional (3D) conformal radiotherapy [1], [2], [3]. Furthermore, beam gating based on the internal markers is expected to be superior to that based on the external markers due to a better correlation with the tumor motion [4], [5], [6], [7]. To date, VMAT using triggered kilovoltage (kV) X-ray imaging to monitor the internal marker position during beam-gating based on external surrogates has been reported [8]. A tool using kV X-ray imaging for monitoring the marker positions throughout treatment has been clinically applied [9]. Although real-time tumor-tracking enabling beam-gating based on the three-dimensional position of internal markers had been conducted with 3D conformal radiotherapy [10], [11], [12], [13], [14], no studies have reported its clinical application in VMAT.
In recent years real-time tumor-tracking using internal fiducial markers and kV X-ray imaging to guide gating in stereotactic body radiation therapy (SBRT) has been initiated for VMAT (called RT-VMAT in this study). To the best of our knowledge, this is the first report of its clinical application. In this study, we aimed to show clinical feasibility of RT-VMAT based on dose-volume characteristics and treatment time.
2. Materials and methods
2.1. Patient characteristics
We evaluated 10 consecutive patients with a single target who underwent liver SBRT with RT-VMAT at our institution between April 2022 and December 2022. The patients were categorized into two groups, the adjacent and non-adjacent groups, based on our institutional protocol [12]. In the non-adjacent group, the tumor was located more than 2 cm away from the intestinal tract and hepatic hilum. In the adjacent group, the tumor was located within 2 cm of the intestinal tract or hepatic hilum. Each group included five patients. A spherical gold marker (iGold, MEDIKIT, Japan) with a diameter of 2 mm was percutaneously implanted near the tumor. The relevant details of the patient characteristics are summarized in Supplementary Table S1.
2.2. Treatment system
All patients were treated using a Linac (TrueBeam; Varian Medical Systems, USA) equipped with a real-time X-ray imaging system (SyncTraX FX4; Shimadzu, Japan). The SyncTraX system (Fig. 1) was used to evaluate the three-dimensional position of internal markers during treatment. It consists of four kV X-ray imaging units. Two X-ray imaging units capable of capturing images without overlapping the gantry head were selected automatically. The X-ray images were obtained at a maximum rate of 30 frames/s. The projected positions of the markers in each X-ray image were automatically recognized using template-pattern matching [15]. Success or failure of image recognition was determined online based on a quantitative score, ranging from 0 to 100, derived from template-pattern matching. The beam irradiation was disabled when the recognition score felt below a threshold value. In our institution, the threshold was empirically set at 30. As the marker recognition performance depends on the type of marker, patient body thickness, marker location, and other factors, it was validated in a phantom test before clinical application [16]. The treatment beam was switched on only when the actual marker position was within the gating window (Fig. 1B). During RT-VMAT, irradiation was performed while rotating intermittently according to the respiratory motion. Because X-ray imaging is blocked by the gantry head if rotational irradiation is continued, gantry angles in the range of , , , and were not used in RT-VMAT. The system latency for the beam-on and beam-off was approximately 150 and 70 ms, respectively [17].
Fig. 1.
(A) Overview of the treatment system which consists of linac and SyncTraX. (B) Snapshot of the SyncTraX software. Position and tolerance width of the gating window are shown as “Plan” and “Delta ()”, respectively.
Before clinical application, dosimetric verification was conducted with an ion chamber and radiochromic film using a dynamic phantom (Supplementary material A).
2.3. Treatment planning
Exhaled breath-hold CT images were used for treatment planning. For treatment plan optimization with a 6MV flattening filter free beam a collapsed cone algorithm was used with a 2 mm grid size, a gantry spacing of 2° and a maximum dose rate of 1400 monitor units (MU)/min. Further details are listed in the Supplementary Table S2. The gross tumor volume –clinical target volume (CTV) margin ranged from 0 to 5 mm, depending on the size and location of the tumor and the patient's liver function. The CTV–planning target volume (PTV) margin was 5 mm. The planning organ at-risk volume (PRV) margin was 5 mm. The prescribed doses for the nonadjacent and adjacent groups were 40 Gy in four fractions and 48 Gy in eight fractions for PTV D95%, respectively. The dose constraints for the targets and OARs are listed in Supplementary Table S3. In the nonadjacent group, one case with an additional dose constraint of Dmax < 12 Gy to the lead of the pacemaker and one case with Dmean < 3 Gy to the past irradiation area were included. The adjacent group included a patient who required an additional constraint of Dmax < 4 Gy to the lung owing to a medical history of interstitial pneumonia. Depending on the case, three to six partial arcs were used to compensate for angles that could not be used for RT-VMAT (Supplementary Table S4).
2.4. Treatment workflow
The initial patient setup was performed by image registration between the orthogonal kV X-ray images obtained using a gantry-mounted onboard imager and digitally reconstructed radiographs with reference to bony structures. CBCT images were also utilized when the rotational deviations were included. Next, kV X-ray imaging with SyncTraX was initiated and stopped during the expiration respiratory phase. The discrepancy between the actual marker position during expiration and the gating window was derived and applied to the patient couch. If the discrepancy, namely the difference between bony structure-based alignment and marker-based alignment, was not clinically acceptable (e.g., 5 mm in a typical case), it was recommended to acquire the CT or CBCT images to exclude marker migration. It is well known that the markers inserted in lung region often dropout [18]. We did not experience marker dropout in the liver cases assessed in this study. Thus, the treatment beam irradiation was enabled while exhaling during free breathing. The tumor position while exhaling could be altered owing to baseline shift/drift during treatment [19]. When baseline shift/drift was observed by visual inspection of the X-ray images, X-ray imaging was stopped during exhalation and couch position was corrected as in the initial patient setup process. A gating window width of ± 2 mm in each direction was applied in all patients.
2.5. Evaluation and statistical analysis
For comparison, treatment plans for real-time tumor-tracking 3D conformal radiotherapy (RT-3D) with nine coplanar beams were created retrospectively by an independent physicist without knowledge of the VMAT plan. RT-3D plans were created to satisfy the target doses and meet the criteria for the OAR dose as much as possible. Wilcoxon signed-rank sum test was used to test the statistical significance of median values of each dose index.
The cumulative imaging time of kV X-ray for each fraction were evaluated using the recorded RT-VMAT data. Treatment time was defined as the time from the first X-ray imaging to the last of treatment beam irradiation. The gating efficiency was defined as the ratio of the gate-on time to the imaging time. The imaging time in RT-3D was estimated by tracing the time-series data of the gate-on/off status, assuming a fixed dose rate of 1400 MU/min. The treatment time in RT-3D was estimated as the sum of the imaging time and interval time between the nine beams. The interval time, defined as the time from the last X-ray imaging to the first imaging in the next field, was estimated from the recorded data to be approximately 28 s. Hence, the total interval time for RT-3D with nine coplanar beams was assumed to be s.
3. Results
3.1. Dosimetric evaluation
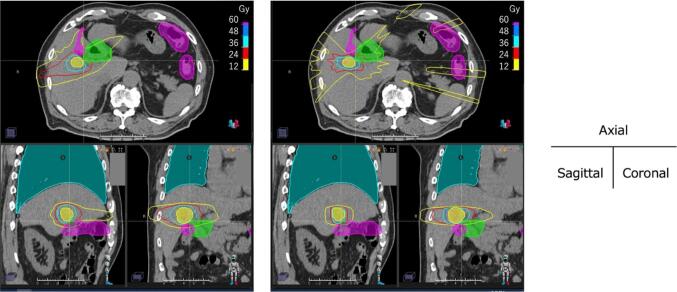
Relatively large difference was found in median dose for the stomach in the non-adjacent group. D0.5cc for stomach in RT-VMAT and RT-3D were 3.5 (2.2–11.7) Gy and 10.7 (2.3–16.9) Gy, respectively. As the direction of the beam was limited for two atypical cases, including additional dose constraints to consider the pacemaker cable and past irradiation for each, a relatively high dose was delivered to the stomach in RT-3D. In the adjacent group, median dose of D0.5cc for intestine was tended to be significantly smaller in RT-VMAT. Details of the dose indices are summarized in Supplementary Table S3. Fig. 2 shows an example of dose distribution in a patient with a medical history of interstitial pneumonia. In RT-3D, as most beams had to be arranged in the lateral direction to spare the lungs, it was difficult to control the high-dose region to the adjacent OARs while maintaining the prescribed doses to the target, and the two-dose constraints for the lung and duodenum PRV were not met. There was no significant difference in MU values between RT-VMAT and RT-3D in either group.
Fig. 2.
Example of dose distributions of (left) RT-VMAT and (right) RT-3D. The ROIs represent the PTV (yellow), duodenum PRV (green), intestine PRV (magenta), and lung (cyan), respectively. The dose constraint for duodenum PRV and intestine PRV was Dmax < 36 Gy. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.2. Imaging time, treatment time, and gating efficiency
In the nonadjacent group, the mean ± SD of imaging time in RT-VMAT and RT-3D were 5.7 ± 2.9 min and 4.8 ± 1.9 min, respectively. Treatment time were 7.5 ± 3.9 min and 8.5 ± 1.9 min, respectively. In the adjacent group, the mean ± SD of imaging time in RT-VMAT and RT-3D were 7.4 ± 2.1 min and 3.7 ± 0.7 min, respectively. Treatment time were 9.6 ± 2.7 min and 7.4 ± 0.7 min, respectively. The imaging and treatment times of RT-VMAT in the adjacent group were longer than those of RT-3D because the dose rate in RT-VMAT was highly modulated to form a complex dose distribution.
All patients were treated without frequent marker recognition failure. The mean score and successful rate of image recognition in daily treatment for ten patients were 79.0 ± 7.6 and 98.8 ± 1.3 %, respectively. The average number, SD and range of baseline shift/drift correction in one fraction was 0.7 ± 1.0 (0–5). Gating efficiency in the nonadjacent and adjacent groups were 34.0 ± 9.6 % and 28.8 ± 4.7 %, respectively.
4. Discussion
In this study, RT-VMAT satisfied most of the dose constraints in all patients, including atypical cases. The target dose may have had to be lowered to spare the OARs if RT-3D had been applied in some cases. In addition, RT-VMAT can be applied to patients with multiple targets, who were excluded from the evaluation in this study because of the difficulty in comparing them with RT-3D. In both RT-VMAT and RT-3D, noncoplanar beams may result in better dose distribution [20], although the treatment time could be prolonged [21].
External surrogates such as an external marker block are used for respiratory beam gating as standard technique at the present time [22], [23], [24], [25]. However, the motion correlation between the internal markers and the target is higher than that between the external surrogate and the target [4], [5], [6], [7]. Therefore, ITV and PTV margins for RT-VMAT could be smaller than that for standard respiratory gating techniques.
One limitation of RT-VMAT is the dose required for X-ray imaging. Assuming the typical X-ray imaging condition of 100 kV, 80 mA, 3 msec pulse width and 15 frames/s for liver SBRT, the accumulated skin dose is estimated to be approximately 0.2 Gy for one hour. Although an additional imaging dose does not induce severe skin reactions, the X-ray tube voltage and current should be minimized while maintaining stable marker recognition. In this study, one marker was used as surrogate. However, deformations or rotations may be included. If multiple markers can be implanted surrounding the tumor, the target localization accuracy will be enhanced [26].
In conclusion, RT-VMAT could be clinically feasible and effective, especially in cases requiring complex dose distributions, such as multiple targets and targets adjacent to OARs.
CRediT authorship contribution statement
Naoki Miyamoto: Conceptualization, Methodology, Investigation, Formal analysis, Writing – original draft. Norio Katoh: Methodology, Investigation, Writing – review & editing. Takahiro Kanehira: Formal analysis. Kohei Yokokawa: Formal analysis. Ryusuke Suzuki: Formal analysis. Yusuke Uchinami: Writing – review & editing. Hiroshi Taguchi: Writing – review & editing. Daisuke Abo: Writing – review & editing. Hidefumi Aoyama: Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This study was partially supported by KAKENHI (20H03612).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.phro.2024.100623.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Xi M., Zhang L., Li Q.Q., Zhao L., Zhang R., Liu M.Z. Assessing the role of volumetric-modulated arc therapy in hepatocellular carcinoma. J Appl Clin Med Phys. 2013;14:4162. doi: 10.1120/jacmp.v14i3.4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuo Y.C., Chiu Y.M., Shih W.P., Yu H.W., Chen C.W., Wong P.F., et al. Volumetric intensity-modulated Arc (RapidArc) therapy for primary hepatocellular carcinoma: comparison with intensity-modulated radiotherapy and 3-D conformal radiotherapy. Radiat Oncol. 2011;6:76. doi: 10.1186/1748-717X-6-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen D., Wang R., Meng X., Liu T., Yan H., Feng R., et al. A comparison of liver protection among 3-D conformal radiotherapy, intensity-modulated radiotherapy and RapidArc for hepatocellular carcinoma. Radiat Oncol. 2014;9:48. doi: 10.1186/1748-717X-9-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Korreman S., Mostafavi H., Le Q.T., Boyer A. Comparison of respiratory surrogates for gated lung radiotherapy without internal fiducials. Acta Oncol. 2006;45:935–942. doi: 10.1080/02841860600917161. [DOI] [PubMed] [Google Scholar]
- 5.Mao W., Kim J., Chetty I.J. Association Between Internal Organ/Liver Tumor and External Surface Motion From Cine MR Images on an MRI-Linac. Front Oncol. 2022;12 doi: 10.3389/fonc.2022.868076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ionascu D., Jiang S.B., Nishioka S., Shirato H., Berbeco R.I. Internal-external correlation investigations of respiratory induced motion of lung tumors. Med Phys. 2007;34:3893–3903. doi: 10.1118/1.2779941. [DOI] [PubMed] [Google Scholar]
- 7.Park S., Farah R., Shea S.M., Tryggestad E., Hales R., Lee J. Simultaneous tumor and surrogate motion tracking with dynamic MRI for radiation therapy planning. Phys Med Biol. 2018;63 doi: 10.1088/1361-6560/aaa20b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Santoso A.P., Vinogradskiy Y., Robin T.P., Goodman K.A., Schefter T.E., Miften M., et al. Clinical and Dosimetric Impact of 2D kV Motion Monitoring and Intervention in Liver Stereotactic Body Radiation Therapy. Adv Radiat Oncol. 2024;9 doi: 10.1016/j.adro.2023.101409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poulsen P.R., Worm E.S., Petersen J.B., Grau C., Fledelius W., Hoyer M. Kilovoltage intrafraction motion monitoring and target dose reconstruction for stereotactic volumetric modulated arc therapy of tumors in the liver. Radiother Oncol. 2014;111:424–430. doi: 10.1016/j.radonc.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 10.Inoue T., Katoh N., Onimaru R., Shimizu S., Tsuchiya K., Suzuki R., et al. Stereotactic body radiotherapy using gated radiotherapy with real-time tumor-tracking for stage I non-small cell lung cancer. Radiat Oncol. 2013;8:69. doi: 10.1186/1748-717X-8-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katoh N., Soda I., Tamamura H., Takahashi S., Uchinami Y., Ishiyama H., et al. Clinical outcomes of stage I and IIA non-small cell lung cancer patients treated with stereotactic body radiotherapy using a real-time tumor-tracking radiotherapy system. Radiat Oncol. 2017;12:3. doi: 10.1186/s13014-016-0742-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uchinami Y., Katoh N., Abo D., Taguchi H., Yasuda K., Nishioka K., et al. Treatment outcomes of stereotactic body radiation therapy using a real-time tumor-tracking radiotherapy system for hepatocellular carcinomas. Hepatol Res. 2021;51:870–879. doi: 10.1111/hepr.13649. [DOI] [PubMed] [Google Scholar]
- 13.Hiroshima Y., Tamaki Y., Sawada T., Ishida T., Yasue K., Shinoda K., et al. Stereotactic Body Radiotherapy for Stage I Lung Cancer With a New Real-time Tumor Tracking System. Anticancer Res. 2022;42:2989–2995. doi: 10.21873/anticanres.15782. [DOI] [PubMed] [Google Scholar]
- 14.Tanabe S., Umetsu O., Sasage T., Utsunomiya S., Kuwabara R., Kuribayashi T., et al. Clinical commissioning of a new patient positioning system, SyncTraX FX4, for intracranial stereotactic radiotherapy. J Appl Clin Med Phys. 2018;19:149–158. doi: 10.1002/acm2.12467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miyamoto N., Ishikawa M., Bengua G., Sutherland K., Suzuki R., Kimura S., et al. Optimization of fluoroscopy parameters using pattern matching prediction in the real-time tumor-tracking radiotherapy system. Phys Med Biol. 2011;56:4803–4813. doi: 10.1088/0031-9155/56/15/011. [DOI] [PubMed] [Google Scholar]
- 16.Miyamoto N., Maeda K., Abo D., Morita R., Takao S., Matsuura T., et al. Quantitative evaluation of image recognition performance of fiducial markers in real-time tumor-tracking radiation therapy. Phys Med. 2019;65:33–39. doi: 10.1016/j.ejmp.2019.08.004. [DOI] [PubMed] [Google Scholar]
- 17.Shiinoki T., Kawamura S., Uehara T., Yuasa Y., Fujimoto K., Koike M., et al. Evaluation of a combined respiratory-gating system comprising the TrueBeam linear accelerator and a new real-time tumor-tracking radiotherapy system: a preliminary study. J Appl Clin Med Phys. 2016;17:202–213. doi: 10.1120/jacmp.v17i4.6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Imura M., Yamazaki K., Shirato H., Onimaru R., Fujino M., Shimizu S., et al. Insertion and fixation of fiducial markers for setup and tracking of lung tumors in radiotherapy. Int J Radiat Oncol Biol Phys. 2005;63:1442–1447. doi: 10.1016/j.ijrobp.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 19.Takao S., Miyamoto N., Matsuura T., Onimaru R., Katoh N., Inoue T., et al. Intrafractional Baseline Shift or Drift of Lung Tumor Motion During Gated Radiation Therapy With a Real-Time Tumor-Tracking System. Int J Radiat Oncol Biol Phys. 2016;94:172–180. doi: 10.1016/j.ijrobp.2015.09.024. [DOI] [PubMed] [Google Scholar]
- 20.Woods K., Nguyen D., Tran A., Yu V.Y., Cao M., Niu T., et al. Viability of Non-Coplanar VMAT for Liver SBRT as Compared to Coplanar VMAT and Beam Orientation Optimized 4pi IMRT. Adv Radiat Oncol. 2016;1:67–75. doi: 10.1016/j.adro.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma M., Niu C., Li M., Chen D., Yan L., Wang H., et al. Noncoplanar Volumetric Modulated Arc Therapy for Hepatocellular Carcinoma Based on a Cage-Like Radiotherapy System: A Simulation Study. Technol Cancer Res Treat. 2023;22 doi: 10.1177/15330338231170495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Snyder J.E., Flynn R.T., Hyer D.E. Implementation of respiratory-gated VMAT on a Versa HD linear accelerator. J Appl Clin Med Phys. 2017;18:152–161. doi: 10.1002/acm2.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Viel F., Lee R., Gete E., Duzenli C. Amplitude gating for a coached breathing approach in respiratory gated 10 MV flattening filter-free VMAT delivery. J Appl Clin Med Phys. 2015;16:5350. doi: 10.1120/jacmp.v16i4.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li R., Mok E., Han B., Koong A., Xing L. Evaluation of the geometric accuracy of surrogate-based gated VMAT using intrafraction kilovoltage x-ray images. Med Phys. 2012;39:2686–2693. doi: 10.1118/1.4704729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qian J., Xing L., Liu W., Luxton G. Dose verification for respiratory-gated volumetric modulated arc therapy. Phys Med Biol. 2011;56:4827–4838. doi: 10.1088/0031-9155/56/15/013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seppenwoolde Y., Wunderink W., Wunderink-van Veen S.R., Storchi P., Mendez Romero A., Heijmen B.J. Treatment precision of image-guided liver SBRT using implanted fiducial markers depends on marker-tumour distance. Phys Med Biol. 2011;56:5445–5468. doi: 10.1088/0031-9155/56/17/001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.