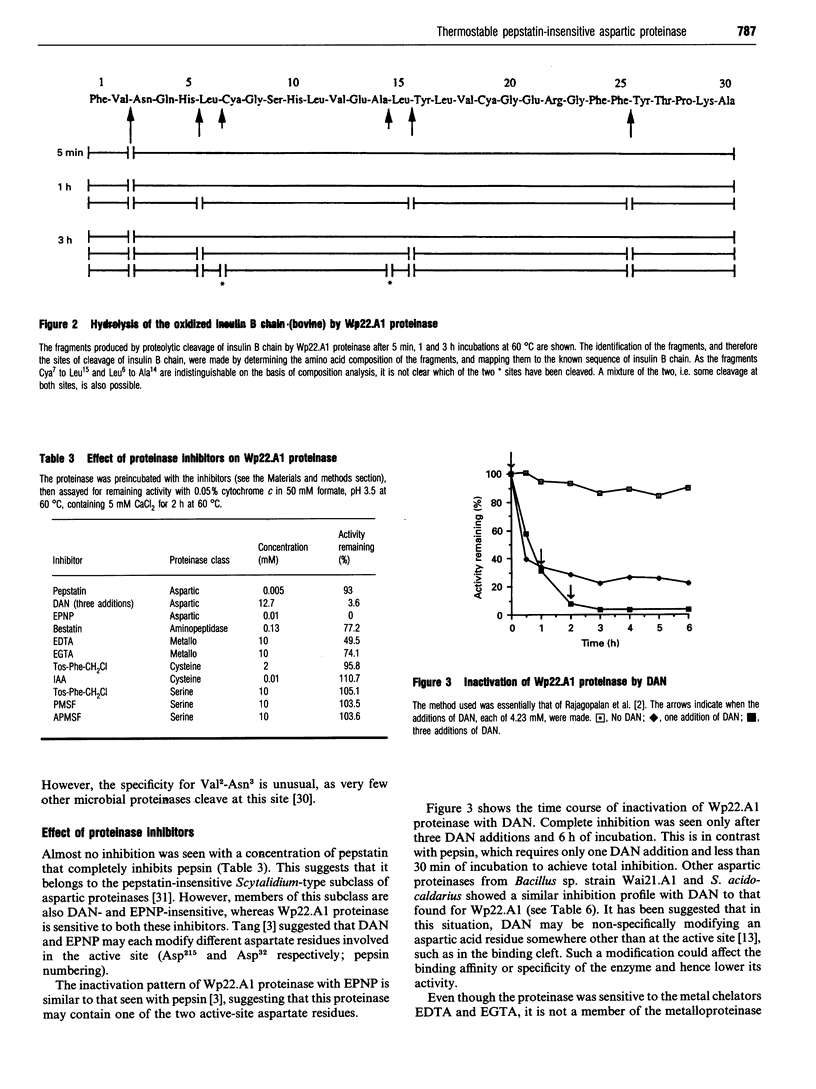
Abstract
Bacillus sp. strain Wp22.A1 produced a cell-associated aspartic proteinase which was purified to homogeneity using phenyl-Sepharose (hydrophobic and affinity chromatography) and Mono Q. The proteinase has a molecular mass of 45 kDa by SDS/PAGE and a pI of 3.8. It is insensitive to pepstatin, but is sensitive to the other aspartic proteinase-specific inhibitors diazoacetyl-DL-norleucine methyl ester (DAN) and 1,2-epoxy-3-(p-nitrophenoxy)propane. Inactivation by DAN was only partial, suggesting that it had non-specifically modified an aspartate residue at a site other than the active site. The enzyme was not inhibited by any of the serine or cysteine proteinase inhibitors tested. Maximum proteolytic activity was observed at pH 3.5. The proteinase had a higher activity with haemoglobin, but was more specific (Vmax./Km) for cytochrome c. Substrate inhibition was observed with both these substrates. The cleavage of oxidized insulin B chain tended to occur at sites where the P1 amino acid was bulky and non-polar, and the P1' amino acid was bulky and polar, such as its primary cleavage site of Val2-Asn3. The proteinase was stable in the pH range 2.5-5.5. Thermostability was increased in the presence of Ca2+, although to a lesser extent at higher temperatures. The thermostabilities at 60, 70, 80 and 90 degrees C were 45 h, 102, 21 and 3 min respectively in the presence of Ca2+.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agarwal N., Rich D. H. An improved cathepsin-D substrate and assay procedure. Anal Biochem. 1983 Apr 1;130(1):158–165. doi: 10.1016/0003-2697(83)90663-2. [DOI] [PubMed] [Google Scholar]
- Fusek M., Lin X. L., Tang J. Enzymic properties of thermopsin. J Biol Chem. 1990 Jan 25;265(3):1496–1501. [PubMed] [Google Scholar]
- Heukeshoven J., Dernick R. Improved silver staining procedure for fast staining in PhastSystem Development Unit. I. Staining of sodium dodecyl sulfate gels. Electrophoresis. 1988 Jan;9(1):28–32. doi: 10.1002/elps.1150090106. [DOI] [PubMed] [Google Scholar]
- Lin X., Tang J. Purification, characterization, and gene cloning of thermopsin, a thermostable acid protease from Sulfolobus acidocaldarius. J Biol Chem. 1990 Jan 25;265(3):1490–1495. [PubMed] [Google Scholar]
- Marciniszyn J., Jr, Hartsuck J. A., Tang J. Mode of inhibition of acid proteases by pepstatin. J Biol Chem. 1976 Nov 25;251(22):7088–7094. [PubMed] [Google Scholar]
- Morihara K. Comparative specificity of microbial proteinases. Adv Enzymol Relat Areas Mol Biol. 1974;41(0):179–243. doi: 10.1002/9780470122860.ch5. [DOI] [PubMed] [Google Scholar]
- Murao S., Ohkuni K., Nagao M., Hirayama K., Fukuhara K., Oda K., Oyama H., Shin T. Purification and characterization of kumamolysin, a novel thermostable pepstatin-insensitive carboxyl proteinase from Bacillus novosp. MN-32. J Biol Chem. 1993 Jan 5;268(1):349–355. [PubMed] [Google Scholar]
- Oda K., Sugitani M., Fukuhara K., Murao S. Purification and properties of a pepstatin-insensitive carboxyl proteinase from a gram-negative bacterium. Biochim Biophys Acta. 1987 Mar 19;923(3):463–469. doi: 10.1016/0304-4165(87)90055-9. [DOI] [PubMed] [Google Scholar]
- Paech C., Christianson T., Maurer K. H. Zymogram of proteases made with developed film from nondenaturing polyacrylamide gels after electrophoresis. Anal Biochem. 1993 Feb 1;208(2):249–254. doi: 10.1006/abio.1993.1041. [DOI] [PubMed] [Google Scholar]
- Peek K., Daniel R. M., Monk C., Parker L., Coolbear T. Purification and characterization of a thermostable proteinase isolated from Thermus sp. strain Rt41A. Eur J Biochem. 1992 Aug 1;207(3):1035–1044. doi: 10.1111/j.1432-1033.1992.tb17140.x. [DOI] [PubMed] [Google Scholar]
- Prescott M., Peek K., Veitch D. P., Daniel R. M. The use of phenyl-Sepharose for the affinity purification of proteinases. J Biochem Biophys Methods. 1993 Feb;26(1):51–60. doi: 10.1016/0165-022x(93)90021-f. [DOI] [PubMed] [Google Scholar]
- Rajagopalan T. G., Stein W. H., Moore S. The inactivation of pepsin by diazoacetylnorleucine methyl ester. J Biol Chem. 1966 Sep 25;241(18):4295–4297. [PubMed] [Google Scholar]
- Read S. M., Northcote D. H. Minimization of variation in the response to different proteins of the Coomassie blue G dye-binding assay for protein. Anal Biochem. 1981 Sep 1;116(1):53–64. doi: 10.1016/0003-2697(81)90321-3. [DOI] [PubMed] [Google Scholar]
- Robinson N. J., Tommey A. M., Kuske C., Jackson P. J. Plant metallothioneins. Biochem J. 1993 Oct 1;295(Pt 1):1–10. doi: 10.1042/bj2950001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saravani G. A., Cowan D. A., Daniel R. M., Morgan H. W. Caldolase, a chelator-insensitive extracellular serine proteinase from a Thermus spp. Biochem J. 1989 Sep 1;262(2):409–416. doi: 10.1042/bj2620409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J. Specific and irreversible inactivation of pepsin by substrate-like epoxides. J Biol Chem. 1971 Jul 25;246(14):4510–4517. [PubMed] [Google Scholar]
- Umezawa H. Low-molecular-weight enzyme inhibitors of microbial origin. Annu Rev Microbiol. 1982;36:75–99. doi: 10.1146/annurev.mi.36.100182.000451. [DOI] [PubMed] [Google Scholar]
- Vellekoop P., Lugones L., van Brederode J. Purification of an UDP-glucose:flavone, 7-O-glucosyltransferase, from Silene latifolia using a specific interaction between the enzyme and phenyl-Sepharose. FEBS Lett. 1993 Sep 6;330(1):36–40. doi: 10.1016/0014-5793(93)80914-g. [DOI] [PubMed] [Google Scholar]
- Voordouw G., Gaucher G. M., Roche R. S. Physiochemical properties of thermomycolase, the thermostable, extracellular, serine protease of the fungus Malbranchea pulchella. Can J Biochem. 1974 Nov;52(11):981–990. doi: 10.1139/o74-137. [DOI] [PubMed] [Google Scholar]
- Zhu H., Guo D. C., Dancik B. P. Purification and Characterization of an Extracellular Acid Proteinase from the Ectomycorrhizal Fungus Hebeloma crustuliniforme. Appl Environ Microbiol. 1990 Apr;56(4):837–843. doi: 10.1128/aem.56.4.837-843.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]




