Abstract
Phospholipase D (PLD), which is present in bacterial, plant and animal cells, can serve as an important element of signal-transduction pathways. This study examined the potential role of this enzyme in the regulation of Saccharomyces cerevisiae. An assay in vitro using a fluorescent 1-acyl-2-alkyl glycerophosphocholine as substrate was used to assess PLD activity in yeast cell extracts. A neutral PLD activity is present in membranes prepared from both haploid and diploid yeast cells, as evidenced by the production of phosphatidic acid and phosphatidylbutanol in the presence of butanol. Alcohols, in addition to serving as substrates for transphosphatidylation, stimulate PLD activity. Increased PLD activity is detected in membranes when either haploid or diploid cells are incubated in the presence of a non-fermentable carbon source. Membrane PLD activity increases within 10 min after diploid cells are placed in a sporulation-inducing medium lacking nitrogen and containing a non-fermentable carbon source. The increased activity persists for 2-3 h, and then declines to control values. This response occurs in the presence of cycloheximide, an inhibitor of protein synthesis. These data indicate that PLD activity is present in yeast, and that activation of PLD is an early event in sporulation in this organism.
Full text
PDF

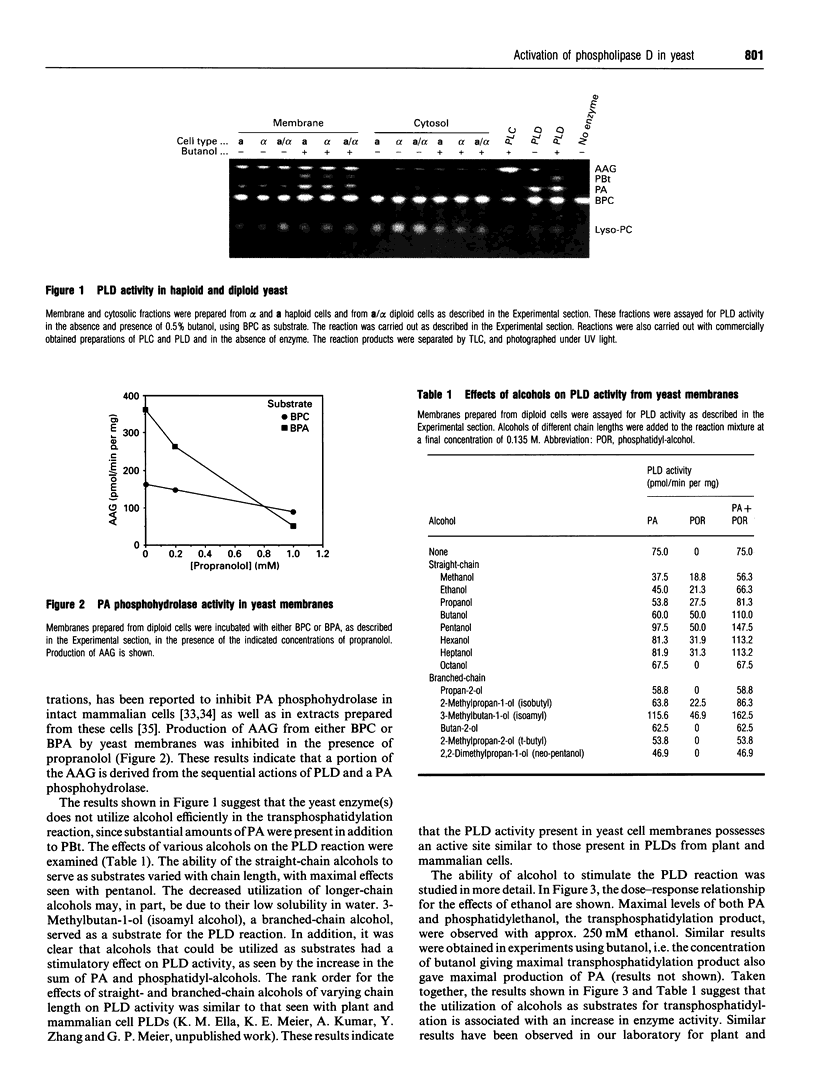
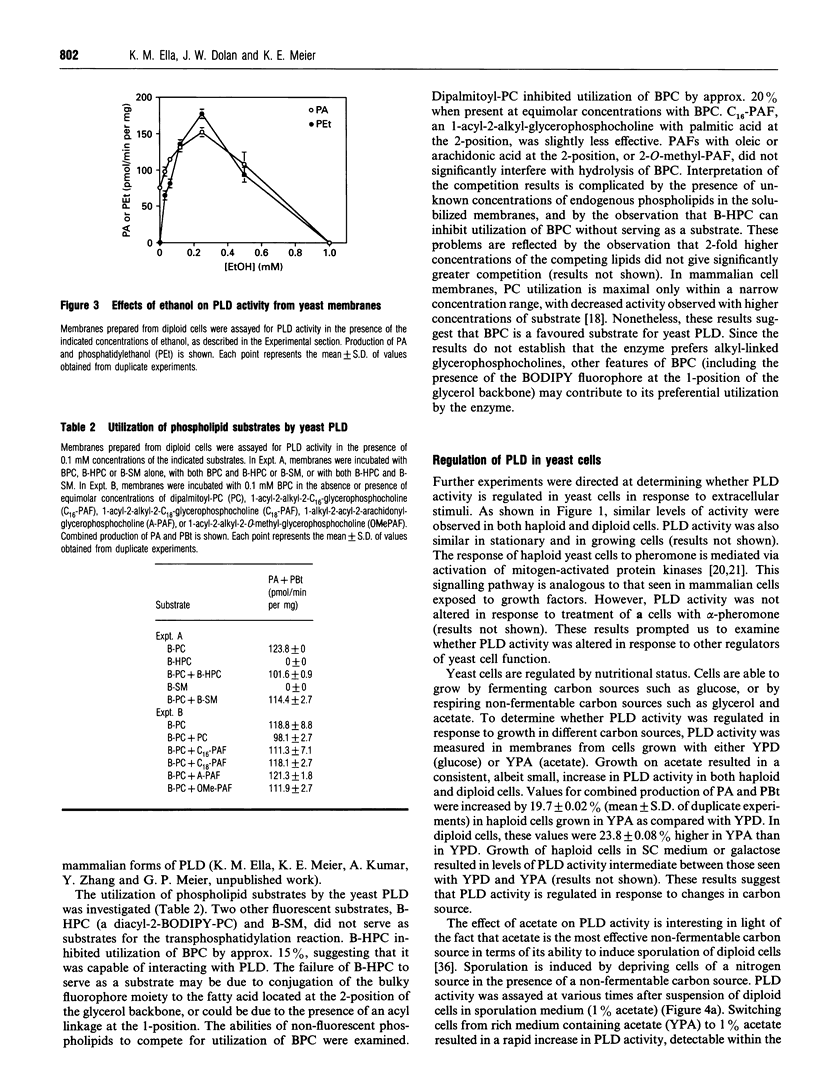
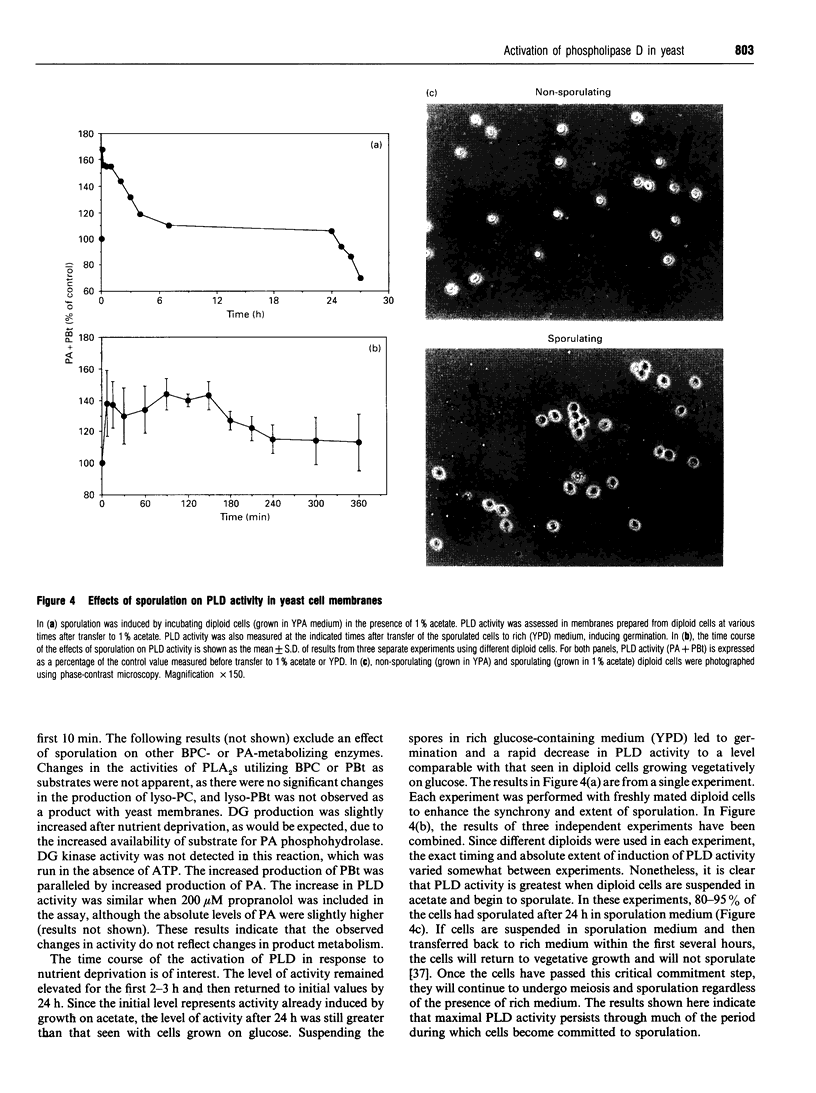
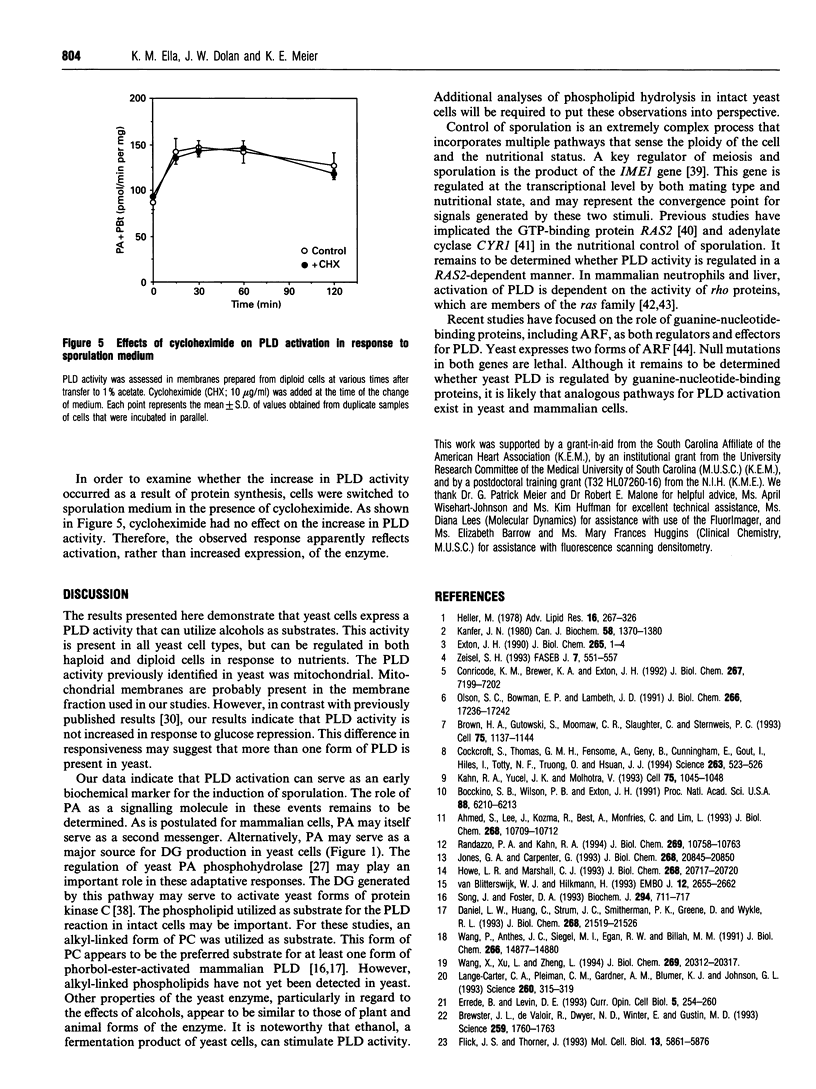

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed S., Lee J., Kozma R., Best A., Monfries C., Lim L. A novel functional target for tumor-promoting phorbol esters and lysophosphatidic acid. The p21rac-GTPase activating protein n-chimaerin. J Biol Chem. 1993 May 25;268(15):10709–10712. [PubMed] [Google Scholar]
- Billah M. M., Eckel S., Mullmann T. J., Egan R. W., Siegel M. I. Phosphatidylcholine hydrolysis by phospholipase D determines phosphatidate and diglyceride levels in chemotactic peptide-stimulated human neutrophils. Involvement of phosphatidate phosphohydrolase in signal transduction. J Biol Chem. 1989 Oct 15;264(29):17069–17077. [PubMed] [Google Scholar]
- Bocckino S. B., Wilson P. B., Exton J. H. Phosphatidate-dependent protein phosphorylation. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6210–6213. doi: 10.1073/pnas.88.14.6210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewster J. L., de Valoir T., Dwyer N. D., Winter E., Gustin M. C. An osmosensing signal transduction pathway in yeast. Science. 1993 Mar 19;259(5102):1760–1763. doi: 10.1126/science.7681220. [DOI] [PubMed] [Google Scholar]
- Brown H. A., Gutowski S., Moomaw C. R., Slaughter C., Sternweis P. C. ADP-ribosylation factor, a small GTP-dependent regulatory protein, stimulates phospholipase D activity. Cell. 1993 Dec 17;75(6):1137–1144. doi: 10.1016/0092-8674(93)90323-i. [DOI] [PubMed] [Google Scholar]
- Cockcroft S., Thomas G. M., Fensome A., Geny B., Cunningham E., Gout I., Hiles I., Totty N. F., Truong O., Hsuan J. J. Phospholipase D: a downstream effector of ARF in granulocytes. Science. 1994 Jan 28;263(5146):523–526. doi: 10.1126/science.8290961. [DOI] [PubMed] [Google Scholar]
- Conricode K. M., Brewer K. A., Exton J. H. Activation of phospholipase D by protein kinase C. Evidence for a phosphorylation-independent mechanism. J Biol Chem. 1992 Apr 15;267(11):7199–7202. [PubMed] [Google Scholar]
- Daniel L. W., Huang C., Strum J. C., Smitherman P. K., Greene D., Wykle R. L. Phospholipase D hydrolysis of choline phosphoglycerides is selective for the alkyl-linked subclass of Madin-Darby canine kidney cells. J Biol Chem. 1993 Oct 15;268(29):21519–21526. [PubMed] [Google Scholar]
- Dharmalingam K., Jayaraman J. Mechanism of glucose repression of mitochondriogenesis: induction of phospholipases. Biochem Biophys Res Commun. 1971 Dec 3;45(5):1115–1118. doi: 10.1016/0006-291x(71)90134-3. [DOI] [PubMed] [Google Scholar]
- Eichberg J., Hauser G. Stimulation by local anesthetics of the metabolism of acidic phospholipids in the rat pineal gland. Biochem Biophys Res Commun. 1974 Oct 23;60(4):1460–1467. doi: 10.1016/0006-291x(74)90362-3. [DOI] [PubMed] [Google Scholar]
- Ella K. M., Meier G. P., Bradshaw C. D., Huffman K. M., Spivey E. C., Meier K. E. A fluorescent assay for agonist-activated phospholipase D in mammalian cell extracts. Anal Biochem. 1994 Apr;218(1):136–142. doi: 10.1006/abio.1994.1152. [DOI] [PubMed] [Google Scholar]
- Errede B., Levin D. E. A conserved kinase cascade for MAP kinase activation in yeast. Curr Opin Cell Biol. 1993 Apr;5(2):254–260. doi: 10.1016/0955-0674(93)90112-4. [DOI] [PubMed] [Google Scholar]
- Exton J. H. Signaling through phosphatidylcholine breakdown. J Biol Chem. 1990 Jan 5;265(1):1–4. [PubMed] [Google Scholar]
- Flick J. S., Thorner J. Genetic and biochemical characterization of a phosphatidylinositol-specific phospholipase C in Saccharomyces cerevisiae. Mol Cell Biol. 1993 Sep;13(9):5861–5876. doi: 10.1128/mcb.13.9.5861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopalan G., Jayaraman J., Rajamanickam C. Effect of exogenous addition of hemin on the biogenesis of mitochondrial membranes during glucose repression in Saccharomyces cerevisiae. Arch Biochem Biophys. 1984 Nov 15;235(1):159–166. doi: 10.1016/0003-9861(84)90264-9. [DOI] [PubMed] [Google Scholar]
- Grossman S., Cobley J., Hogue P. K., Kearney E. B., Singer T. P. Relation of phospholipase D activity to the decay of succinate dehydrogenase and of covalently bound flavin in yeast cells undergoing glucose repression. Arch Biochem Biophys. 1973 Oct;158(2):744–753. doi: 10.1016/0003-9861(73)90569-9. [DOI] [PubMed] [Google Scholar]
- HALPERN C., MILLER J. J. The metabolism of yeast sporulation. I. Effect of certain metabolites and inhibitors. Can J Microbiol. 1956 Oct;2(6):519–537. doi: 10.1139/m56-064. [DOI] [PubMed] [Google Scholar]
- Heller M. Phospholipase D. Adv Lipid Res. 1978;16:267–326. doi: 10.1016/b978-0-12-024916-9.50011-1. [DOI] [PubMed] [Google Scholar]
- Howe L. R., Marshall C. J. Lysophosphatidic acid stimulates mitogen-activated protein kinase activation via a G-protein-coupled pathway requiring p21ras and p74raf-1. J Biol Chem. 1993 Oct 5;268(28):20717–20720. [PubMed] [Google Scholar]
- Jamal Z., Martin A., Gomez-Muñoz A., Brindley D. N. Plasma membrane fractions from rat liver contain a phosphatidate phosphohydrolase distinct from that in the endoplasmic reticulum and cytosol. J Biol Chem. 1991 Feb 15;266(5):2988–2996. [PubMed] [Google Scholar]
- Jones G. A., Carpenter G. The regulation of phospholipase C-gamma 1 by phosphatidic acid. Assessment of kinetic parameters. J Biol Chem. 1993 Oct 5;268(28):20845–20850. [PubMed] [Google Scholar]
- Kahn R. A., Yucel J. K., Malhotra V. ARF signaling: a potential role for phospholipase D in membrane traffic. Cell. 1993 Dec 17;75(6):1045–1048. doi: 10.1016/0092-8674(93)90314-g. [DOI] [PubMed] [Google Scholar]
- Kanfer J. N. The base exchange enzymes and phospholipase D of mammalian tissue. Can J Biochem. 1980 Dec;58(12):1370–1380. doi: 10.1139/o80-186. [DOI] [PubMed] [Google Scholar]
- Lange-Carter C. A., Pleiman C. M., Gardner A. M., Blumer K. J., Johnson G. L. A divergence in the MAP kinase regulatory network defined by MEK kinase and Raf. Science. 1993 Apr 16;260(5106):315–319. doi: 10.1126/science.8385802. [DOI] [PubMed] [Google Scholar]
- Levin D. E., Fields F. O., Kunisawa R., Bishop J. M., Thorner J. A candidate protein kinase C gene, PKC1, is required for the S. cerevisiae cell cycle. Cell. 1990 Jul 27;62(2):213–224. doi: 10.1016/0092-8674(90)90360-q. [DOI] [PubMed] [Google Scholar]
- Malcolm K. C., Ross A. H., Qiu R. G., Symons M., Exton J. H. Activation of rat liver phospholipase D by the small GTP-binding protein RhoA. J Biol Chem. 1994 Oct 21;269(42):25951–25954. [PubMed] [Google Scholar]
- Matsumoto K., Uno I., Ishikawa T. Initiation of meiosis in yeast mutants defective in adenylate cyclase and cyclic AMP-dependent protein kinase. Cell. 1983 Feb;32(2):417–423. doi: 10.1016/0092-8674(83)90461-0. [DOI] [PubMed] [Google Scholar]
- Morlock K. R., McLaughlin J. J., Lin Y. P., Carman G. M. Phosphatidate phosphatase from Saccharomyces cerevisiae. Isolation of 45- and 104-kDa forms of the enzyme that are differentially regulated by inositol. J Biol Chem. 1991 Feb 25;266(6):3586–3593. [PubMed] [Google Scholar]
- Olson S. C., Bowman E. P., Lambeth J. D. Phospholipase D activation in a cell-free system from human neutrophils by phorbol 12-myristate 13-acetate and guanosine 5'-O-(3-thiotriphosphate). Activation is calcium dependent and requires protein factors in both the plasma membrane and cytosol. J Biol Chem. 1991 Sep 15;266(26):17236–17242. [PubMed] [Google Scholar]
- Payne W. E., Fitzgerald-Hayes M. A mutation in PLC1, a candidate phosphoinositide-specific phospholipase C gene from Saccharomyces cerevisiae, causes aberrant mitotic chromosome segregation. Mol Cell Biol. 1993 Jul;13(7):4351–4364. doi: 10.1128/mcb.13.7.4351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan J. J., Nickels J. T., Jr, Wu W. I., Lin Y. P., Broach J. R., Carman G. M. The 45- and 104-kDa forms of phosphatidate phosphatase from Saccharomyces cerevisiae are regulated differentially by phosphorylation via cAMP-dependent protein kinase. J Biol Chem. 1992 Sep 5;267(25):18013–18020. [PubMed] [Google Scholar]
- Randazzo P. A., Kahn R. A. GTP hydrolysis by ADP-ribosylation factor is dependent on both an ADP-ribosylation factor GTPase-activating protein and acid phospholipids. J Biol Chem. 1994 Apr 8;269(14):10758–10763. [PubMed] [Google Scholar]
- Sherman F. Getting started with yeast. Methods Enzymol. 1991;194:3–21. doi: 10.1016/0076-6879(91)94004-v. [DOI] [PubMed] [Google Scholar]
- Simchen G., Piñon R., Salts Y. Sporulation in Saccharomyces cerevisiae: premeiotic DNA synthesis, readiness and commitment. Exp Cell Res. 1972 Nov;75(1):207–218. doi: 10.1016/0014-4827(72)90538-1. [DOI] [PubMed] [Google Scholar]
- Smith H. E., Driscoll S. E., Sia R. A., Yuan H. E., Mitchell A. P. Genetic evidence for transcriptional activation by the yeast IME1 gene product. Genetics. 1993 Apr;133(4):775–784. doi: 10.1093/genetics/133.4.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J., Foster D. A. v-Src activates a unique phospholipase D activity that can be distinguished from the phospholipase D activity activated by phorbol esters. Biochem J. 1993 Sep 15;294(Pt 3):711–717. doi: 10.1042/bj2940711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stearns T., Kahn R. A., Botstein D., Hoyt M. A. ADP ribosylation factor is an essential protein in Saccharomyces cerevisiae and is encoded by two genes. Mol Cell Biol. 1990 Dec;10(12):6690–6699. doi: 10.1128/mcb.10.12.6690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatchell K. RAS genes and growth control in Saccharomyces cerevisiae. J Bacteriol. 1986 May;166(2):364–367. doi: 10.1128/jb.166.2.364-367.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P., Anthes J. C., Siegel M. I., Egan R. W., Billah M. M. Existence of cytosolic phospholipase D. Identification and comparison with membrane-bound enzyme. J Biol Chem. 1991 Aug 15;266(23):14877–14880. [PubMed] [Google Scholar]
- Wang X., Xu L., Zheng L. Cloning and expression of phosphatidylcholine-hydrolyzing phospholipase D from Ricinus communis L. J Biol Chem. 1994 Aug 12;269(32):20312–20317. [PubMed] [Google Scholar]
- Wu W. I., Lin Y. P., Wang E., Merrill A. H., Jr, Carman G. M. Regulation of phosphatidate phosphatase activity from the yeast Saccharomyces cerevisiae by sphingoid bases. J Biol Chem. 1993 Jul 5;268(19):13830–13837. [PubMed] [Google Scholar]
- Zeisel S. H. Choline phospholipids: signal transduction and carcinogenesis. FASEB J. 1993 Apr 1;7(6):551–557. doi: 10.1096/fasebj.7.6.8472893. [DOI] [PubMed] [Google Scholar]
- van Blitterswijk W. J., Hilkmann H. Rapid attenuation of receptor-induced diacylglycerol and phosphatidic acid by phospholipase D-mediated transphosphatidylation: formation of bisphosphatidic acid. EMBO J. 1993 Jul;12(7):2655–2662. doi: 10.1002/j.1460-2075.1993.tb05926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]