Abstract
Introduction
There is a lack of evidence to support the effectiveness of prolonged β-blocker therapy after stabilisation of patients with acute myocardial infarction (AMI) without heart failure (HF) or left ventricular systolic dysfunction.
Methods and analysis
The SMart Angioplasty Research Team: DEcision on Medical Therapy in Patients with Coronary Artery DIsease or Structural Heart Disease Undergoing InterventiON (SMART-DECISION) trial is a multicentre, prospective, open-label, randomised, non-inferiority trial designed to determine whether discontinuing β-blocker therapy after ≥1 year of maintenance in stabilised patients after AMI is non-inferior to continuing it. Patients eligible for participation are those without HF or left ventricular systolic dysfunction (ejection fraction >40%) who have been continuing β-blocker therapy for ≥1 year after AMI. A total of 2540 patients will be randomised 1:1 to continuation of β-blocker therapy or not. Randomisation will be stratified according to the type of AMI (ie, ST-segment-elevation MI or non-ST-segment-elevation MI), type of β-blocker (carvedilol, bisoprolol, nebivolol or other) and participating centre. The primary study endpoint is a composite of all-cause death, MI and hospitalisation for HF over a median follow-up period of 3.5 years (minimum, 2.5 years; maximum, 4.5 years). Adverse effects related to β-blocker therapy, the occurrence of atrial fibrillation, medical costs and Patient-reported Outcomes Measurement Information system-29 questionnaire responses will also be collected as secondary endpoints.
Ethics and dissemination
Ethics approval for this study was granted by the Institutional Review Board of Samsung Medical Center (no. 2020-10-176). Informed consent is obtained from every participant before randomisation. The results of this study will be submitted for publication in international peer-reviewed journals and the key findings will be presented at international scientific conferences.
Trial registration number
ClinicalTrials.gov, NCT04769362.
Keywords: myocardial infarction, clinical trial, patient reported outcome measures, heart failure
Strengths and limitations of this study.
This is a new concept of study to demonstrate the efficacy and safety of β-blocker discontinuation for patients without heart failure or left ventricular systolic dysfunction after acute myocardial infarction.
Detailed secondary endpoints including cost-effectiveness analysis and patient-reported outcome will provide the impact of β-blocker discontinuation for healthcare costs and the patient’s change of symptoms.
The potential for bias in event detection cannot be dismissed due to the open-label trial design.
One of the limitations is the enrolment of patients from a single ethnic background.
Introduction
β-blockers are a crucial part of secondary prevention after acute myocardial infarction (AMI) to attenuate sympathetic activation, thereby reducing myocardial oxygen consumption, preventing fatal cardiac arrhythmias and limiting adverse cardiac remodelling.1 The clinical benefits of using β-blockers in patients with reduced left ventricular ejection fraction (LVEF) after the development of AMI are well-established.2,4 However, evidence of the therapeutic effects of β-blockers in patients with AMI without heart failure (HF) or LV systolic dysfunction is relatively sparse.5 More importantly, most existing evidence for the benefits of β-blockers in patients with AMI was generated when primary percutaneous coronary intervention was not the standard of care for AMI. Given that appropriate revascularisation improves prognosis by increasing myocardial salvage, reducing infarct size and decreasing the risk of arrhythmia,6 7 the role of β-blockers in AMI needs to be redefined in contemporary practice.
Although the current guidelines recommend the long-term use of β-blockers in all patients with AMI without contraindications to β-blocker therapy,8 9 the optimal duration of β-blocker treatment after stabilised AMI without reduced LVEF or HF remains uncertain. Theoretically, discontinuing β-blockers in survivors of AMI without a reduced LVEF or HF may prevent unnecessary overtreatment, save medical costs, limit potential side effects and improve quality of life or adherence to other medications. In this regard, some observational studies have been conducted to evaluate the long-term maintenance effects of β-blockers beyond 1 year after AMI, but the non-randomised nature of such studies limits their conclusions due to the presence of selection bias.10,13 Therefore, the SMart Angioplasty Research Team: DEcision on Medical Therapy in Patients with Coronary Artery DIsease or Structural Heart Disease Undergoing InterventiON (SMART-DECISION) trial will investigate whether the discontinuation of β-blocker therapy after ≥1 year of β-blocker therapy is non-inferior to the continuation of β-blocker therapy in patients without HF or LV systolic dysfunction after AMI.
Methods and analysis
Trial design, objectives and hypothesis
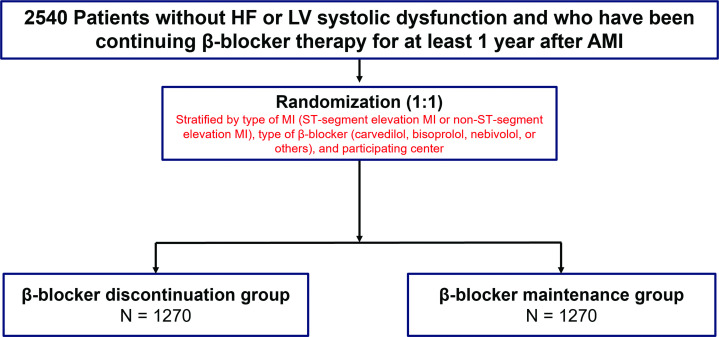
The SMART-DECISION trial is a prospective, randomised, open, multicentre, non-inferiority study that will be conducted by 25 centres in the Republic of Korea. A study overview is presented in figure 1. The primary objective of this trial will be to determine whether the discontinuation of β-blocker therapy after ≥1 year of β-blocker treatment is non-inferior to the continuation of these medications in patients without HF or LV systolic dysfunction after AMI. We hypothesised that discontinuing β-blocker therapy after AMI stabilisation would be non-inferior to continuing β-blocker therapy in terms of a composite of all-cause death, MI or hospitalisation for HF in patients with AMI without HF or LV systolic dysfunction. We will also assess whether the discontinuation of β-blocker therapy after ≥1 year of β-blocker therapy influences quality of life, the occurrence of new atrial fibrillation and medical costs, respectively.
Figure 1. Study flowchart. AMI, acute myocardial infarction; HF, heart failure; LV, left ventricular.
Study population
Patients with stabilised AMI who have been continuing β-blocker therapy for ≥1 year will be screened and invited to participate in this trial. The definition of AMI used in this trial has been adapted from the Fourth Universal Definition of MI.14 A full list of inclusion and exclusion criteria is provided in box 1. In brief, consenting adult patients (≥19 years old) without HF or LV systolic dysfunction who have continued with β-blocker therapy for ≥1 year after the development of AMI are eligible for participation in the study. Patients will be excluded if they have a reduced LVEF (<40%), sustained treatment for HF, contraindication to β-blocker therapy or history of atrial fibrillation.
Box 1. Study inclusion and exclusion criteria.
Inclusion criteria
Subject is ≥19 years of age.
Subject has continued β-blocker therapy for ≥1 year after acute myocardial infarction regardless of the time of diagnosis.
Subject can verbally confirm their understanding of the risks and benefits of this trial, and they or their legally authorised representative has provided written informed consent prior to any study-related procedure.
Exclusion criteria
Subject has a left ventricle ejection fraction of <40% according to echocardiography performed after acute myocardial infarction or they never received echocardiography.
Subject is continuing treatment for heart failure.
Subject has a contraindication to β-blocker therapy (eg, history of uncontrolled bronchial asthma or chronic obstructive pulmonary disease, second-degree or third-degree atrioventricular block, cardiac pacemaker implantation or another case where β-blocker therapy cannot be used per the judgement of the clinician).
Subject has a non-cardiac comorbid condition with a life expectancy of <1 year or that may result in protocol non-compliance (per site investigator’s medical judgement).
Subject has a history of atrial fibrillation.
Subject is pregnant or breastfeeding.
Randomisation and interventions in allocated arms
Eligible patients who agreed to participate in this study by signing the informed consent form will be randomised to either the β-blocker discontinuation group or the β-blocker maintenance group at the time of enrolment in a 1:1 ratio using a web-based case report form in the Clinical Data Management System from the Korea National Institute of Health. Randomisation will be stratified according to the type of MI (ST-segment-elevation MI or non-ST-segment-elevation MI), the type of β-blocker (carvedilol, bisoprolol, nebivolol or other) and the participating centre. According to randomisation, the β-blocker discontinuation group will stop β-blocker treatment, while the β-blocker maintenance group will continue with β-blocker treatment.
Study endpoints
The primary endpoint of this study is a composite of all-cause death, MI or hospitalisation for HF, over the total individual follow-up period. Secondary endpoints will include individual components of the primary endpoint; cardiac death; any hospitalisation; hospitalisation specifically for acute coronary syndrome; all-cause death or MI; cardiac death or MI; MI or hospitalisation for HF; any revascularisation; MI or any revascularisation; a composite of cardiac death, MI or hospitalisation for HF; a composite of cardiac death, MI or any revascularisation; changes in LVEF; changes in N-terminal pro-brain natriuretic peptide level; the occurrence of new atrial fibrillation; medical cost; Patient-reported Outcome Measurement Information System-29 Profile (PROMIS-29) questionnaire results; and adverse effects related to β-blocker therapy.
All deaths will be considered cardiac in nature unless an undisputed non-cardiac cause can be identified. For this study, MI will be defined by elevated cardiac enzymes (troponin or myocardial band fraction of creatine kinase) above the upper reference limit with ischaemic symptoms or electrocardiography findings indicative of ischaemia. HF hospitalisation will be defined by admission for ≥24 hours with a primary diagnosis of HF with at least one symptom of HF; at least two physical examinations, laboratory or invasive findings of HF; and the current status of receiving an HF-specific treatment.15 Revascularisation will be considered clinically driven if the diameter stenosis of the revascularised coronary segment is ≥50% by quantitative coronary angiography and any of the following criteria for ischaemia are met: (1) a positive functional study; (2) ischaemic electrocardiography changes at rest; (3) typical ischaemic symptoms; (4) positive invasive physiological test (fractional flow reserve ≤0.80 or instantaneous wave-free ratio ≤0.89); or (5) presence of stenosis with ≥70% diameter stenosis, even in the absence of other criteria. Patient-reported outcomes will be collected at baseline prior to randomisation; after 6, 12 and 24 months; and at the end of follow-up through the PROMIS-29 questionnaire.16
Data collection and follow-up
An overview of the data-collection process during screening, enrolment and treatment follow-up to the study end is presented in table 1. All patients will be surveyed at 6, 12, 24 and 30 months, then yearly thereafter. The follow-up duration of the study will be ≥2.5 years after the last patient enrolment (the expected median follow-up will be 3.5 years, assuming a constant inclusion rate).
Table 1. Schedule of measurements.
| Visit | Screening and baseline | Follow-up | ||||
| 6 months ±90 days | 12 months ±90 days | 24 months ±90 days | 30 months ±90 days and annually thereafter | End of treatment ±90 days | ||
| Medical history | ο | |||||
| Inclusion and exclusion criteria | ο | |||||
| Informed consent | ο | |||||
| Weight, height | ο | |||||
| Echocardiography | ο | ο | ο | |||
| Randomisation | ο | |||||
| Angiographic and procedural data | ο | |||||
| Vital status | ο | ο | ο | ο | ο | o |
| Laboratory measurement | ο | ο | ο | |||
| ECG | ο | ο | ο | ο | ο | ο |
| Prescription and adherence to β-blocker therapy | ο | ο | ο | ο | ο | ο |
| Other medications | ο | ο | ο | ο | ο | ο |
| Complications and adverse events | ο | ο | ο | ο | ο | ο |
| Endpoint events | ο | ο | ο | ο | ο | |
| Medical cost | ο | ο | ο | ο | ο | |
| PROMIS-29 | ο | ο | ο | ο | ||
PROMIS-29Patient-reported Outcome Measurement Information System 29 Profile
Sample size calculations
Originally, we assumed that the recruitment of study patients would occur at a constant rate over 3 years and that study patients would be followed-up with for an additional 2 years after the recruitment of the last patient (median follow-up of 3.5 years; range, 2–5 years). However, the enrolment rate has already exceeded our initial expectations (actual accrual period of 2 years), prompting us to perform a revised sample size calculation. To maintain a median follow-up duration of 3.5 years (range, 2.5–4.5 years), we have decided to extend the follow-up period to 2.5 years after the recruitment of the last patient. According to data from previous Korean nationwide observational studies, the annual incidence of the primary endpoint is estimated to be 3% in the β-blocker maintenance group.12 The non-inferiority margin of the HR selected was 1.4. With a sampling ratio of 1:1, we estimated that 2540 patients (1270 per group) would result in ≥80% power at a one-sided type I error of 2.5% and a 2% attrition rate.
Statistical analyses
All primary and secondary endpoints will be analysed on an intention-to-treat basis. No imputation methods will be used to infer missing values of baseline variables. Continuous variables will be presented using mean±SD values and compared using the independent t-test or the Wilcoxon rank-sum test, as appropriate. Categorical variables will be presented as counts and percentages and compared with the χ2 or Fisher’s exact test as appropriate. Cumulative event rates will be estimated with the Kaplan-Meier method and compared using log-rank tests. HRs with 95% CIs will be estimated with the Cox proportional hazards method. If the upper limit of the one-sided 97.5% CI of the HR of the discontinuation group is less than the prespecified non-inferiority margin, discontinuation of β-blocker therapy will be considered non-inferior to β-blocker therapy continuation. All models will be adjusted for the stratification factors (type of MI, type of β-blocker and participating centre). Per-protocol analysis among patients who adhere to the study protocol will be performed as a sensitivity analysis. For per-protocol analyses, participants will be censored if they deviate from their original treatment plan (whether or not to use β-blockers) without any clinical reason, as defined by Hernan and Robins.17 For the secondary outcomes, such as LVEF, N-terminal pro-brain natriuretic peptide level and quality of life per PROMIS-29, we will use linear mixed-effect models including main effects for visits (indicator terms for each visit) and visit—group interaction terms to assess differences in outcome alterations between the intervention and control groups. Random intercepts will be included to allow for variations in outcomes across study participants at baseline. Any cost-effective analysis performed will follow the Consolidated Health Economic Evaluation Reporting Standards guidelines.18 The outcome of the cost-effectiveness analysis will be expressed as the difference in quality-adjusted life years (QALYs) between the two strategies. QALYs represent a patient’s survival time weighted by the quality of life, represented by a utility weight. The cost-effectiveness of discontinuation of β-blocker therapy will be expressed as the incremental cost-effectiveness ratio, defined as the difference in the cumulative costs divided by the difference in cumulative QALYs.
Prespecified subgroup analysis
A set of 13 prespecified subgroup analyses will be performed using interaction models based on the primary endpoint. Detailed prespecified subgroups are listed in table 2. Estimates will be presented as HRs for each subgroup alongside p values for interaction.
Table 2. Prespecified subgroups.
| Prespecified subgroup | |
| Age | ≥65 vs <65 years |
| Sex | Male vs female |
| Body mass index | ≥25 vs <25 kg/m2 |
| Diabetes mellitus | Presence vs absence |
| Hypertension | Presence vs absence |
| Chronic kidney disease* | Presence vs absence |
| LVEF | 41–49% vs ≥50% |
| Systolic blood pressure | ≥140 vs <140 mm Hg |
| Heart rate | ≥60 vs <60 beats/min |
| Type of MI | STEMI vs NSTEMI |
| Type of β-blocker | Carvedilol vs bisoprolol vs nebivolol vs other |
| Revascularisation strategy | PCI vs CABG |
| Time from index MI to randomisation | 1–2 vs 2–3 vs ≥3 years |
Chronic kidney disease was defined by estimated glomerular filtration rate <60 mL/min/1.73 m2.
CABG, coronary artery bypass grafting; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NSTEMI, non-ST-segment-elevation MI; PCI, percutaneous coronary intervention; STEMI, ST-segment-elevation MI
Patient and public involvement
There was no patient or public involvement in this trial.
Study organisation
Steering committee and Data Safety Monitoring Board
The steering committee, composed of this study’s chairperson and the principal investigators of the main investigating centres, has approved the study design, protocol and amendments issued to the Data Safety Monitoring Board (DSMB) and the participating centres. An independent DSMB will review the safety data from the study and construct recommendations for adverse events/serious adverse events, protocol deviation and follow-up case reports. No interim analysis is planned, but scheduled DSMB meetings will include discussions of safety or compliance issues and generate advice on modifying or stopping the study as needed. However, the final decisions regarding changes in the study protocol will remain in the hands of the steering committee. In addition, the DSMB will help to conduct the trial appropriately by reviewing and reporting the cumulative investigational data for accuracy and completeness, ensuring protocol compliance. The DSMB will develop a consensus understanding of all trial endpoints and definitions used in the event-adjudication process.
Clinical event adjudication committee
The study clinical event adjudication committee (CEAC) is composed of interventional cardiologists who are not participants in the study. This committee is charged with the development of specific criteria based on protocol that will be used for the categorisation of clinical events and clinical endpoints in the study. At the onset of the trial, the CEAC established explicit rules outlining the minimum amount of data required as well as the algorithm to be followed to classify a clinical event. All members of the CEAC will be blinded to the primary results of the trial.
Ethical and dissemination
The trial protocol has been approved by the relevant ethics committees (institutional review board no. 2020-10-176) and registered with ClinicalTrials.gov and the Clinical Research Information Service in Korea (KCT0005903). The current protocol V.4.3, dated 19 February 2024. Written informed consent for participation in the study will be obtained from all participating patients. The results of this study will be submitted for publication in international peer-reviewed journals and the key findings will be presented at international scientific conferences.
Trial status and timeline
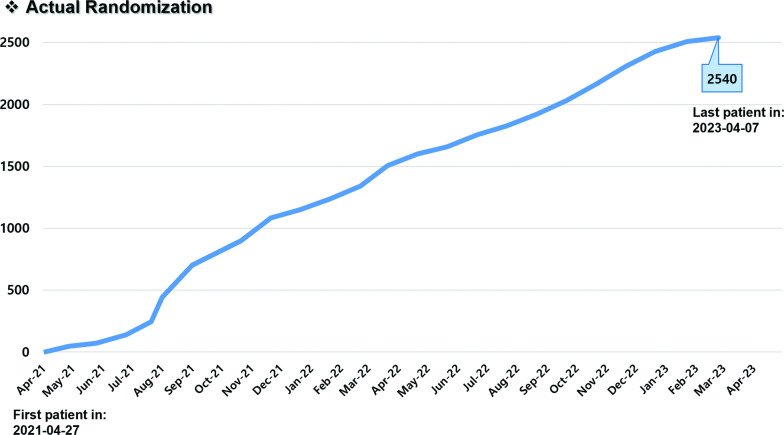
The first patient was randomised on 27 April 2021, and the last patient (no. 2540) was randomised on 7 April 2023 (figure 2). The follow-up period is planned to last for ≥2.5 years after the last patient enrolment; therefore, it will end in October 2025.
Figure 2. Enrolment curve over the 24 months of the study accrual period.
Discussion
The SMART-DECISION trial aims to evaluate the efficacy and safety of β-blocker discontinuation after ≥1 year of β-blocker therapy in stabilised patients with AMI without HF or LV systolic dysfunction. If it is proven that discontinuation of β-blockers is non-inferior to maintenance of β-blockers in this population, socioeconomic benefits could be achieved by reducing unnecessary drug use and patient quality of life could be improved by achieving freedom from β-blocker side effects. If the study does not demonstrate non-inferiority for the primary endpoint in the β-blocker discontinuation group, this will strengthen the recommendation for long-term maintenance of β-blockers, even in stabilised patients with AMI without HF or LV systolic dysfunction.
β-blockers have been widely used and recommended after AMI on the basis of pivotal trials conducted in the pre-reperfusion era.19,21 However, clinical practice patterns have changed significantly in the current era given the introduction of high-sensitivity troponin for faster diagnosis of AMI; timely revascularisation strategies of occluded coronary arteries; and improved medical therapies for secondary prevention, such as high-intensity statins.22,24 In addition, unlike the abundant evidence for the benefits of β-blockers in patients with AMI with reduced LVEF, there has been a lack of data for the treatment effect of β-blockers in those without LV systolic dysfunction. The Carvedilol Post-intervention Long-term Administration in Large-scale Randomised Controlled Trial (CAPITAL-RCT) investigated the long-term efficacy of β-blockers in 801 patients with AMI with LVEF ≥40% and found no beneficial effects of β-blockers during a median follow-up of 3.9 years.5 However, this trial was prematurely terminated and underpowered to identify a difference in the treatment effect concerning adverse cardiac events. In this regard, several large-sized randomised trials are currently ongoing to test the effects of long-term treatment with β-blockers in patients with AMI without reduced LVEF, including BEtablocker Treatment After acute Myocardial Infarction in revascularised patients without reduced LVEF (BETAMI) (NCT03646357), the Danish trial of β-blocker treatment after myocardial infarction without reduced ejection fraction (DANBLOCK) (NCT03778554), TREatment With Beta-blockers After myOcardial Infarction withOut Reduced Ejection fraction (REBOOT) (NCT03596385) and a randomised evaluation of decreased usage of β-blockers after acute myocardial infarction (REDUCE-AMI) (NCT03278509).25,29 The results of these trials will provide robust evidence to guide early-phase prescription of β-blockers to patients discharged after AMI without reduced LVEF.
However, a clinically important but difficult decision about β-blocker therapy after AMI not covered by the above four trials concerns the duration of β-blocker therapy in patients without HF or LV systolic dysfunction. Using data from a nationwide prospective French registry, Puymirat et al previously reported that early use of β-blockers was associated with reduced 30-day mortality, but discontinuation of β-blockers at 1-year was not associated with higher 5-year mortality in patients with AMI without HF or LV systolic dysfunction.11 This result might suggest a progressively decreasing benefit of β-blocker treatment over time. On the other hand, retrospective nationwide cohort studies from France and Korea have identified that discontinuation of β-blockers beyond 1-year after AMI was associated with an increased risk of death or recurrent MI in stabilised patients without HF.12 13 Therefore, we are conducting the SMART-DECISION trial to confirm the safety of β-blocker discontinuation after ≥1 year of β-blocker therapy in stabilised patients with AMI without HF or LV systolic dysfunction. The Assessment of β-blocker interruption 1 Year after an uncomplicated myocardial infarction on Safety and Symptomatic cardiac events requiring hospitalisation (AβYSS) (NCT03498066) trial is also currently ongoing with a concept similar to that of our study.30 These two trials will be helpful in determining the optimal duration of β-blocker therapy after post-MI patients without HF or LV systolic dysfunction are stabilised.
This trial has an open-label design with no placebo treatment; however, this is expected to have a limited impact on the hard outcomes of the primary composite endpoint, and t a blinded adjudication of the endpoints by an independent CEAC may overcome the potential for overestimation of the treatment effects. Patients with ST-segment-elevation or non-ST-segment-elevation MI are included in this trial despite the expectation of some differences in the pathophysiology and prognosis between them. However, stratified randomisation is planned to minimise the potential selection bias. Furthermore, the time from index MI to randomisation is not the same for all patients. However, to evaluate the efficacy and safety of β-blocker discontinuation according to the differential period from the index MI, a prespecified subgroup analysis will be performed among patients randomised at 1–2 years from index MI versus 2–3 years versus ≥3 years.
SMART-DECISION is a non-inferiority trial to test the efficacy and safety of β-blocker therapy discontinuation in stabilised patients with AMI without HF or LV systolic dysfunction. Differences in the risk for a composite of all-cause death, MI or hospitalisation for HF will be assessed over a median follow-up period of 3.5 years. The results of the SMART-DECISION trial will add important scientific evidence about the optimal duration of β-blocker therapy after stabilisation of AMI without HF or LV systolic dysfunction.
Footnotes
Funding: This research was supported by a grant from the Patient-centered Clinical Research Coordinating Center (PACEN) funded by the Ministry of Health & Welfare, Republic of Korea (grant no. RS-2020-KH096065).
Prepub: Prepublication history for this paper is available online. To view these files, please visit the journal online (https://doi.org/10.1136/bmjopen-2024-086971 ).
Patient consent for publication: Consent obtained directly from patient(s).
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Collaborators: Cheol Whan Lee (DSMB chair), Sun Woo Kim (DSMB member), Hyun-Jong Lee (DSMB member), Kyeong Ho Yun (CEAC chair), Woo Jung Chun (CEAC member), Hyun Sung Joh (CEAC member)
Contributor Information
Ki Hong Choi, Email: cardiokh@gmail.com.
Juwon Kim, Email: abcd186a@naver.com.
Danbee Kang, Email: dbee.kang@skku.edu.
Joon-Hyung Doh, Email: joon.doh@gmail.com.
Juhan Kim, Email: kim@juhan.com.
Yong Hwan Park, Email: hippomac@hanmail.net.
Sung Gyun Ahn, Email: sgahn@yonsei.ac.kr.
Weon Kim, Email: mylovekw@hanmail.net.
Jong Pil Park, Email: jppark74@gmail.com.
Sang Min Kim, Email: masuri75@hanmail.net.
Byung-Ryul Cho, Email: heartcho@kangwon.ac.kr.
Chang-Wook Nam, Email: ncwcv@dsmc.or.kr.
Jang Hyun Cho, Email: goodnew8@naver.com.
Seung-Jae Joo, Email: sejjoo@jejunu.ac.kr.
Jon Suh, Email: sirjon@daum.net.
Jin-Ok Jeong, Email: jojeong@cnu.ac.kr.
Woo Jang, Email: wj78914@gmail.com.
Chang-Hwan Yoon, Email: hippsons@gmail.com.
Jin-Yong Hwang, Email: jyhwang@gnu.ac.kr.
Seong-Hoon Lim, Email: shlimd@gmail.com.
Sang-Rok Lee, Email: medorche@jbnu.ac.kr.
Eun-Seok Shin, Email: sesim1989@gmail.com.
Byung Jin Kim, Email: bjjake.kim@samsung.com.
Cheol Woong Yu, Email: ycw717@naver.com.
Sung-Ho Her, Email: hhhsungho@hanmail.net.
Hyun Kuk Kim, Email: sale38@hanmail.net.
Kyu Tae Park, Email: jamems99@hanmail.net.
Jihoon Kim, Email: novolley@naver.com.
Taek Kyu Park, Email: taekkyu.park@samsung.com.
Joo-Myung Lee, Email: drone80@hanmail.net.
Juhee Cho, Email: jcho@skku.edu.
Jeong Hoon Yang, Email: jhysmc@gmail.com.
Young Bin Song, Email: youngbin.song@gmail.com.
Seung Hyuk Choi, Email: sh1214.choi@samsung.com.
Hyeon-Cheol Gwon, Email: hcgwon@naver.com.
Eliseo Guallar, Email: eguallar@jhu.edu.
Joo-Yong Hahn, Email: jyhahn@skku.edu.
References
- 1.Pepper GS, Lee RW. Sympathetic activation in heart failure and its treatment with beta-blockade. Arch Intern Med. 1999;159:225–34. doi: 10.1001/archinte.159.3.225. [DOI] [PubMed] [Google Scholar]
- 2.Dargie HJ. Effect of carvedilol on outcome after myocardial infarction in patients with left-ventricular dysfunction: the CAPRICORN randomised trial. Lancet. 2001;357:1385–90. doi: 10.1016/s0140-6736(00)04560-8. [DOI] [PubMed] [Google Scholar]
- 3.Flather MD, Shibata MC, Coats AJS, et al. Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS) Eur Heart J. 2005;26:215–25. doi: 10.1093/eurheartj/ehi115. [DOI] [PubMed] [Google Scholar]
- 4.The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. The Lancet. 1999;353:9–13. doi: 10.1016/S0140-6736(98)11181-9. [DOI] [PubMed] [Google Scholar]
- 5.Watanabe H, Ozasa N, Morimoto T, et al. Long-term use of carvedilol in patients with ST-segment elevation myocardial infarction treated with primary percutaneous coronary intervention. PLoS One. 2018;13:e0199347. doi: 10.1371/journal.pone.0199347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huynh T, Perron S, O’Loughlin J, et al. Comparison of primary percutaneous coronary intervention and fibrinolytic therapy in ST-segment-elevation myocardial infarction: bayesian hierarchical meta-analyses of randomized controlled trials and observational studies. Circulation. 2009;119:3101–9. doi: 10.1161/CIRCULATIONAHA.108.793745. [DOI] [PubMed] [Google Scholar]
- 7.Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet. 2003;361:13–20. doi: 10.1016/S0140-6736(03)12113-7. [DOI] [PubMed] [Google Scholar]
- 8.Byrne RA, Rossello X, Coughlan JJ, et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur Heart J. 2023;44:3720–826. doi: 10.1093/eurheartj/ehad191. [DOI] [PubMed] [Google Scholar]
- 9.Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC guideline for the management of patients with Non-ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64:e139–228. doi: 10.1016/j.jacc.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 10.Dondo TB, Hall M, West RM, et al. β-Blockers and mortality after acute myocardial infarction in patients without heart failure or ventricular dysfunction. J Am Coll Cardiol. 2017;69:2710–20. doi: 10.1016/j.jacc.2017.03.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Puymirat E, Riant E, Aissaoui N, et al. β blockers and mortality after myocardial infarction in patients without heart failure: multicentre prospective cohort study. BMJ. 2016;354:i4801. doi: 10.1136/bmj.i4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim J, Kang D, Park H, et al. Long-term β-blocker therapy and clinical outcomes after acute myocardial infarction in patients without heart failure: nationwide cohort study. Eur Heart J. 2020;41:3521–9. doi: 10.1093/eurheartj/ehaa376. [DOI] [PubMed] [Google Scholar]
- 13.Neumann A, Maura G, Weill A, et al. Clinical events after discontinuation of β-blockers in patients without heart failure optimally treated after acute myocardial infarction: A cohort study on the French healthcare databases. Circ Cardiovasc Qual Outcomes. 2018;11:e004356. doi: 10.1161/CIRCOUTCOMES.117.004356. [DOI] [PubMed] [Google Scholar]
- 14.Thygesen K, Alpert JS, Jaffe AS, et al. Fourth universal definition of myocardial infarction (2018) Circulation. 2018;138:e618–51. doi: 10.1161/CIR.0000000000000617. [DOI] [PubMed] [Google Scholar]
- 15.Abraham WT, Psotka MA, Fiuzat M, et al. Standardized definitions for evaluation of heart failure therapies: scientific expert panel from the heart failure collaboratory and academic research consortium. JACC Heart Fail. 2020;8:961–72. doi: 10.1016/j.jchf.2020.10.002. [DOI] [PubMed] [Google Scholar]
- 16.Craig BM, Reeve BB, Brown PM, et al. US valuation of health outcomes measured using the PROMIS-29. Val Health. 2014;17:846–53. doi: 10.1016/j.jval.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hernán MA, Robins JM. Per-protocol analyses of pragmatic trials. N Engl J Med. 2017;377:1391–8. doi: 10.1056/NEJMsm1605385. [DOI] [PubMed] [Google Scholar]
- 18.Husereau D, Drummond M, Petrou S, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS)-explanation and elaboration: a report of the ISPOR health economic evaluation publication guidelines good reporting practices task force. Val Health. 2013;16:231–50. doi: 10.1016/j.jval.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 19.Hjalmarson A, Elmfeldt D, Herlitz J, et al. Effect on mortality of metoprolol in acute myocardial infarction. A double-blind randomised trial. Lancet. 1981;2:823–7. doi: 10.1016/s0140-6736(81)91101-6. [DOI] [PubMed] [Google Scholar]
- 20.A randomized trial of propranolol in patients with acute myocardial infarction. JAMA. 1982;247:1707. doi: 10.1001/jama.1982.03320370021023. [DOI] [PubMed] [Google Scholar]
- 21.The Norwegian Multicenter Study Group Timolol-induced reduction in mortality and reinfarction in patients surviving acute myocardial infarction. N Engl J Med. 1981;304:801–7. doi: 10.1056/NEJM198104023041401. [DOI] [PubMed] [Google Scholar]
- 22.McCarthy C, Li S, Wang TY, et al. Implementation of high-sensitivity cardiac troponin assays in the United States. J Am Coll Cardiol. 2023;81:207–19. doi: 10.1016/j.jacc.2022.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwartz GG, Olsson AG, Ezekowitz MD, et al. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: the MIRACL study: a randomized controlled trial. JAMA. 2001;285:1711–8. doi: 10.1001/jama.285.13.1711. [DOI] [PubMed] [Google Scholar]
- 24.Hochman JS, Sleeper LA, Webb JG, et al. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. N Engl J Med. 1999;341:625–34. doi: 10.1056/NEJM199908263410901. [DOI] [PubMed] [Google Scholar]
- 25.Munkhaugen J, Ruddox V, Halvorsen S, et al. BEtablocker treatment after acute myocardial infarction in revascularized patients without reduced left ventricular ejection fraction (BETAMI): Rationale and design of a prospective, randomized, open, blinded end point study. Am Heart J. 2019;208:37–46. doi: 10.1016/j.ahj.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 26.Kristensen AMD, Bovin A, Zwisler AD, et al. Design and rationale of the Danish trial of beta-blocker treatment after myocardial infarction without reduced ejection fraction: study protocol for a randomized controlled trial. Trials. 2020;21:415. doi: 10.1186/s13063-020-4214-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rossello X, Raposeiras-Roubin S, Latini R, et al. Rationale and design of the pragmatic clinical trial tREatment with Beta-blockers after myOcardial infarction withOut reduced ejection fracTion (REBOOT) Eur Heart J Cardiovasc Pharmacother. 2022;8:291–301. doi: 10.1093/ehjcvp/pvab060. [DOI] [PubMed] [Google Scholar]
- 28.Yndigegn T, Lindahl B, Alfredsson J, et al. Design and rationale of randomized evaluation of decreased usage of beta-blockers after acute myocardial infarction (REDUCE-AMI) Eur Heart J Cardiovasc Pharmacother. 2023;9:192–7. doi: 10.1093/ehjcvp/pvac070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kristensen AMD, Munkhaugen J, Halvorsen S, et al. The Danish-Norwegian randomized trial on beta-blocker therapy after myocardial infarction: Design, rationale, and baseline characteristics. Eur Heart J Cardiovasc Pharmacother. 2024;10:175–83. doi: 10.1093/ehjcvp/pvad093. [DOI] [PubMed] [Google Scholar]
- 30.Silvain J, Cayla G, Ferrari E, et al. βeta blocker interruption after uncomplicated myocardial infarction: rationale and design of the randomized ABYSS trial. Am Heart J. 2023;258:168–76. doi: 10.1016/j.ahj.2023.01.014. [DOI] [PubMed] [Google Scholar]