Abstract
Immune checkpoint inhibitors (ICI) have transformed the management of cancer, particularly for older adults, who constitute a majority of the global cancer patient population. This study aimed to assess the inclusion, characteristics, and reporting of older adults enrolled in Food and Drug Administration (FDA) registration clinical trials of ICI between 2018 and 2022. Clinical trials of ICI leading to an FDA approval in solid tumor oncology between 2018 and 2022 were included. Primary study reports and all available secondary publications were assessed. The availability and completeness of older subgroup data for protocol-defined clinical efficacy endpoints, health-related quality of life (HRQOL) and toxicity outcomes, and baseline characteristics were assessed according to predefined criteria which categorized reporting completeness hierarchically in relation to the availability of published data, including effect size, sample size, and measures of precision. 53 registration trials were included, involving a total of 37,094 participants. Most trials (64.2%) were of ICI combination therapy. 42.3% of patients were aged≥65 years; 11.1% were aged≥75. No trials specified an upper age limit for eligibility. 98.1% of trials excluded patients with European Cooperative Oncology Group performance status>1. 87.2% of primary efficacy endpoints and 17.9% of secondary efficacy endpoints were reported completely for older adults. Five studies (9.4%) reported baseline characteristics, three (6.1%) reported HRQOL assessments, and four (7.5%) reported toxicity outcomes completely among older subgroups. No trials conducted baseline geriatric assessments or reported geriatric-specific symptoms or quality of life scales. This analysis highlights significant deficits in the enrollment and reporting of older subgroups in pivotal trials of ICI therapy. The findings highlight an urgent need for improved reporting and inclusion standards in clinical trials of ICI to better inform treatment decisions for older adults.
Keywords: Immune Checkpoint Inhibitor, Immunotherapy, Geriatric
Introduction
Immune checkpoint inhibitors (ICI) have transformed the treatment landscape for many cancers, and are approved for use in as many as 44% of patients with cancer.1 Older adults (broadly defined as those aged 65 and above) already comprise a majority of patients diagnosed with cancer,2 and will likely represent an ever-greater proportion of patients receiving ICI therapy over the coming decades, in line with global aging, the rising prevalence of cancer among this age cohort,3 and the rapidly expanding number of indications for this class of therapy.
Immunosenescence4 refers to age-related changes in the immune system, including both structural and functional decline of immune organs, and is associated with increased susceptibility to infections, age-related diseases, and malignancy. Aging is associated with gradual thymic involution and atrophy, a reduction in CD8+naïve T-cell populations,5 increases in terminally differentiated memory T-cells, and impaired migration of naïve T-cells to peripheral tissues. Aging is also associated with changes in the proportion and activity of CD4+naïve T-cells, B-cells, and antigen-presenting cells including dendritic cells.4 The reduction in naïve T-cell populations reduces the immune system’s capacity to respond to novel antigens, while chronic activation of the innate immune system6 and the accumulation of autoreactive T-cells7 contributes to a state of chronic low-grade inflammation termed “inflammaging”.8 Furthermore, the loss with age of the costimulatory molecule CD28,9 which is required for complete T-cell activation and cytokine production, results in an attenuated response to vaccines and pathogens.
These factors have led to preclinical concerns that such age-related changes in the immune system may impair the ability of older patients to respond to checkpoint inhibitors.10 These concerns are corroborated by recent clinical evidence suggesting a response rate of 0% to ICI for older patients with non-small cell lung cancer with a senescent immune phenotype.11 Despite these concerns, much clinical evidence and multiple recent meta-analyses appear to suggest that older patients derive at least equal benefit from immunotherapy, relative to their younger counterparts.12,15 This discordance may be at least partially explained by the significant heterogeneity in immune profiles of “fit” older patients, who typically comprise the older population enrolled to clinical trials, relative to phenotypically frail individuals.
This uncertainty underscores the need for a greater understanding of the characteristics of older patients enrolled in major immunotherapy trials. Although prospective randomized clinical trials remain the gold standard evidence base on which treatment paradigms should be based, most data relating to toxicity and health-related quality of life (HRQOL) outcomes among older adults undergoing ICI treatment come from retrospective and observational studies, which have acknowledged bias16 and often poor internal validity.17 Recent trends towards the use of checkpoint inhibitors as part of multidrug combination treatment regimens, and in adjuvant settings, further underscore the need for greater understanding and reporting of older patients enrolled in pivotal trials, since real-world and observational data is particularly sparse for these treatment approaches.18
Older adults remain significantly under-represented and under-reported in oncology clinical trials generally,19,22 however, their enrollment and reporting have not been systematically assessed in trials of ICI. Substantial barriers to the recruitment and retention of older adults in clinical trials remain.23 The underlying causes for this are complex, but include systemic factors (such as trial design and eligibility criteria), provider concerns (including apprehension regarding toxicity, the perceived burden of study participation, and a bias towards established treatments), and both patient and caregiver factors (such as individual preference, and the perception of additional burdens associated with study participation).23 Therefore, evidence on the efficacy, safety and impacts on quality of life and aging-related health domains of immunotherapy in older individuals is often limited, and dedicated reporting of this subgroup in registration trials is especially necessary.
This paper aims to describe the inclusion, characteristics, and reporting of data relating to older adults enrolled in recent registration clinical trials of ICI.
Methods
The study methods were prospectively registered at protocols.io.24 US Food and Drug Administration (FDA) approvals (n=63) between 2018 and 2022 for checkpoint inhibitor immunotherapy (anti-programmed cell death protein-1, anti-programmed death-ligand 1 (PD-L1), anti-cytotoxic T-lymphocytes-associated protein-4, anti-lymphocyte-activation gene 3 (LAG-3) in solid tumor oncology were retrieved from FDA Drugs Approvals and Databases records.25 Approvals based on umbrella/basket studies, and approvals based on subgroups of multiple clinical trials were excluded (n=10, online supplemental S1). A total of 53 drug approvals were included, each corresponding to an individual clinical trial.
Individual trial-level PubMed searches, according to trial name, National Clinical Trial registration number, and drug name, were conducted on December 31, 2023, for all publications relating to included trials. Individual trial search terms are presented in online supplemental S2. Search results were assessed using Covidence by pairs of reviewers, according to trial of origin, for inclusion and exclusion by title and abstract, followed by full-text and reference review for included publications. Conflicts were resolved by the corresponding author (CME). Study efficacy endpoints were extracted from the most recent available study protocol or statistical analysis plan (online supplemental S3), and cross-referenced with ClinicalTrials.gov registry data and published descriptions of trial endpoints.
Primary and secondary clinical efficacy endpoints were included; exploratory endpoints, those relating exclusively to pharmacokinetics and basic science, and primary/secondary endpoints relating to toxicity, safety, and HRQOL, were excluded. HRQOL and toxicity data were examined separately.
Where individual endpoints were assessed both as primary and secondary endpoints (ie, primary endpoint: Overall survival (OS) in the intention-to-treat population; secondary endpoint: OS in the PD-L1 positive population), these endpoints were assessed separately. Conversely, where individual endpoints were assessed according to multiple definitions as a primary or a secondary endpoint (ie, secondary endpoint: Progression-free survival (PFS) at 12, 24 and 36 months), these were considered as a single endpoint, and considered assessable where data was available for any component of the aggregate definition.
Immature efficacy endpoints, and studies not reporting older subgroup HRQOL endpoints by design (ie, where such data was not measured according to the trial protocol, n=4), were excluded from relevant analyses. Interim OS data was considered unavailable if reported for the full study cohort but not for the older subgroup. Conversely, where OS data was not reported at all, it was deemed immature, and excluded from relevant analyses.
Assessment of data completeness
Reporting of data completeness was appraised according to hierarchical criteria for the assessment of subgroup reporting established by Chan et al and further developed by Mac Eochagain and Battisti.21 26 Data completeness was categorized as complete, partial, qualitative, or unreported, according to the availability of data relating to effect size and measure of precision, sample size, and includability in meta-analysis, the criteria for which differ according to the statistical characteristics of the data type (paired, unpaired, continuous, etc) (table 1).
Table 1. Efficacy endpoint assessment.
| Level of reporting | Reported data | Sufficient for inclusion in meta-analysis* |
| Complete | Number of participants per groupEffect sizePrecision or precise p value for continuous data | Yes |
| Partial | Effect size or precision (±p value+/−sample size) | No |
| Qualitative | P value±sample size | No |
| Unreported | Not available | No |
Table adapted from. Chan et al, JAMA 2004.26
See online supplemental table 1 for detailed definitions according to data type.
Multidimensional domains, including baseline data, HRQOL, and toxicity, were assessed with reference to minimum data thresholds (table 2) so that data with complete reporting of a single component dimension (ie, statistically full reporting of a single baseline characteristic or individual toxicity domain) would not meet the threshold for aggregate full reporting. Unidimensional domains, including all primary and secondary efficacy endpoints, were assessed for completeness without the application of minimum data thresholds.
Table 2. Baseline characteristics, toxicity, and HRQOL endpoint assessment.
| Domain | Minimum threshold |
| Baseline characteristics | Performance status OR comorbiditiesAND≥1 key prognostic OR predictive factor(s) |
| Toxicity | ≥3 key toxicity domain(s) by organ siteOROverall G3 toxicity AND ≽1 key toxicity domain |
| HRQOL | ≥1 validated HRQOL instrument(s) |
| Level of reporting | Reported data |
| Complete | Meets minimum threshold; including numerical data sufficient for inclusion in meta-analysis* |
| Partial | Meets minimum threshold; including numerical data insufficient for inclusion in meta-analysis* |
| Qualitative | Meets minimum threshold; without numerical data |
| Unreported | Does not meet minimum threshold |
See online supplemental table 1online supplemental table 1 for detailed definitions according to data type.
HRQOLhealth-related quality of life
Data extraction
Data relating to older subgroups (defined as subgroups with a minimum age of 65) was extracted individually for each included trial by two reviewers using Microsoft Forms and imported into Excel (Microsoft, V.16.16.27). Conflicts were resolved by the corresponding author (CME). Briefly,24 data was collected with respect to the following domains: Trial characteristics and enrollment, baseline patient characteristics, performance status, primary and secondary efficacy outcomes, HRQOL assessments, toxicity, and conduct and reporting of baseline geriatric assessments or geriatric-specific patient-reported outcomes.
Studies (n=2)27 28 reporting performance status (PS) data using the Karnofsky Performance Status (KPS) scale rather than the European Cooperative Oncology Group (ECOG) scale were approximated according to European Society of Medical Oncology guidelines as follows: KPS 90–100=ECOG 0; KPS 70–80=ECOG 1.29
Results
Trial characteristics
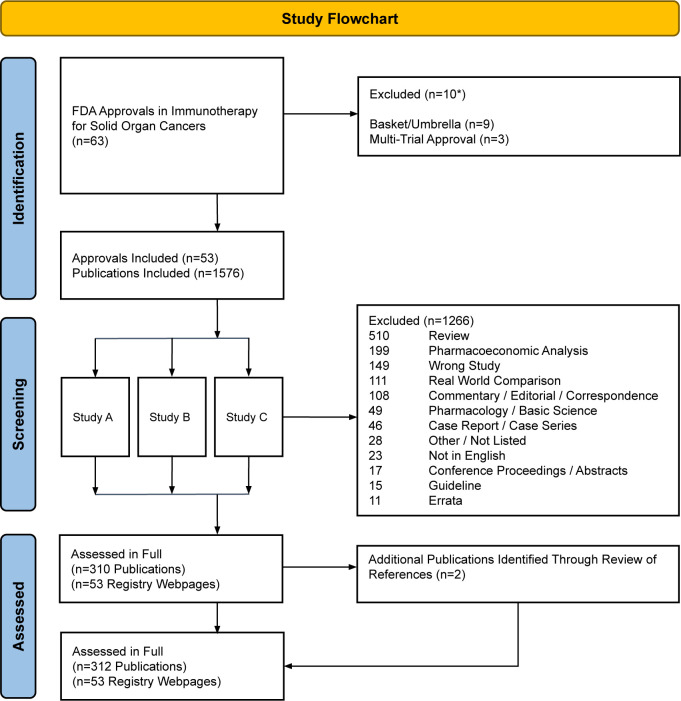
53 FDA approvals, corresponding to 53 clinical trials, in solid tumor oncology ICI immunotherapy were included. Across all trials, a total of 1576 search results were assessed in duplicate by paired reviewers according to title and abstract. Inter-rater reliability was high (Cohen’s kappa 0.82). 310 publications were included following title/abstract review (median: 5 publications per trial; online supplemental S4). 1266 publications were excluded (median: 19 publications per trial; online supplemental S5); reasons for exclusion are shown in figure 1. Two further publications identified through a review of references from included publications were included. A total of 312 publications and 53 ClinicalTrials.gov registry datasets were included in the final analysis.
Figure 1. Study flow diagram. FDA, Food and Drug Administration.
Trial characteristics are shown in table 3. Most included studies were phase 3 trials (n=44, 83.0%); most studies were in the palliative setting (n=45; 84.9%). A total of 37,094 participants were enrolled across all trials. Median overall trial enrollment was 713 (IQR 501–970). 19 (36.1%) trials were of single-agent checkpoint inhibitor therapy; a further 7 (13.1%) trials were of dual checkpoint inhibitor therapy. The remaining trials (n=27, 50.9%) involved combination therapy with chemotherapy, tyrosine kinase inhibitors, monoclonal antibodies, or v-raf murine sarcoma viral oncogene homolog B1 (BRAF) inhibitors.
Table 3. Characteristics of included studies.
| n | % | |
| Disease site | ||
| Lung | 17 | 32.1 |
| Skin | 7 | 13.2 |
| Renal | 6 | 11.3 |
| Upper GI | 6 | 11.3 |
| Liver/hepatobiliary | 5 | 9.4 |
| Breast | 3 | 5.7 |
| Urothelial/bladder | 3 | 5.7 |
| Colorectal | 2 | 3.8 |
| Cervical | 1 | 1.9 |
| Endometrial | 1 | 1.9 |
| Head and neck | 1 | 1.9 |
| Sarcoma | 1 | 1.9 |
| Phase | ||
| Phase 1/2 | 1 | 1.9 |
| Phase 2 | 7 | 13.2 |
| Phase 2/3 | 1 | 1.9 |
| Phase 3 | 44 | 83.0 |
| Setting | ||
| Adjuvant | 8 | 15.1 |
| Palliative | 45 | 84.9 |
| Enrollment | ||
| N (Median, IQR) | 713 | 501–970 |
| Age>65 (% Median, IQR) | 46.5 | 38.2–53.4 |
| ECOG 0 (% Median, IQR) | 50.7 | 39.7–62.4 |
| ECOG 1 (% Median, IQR) | 49.3 | 37.5–60.1 |
| Intervention | ||
| ICI monotherapy | 19 | 35.8 |
| ICI/chemotherapy | 16 | 30.2 |
| ICI/ICI | 7 | 13.2 |
| ICI/TKI | 5 | 9.4 |
| ICI/MAb/chemotherapy | 2 | 3.8 |
| ICI/ICI/chemotherapy | 2 | 3.8 |
| ICI/BRAF | 1 | 1.9 |
| ICI/MAb | 1 | 1.9 |
BRAF, v-raf murine sarcoma viral oncogene homolog B1ECOG, Eastern Cooperative Oncology Group; GI, Gastrointestinal; ICI, Immune checkpoint Inhibitor; MAb, monoclonal antibody; TKI, tyrosine kinase Inhibitor
Three trials presented unpooled older subgroup findings in a dedicated secondary publication.30,32 Two further trials reported pooled data in dedicated secondary publications among older populations with non-small cell lung cancer enrolled in studies of pembrolizumab,33 and nivolumab/ipilimumab34 respectively.
Efficacy endpoint reporting
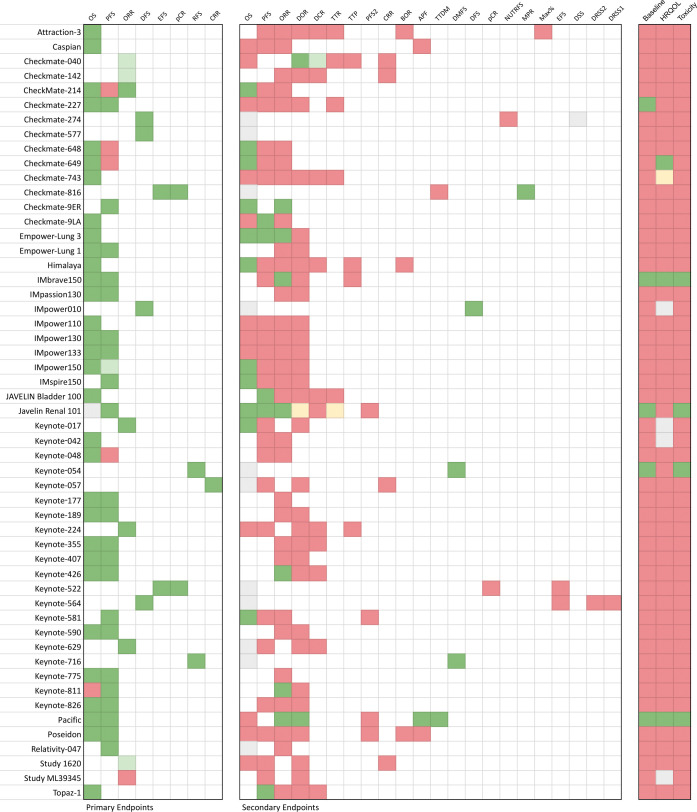
A total of 79 primary endpoints, and 185 secondary endpoints were included across all studies. Data was immature for one primary endpoint and for 12 secondary endpoints. The total number of evaluable primary endpoints was 78. The total number of evaluable secondary endpoints was 173 (table 4, figure 2).
Table 4. Reporting of older adult subgroups.
| n | Complete | Partial | Qualitative | Unreported | |
| Primary endpoints | 78 | 68 (87.2) | 4 (5.1) | 0 (0.0) | 6 (7.7) |
| Secondary endpoints | 173 | 31 (17.9) | 1 (0.6) | 2 (1.2) | 139 (80.3) |
| Baseline characteristics | 53 | 5 (9.4) | 0 (0.0) | 1 (1.9) | 47 (88.7) |
| HRQOL | 49 | 3 (6.1) | 0 (0.0) | 0 (0.0) | 46 (93.9) |
| Toxicity | 53 | 4 (7.5) | 0 (0.0) | 0 (0.0) | 49 (92.5) |
HRQOL, health-related quality of life
Figure 2. Completeness of reporting of older subgroups according to individual trials. APF, alive and progression-free; BOR, best overall response; CRR, complete response rate; DCR, disease control rate; DFS, disease-free survival; DMFS, distant metastasis-free survival; DOR, duration of response; DRSS1, disease recurrence-specific survival 1; DRSS2, disease recurrence–specific survival 2; DSS, disease-specific survival; EFS, event-free survival; HRQOL, health-related quality of life; Max%, maximum percent change from baseline in the sum of diameters of the target lesion; MPR, major pathological response; NUTRFS, non-urothelial tract recurrence-free survival; ORR, objective response rate; OS, overall survival; pCR, pathologic complete response; PFS, progression-free survival; PFS2, progression-free survival 2; RFS, recurrence-free survival; TTDM, time to distant metastasis; TTP, time to progression; TTR, time to response.
68 (87.2%) primary endpoints were reported completely, 4 (5.1%) were reported partially, and 6 (7.7%) were unreported among older populations (figure 2). 49 trials (92.5%) reported at least one primary endpoint completely among older populations. 31 secondary endpoints (17.9%) were reported completely, 1 (0.6%) was reported partially, 2 (1.2%) were reported qualitatively, and 139 (80.3%) were unreported among older populations. Five studies (9.4%) completely reported all mature primary and secondary efficacy endpoints. Three trials (5.7%) did not report any primary or secondary endpoints completely among older populations.
51 trials assessed OS, of which 11 trials were immature for reporting. Of the 40 evaluable trials, OS among older populations was fully reported for 36 studies (90%), and unreported for 4 (10%). 43 trials assessed PFS. Of these, 26 trials (60.5%) reported PFS completely, 1 (2.3%) reported PFS partially, and 16 (37.2%) did not report PFS among older populations.
Baseline characteristics
None of the included studies specified an upper age cut-off for eligibility. All included studies presented quantitative summaries of participant ages, using either mean and SD or median and range or IQR. The median size of older patient cohorts was 309 (IQR 185–374). 48 studies (90.6%) provided age-stratified enrollment data according to at least one age cut-off (typically≥65 years). Among the studies for which age-stratification was reported, 42.3% of patients were aged≥65. 10 studies (18.9%) additionally reported enrollment according to a stratification of age≥75. Among studies reporting data for this cohort, 11.1% of patients were aged≥75. One study additionally reported enrollment according to an age stratification of≥70; another additionally reported enrollment according to age≥85.
Reporting of baseline characteristics was completed in five studies (9.4%). 48 studies (90.6%) did not report baseline characteristics of the older subgroup. None of the included studies conducted geriatric-specific baseline assessments or frailty screening.
49 studies (92.5%) conducted HRQOL assessments using a validated instrument, of which none were geriatric-specific HRQOL tools. Three studies (6.1%) reported HRQOL outcomes for at least one validated HRQOL tool completely among the older population. One study (2.0%) reported HRQOL data qualitatively. 45 studies (91.8%) did not report HRQOL assessments among older populations.
Four studies (7.5%) reported toxicity outcomes completely among older populations. 49 studies (92.5%) did not report toxicity outcomes among older populations.
Performance status
52 studies (98.1%) excluded patients with ECOG PS>1. One study (n=1096 patients)35 reported KPS data dichotomized above and below KPS=70; this data could not be approximated to ECOG PS, and was excluded from PS analyses. ECOG PS data was unavailable or missing for a further 65 participants across the remaining 52 studies. Among the 35,936 assessable patients, ECOG was 0 in 19,549 (54.4%), 1 in 16,340 (45.5%) and 2 in 44 (0.1%).
Discussion
This analysis identifies significant deficits in the reporting of older populations enrolled in FDA registration trials of ICI therapy, particularly with regard to baseline characteristics, toxicity, and HRQOL outcomes. Reporting of primary efficacy endpoint data, including OS, was generally good across the examined trials. Reporting of secondary efficacy endpoint outcomes was poor.
Although 42.3% of patients enrolled in the trials were aged≥65, the substantially lower numbers of patients aged 75 and above (11.1%) suggest clustering or concentration of patients aged around 60 years in these trials. Older adults were under-represented among the included studies relative to global demographics of patients diagnosed with cancer among the majority of tumor types addressed by these studies, particularly with regard to patients aged≥75 years, who represent approximately 25% of all patients diagnosed with cancer globally, and (for instance) 36.3% of patients diagnosed with lung cancer.2 36 37
Enrollment was restricted in 52 trials (98.1%) to patients with ECOG PS of 0–1, which implies a level of health, functional status, and general well-being frequently not seen in patients with cancer aged≥75 years. It is acknowledged that older patients with equivalent objective functional status are routinely ascribed to lower ECOG PS scores than their younger counterparts.38 This in practice likely results in the selection of only the fittest older patients for participation in registration studies, resulting in highly selected trial populations which are unlikely to be reflective of a significant proportion of older patients who ultimately receive these treatments. The use of age and ECOG PS as single measures of functional status or of aging phenotype in clinical trials has well-described limitations,39 and these measures are known to perform poorly in the prediction of toxicity or treatment tolerability among patients undergoing other systemic therapies, particularly chemotherapy.40 The adequacy and performance of ECOG as a predictor of toxicity or of clinical benefit in ICI therapy specifically remains inadequately described, in large part due to the low numbers of ECOG PS≥2 patients enrolled to prospective trials,41,43 and its widespread use as an exclusion criterion among these trials substantially limits the external validity of these studies among older real-world populations.
Reporting on the baseline composition of older patients recruited to these studies, including the distribution of clinical stage and prognostic biomarker data (ie, PD-L1 status), was poor, making it difficult to reliably interpret and contextualize older subgroup efficacy data either individually or in meta-analyses. Although baseline oncogeriatric assessment is considered a standard of care for patients aged≥65 years commencing a new line of systemic anticancer therapy,44,46 and is supported by major international societies including the American Society of Clinical Oncology (ASCO),44 the International Society of Geriatric Oncology46 and the National Comprehensive Cancer Network,45 no trials reported undertaking such assessments. Such assessments have been demonstrated to reduce toxicity and healthcare resource use, and to improve quality of life in multiple large randomized control trials,47,49 and should be undertaken both within and outside of clinical trial settings.
Reporting of OS was generally good across the examined trials. However, reporting of other key efficacy outcomes, toxicity, and HRQOL data for older adults was poor across a significant majority of studies. Although ICIs are acknowledged to have a generally favorable risk and toxicity profile relative to many alternative treatments, particularly chemotherapy,50 64.2% of immunotherapy approvals between 2018 and 2022 were of combination therapy, the tolerability and HRQOL impacts of which are significantly less clear among older adults.18 51 Given the large body of evidence suggesting that HRQOL and toxicity outcomes are of equal or greater significance to older adults compared with survival outcomes,52 53 the utility and validity of reporting OS as an isolated endpoint among older patients, particularly those treated in the palliative setting, remains unclear. Similarly, in the adjuvant setting, where the intention of treatment is to prevent the future relapse of cancer following curative surgery, the number needed to treat to avert one cancer-related mortality event is likely to be substantially greater among older patients due to competing causes of mortality. Primary data describing the relative risks and benefits of such approaches among older patients are sparse, but much needed, since the risk of fatal toxicity from ICI is significantly higher among older patients,54 and recovery from any-grade toxicity is generally slower and less complete.55
Dedicated publications relating to outcomes for older adults enrolled to the included studies were available for just three trials (5.7%). By contrast, in an exploratory analysis, 24 (45.3%) of the examined trials published dedicated reports relating to the characteristics and outcomes of subgroups differentiated by demographics, ethnicity, or geographical location; the median size of these analyses was n=72 (IQR 58–143) (online supplemental S6). This demonstrates the value and validity of detailed reporting on study subgroups, even where population sizes are modest and findings can be regarded as only exploratory in nature, and suggests that similar analyses among older subgroups should be feasible.
Regulatory authorities and major societies, including the EMA (European Medicines Agency), FDA and ASCO, have advocated for the increased recruitment of older adults in oncology clinical trials and the establishment of reporting standards for older adult subgroups in registration trials.56,59 These initiatives are in line with the broader goals of precision medicine, health equity and research inclusivity, and the development of an evidence base which both reflects real-world populations and which examines and reports endpoints that are meaningful to patients.60 However, our findings indicate that adherence to such voluntary reporting standards is poor and underscores the necessity for mandatory reporting of older patients, particularly in trials that lead to licensing approvals with major regulatory bodies.
Limitations
This analysis has some limitations. Four studies (Keynote 017, CheckMate 743, Keynote 629, and Keynote 057) included high proportions of older patients (≥70% aged over 65, median age over 70, or both). Although these studies did not report subgroup analyses according to age across all examined endpoints, it may be reasonable to accept these data as being generally reflective of (fit) older populations. This study did not assess real-world or retrospective data, as this was not the focus of this analysis. Similarly, this study did not include unpublished findings that may be available to FDA at the time of licensing nor abstract or conference proceedings data which were not otherwise available as a peer-reviewed publication. Finally, intended future publications of older subgroup data may not yet have been completed among more recent trials, although all included studies were assessed at a minimum of 1 year following licensing approval.
Conclusion
This analysis underscores significant deficits in the reporting of older adults enrolled in registration clinical trials of cancer immunotherapy. Enrollment of older adults, particularly those aged≥75 years, was not reflective of global cancer patient demographics. With the exception of OS, reporting on older subgroups with regard to key secondary efficacy endpoints, baseline characteristics, toxicity, and HRQOL outcomes was poor. 98.1% of trials excluded patients with ECOG>1. No studies conducted oncogeriatric assessments or examined geriatric-specific HRQOL scales. Dedicated secondary publications reporting on older adults enrolled in the included trials were available for only 5.7% of studies.
The significant limitations of these data should be considered in the interpretation of meta-analyses which are derived from this evidence base. The clinical utility of such evidence to guide treatment decisions among older adults with frailty or geriatric syndromes remains limited. Considering the aging of the global general population and the rising incidence of cancer among older individuals, there is a compelling need for mandatory regulatory guidelines to ensure comprehensive reporting on older adult subgroups in clinical trials of cancer immunotherapy.
supplementary material
Acknowledgements
The authors would like to thank Mr Paul Howell, librarian at The Royal Marsden Hospital, for his contribution in defining the study’s search methods and terms.
Footnotes
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Provenance and peer review: Commissioned; externally peer reviewed.
Patient consent for publication: Not applicable.
Contributor Information
Colm Mac Eochagain, Email: colmme@gmail.com.
Robert Power, Email: powerr8@tcd.ie.
Christine Sam, Email: christine.sam@moffitt.org.
Nicolas M Gonzalez-Senac, Email: nic.gsenac@gmail.com.
Darren Walsh, Email: darren.walsh@hse.ie.
Mukul Roy, Email: dr.roy.mukul@gmail.com.
Nicolò Matteo Luca Battisti, Email: Nicolo.Battisti@rmh.nhs.uk.
References
- 1.Haslam A, Prasad V. Estimation of the Percentage of US Patients With Cancer Who Are Eligible for and Respond to Checkpoint Inhibitor Immunotherapy Drugs. JAMA Netw Open . 2019;2:e192535. doi: 10.1001/jamanetworkopen.2019.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.International Agency for Research on Cancer Globocan 2020 global cancer observatory. [-Oct-2023]. http://gco.iarc.fr Available. Accessed.
- 3.Pilleron S, Sarfati D, Janssen-Heijnen M, et al. Global cancer incidence in older adults, 2012 and 2035: A population-based study. Int J Cancer. 2019;144:49–58. doi: 10.1002/ijc.31664. [DOI] [PubMed] [Google Scholar]
- 4.Pawelec G. Age and immunity: What is “immunosenescence”? Exp Gerontol. 2018;105:4–9. doi: 10.1016/j.exger.2017.10.024. [DOI] [PubMed] [Google Scholar]
- 5.Thomas R, Wang W, Su D-M. Contributions of Age-Related Thymic Involution to Immunosenescence and Inflammaging. Immun Ageing. 2020;17:2. doi: 10.1186/s12979-020-0173-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coppé J-P, Desprez P-Y, Krtolica A, et al. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol. 2010;5:99–118. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coder BD, Wang H, Ruan L, et al. Thymic involution perturbs negative selection leading to autoreactive T cells that induce chronic inflammation. J Immunol. 2015;194:5825–37. doi: 10.4049/jimmunol.1500082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Franceschi C, Garagnani P, Parini P, et al. Inflammaging: a new immune-metabolic viewpoint for age-related diseases. Nat Rev Endocrinol. 2018;14:576–90. doi: 10.1038/s41574-018-0059-4. [DOI] [PubMed] [Google Scholar]
- 9.Torres L, Telles O Lima J, Thuler LCS, et al. Loss of the CD28 costimulatory molecules on the immune subsets of TCD4+ cells in prostate cancer elderly patients. J C O. 2016;34:e16612. doi: 10.1200/JCO.2016.34.15_suppl.e16612. [DOI] [Google Scholar]
- 10.Daste A, Domblides C, Gross-Goupil M, et al. Immune check point inhibitors and elderly people: A review. Eur J Cancer. 2017;82:155–66. doi: 10.1016/j.ejca.2017.05.044. [DOI] [PubMed] [Google Scholar]
- 11.Ferrara R, Naigeon M, Auclin E, et al. Circulating T-cell Immunosenescence in Patients with Advanced Non-small Cell Lung Cancer Treated with Single-agent PD-1/PD-L1 Inhibitors or Platinum-based Chemotherapy. Clin Cancer Res. 2021;27:492–503. doi: 10.1158/1078-0432.CCR-20-1420. [DOI] [PubMed] [Google Scholar]
- 12.Elias R, Giobbie-Hurder A, McCleary NJ, et al. Efficacy of PD-1 & PD-L1 inhibitors in older adults: a meta-analysis. J Immunother Cancer. 2018;6:26. doi: 10.1186/s40425-018-0336-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang L, Sun L, Yu J, et al. Comparison of Immune Checkpoint Inhibitors between Older and Younger Patients with Advanced or Metastatic Lung Cancer: A Systematic Review and Meta-Analysis. Biomed Res Int. 2019;2019:1–13. doi: 10.1155/2019/9853701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim CM, Lee JB, Shin SJ, et al. The efficacy of immune checkpoint inhibitors in elderly patients: a meta-analysis and meta-regression. ESMO Open. 2022;7:100577. doi: 10.1016/j.esmoop.2022.100577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yan X, Tian X, Wu Z, et al. Impact of Age on the Efficacy of Immune Checkpoint Inhibitor-Based Combination Therapy for Non-small-Cell Lung Cancer: A Systematic Review and Meta-Analysis. Front Oncol. 2020;10:1671. doi: 10.3389/fonc.2020.01671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geneletti S, Richardson S, Best N. Adjusting for selection bias in retrospective, case-control studies. Biostatistics. 2009;10:17–31. doi: 10.1093/biostatistics/kxn010. [DOI] [PubMed] [Google Scholar]
- 17.Booth CM, Tannock IF. Randomised controlled trials and population-based observational research: partners in the evolution of medical evidence. Br J Cancer. 2014;110:551–5. doi: 10.1038/bjc.2013.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dottorini L, Ghidini A, Deda R, et al. Immune checkpoint inhibitor doublets: Are they beneficial for older patients? A systematic review and meta-analysis. J Geriatr Oncol. 2024;15:101741. doi: 10.1016/j.jgo.2024.101741. [DOI] [PubMed] [Google Scholar]
- 19.Talarico L, Chen G, Pazdur R. Enrollment of elderly patients in clinical trials for cancer drug registration: a 7-year experience by the US Food and Drug Administration. J Clin Oncol. 2004;22:4626–31. doi: 10.1200/JCO.2004.02.175. [DOI] [PubMed] [Google Scholar]
- 20.Singh H, Kanapuru B, Smith C, et al. FDA analysis of enrollment of older adults in clinical trials for cancer drug registration: A 10-year experience by the U.S. Food and Drug Administration. J C O. 2017;35:10009. doi: 10.1200/JCO.2017.35.15_suppl.10009. [DOI] [Google Scholar]
- 21.Eochagain CM, Battisti NML. Reporting of older subgroups in registration breast cancer trials 2012-2021. Breast Cancer Res Treat. 2023;202:411–21. doi: 10.1007/s10549-023-07081-0. [DOI] [PubMed] [Google Scholar]
- 22.BrintzenhofeSzoc K, Krok-Schoen JL, Canin B, et al. The underreporting of phase III chemo-therapeutic clinical trial data of older patients with cancer: A systematic review. J Geriatr Oncol. 2020;11:369–79. doi: 10.1016/j.jgo.2019.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sedrak MS, Freedman RA, Cohen HJ, et al. Older adult participation in cancer clinical trials: A systematic review of barriers and interventions. CA Cancer J Clin. 2021;71:78–92. doi: 10.3322/caac.21638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eochagain Colm M. SIOG-IO reporting of older subgroups enrolled to pivotal immunotherapy trials 2018-2022 v1. 2023. Available. [DOI]
- 25.Drugs@FDA: FDA-approved drugs. [03-Mar-2024]. https://www.fda.gov/drugs/development-approval-process-drugs/drug-approvals-and-databases Available. Accessed.
- 26.Chan A-W, Hróbjartsson A, Haahr MT, et al. Empirical evidence for selective reporting of outcomes in randomized trials: comparison of protocols to published articles. JAMA. 2004;291:2457–65. doi: 10.1001/jama.291.20.2457. [DOI] [PubMed] [Google Scholar]
- 27.Choueiri TK, Powles T, Burotto M, et al. Nivolumab plus Cabozantinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med. 2021;384:829–41. doi: 10.1056/NEJMoa2026982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rini BI, Plimack ER, Stus V, et al. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med. 2019;380:1116–27. doi: 10.1056/NEJMoa1816714. [DOI] [PubMed] [Google Scholar]
- 29.Peyret L-E. Performance Scales: Karnofsky & ECOG Scores. 2022. [12-Mar-2024]. https://oncologypro.esmo.org/oncology-in-practice/practice-tools/performance-scales Available. Accessed.
- 30.Socinski MA, Özgüroğlu M, Villegas A, et al. Durvalumab After Concurrent Chemoradiotherapy in Elderly Patients With Unresectable Stage III Non–Small–Cell Lung Cancer (PACIFIC) Clin Lung Cancer. 2021;22:549–61. doi: 10.1016/j.cllc.2021.05.009. [DOI] [PubMed] [Google Scholar]
- 31.Tomita Y, Motzer RJ, Choueiri TK, et al. Efficacy and safety of avelumab plus axitinib in elderly patients with advanced renal cell carcinoma: extended follow-up results from JAVELIN Renal 101. ESMO Open. 2022;7:100450. doi: 10.1016/j.esmoop.2022.100450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li D, Toh HC, Merle P, et al. Atezolizumab plus Bevacizumab versus Sorafenib for Unresectable Hepatocellular Carcinoma: Results from Older Adults Enrolled in the IMbrave150 Randomized Clinical Trial. Liver Cancer. 2022;11:558–71. doi: 10.1159/000525671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nosaki K, Saka H, Hosomi Y, et al. Safety and efficacy of pembrolizumab monotherapy in elderly patients with PD-L1-positive advanced non-small-cell lung cancer: Pooled analysis from the KEYNOTE-010, KEYNOTE-024, and KEYNOTE-042 studies. Lung Cancer (Auckl) 2019;135:188–95. doi: 10.1016/j.lungcan.2019.07.004. [DOI] [PubMed] [Google Scholar]
- 34.Paz-Ares LG, Ciuleanu T-E, Pluzanski A, et al. Safety of First-Line Nivolumab Plus Ipilimumab in Patients With Metastatic NSCLC: A Pooled Analysis of CheckMate 227, CheckMate 568, and CheckMate 817. J Thorac Oncol. 2023;18:79–92. doi: 10.1016/j.jtho.2022.08.014. [DOI] [PubMed] [Google Scholar]
- 35.Motzer RJ, Rini BI, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in first-line treatment for advanced renal cell carcinoma: extended follow-up of efficacy and safety results from a randomised, controlled, phase 3 trial. Lancet Oncol. 2019;20:1370–85. doi: 10.1016/S1470-2045(19)30413-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.SEER Cancer of the lung and bronchus - cancer stat facts. [22-Feb-2024]. https://seer.cancer.gov/statfacts/html/lungb.html Available. Accessed.
- 37.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 38.Broderick JM, Hussey J, Kennedy MJ, et al. Patients over 65 years are assigned lower ECOG PS scores than younger patients, although objectively measured physical activity is no different. J Geriatr Oncol. 2014;5:49–56. doi: 10.1016/j.jgo.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 39.Jolly TA, Deal AM, Nyrop KA, et al. Geriatric assessment-identified deficits in older cancer patients with normal performance status. Oncologist. 2015;20:379–85. doi: 10.1634/theoncologist.2014-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hurria A, Togawa K, Mohile SG, et al. Predicting Chemotherapy Toxicity in Older Adults With Cancer: A Prospective Multicenter Study. JCO. 2011;29:3457–65. doi: 10.1200/JCO.2011.34.7625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mojsak D, Kuklińska B, Minarowski Ł, et al. Current state of knowledge on immunotherapy in ECOG PS 2 patients. A systematic review. Adv Med Sci. 2021;66:381–7. doi: 10.1016/j.advms.2021.07.005. [DOI] [PubMed] [Google Scholar]
- 42.Passaro A, Spitaleri G, Gyawali B, et al. Immunotherapy in Non-Small-Cell Lung Cancer Patients With Performance Status 2: Clinical Decision Making With Scant Evidence. J Clin Oncol. 2019;37:1863–7. doi: 10.1200/JCO.18.02118. [DOI] [PubMed] [Google Scholar]
- 43.Tomasik B, Bieńkowski M, Braun M, et al. Effectiveness and safety of immunotherapy in NSCLC patients with ECOG PS score ≥2 - Systematic review and meta-analysis. Lung Cancer (Auckl) 2021;158:97–106. doi: 10.1016/j.lungcan.2021.06.004. [DOI] [PubMed] [Google Scholar]
- 44.Dale W, Klepin HD, Williams GR, et al. Practical Assessment and Management of Vulnerabilities in Older Patients Receiving Systemic Cancer Therapy: ASCO Guideline Update. J Clin Oncol. 2023;41:4293–312. doi: 10.1200/JCO.23.00933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dotan E, Walter LC, Browner IS, et al. NCCN Guidelines® Insights: Older Adult Oncology, Version 1.2021. J Natl Compr Canc Netw. 2021;19:1006–19. doi: 10.6004/jnccn.2021.0043. [DOI] [PubMed] [Google Scholar]
- 46.Wildiers H, Heeren P, Puts M, et al. International Society of Geriatric Oncology consensus on geriatric assessment in older patients with cancer. J Clin Oncol. 2014;32:2595–603. doi: 10.1200/JCO.2013.54.8347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mohile SG, Mohamed MR, Xu H, et al. Evaluation of geriatric assessment and management on the toxic effects of cancer treatment (GAP70+): a cluster-randomised study. Lancet. 2021;398:1894–904. doi: 10.1016/S0140-6736(21)01789-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li D, Sun C-L, Kim H, et al. Geriatric Assessment-Driven Intervention (GAIN) on Chemotherapy-Related Toxic Effects in Older Adults With Cancer: A Randomized Clinical Trial. JAMA Oncol. 2021;7:e214158. doi: 10.1001/jamaoncol.2021.4158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Soo WK, King MT, Pope A, et al. Integrated Geriatric Assessment and Treatment Effectiveness (INTEGERATE) in older people with cancer starting systemic anticancer treatment in Australia: a multicentre, open-label, randomised controlled trial. Lancet Healthy Longev. 2022;3:e617–27. doi: 10.1016/S2666-7568(22)00169-6. [DOI] [PubMed] [Google Scholar]
- 50.Magee DE, Hird AE, Klaassen Z, et al. Adverse event profile for immunotherapy agents compared with chemotherapy in solid organ tumors: a systematic review and meta-analysis of randomized clinical trials. Ann Oncol. 2020;31:50–60. doi: 10.1016/j.annonc.2019.10.008. [DOI] [PubMed] [Google Scholar]
- 51.Wildiers H, de Glas NA. Anticancer drugs are not well tolerated in all older patients with cancer. Lancet Healthy Longev. 2020;1:e43–7. doi: 10.1016/S2666-7568(20)30001-5. [DOI] [PubMed] [Google Scholar]
- 52.Mac Eochagain C, Barrell A, Murphy J, et al. “What matters to you?” Patient-reported treatment goals in geriatric oncology: A cross-sectional survey. J Geriatr Oncol. 2024;15:101641. doi: 10.1016/j.jgo.2023.101641. [DOI] [PubMed] [Google Scholar]
- 53.Seghers PALN, Wiersma A, Festen S, et al. Patient Preferences for Treatment Outcomes in Oncology with A Focus on the Older Patient-A Systematic Review. Cancers (Basel) 2022;14:1147. doi: 10.3390/cancers14051147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang DY, Salem J-E, Cohen JV, et al. Fatal Toxic Effects Associated With Immune Checkpoint Inhibitors. JAMA Oncol. 2018;4:1721. doi: 10.1001/jamaoncol.2018.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Godby RC, Johnson DB, Williams GR. Immunotherapy in Older Adults with Cancer. Curr Oncol Rep. 2019;21:56. doi: 10.1007/s11912-019-0806-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Office of the Commissioner Inclusion of older adults in cancer clinical trials. U.S. Food and Drug Administration. 2022. [11-Mar-2024]. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/inclusion-older-adults-cancer-clinical-trials Available. Accessed.
- 57.Medicines for older people. [11-Mar-2024]. https://www.ema.europa.eu/en/human-regulatory-overview/research-development/medicines-older-people Available. Accessed.
- 58.Hurria A, Levit LA, Dale W, et al. Improving the Evidence Base for Treating Older Adults With Cancer: American Society of Clinical Oncology Statement. J Clin Oncol. 2015;33:3826–33. doi: 10.1200/JCO.2015.63.0319. [DOI] [PubMed] [Google Scholar]
- 59.Food and Drug Administration US . Silver Spring, MD: US Food and Drug; FDA action plan to enhance the collection and availability of demographic subgroup data. [Google Scholar]
- 60.Booth CM, Sengar M, Goodman A, et al. Common Sense Oncology: outcomes that matter. Lancet Oncol. 2023;24:833–5. doi: 10.1016/S1470-2045(23)00319-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.