Summary
Since the 1970s, influential literature has been using famines as natural experiments to examine the long-term health impact of prenatal famine exposure at the individual level. Although studies based on various famines have consistently shown that prenatal famine exposure is associated with an increased risk of type 2 diabetes (T2D), no studies have yet quantified the contribution of famines to later-life T2D at the population level. We, therefore, synthesised findings from the famines in Ukraine 1932–1933, the Western Netherlands 1944–1945 and China 1959–1961 to make preliminary estimates of T2D cases attributable to prenatal famine exposure. These famines were selected because they provide the most extensive and reliable data from an epidemiological perspective. We observed a consistent increase in T2D risk among prenatally exposed individuals in these famines, which translated into about 21 000, 400 and 0.9 million additional T2D cases due to prenatal famine exposure in Ukraine, Western Netherlands and China, respectively. The T2D increase related to famine exposure represented only around 1% of prevalent T2D cases in these countries. Our observations highlight the significant increase in later-life T2D risk among individuals with prenatal famine exposure but also the limited contribution of prenatal famine exposure to T2D epidemics at the population level.
Keywords: Diabetes, Epidemiology, Public Health, Nutrition
Summary box.
We were not able to find any studies on the contribution of pre-natal famine exposure to later-life T2D at the population level.
We estimated that the later-life T2D increase related to prenatal exposure to the Ukrainian, Dutch, or Chinese famine represented around 1% of prevalent T2D cases in these countries.
Established T2D factors including obesity, poor diets, and sedentary life styles should remain the primary targets for T2D prevention even in populations exposed to famine in early life.
Overview of famine research and unanswered questions
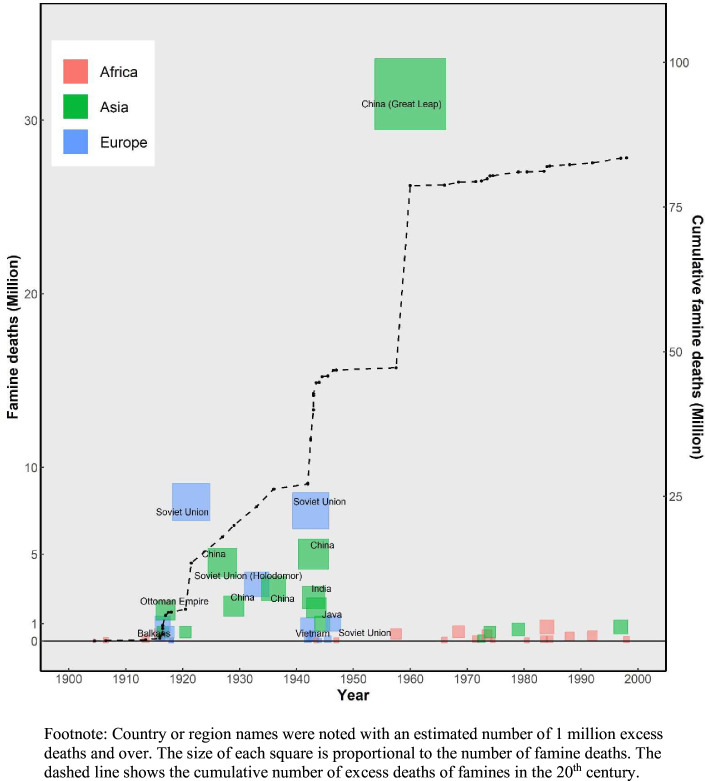
Over the course of the 20th century, famines resulted in 80–90 million deaths globally (figure 1), more than the total number of deaths from all causes during the two world wars.1,3 Famines in the former Soviet Union and the Great Chinese Famine of 1959–1961 were responsible for over half of all famine deaths (online supplemental table 1). Many scholars have studied the immediate demographic impact of these famines and their causes. Human agency in the form of warfare, autocracy and policy errors rather than Malthusian population pressure was the root cause for most of those famine deaths and the myriad horrors they entailed.2 In the new millennium, famines caused by conflicts and violence continue to threaten millions of lives across the world.4 And the ongoing climate change and future extraordinary events such as volcanic eruptions or a regional nuclear conflict could affect food security at the global level.5
Figure 1. Excess deaths caused by famines in the 20th century.
The costs imposed by famines outlasted their immediate demographic impact. Their consequences had an afterlife in the form of the physical and mental damage inflicted on those born in their wake. In the 1970s, epidemiologists Stein and Susser were the first to use a famine setting as a natural experiment to examine the long-term health impact of a prenatal environmental shock.6 Later, the important potential use of famines as a human laboratory to study biomedical mechanisms has become more widely recognised.7 The number of famine studies has grown rapidly over the past decades. For example, around 160 studies based on the Dutch famine of 1944–1945 had been published by 2010,8 and that number has now exceeded 250. An exponential increase in the number of studies on the Chinese famine of 1959–1961 has been observed in the past decade, with over 300 studies to date (online supplemental figure 1).9 There is also a growing interest in studying the Ukraine famine of 1932–1933 for this purpose.10 11 This fast-growing literature has mainly concentrated on the potential impact of early-life famine exposure on later health, possibly decades later. Despite a consensus on the increased risks of diseases among individuals prenatally exposed to famines, no studies to date have yet examined the additional disease cases attributable to the famines in relation to famine mortality or the overall disease burden at the population level. While the estimates of additional disease cases and excess deaths bear very different meanings, comparing such estimates side by side serves illustrative purposes and can help us to better understand the short-term and long-term health costs associated with early-life famine exposure.12
Epidemiological studies on Ukraine, Dutch, Chinese and other famines show an increased risk of obesity and type 2 diabetes (T2D) after prenatal famine exposure.9,1113 Some researchers, therefore, have discussed a potential relation between early-life famine exposure and diabetes epidemics in several reviews and commentaries.16,22 They propose that many adults in Asia, having experienced undernutrition early in life and now adopting Westernised diets and lifestyles, might face a significantly increased risk of diabetes and other metabolic disorders. They also state a mismatch between the intrauterine and adult life environment might explain the current diabetes epidemic in some developing countries17 23; and that the potential effect of famines, natural disasters and malnutrition on trends in T2D could show many decades after the events22 24; that the Chinese famine could trigger a diabetes ‘avalanche’ many years later25; that future natural disasters such as famines will result in the perpetuation of the T2D epidemic21; and that children born during a famine who live in an obesogenic environment as an adult have an increased risk of chronic diseases and even transgenerational risk transmission.20 26 The idea that early-life famine could drive current and future diabetes epidemics seems plausible. However, no studies to date have attempted to estimate the possible contribution of famines to the burden of later-life T2D at the population level. Such estimates could be obtained by estimating the number of additional T2D cases caused by the famine and comparing this figure to the number of prevalent T2D cases, as illustrated below.
A quantitative answer to these questions is needed to better understand the possible short-term and long-term costs of famines and to provide empirical evidence for health policies. Epidemiological studies in a variety of settings, including Ukraine, the Netherlands, Russia, Austria, China and others, have examined the relationship between prenatal famine exposure and later-life T2D. 9,1113 Here, we synthesise reported findings from three well-documented famines: the Ukrainian famine of 1932–1933, the Dutch famine of 1944–1945 and the Chinese famine of 1959–1961. We selected these famines for several reasons. First, available studies on this relationship in the three countries provide a solid foundation for reliable estimates. Second, the Dutch and Chinese famines are among the most thoroughly studied famines in the epidemiological literature, providing a wealth of comparative data. Third, the distinct characteristics of the three famines—including differences in their causes, duration, severity and magnitude in terms of lives lost—offer a unique opportunity to explore commonalities and differences in their immediate and long-term impact.
For short-term costs, we focused on excess deaths caused by the famines; for long-term costs, we examined the increased risk of T2D at the age of 60 years and older after prenatal famine exposure and the corresponding number of additional T2D cases. We further compared the number of additional T2D cases to the number of prevalent T2D cases to estimate the specific contribution of famines at the population level. We also applied this approach to assess the potential long-term health costs of famines during World War II (WWII) in relation to their immediate impact. We aimed to identify potential commonalities in the relation between the short-term and long-term costs comparing famines under highly variable conditions.
Short-term costs of famines: excess deaths and prenatally exposed births
For the short-term costs of the three famines, we reviewed studies on the number of excess deaths and prenatally exposed births. We provide the range of available estimates from representative studies for each famine (online supplemental table 2). A more comprehensive summary of these estimates across famines can be found in other studies.29,31
In Ukraine, estimates of excess deaths caused by the famine ranged between 2.6 million and 4.6 million because of different assumptions about emigration when reconstructing demographic data.29 32 33 For our purpose, the variation is not critical as these estimates are of the same order of magnitude. For further calculations, we used an intermediate estimate of 3.5 million excess deaths in the absence of definitive data (table 1, row c). The estimates of famine births from available studies were in close agreement, suggesting that the number of prenatally exposed births was around 0.6 million (table 1, row d).29 32 This number was used in later calculations to estimate the number of additional T2D cases attributable to prenatal famine exposure.
Table 1. Short-term and long-term costs of three famines.
| Famine | Holodomor or Great Ukrainian Famine of 1932–1933 | Dutch Hunger Winter Famine of 1944–1945 | Great Leap Forward Chinese Famine of 1959–1961 |
|---|---|---|---|
| a. Region | Nationwide | Western Netherlands | Nationwide |
| b. Circumstances that led to famine | Natural causes with poor economic policies and political repression | WWII blockade by German army and national railway strike | Great Leap Forward economic and social plan |
| c. Excess deaths of famine* | 2.6–4.6 million (3.5 million) |
10 000–30 000 (23 000) |
15 million–50 million (33 million) |
| d. Prenatally exposed births | 0.6 million | 13 000 | 35 million |
| e. Prevalent T2D cases in 2010† | 1.2 million | 0.1 million | 90 million |
| f. T2D prevalence among unexposed in 2010† | 2.9% | 5.3% | 8.8% |
| g. OR of T2D due to prenatal famine exposure | 2.2 | 1.6 | 1.3 |
| h. Increased odds of T2D (g−1) | 1.2 | 0.6 | 0.3 |
| i. Additional cases of T2D due to famine exposure in 2010 (d×f×h)‡ | 20 880 | 413 | 924 000 |
| j. Proportion of T2D cases (%) related to prenatal famine exposure (i/e) | 1.7% | 0.4% | 1.0% |
Estimates of excess deaths varied by studies with more details presented in online supplemental table 2. Figures used for preliminary estimates were presented in brackets.
The number of prevalent T2D cases and the prevalence among unexposed individuals in 2010 were estimated from the International Diabetes Federation Atlas (10th edition 2021). Since no specific data were available for the Western Netherlands, the number of T2D cases in this region was calculated in proportion to its population size, based on the overall number of T2D cases throughout the Netherlands.
This estimate of additional cases of T2D was made without considering survivorship from birth to 2010.
T2D, type 2 diabetes.
In the western Netherlands, plausible estimates of famine-related excess deaths ranged from 10 000 to 30 000.3034,36 With ongoing improvements in data availability and analytic methods, recent studies estimated the number of excess deaths to be around 23 000 (table 1, row c). The number of prenatally exposed births, conceived between November 1944 and May 1945 in the western Netherlands, was estimated to be about 13 000 based on vital statistics data as reported by Stein et al (table 1, row d).6
In China, estimates of excess deaths mainly ranged from around 20 to over 40 million with 30 million excess deaths as a widely accepted number.37,41 The large variation was due to the limited availability of demographic data and concerns over data quality in China. The study by Cao that combined population census data from the 1980s with information from local archives in different provinces is considered the most reliable for both excess deaths and prenatally exposed births.39 Therefore, we used his number of 33 million excess deaths and 35 million prenatally exposed births for our further calculations (table 1, rows c and d). Because of less clearly defined famine periods and lower-quality data, the estimates for China and Ukraine are less precise than those in the Netherlands.
Long-term costs of famines: additional T2D cases
To estimate the number of additional T2D cases attributable to prenatal famine exposure, information is needed on the number of prenatally exposed births, the prevalence of T2D among unexposed births and the increased odds of T2D comparing exposed to unexposed births. Multiplying these figures will give an upper-bound estimate for the additional T2D cases because the number of deaths between birth and late adulthood in the cohorts at risk is unknown and could not be taken into account.12
As noted in the previous section, we estimated the number of prenatally exposed births to be 0.6 million in Ukraine, 13 000 in the western Netherlands, and 35 million in China (table 1, row d). Information on the number of prevalent T2D cases was added from the 10th edition of the International Diabetes Federation Atlas (IDF Atlas) (table 1, row e).42 Information on T2D prevalence among unexposed births was taken from the same source. The prevalence for the year 2010 was 2.9% in Ukraine, 5.3% in the western Netherlands and 8.8% in China (table 1, row f). We selected estimates from this edition of the IDF Atlas for three reasons: First, most T2D famine studies in the three selected countries were conducted between 2005 and 2015, aligning the increased T2D odds in individuals and the national T2D prevalence estimates for the year 2010 to the same period. Second, the IDF Atlas used uniform methods to estimate the prevalence and the number of diabetes cases in our selected countries. Third, all estimates provided by the IDF Atlas took undiagnosed T2D cases into consideration and were consistent with available national studies.43,46
The increased odds of T2D due to prenatal famine exposure were derived from a literature review of epidemiological studies. To date, over 40 studies and multiple reviews have examined the relationship between prenatal famine exposure and later-life T2D.921 47,50 We used results from high-quality studies in our selected countries to estimate the increased odds of T2D after prenatal famine exposure. In Ukraine, a study from 2015 covering 9 out of 24 oblasts reported an OR of 1.6 for T2D for births in famine regions in the first half-year of 1934 compared with births in non-famine regions.10 51 The most recent study, covering all 24 oblasts, reported an OR of 2.2 (95% CI 2.0 to 2.5).27 In the Western Netherlands, the most reliable estimate to date showed an OR of 1.6 (95% CI 1.1 to 2.4).24 51 Other Dutch famine studies have focused on glucose dysregulation and did not report on T2D.52 53 In China, over 30 studies examined the relationship between prenatal famine and T2D.9 48 54 Many had methodological limitations related to the use of inappropriate controls, ignoring important age differences between famine-exposed individuals and the selected controls.55 56 Consistent findings have emerged however from high-quality studies in China by Meng et al who found that prenatally exposed participants had an increased T2D risk (OR 1.3, 95% CI 1.1 to 1.5) in the China Kadoorie Biobank13 and from studies based on the China Health and Retirement Longitudinal Study and the China Cardiometabolic Disease and Cancer Cohort.31 57 58 We, therefore, used ORs of 2.2, 1.6 and 1.3, respectively, to calculate the increased odds of T2D in Ukraine, Western Netherlands and China after prenatal famine (table 1, rows g and h).
An estimate of the number of additional T2D cases attributable to prenatal famine exposure was made by multiplying the number of prenatally exposed births, the prevalence of T2D among the unexposed, and the increased odds of T2D due to exposure (table 1, rows d, f, h). With these figures, we estimated that there were likely 20 880 additional T2D cases in Ukraine, 413 in the Western Netherlands and 924 000 in China, attributable to prenatal exposure to the respective famines (table 1, row i). Comparing the number of additional T2D cases to the number of prevalent T2D cases, we calculated the proportion of famine-attributable cases was 1.7%, 0.4% and 1.0%, respectively, in Ukraine, Western Netherlands and China (table 1, row j). The proportion is lowest in the Western Netherlands because of the short duration of the famine and the smaller number of exposed births compared with the two other settings. Some variation in the number of prenatally exposed births, the prevalence of T2D among the unexposed, or the increased odds of T2D due to prenatal famine exposure could lead to changes in the estimates of additional T2D cases for each of the famines but this will not affect their order of magnitude.
The number of additional T2D cases was much smaller than the number of excess deaths in all three famine settings. The ratio of excess deaths (3.5 million) to additional T2D cases (20 880) is the largest in Ukraine (168 to one compared with 56 to one in the western Netherlands and 36 to one in China) (table 1, rows c and i). This can be explained by the high number of deaths compressed in a short period of time in Ukraine.
Famines during the WWII
We further examined the short-term and long-term costs of famines during WWII. Together these famines were associated with 17–19 million excess deaths. Expanding our approach as set out above, using comparable added risk estimates for T2D after prenatal famine and assuming an average birth rate of 20 per 1000 across all famines, we estimated that 28 million people with prenatal famine exposure potentially generated 0.8 million additional T2D cases given a difference in the T2D prevalence of 3% between exposed and unexposed births.
Discussion
There is a rapidly expanding biomedical literature on the relationship between prenatal environmental shocks and health in later life.59 60 Famines are a large part of this literature because of their use as natural experiments.59 Surprisingly, this body of literature has not yet linked long-term health costs in terms of additional morbidity to short-term costs of excess deaths. Nor has it quantified the contribution of famine to later health at the population level. To answer these questions, we synthesised results from earlier studies, conducted straightforward calculations and made some preliminary estimates. Excess deaths outnumbered additional T2D cases attributable to prenatal famine exposure by a large margin across all examined famines. While excess deaths and additional T2D cases signify different aspects of impact, these figures enhance our understanding of both the immediate and enduring costs of famines.
We also quantified the T2D increase that could be attributed to famine exposure in early life. Despite a notable rise in T2D risk among those exposed to famine in utero, such exposure accounted for only a small fraction of T2D cases at the population level. These findings hold in all three famine settings, despite their varying durations, death tolls and severity. Specifically, in 2010, the proportion of T2D cases attributable to prenatal famine exposure was only 1.7% of prevalent cases in Ukraine, 0.4% in Western Netherlands and 1.0% in China. These estimates may even be a little high as they do not account for deaths prior to 2010 among famine-exposed individuals. The long-term health impact at the population level, in terms of additional morbidity, was much lower than suggested by recent reviews and commentaries. The small proportion of disease attributable to famine exposure can be understood considering the limited number of individuals who were exposed to famine during gestation relative to the size of the population at large facing T2D risks from common causes including obesity and other risk factors.
In earlier centuries, famines and malnutrition were much more frequent and extensive than in current times. Before industrialisation and modern growth, European evidence suggests that serious back-to-back harvest shortfalls likely resulted in famines that occurred on average one time every fifteen years. In such contexts, the aggregate cost of prenatal shocks on T2D could have been greater than today.
The relative importance of famine exposure contributing to long-term disease at the population level is likely to diminish over time because individuals with prenatal famine exposure in Ukraine, Western Netherlands and China if still alive will now be in their early 90s, late 70s and early 60s, respectively. While some Chinese famine studies suggest intergenerational effects on T2D, many of these have considerable methodological limitations.21 48 Animal studies indicate that such intergenerational effects might exhibit sex-specific patterns and diminish in subsequent generations. Therefore, even if we account for the potential intergenerational impact of famine on T2D, its additional contribution to the T2D burden in future generations is likely to be limited.
Our analysis has some limitations. First, our estimates of additional T2D cases attributable to the famines are based on reported findings from prior studies, using available data. This results in provisional estimates. For more rigorous estimates, individual-level data from representative populations are necessary to construct counterfactual estimates in a control group that mirrors the observed population in all relevant aspects except for events occurring during the famine.61 62 Second, prenatal famine exposure may interact with factors like obesity and other socioeconomic factors in later life, which potentially increase the risk of T2D.13 63 64 Incorporating this information could refine our estimates across different famine contexts, although it is unlikely to fundamentally change our findings. Third, we only estimated the potential contribution of famine to T2D in 2010 while the risks are likely to change over time as a function of population ageing and other disease risk factors.65 Fourth, our study did not examine the potential impact of early-childhood famine exposure on later-life T2D which may have underestimated the long-term costs of famine. This was not examined mainly because of conflicting findings on the impact of early-childhood famine exposure on later-life T2D. There is a growing literature, however, on the potential impact of childhood famine exposure on later-life non-communicable diseases.60 66 Last, while we have made extrapolations to famines during WWII, such statements at this stage are still speculative. Each of the famines had unique characteristics that could lead to different health later-life outcomes. To more accurately examine the costs of these famines, future research should take their heterogeneity into account based on data relevant to each famine.
These limitations do not affect the overall picture that the famines under study increased risks of T2D among famine-exposed individuals and did not significantly contribute to the T2D burden at the population level.67 68 In 2010, only about 1.0% of T2D cases in China could be attributed to prenatal famine exposure, in contrast to the 30%–40% attributable risk of excess weight and physical inactivity.69 70 This observation casts doubt on the widely cited claims that prenatal famine exposure is a major factor in the ongoing T2D epidemic in China and other countries, and in potentially fuelling a future epidemic.16 20 21 26 We think that the main reason behind these claims is that researchers frequently extrapolate findings of famine studies in individuals to risk estimates at the population level.21 24 68 It is also important to recognise that while chronic undernutrition and famine share many similarities, famine represents an extreme form of undernutrition and early-life exposure. It is also more rare than chronic undernutrition. Consequently, the contributions of chronic undernutrition and famine to T2D epidemics may vary significantly.
We do not dispute the important findings by many, ourselves included, that famine exposure at the individual level increases the risk of T2D and some other conditions. It is important, however, to provide estimates of the impact of early famine on T2D at the population level, as this was never attempted to date. The study of a famine’s impact on T2D is not merely of academic interest but carries profound public health implications. Research in this area underscores the urgency of addressing the nutritional needs of populations worldwide and demonstrates the long-term risks associated with early-life nutritional deficits. It also strengthens the argument for comprehensive health and economic policies to prevent famine conditions and malnutrition—a moral imperative because the costs of preventing and treating malnutrition should be viewed as an investment in the long-term health and well-being of populations.
Famine studies have greatly advanced biomedical research by offering a model to study the selected biological effects of early-life environmental shocks on human development and health in a quasi-experimental setting.59 This framework has enabled further exploration into the biological mechanisms, including genetic and epigenetic changes, which may explain how prenatal famine exposure could lead to later adverse health outcomes.4871,73 Our findings across the famines show consistent results on the relation between prenatal famine exposure and increased T2D in adulthood.9 10 51 The increase in T2D prevalence among cohorts exposed to prenatal famine was remarkably similar across countries (3.5% in Ukraine, 3.2% in Western Netherlands and 2.6% in China). This demonstrates the potential utility of using famine settings to examine important study questions that otherwise could not be addressed.
Conclusion
Our study has generated important insights on two counts. First, it demonstrated that famine exposure in the three well-documented settings carries a comparable increase in risk for later-life T2D among those exposed prenatally. The effect of famine is related to biological mechanisms that are now being increasingly understood. Second, it suggested that the impact of early-life famine exposure on T2D at the population level is limited in relation to established risk factors such as obesity, poor diets and sedentary lifestyles. It is important, however, to recognise that the broader social and political factors contributing to famines can also affect established T2D risk factors. This understanding should inform public health efforts and policies, ensuring a comprehensive approach to combating the T2D epidemics and improving global health.
Supplementary material
Acknowledgements
We thank Morgan Kelly, Joel Mokyr, Natalya Naumenko, Peter Solar, Peter Ekamper and Guohua Li, for their comments and suggestions.
Footnotes
Funding: National Institute of Aging R01 AG028593 (LHL), National Institute of Aging R01 AG06687 (LHL), National Institute of Aging R01 AG070953 (CL) and National Institute of Aging R01AG075719 (CL).
Provenance and peer review: Not commissioned; externally peer reviewed.
Handling editor: Helen J Surana
Patient consent for publication: Not applicable.
References
- 1.Devereux S. Famine in the twentieth century. IDS Working Paper. 2000;105 [Google Scholar]
- 2.Ó Gráda C. Famine: a short history. New Jersey: Princeton University Press; 2009. [Google Scholar]
- 3.Ó Gráda C. The hidden victims: civilian casualties of the two world wars. New Jersey: Princeton University Press; 2024. [Google Scholar]
- 4.Maxwell D, Khalif A, Hailey P, et al. Viewpoint: Determining famine: Multi-dimensional analysis for the twenty-first century. Food Policy. 2020;92:101832. doi: 10.1016/j.foodpol.2020.101832. [DOI] [Google Scholar]
- 5.Xia L, Robock A, Scherrer K, et al. Global food insecurity and famine from reduced crop, marine fishery and livestock production due to climate disruption from nuclear war soot injection. Nat Food . 2022;3:586–96. doi: 10.1038/s43016-022-00573-0. [DOI] [PubMed] [Google Scholar]
- 6.Stein Z, Susser M, Saenger G, et al. Famine and human development: the dutch hunger winter of 1944-1945. New York, NY, US: Oxford University Press; 1975. [Google Scholar]
- 7.Lumey LH, Vaiserman AM. The Dutch famine of 1944-45 as a human laboratory: changes in the early life environment and adult health. Early life nutrition and adult health and development. New York: Nova Science Publisher; 2013. pp. 59–70. [Google Scholar]
- 8.O’Grada C. Fetal Origins, Childhood Development, and Famine: A Bibliography and Literature Review. SSRN J. 2011 doi: 10.2139/ssrn.1980709. [DOI] [Google Scholar]
- 9.Li C, Lumey LH. Early-Life Exposure to the Chinese Famine of 1959-1961 and Type 2 Diabetes in Adulthood: A Systematic Review and Meta-Analysis. Nutrients. 2022;14:2855. doi: 10.3390/nu14142855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lumey LH, Khalangot MD, Vaiserman AM. Association between type 2 diabetes and prenatal exposure to the Ukraine famine of 1932-33: a retrospective cohort study. Lancet Diabetes Endocrinol. 2015;3:787–94. doi: 10.1016/S2213-8587(15)00279-X. [DOI] [PubMed] [Google Scholar]
- 11.Vaĭserman AM, Khalangot ND, Pisaruk AV, et al. Predisposition to type II diabetes in Ukraine residents exposed to famine 1932-1933 during prenatal development. Adv Gerontol. 2010;23:588–92. [PubMed] [Google Scholar]
- 12.Ó Gráda C, Li C, Lumey LH. How much schizophrenia do famines cause? Schizophrenia (Heidelb) . 2023;9:90. doi: 10.1038/s41537-023-00416-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meng R, Lv J, Yu C, et al. Prenatal famine exposure, adulthood obesity patterns and risk of type 2 diabetes. Int J Epidemiol. 2018;47:399–408. doi: 10.1093/ije/dyx228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaleta M, Leutner M, Thurner S, et al. Diabetes incidence in Austria: the role of famines on diabetes and related NCDs. Heliyon. 2023;9:e17570. doi: 10.1016/j.heliyon.2023.e17570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thurner S, Klimek P, Szell M, et al. Quantification of excess risk for diabetes for those born in times of hunger, in an entire population of a nation, across a century. Proc Natl Acad Sci U S A. 2013;110:4703–7. doi: 10.1073/pnas.1215626110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chan JCN, Zhang Y, Ning G. Diabetes in China: a societal solution for a personal challenge. Lancet Diabetes Endocrinol. 2014;2:969–79. doi: 10.1016/S2213-8587(14)70144-5. [DOI] [PubMed] [Google Scholar]
- 17.Chen L, Magliano DJ, Zimmet PZ. The worldwide epidemiology of type 2 diabetes mellitus--present and future perspectives. Nat Rev Endocrinol. 2011;8:228–36. doi: 10.1038/nrendo.2011.183. [DOI] [PubMed] [Google Scholar]
- 18.Hu C, Jia W. Diabetes in China: Epidemiology and Genetic Risk Factors and Their Clinical Utility in Personalized Medication. Diabetes. 2018;67:3–11. doi: 10.2337/dbi17-0013. [DOI] [PubMed] [Google Scholar]
- 19.Hu FB. Globalization of diabetes: the role of diet, lifestyle, and genes. Diabetes Care. 2011;34:1249–57. doi: 10.2337/dc11-0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14:88–98. doi: 10.1038/nrendo.2017.151. [DOI] [PubMed] [Google Scholar]
- 21.Zimmet P, Shi Z, El-Osta A, et al. Epidemic T2DM, early development and epigenetics: implications of the Chinese Famine. Nat Rev Endocrinol. 2018;14:738–46. doi: 10.1038/s41574-018-0106-1. [DOI] [PubMed] [Google Scholar]
- 22.Zimmet PZ, Magliano DJ, Herman WH, et al. Diabetes: a 21st century challenge. Lancet Diabetes Endocrinol. 2014;2:56–64. doi: 10.1016/S2213-8587(13)70112-8. [DOI] [PubMed] [Google Scholar]
- 23.Zimmet PZ, Alberti K. Epidemiology of Diabetes-Status of a Pandemic and Issues Around Metabolic Surgery. Diabetes Care. 2016;39:878–83. doi: 10.2337/dc16-0273. [DOI] [PubMed] [Google Scholar]
- 24.Ke C, Narayan KMV, Chan JCN, et al. Pathophysiology, phenotypes and management of type 2 diabetes mellitus in Indian and Chinese populations. Nat Rev Endocrinol. 2022;18:413–32. doi: 10.1038/s41574-022-00669-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zimmet PZ. Diabetes and its drivers: the largest epidemic in human history? Clin Diabetes Endocrinol. 2017;3:1. doi: 10.1186/s40842-016-0039-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zimmet PZ, El-Osta A, Shi Z. The diabetes epidemic in China is a public health emergency: the potential role of prenatal exposure. J Public Health Emerg. 2017;1:80. doi: 10.21037/jphe.2017.10.01. [DOI] [Google Scholar]
- 27.Lumey LH, Li C, Khalangot M. Long-term impact of pre-natal exposure to the Ukraine famine of 1932-1933 on adult type 2 diabetes mellitus. Science. 2024;385:667–71. doi: 10.1126/science.adn4614. [DOI] [PubMed] [Google Scholar]
- 28.Sparén P, Vågerö D, Shestov DB, et al. Long term mortality after severe starvation during the siege of Leningrad: prospective cohort study. BMJ. 2004;328:11. doi: 10.1136/bmj.37942.603970.9A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rudnytskyi O, Levchuk N, Wolowyna O, et al. Demography of a man-made human catastrophe: The case of massive famine in Ukraine 1932-1933. Can Stud Popul. 2015;42:53. doi: 10.25336/P6FC7G. [DOI] [Google Scholar]
- 30.Ekamper P, Bijwaard G, van Poppel F, et al. War-related excess mortality in The Netherlands, 1944-45: New estimates of famine- and non-famine-related deaths from national death records. Hist Methods. 2017;50:113–28. doi: 10.1080/01615440.2017.1285260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li C. Prenatal famine exposure and later-life risk of type 2 diabetes: examining the relationship in a national longitudinal study in China (Thesis) New York: Columbia University; 2022. [Google Scholar]
- 32.Meslé F, Vallin J, Andreev E. Demographic consequences of the great famine: then and now. Harv Ukr Stud. 2008;30:217–41. [Google Scholar]
- 33.Vallin J, Meslé F, Adamets S, et al. A new estimate of Ukrainian population losses during the crises of the 1930s and 1940s. Popul Stud (Camb) 2002;56:249–63. doi: 10.1080/00324720215934. [DOI] [PubMed] [Google Scholar]
- 34.Banning C. Food Shortage and Public Health, First Half of 1945. Ann Am Acad Pol Soc Sci. 1946;245:93–110. doi: 10.1177/000271624624500114. [DOI] [Google Scholar]
- 35.Burger GCE, Drummond JCS. Malnutrition and starvation in Western Netherlands: September 1944-July 1945. General State Print Office; 1948. [Google Scholar]
- 36.Trienekens GMT. Tussen ons volk en de honger: de voedselvoorziening, 1940-1945 (in Dutch) Matrijs, Utrecht; 1985. [Google Scholar]
- 37.Aird JS. Population studies and population policy in China. Popul Dev Rev. 1982;8:267. doi: 10.2307/1972987. [DOI] [Google Scholar]
- 38.Ashton B, Hill K, Piazza A, et al. Famine in China, 1958-61. Popul Dev Rev. 1984;10:613. doi: 10.2307/1973284. [DOI] [Google Scholar]
- 39.Cao S. The deaths of China’s population and its root cause during 1959-1961 (in Chinese) Popul Sci Chin. 2005;1:3. [Google Scholar]
- 40.Jin H. Memorandum on the ‘Three-year Natural Disaster’ (in Chinese) Society. 1993;4–5:13–22. [Google Scholar]
- 41.Peng X. Demographic consequences of the Great Leap Forward in China’s provinces. Popul Dev Rev. 1987;13:639. doi: 10.2307/1973026. [DOI] [Google Scholar]
- 42.IDF IDF diabetes atlas: diabetes data portal. 2021. [16-Apr-2023]. https://www.diabetesatlas.org/data/en/ Available. Accessed.
- 43.Khalangot M, Kravchenko V, Tronko M, et al. Assessment of current insulin usage in type 2 diabetics according to diabetes type distribution among insulin-treated patients in Ukraine. Diabetol Croat. 2007;36:15–21. doi: 10.1016/j.pcd.2007.10.014. [DOI] [Google Scholar]
- 44.Overbeek JA, Nijpels G, Swart KMA, et al. Type 2 Diabetes, the Epidemic: Trends in Prevalence and Incidence, 2004-2020. Diabetes Metab Syndr Obes. 2024;17:1503–9. doi: 10.2147/DMSO.S445288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang W, Lu J, Weng J, et al. Prevalence of diabetes among men and women in China. N Engl J Med. 2010;362:1090–101. doi: 10.1056/NEJMoa0908292. [DOI] [PubMed] [Google Scholar]
- 46.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 47.Hidayat K, Du X, Shi B-M, et al. Foetal and childhood exposure to famine and the risks of cardiometabolic conditions in adulthood: a systematic review and meta-analysis of observational studies. Obes Rev. 2020;21:e12981. doi: 10.1111/obr.12981. [DOI] [PubMed] [Google Scholar]
- 48.Li C, Tobi EW, Heijmans BT, et al. The effect of the Chinese Famine on type 2 diabetes mellitus epidemics. Nat Rev Endocrinol. 2019;15:313–4. doi: 10.1038/s41574-019-0195-5. [DOI] [PubMed] [Google Scholar]
- 49.Liu H, Chen X, Shi T, et al. Association of famine exposure with the risk of type 2 diabetes: a meta-analysis. Clin Nutr. 2020;39:1717–23. doi: 10.1016/j.clnu.2019.08.002. [DOI] [PubMed] [Google Scholar]
- 50.Liu L, Wang W, Sun J, et al. Association of famine exposure during early life with the risk of type 2 diabetes in adulthood: a meta-analysis. Eur J Nutr. 2018;57:741–9. doi: 10.1007/s00394-016-1363-1. [DOI] [PubMed] [Google Scholar]
- 51.Lumey LH, Li C, Stein AD, et al. Adult glucose dysregulation after severe prenatal food restriction in the Dutch Hunger Winter: Only partial mediation by current body size. J Dev Orig Health Dis. 2017;8 [Google Scholar]
- 52.De Rooij SR, Bleker LS, Painter RC, et al. Lessons learned from 25 Years of Research into Long term Consequences of Prenatal Exposure to the Dutch famine 1944-45: The Dutch famine Birth Cohort. Int J Environ Health Res. 2022;32:1432–46. doi: 10.1080/09603123.2021.1888894. [DOI] [PubMed] [Google Scholar]
- 53.Ravelli AC, van der Meulen JH, Michels RP, et al. Glucose tolerance in adults after prenatal exposure to famine. Lancet. 1998;351:173–7. doi: 10.1016/s0140-6736(97)07244-9. [DOI] [PubMed] [Google Scholar]
- 54.Li C, Lumey LH. Exposure to the Chinese famine of 1959-61 in early life and long-term health conditions: a systematic review and meta-analysis. Int J Epidemiol. 2017;46:1157–70. doi: 10.1093/ije/dyx013. [DOI] [PubMed] [Google Scholar]
- 55.Li C, Lumey LH. Studies into severe famine in early life and diabetes in adulthood: the need to control for differences in participant age and location. Diabetologia. 2017;60:1359–60. doi: 10.1007/s00125-017-4300-9. [DOI] [PubMed] [Google Scholar]
- 56.Liu C, Li C. The need for appropriate “age-balanced” controls and transparent reporting in Chinese famine studies: a re-analysis of the China Patient-centred Evaluative Assessment of Cardiac Events million persons project. Eur J Prev Cardiol. 2023;30:e16–7. doi: 10.1093/eurjpc/zwac254. [DOI] [PubMed] [Google Scholar]
- 57.Wang Z, Zou Z, Yang Z, et al. The association between fetal-stage exposure to the China famine and risk of diabetes mellitus in adulthood: results from the China health and retirement longitudinal study. BMC Public Health. 2018;18:1205. doi: 10.1186/s12889-018-6134-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lu J, Li M, Xu Y, et al. Early Life Famine Exposure, Ideal Cardiovascular Health Metrics, and Risk of Incident Diabetes: Findings From the 4C Study. Diabetes Care. 2020;43:1902–9. doi: 10.2337/dc19-2325. [DOI] [PubMed] [Google Scholar]
- 59.Lumey LH, Stein AD, Susser E. Prenatal famine and adult health. Annu Rev Public Health. 2011;32:237–62. doi: 10.1146/annurev-publhealth-031210-101230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Grey K, Gonzales GB, Abera M, et al. Severe malnutrition or famine exposure in childhood and cardiometabolic non-communicable disease later in life: a systematic review. BMJ Glob Health. 2021;6:e003161. doi: 10.1136/bmjgh-2020-003161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cheng Q, Trangucci R, Nelson KN, et al. Prenatal and early-life exposure to the Great Chinese Famine increased the risk of tuberculosis in adulthood across two generations. Proc Natl Acad Sci U S A. 2020;117:27549–55. doi: 10.1073/pnas.2008336117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li C, Zhou Z, Lumey LH. Early-life exposure to the Chinese famine and tuberculosis risk: Unrecognized biases from different measures of famine intensity. Proc Natl Acad Sci USA. 2021;118:16. doi: 10.1073/pnas.2102809118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li C, Lumey LH. Interaction or mediation by adult obesity of the relation between fetal famine exposure and type 2 diabetes? Int J Epidemiol. 2019;48:654–6. doi: 10.1093/ije/dyy293. [DOI] [PubMed] [Google Scholar]
- 64.Wang N, Wang X, Han B, et al. Is Exposure to Famine in Childhood and Economic Development in Adulthood Associated With Diabetes? J Clin Endocrinol Metab. 2015;100:4514–23. doi: 10.1210/jc.2015-2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wells JCK. The diabesity epidemic in the light of evolution: insights from the capacity-load model. Diabetologia. 2019;62:1740–50. doi: 10.1007/s00125-019-4944-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cheng M, Sommet N, Kerac M, et al. Exposure to the 1959-1961 Chinese famine and risk of non-communicable diseases in later life: A life course perspective. PLOS Glob Public Health. 2023;3:e0002161. doi: 10.1371/journal.pgph.0002161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li C, Tobi EW, Heijmans BT, et al. Reply to ‘Chinese famine and the diabetes mellitus epidemic.’. Nat Rev Endocrinol. 2020;16:123–4. doi: 10.1038/s41574-019-0301-8. [DOI] [PubMed] [Google Scholar]
- 68.Zimmet P, Shi Z, El-Osta A, et al. Chinese Famine and the diabetes mellitus epidemic. Nat Rev Endocrinol. 2020;16:123. doi: 10.1038/s41574-019-0300-9. [DOI] [PubMed] [Google Scholar]
- 69.Feng G-S, Li H-L, Shen Q-M, et al. Population attributable risk of excess weight, abdominal obesity and physical inactivity for type 2 diabetes in Chinese men and women. Ann Transl Med. 2021;9:326. doi: 10.21037/atm-20-6121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lv J, Yu C, Guo Y, et al. Adherence to a healthy lifestyle and the risk of type 2 diabetes in Chinese adults. Int J Epidemiol. 2017;46:1410–20. doi: 10.1093/ije/dyx074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Heijmans BT, Tobi EW, Stein AD, et al. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc Natl Acad Sci U S A. 2008;105:17046–9. doi: 10.1073/pnas.0806560105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tobi EW, Slieker RC, Luijk R, et al. DNA methylation as a mediator of the association between prenatal adversity and risk factors for metabolic disease in adulthood. Sci Adv. 2018;4:eaao4364. doi: 10.1126/sciadv.aao4364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang B, Cheng J, Wan H, et al. Early-life exposure to the Chinese famine, genetic susceptibility and the risk of type 2 diabetes in adulthood. Diabetologia. 2021;64:1766–74. doi: 10.1007/s00125-021-05455-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.