Abstract
Objective
Previous technical limitations prevented the proof of Fcγ-receptor (FcγR)-activation by soluble immune complexes (sICs) in patients. FcγRIIIa (CD16) is a risk factor in rheumatoid arthritis (RA). We aimed at determining the presence of CD16-activating sICs in RA and control diseases.
Methods
Sera from an exploratory cohort (n=50 patients with RA) and a validation cohort (n=106 patients with RA, 20 patients with psoriasis arthritis (PsA), 22 patients with systemic lupus erythematosus (SLE) and 31 healthy controls) were analysed using a new reporter cell assay. Additionally, 26 synovial fluid samples were analysed, including paired serum/synovial samples.
Results
For the first time using a reliable and sensitive functional assay, the presence of sICs in RA sera was confirmed. sICs possess an intrinsic capacity to activate CD16 and can be found in both synovial fluid and in blood. In low experimental dilutions, circulating sICs were also detected in a subset of healthy people and in PsA. However, we report a significantly increased frequency of bioactive circulating sICs in RA. While the bioactivity of circulating sICs was low and did not correlate with clinical parameters, synovial sICs were highly bioactive and correlated with serum autoantibody levels. Receiver operator curves indicated that sICs bioactivity in synovial fluid could be used to discriminate immune complex-associated arthritis from non-associated forms. Finally, circulating sICs were more frequently found in SLE than in RA. The degree of CD16 bioactivity showed strong donor-dependent differences, especially in SLE.
Conclusions
RA is characterised by the presence of circulating and synovial sICs that can engage and activate CD16.
Keywords: rheumatoid arthritis, immune complex diseases, risk factors, synovial fluid, autoimmunity
WHAT IS ALREADY KNOWN ON THIS TOPIC.
WHAT THIS STUDY ADDS
A reporter cell assay demonstrates that RA is characterised by the presence of circulating and synovial soluble immune complexes that can engage and activate CD16.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
The CD16 bioactivity of synovial soluble immune complexes may help to stratify patients with arthritis.
Closer biochemical characterisation of immune complexes with proven bioactivity at FcγRs will lead to a better understanding of autoimmunity in RA.
Introduction
Rheumatoid arthritis (RA) is a rheumatic disease characterised by autoimmunity, with the focus of inflammation in the synovium. Systemic manifestations such as interstitial lung disease, osteoporosis and vasculitis can also occur. The pathophysiology of RA is not fully understood.1 At least its seropositive form is in part mediated by autoantibodies such as rheumatoid factor (RF) and anticitrullinated peptide antibodies (ACPA).2 Interestingly, autoantibodies are associated with extra-articular manifestations and erosiveness but can emerge prior to the onset of symptoms,3 4 indicating further factors driving arthritis than the mere presence of antigen-specific autoantibodies. Indeed, RA pathophysiology is a multistep process,5 and RF and ACPA poorly correlate with disease activity and treatment response and are not used as clinical biomarkers once the diagnosis of RA being established. As autoantibodies are nonetheless specific, for example, targeting citrullinated residues of several proteins, and associated with worse outcomes,6 7 an explanation for the incomplete pathogenicity of autoantibodies is needed. We hypothesise that this explanation may be found in regulatory or functional downstream processes aside antigen specificity or titres.
Through their Fc regions, IgG autoantibodies can engage FcγRs such as FcγRIIIA/CD16. A function of human CD16 in ACPA-positive RA can be derived from polymorphisms identified as genetic risk factors. Mouse models likewise propose a role of CD16 in autoantibody-mediated arthritis.8,12 Nevertheless, recently identified differences between murine and human CD16 warrants further study to understand the role of this FcγR in human disease.13
Importantly, monomeric IgG (auto)antibodies do not per se trigger CD16 activation—CD16 is a low affinity receptor requiring crosslinking and clustering for activation.14 15 As such, CD16 is mainly activated by immobilised IgG, cell-bound antibodies or soluble immune complexes (sICs).16 17 Most likely, CD16-mediated downstream effects of autoantibodies therefore depend on the three-dimensional space surrounding monomer autoantibodies, for example, on its organisation in sICs.
Indeed, preliminary studies from the 1980s suggested the presence of sICs in autoimmune diseases such as RA.2 18 19 Later on, several analyses of enriched protein complexes supposedly containing sICs using chromatography, precipitation and binding to C1q or protein G, for instance, suggested citrullinated antigens within sICs in plasma and synovial fluid in RA.20,22 On the functional level, it was suggested that sICs activate various cell types, including macrophages to produce tumour necrosis factor,23 neutrophils to produce reactive oxygen species products24 and osteoclasts to promote erosions.25 26 It should be mentioned, though, that in some of these studies on sIC function, immune complexes were artificially generated by adding antigens or performing heat aggregation.23 24 26 Other studies enriched sICs prior to in vitro analysis, which alters sIC concentration and cannot be differentiated from more artificial protein aggregates.25 Newer studies used RNA-based cellular pathway signatures to indirectly illustrate the activation of joint neutrophils via IgG, but this may also result from deposited ICs.24 Thus, the exact functions of untreated, native circulating and synovial sICs in vivo in RA remain elusive. In the past, detection of sICs in patient samples was limited by available technology. Previous assays designed to quantify sICs have failed to gain relevance in clinical application because of low sensitivity and specificity and low intermethod reproducibility. Furthermore, these methods are not able to measure sIC bioactivity, meaning their potential to activate FcγRs on expressing cells,18 27 leaving a gap in our understanding of the role of sICs.17 sIC bioactivity includes all parameters relevant to FcγR activation, such as sIC size, sIC concentration, IgG glycan profiles, IgG subclasses and IgG affinity.14 17 26 28 In RA specifically, the Fc glycosylation pattern influences the pro-inflammatory capacity of autoantibodies.1029,32
We have recently developed a novel cell-based reporter assay, which allows for the quantification of sIC bioactivity regarding host FcγR activation.17 33 This enabled us to demonstrate the presence of bioactive circulating sICs in SLE and severe COVID-19, where bioactivity was linked to disease severity.17 34 In addition, we were able to demonstrate a high CD16 bioactivity of synovial sICs in a pilot study in RA.35 In the present study, we investigated the CD16 bioactivity of circulating sICs in a large RA cohort and control cohorts. Furthermore, we compared local CD16 bioactivity in synovial fluid from patients with RA and patients without RA. Finally, we compared local with systemic CD16 bioactivity in paired and unpaired patient samples.
Methods
Patients and materials
Written informed consent was obtained from every blood and synovial fluid donor. Clinical data were extracted from patient records. Data and samples were pseudomised. Patient characteristics are provided in table 1 and online supplemental tables 1,2. Healthy controls (HCs) were volunteers without obvious signs of infection and without a known history of systemic autoimmune disease. These individuals were recruited within the environment of our university clinic and mostly employees or friends and relatives from our scientists. More characteristics of HCs are provided in table 1.
Table 1. Patient characteristics (validation cohort).
| HC | PsA | RA | SLE | |
| Number of samples | 31 | 20 | 106 | 22 |
| Per cent female | 58% | 55% | 72% | 100% |
| Age, mean | 35 | 55 | 61 | 36 |
| Age, range | (23; 76) | (32; 74) | (19; 88) | (19; 68) |
| Per cent RF positive | nd | 10% | 70% | nd |
| Per cent ACPA positive | nd | 10% | 75% | nd |
| Patients on corticosteroids | nd | 5 | 37 | 14 |
| Mean steroid dose | nd | 7.6 mg | 6.4 mg | 9.2 mg |
| Patients on DMARDs | nd | 14 | 71 | 22 |
| Methotrexate | nd | 8 | 43 | 3 |
| Leflunomide | nd | 3 | 25 | 0 |
| Sulfasalazine | nd | 4 | 3 | 0 |
| Hydroxychloroquine | nd | 0 | 1 | 17 |
| Ciclosporin | nd | 0 | 0 | 1 |
| Mycophenolate mofetil | nd | 0 | 0 | 8 |
| Azathioprine | nd | 1 | 2 | 4 |
| Patients on biologicals | nd | 7 | 24 | 6 |
| Tumour necrosis factor inhibitors | nd | 4 | 14 | 0 |
| Anti-IL-6 antibody | nd | 0 | 5 | 0 |
| Anti-IL-12/23 antibody | nd | 1 | 0 | 0 |
| Anti-IL-17A antibody | nd | 2 | 0 | 0 |
| Abatacept | nd | 0 | 4 | 0 |
| Belimumab | nd | 0 | 0 | 4 |
| Rituximab/Obinutuzumab | nd | 0 | 1 | 2 |
| Patients on JAK inhibitors | nd | 0 | 20 | 0 |
Numbers represent absolute numbers except for the the gender row; numbers in the latter represent the percentage of female patients. Of note, two female patients (aged 36 years and 66 years) were punctured twice.
DMARDsdisease-modifying antirheumatic drugsHChealthy controlILinterleukinJAKJanus kinase ndnot determinedPsApsoriatic arthritisRArheumatoid arthritisSLEsystemic lupus erythematosus
Generation and culture of CD16+ BW5147 reporter cells
BW5147 reporter cells were kept in Roswell Park Memorial Institute medium supplemented with 10% fetal calf serum (FCS, Invitrogen), 4 mM glutamin (1/100, Sigma-Aldrich), 1x penicillin/streptomycin (Sigma-Aldrich), 1x sodium pyruvate (Gibco) and 0.1 mM beta-mercaptoethanol (Gibco). BW5147 reporter cells expressing human FcγRIIIA/CD16 ectodomains genetically fused to the transmembrane and cytosolic domains of mouse CD3-zeta were newly prepared for this study. Lentiviral transduction and selection were performed as described previously.17 34 35 In brief, chimeric FcγR-mCD3-zeta constructs encoded on a lentiviral packaging vector and supplementing vectors providing the VSV gag/pol and VSV-G-env proteins were transfected into a 293T packaging cell line at roughly 70% density in a 10 cm dish format. After 3 days, supernatant was collected, filtered (0.45 nM) and incubated with parental BW5147 cells (3.5 mL of supernatant on 106 target cells) followed by culture expansion and antibiotics pool selection using complete medium supplemented with 2 μg/mL of puromycin (Sigma) over a 1-week culture period. Parental cell selection served as a pint of reference. Cell sorting was performed at the Lighthouse core facility of the University Hospital Freiburg using receptor staining (clone 3G8, BD Pharmingen, PE-conjugated). Receptor expression was evaluated regularly via flow cytometry using a fluorescein isothiocyanate (FITC)-conjugated antihuman CD16 antibody (clone 3G8, BioLegend). Parental cells served as a point of reference and staining using FITC-conjugated antihuman CD55 (Cat. 311305; BioLegend) served as isotype control. Flow cytometry was performed on a BD LSR II flow cytometer. Data were analysed using FlowJo software V.10.7.1.
FcγR reporter activation assay
FcγR activation was measured using a previously described cell-based assay.17 34 35 Briefly, 2×105 reporter cells expressing human FcγRIIIA/CD16 or parental cells lacking receptor expression were incubated with graded amounts of patient sample in a 96-well format in a total volume of 100 µL. Incubation on immobilised human intravenous IgG served as positive control for receptor activation (20 µg/mL Octagam, Octapharma on NUNC MaxiSorp plate at 4°C, 16 hours incubation). Incubation with medium alone served as a negative control for receptor activation. Incubation was performed in a 96-well ELISA plate (NUNC MaxiSorp) pretreated with phosphate-buffered saline (PBS)/10% FCS (v/v) for 1 hour. Reporter cell mouse interleukin (IL)-2 secretion was quantified after 16 hours of incubation via anti-IL-2 ELISA (Mouse IL-2 DuoSet, R&D). IL-2 concentrations normalised to the intra-experimental negative control via subtraction and area under the curve (AUC) index values were calculated from titrations (GraphPad Prism). Alternatively, reporter cells were analysed for mouse CD69 expression using a BD LSR II flow cytometer. Staining was performed in PBS/2% FCS using an APC-labelled antimouse CD69 antibody (Clone H1.253, BioLegend). Armenian Hamster IgG (BioLegend) served as a staining control. Additionally, PE-labelled antihuman IgG Fc (BioLegend) was used to control for the binding of human IgG to mouse reporter cells. Data were analysed using FlowJo software V.10.7.1.
Statistical analysis
Statistical analysis was performed using GraphPad Prism software V.9 and V.10. P values <0.05 were considered significant and were interpreted descriptively. Unless stated otherwise, normal distribution of the data was not assumed, and tests were two-sided. The specific tests used are indicated in the figure legends. Graphics were in part created with BioRender.com. If not otherwise indicated, outliers were identified and excluded using ROUT.
Results
As outlined in the ‘Introduction’ section, there is compelling evidence of the importance of autoantibodies and CD16 (FcRɣIIIA) in the pathophysiology of RA. Still, the presumable functional link between autoantibodies and the activation of the low affinity receptor CD16 via the formation of sIC remains to be proven. At present, it is unclear whether sIC in RA are indeed bioactive and can engage FcγRs such as CD16.
CD16+ bioactivity measurement identifies systemic sICs in patients with RA
To overcome drawbacks of previously available methods, we applied a new CD16 reporter cell model that directly determines the bioactivity of body liquids at the CD16 receptor (figure 1A,B). The CD16+ reporter cells produce mouse IL-2 on binding of complexed human IgG to CD16. Thus, they detect sICs in liquids such as synovial fluid and serum without necessitating isolation steps and allow for conclusions of both the presence of immune complexes and their bioactivity in these liquids.17 34 35
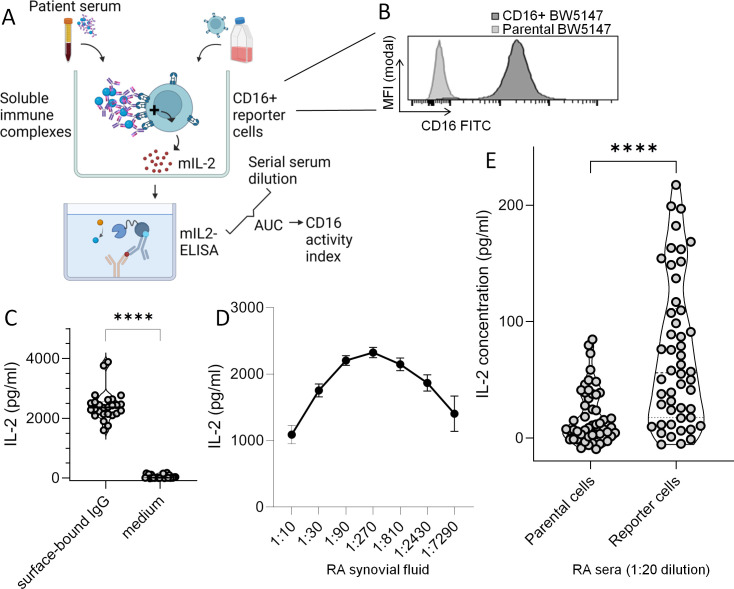
Figure 1. Evaluation of CD16 activation by surface-bound IgG and soluble immune complexes in patient samples using CD16+ reporter BW5147 cells. (A) Graphic experimental design. Patient samples (serum or synovial fluid) was added to CD16+ reporter cells (mouse BW5147 thymoma cells transduced with a construct containing extracellular human CD16) and incubated for 16 hours. CD16 engagement was measured by quantification of interleukin (IL)-2 secretion using ELISA. (B) Expression of CD16 on non-transfected parental and CD16+ reporter cells. The histogram shows an overlay of parental BW5147 cells and successfully transduced CD16+ reporter cells as determined by flow cytometry. (C) IgG complexed to plastic surface activates CD16+ reporter cells. IL-2 concentration produced by CD16+ reporter cells cultured overnight in intravenous immunoglobulin (IVIG)-coated wells across 26 experiments; IVIG-coated wells and culture medium were used as positive and negative controls in experiments with patient samples throughout this study. (D) Soluble immune complexes (sICs) activate CD16+ reporter cells. Synovial fluid from a patient with rheumatoid arthritis (RA) known to contain sIC35 was tested in all n=26 experiments with patient sera as an additional positive control. Error bars indicate SEM. With stable CD16 expression on reporter cells, serial dilution of serum lead to a bioactivity-response curve that mimics the Heidelberger-Kendall curve, which describes the size of immune complexes depending on antigen/antibody ratios. (E) Circulating immune complexes (cICs) activate CD16+ reporter cells. In an exploratory study, parental and CD16+ reporter BW5147 cells were stimulated with serum samples from patients with RA (n=50) at a dilution of 1:20. The violin plot depicts individual patients using the average IL-2 concentration from technical duplicates. Statistical analysis with Wilcoxon matched-pairs test. ****P (two-tailed)<0.0001.
Immobilised IgG is used as a positive control in all the experiments performed with patient liquids in this study (figure 1C). Culture medium served as a negative control. As shown in our previous study, CD16+ reporter cells can detect sICs in synovial fluid.35 As we slightly adapted the protocol, we confirmed the bioactivity of the same synovial fluid samples as used in the previous study.35 We employed this synovial fluid sample as a second positive control throughout this study (figure 1D). Interestingly, serial dilution of this synovial fluid sample resulted in a bioactivity curve that resembled the Heidelberger-Kendall curve (figure 1D). The dilution of patient samples thus affects outcome of the reporter cell model in a complex, non-linear way.
Wondering whether CD16-activating sICs may be also be found in circulation, we conducted an exploratory study using a single dilution (1:20) to screen banked sera from patients with RA (n=50). As shown in figure 1E, RA sera activated CD16+ reporter cells, while the parental cells lacking CD16 were not activated.
Taken together, these data show that CD16+ reporter cells can detect IgG complexed on surfaces or in sICs. The results from the exploratory study suggest the presence of circulating sICs in sera of patients with RA with an intrinsic capacity to activate CD16.
Cohort analysis reveals significant elevation of systemic sICs in RA
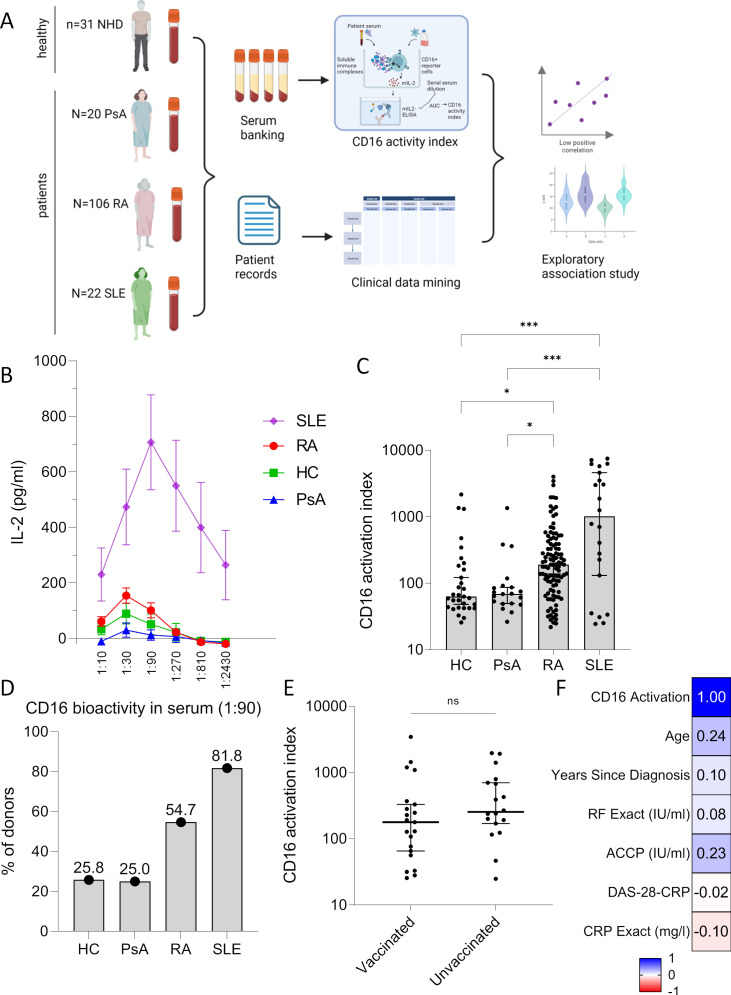
Having observed bioactivity of RA serum on the CD16 receptor in the exploratory screening study using a 1:20 dilution (figure 1D), we next compared the bioactivity of serum from patients with RA with HCs as well as with control rheumatic diseases. Psoriasis arthritis (PsA) is an important differential diagnosis of RA lacking IgG autoantibodies, while systemic lupus erythematosus (SLE) is associated with IgG autoantibodies targeting multiple antigens. SLE can be regarded as a positive control for the presence of circulating sICs, as we had discovered bioactivity at the CD16 receptor in our previous study.17 Patients characteristics are described in table 1. As repeated experiments with synovial fluid indicated variable bioactivity at CD1617 depending on the degree of experimental dilutions (figure 1D), we performed the reporter cell assay using serial dilutions of serum from SLE, RA, HCs and PsA (figure 2A). Circulating sICs in SLE were confirmed to possess high CD16 bioactivity (figure 2B). Similar to what was observed with sIC-containing synovial fluid, serial dilutions of SLE serum resulted in a bioactivity curve indicating an influence of sIC concentrations on CD16 activation on reporter cells, with an optimal activation at 1:90 dilutions. In contrast to SLE, serum from RA, HC and PsA showed much lower activation through CD16. RA serum reached an optimal CD16 activation at a lower dilution (1:30) as compared with SLE (figure 2B).
Figure 2. Detection of bioactive soluble immune complexes in serum from patients with rheumatoid arthritis (RA) using CD16 activity index. (A) Graphic experimental design. Serum samples from the following groups were enrolled: healthy controls (HC; n=31), patients with psoriatic arthritis (PsA; n=20), RA (n=106) and systemic lupus erythematosus (SLE; n=22). (B) Interleukin (IL)-2 secreted by CD16+ reporter BW5147 cells cultured with serial dilutions of serum overnight was determined by ELISA. (C) CD16 activation index (=area under the curve of (IL-2 concentration of the sample–IL-2 concentration in medium control)) induced by serum. Statistical analysis performed with Kruskal-Wallis test, significant post-tests are indicated by stars. (D) Percentage of individuals within each group tested positive for CD16 bioactivity. As a cut-off, a value of ‘1’ pg/mL IL-2 was chosen, as serum from RA, HC and PsA yielded values below 1 in very high dilutions (see B). (E) CD16 activation index in patients with RA who were vaccinated (n=21) and unvaccinated (n=18) against the novel SARS‑CoV‑2 virus. Sample size is limited to patients with RA whose serum was collected after start of the SARS‑CoV‑2 pandemic in 2020. Graph depicts individual patients and medians. Statistical analysis performed with Mann-Whitney U test. (F) Correlation matrix showing Spearman’s r for comparisons with CD16 activation index. CRP, C reactive protein; DAS-28, disease-activity index-28; ns, not significant; RF, rheumatoid factor. *P<0.05; ***p<0.001.
For statistical analysis, we calculated the AUC of these serial dilutions, resulting in the ‘CD16 activation index’ (figure 2C). CD16 activation was variable within groups. While RA had a significantly higher index compared with HC and PsA, we also noted CD16 activation in several HCs.
Diagnostic autoantibody tests frequently use dilutions between 1:80 and 1:100. We used the 1:90 dilution to determine the percentage of individuals within each group that were tested positive for CD16 bioactivity (figure 2D). As a cut-off for being positive, we used a value of ‘1’ pg/mL IL-2 (nota bene: each IL-2 value refers to the result after subtraction of the background IL-2 concentration in wells with culture medium lacking any human serum). This cut-off was chosen, as serum from RA, HC and PsA reached values below 1 in very high dilutions (figure 2B). This analysis confirmed the presence of CD16 bioactivity in about every fourth HC and patient with PsA. It was more frequent in RA and SLE (54.7% and 81.8%). Fisher’s exact tests confirmed that CD16 bioactivity was significantly more frequent in RA compared with HC and PsA, but less frequent compared with SLE (p=0.007, 0.026 and 0.030, respectively).
As the study had been performed during the COVID-19 pandemic, we excluded a significant impact of COVID-19 vaccination on the CD16 activation index (figure 2E).
A comparison with clinical data indicated no correlation of clinical parameters on CD16 activation index, including age, sex, duration of the disease, the concentration of RF and ACPA, disease-activity index-28 (DAS-28) or C reactive protein (CRP) levels (table 1, figure 2F and online supplemental figure S1). No association to specific therapeutic agents was found either (online supplemental figure S2).
In summary, the validation cohort confirmed that circulating sICs possess CD16 bioactivity in RA. CD16 bioactivity is more frequently observed in RA and in SLE compared with HC and PsA. While overall systemic CD16 bioactivity can be very high in patients with SLE, it is rather low in RA, HC and PsA, however, with an important interindividual variability.
CD16 bioactivity in synovial fluid can identify types of arthritis
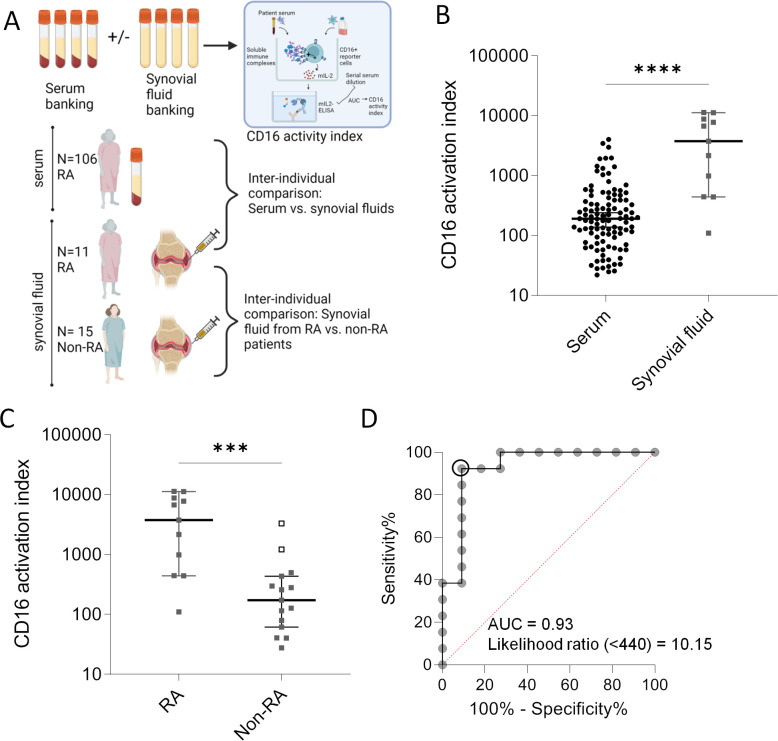
In the previous section, we reported an increased frequency of bioactive sIC in RA serum, yet in low quantities in most cases and without correlation to clinical parameters. As it had been reported that autoantibodies and immune complexes accumulate in joints in RA, we next analysed CD16 bioactivity of RA synovial fluid on several levels (figure 3A).
Figure 3. CD16 bioactivity in synovial fluid in patients with arthritis. (A) Graphic experimental design. Unpaired serum and synovial fluid samples used to determine CD16 bioactivity in these liquids using the reporter cell model. (B) Comparison of unpaired rheumatoid arthritis (RA) serum (n=106) and synovial fluid (n=11) samples. The y-axis represents the CD16 activation index (area under the curve (AUC) for the interleukin (IL)-2 concentrations of six dilution steps of each sample). Graph depicts individual patients as well as medians and 95% CI. Statistical analysis with Mann-Whitney U test. ****P (two-tailed)<0.0001. (C) Comparison of RA (n=11) and non-RA (n=15) synovial fluid samples. Non-RA diagnoses include osteoarthritis, psoriatic arthritis, spondyloarthritis, immune-related adverse event-associated arthritis, gout, polymyalgia rheumatica. For patient details, see online supplemental tables 1–3. Two patients without RA (non-RA) were punctured twice. The two empty squares correspond to the same patient included at two different time points suffering from juvenile idiopathic oligoarthritis; this patient had high antinuclear antibodies (ANA 1:5120) and was identified as an outlier using the ROUT method. Graph depicts individual patients as well as medians and 95% CI. Statistical analysis with Mann-Whitney U test. ***P (two-tailed)=0.0005. (D) Receiver operator curve resulting from data shown in (C); the outliers were not included. Using CD16 activation index of <440 as a threshold yielded in a likelihood ratio of 10.15.
The comparison between serum and synovial fluid from different patients with RA indicated significantly higher bioactivity of synovial sIC compared with circulating sICs (figure 3B).
Synovial fluid from RA possessed a significantly higher CD16 bioactivity than synovial fluid from patients without RA (non-RA) (figure 3C). In the non-RA synovial fluid group, two samples were recognised as outliers using the ROUT method—these samples stemmed from the same patient who underwent arthrocentesis at two different time points and had high levels of antinuclear antibodies (ANAs) indicating that ANA-positive arthritis is associated with synovial sICs. When eliminating these outlier values from the group and creating a receiver operator curve (ROC), an AUC of 0.93 was obtained (figure 3D). This value of 0.93 indicated that synovial sICs could be used to discriminate RA from non-RA. Alternatively, it indicates that the reporter cell model could be used to group distinctive causes of arthritis. The latter interpretation allows re-allocation of the ANA+ outliers to the ‘RA’ group resulting in a combined group of autoantibody-positive arthritis that can be separated from a group of autoantibody-negative arthritis by determining synovial CD16 bioactivity. The ideal cut-off value resulting from ROC was a CD16 activation index of 440, yielding a likelihood ratio of 10.15 (sensitivity of 92.3% and specificity of 90.9%) (figure 3D).
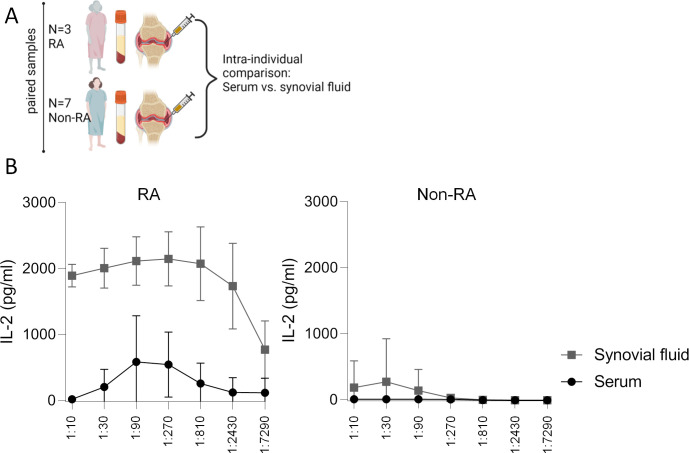
The comparison between paired serum and synovial fluid samples from the same RA donors (n=3, all were seropositive) confirmed that synovial fluid possessed a significantly higher CD16 bioactivity than serum (figure 4A and B). This was the case despite relatively high serum CD16 bioactivity in these patients, the serial dilution curves of which, additionally, described a bioactivity curve (figure 4B). Paired samples from non-RA showed no differences between synovial fluid and serum, but were anyway negative regarding CD16 bioactivity (figure 4B).
Figure 4. CD16 bioactivity in synovial fluid versus serum in patients with arthritis. (A) Graphic experimental design. Paired serum and synovial fluid samples were sampled and used to determine CD16 activation indices in these liquids using the reporter cell model. (B) Comparison of paired serum and synovial fluid samples from patient with seropositive rheumatoid arthritis (RA, n=3, left) and control diseases (n=7 patients without RA (non-RA) with joint effusions including two patients with psoriasis arthritis and each one patient with reactive arthritis, juvenile idiopathic arthritis, spondyloarthritis and immune-related adverse event arthritis). The graphs show mean and SD of interleukin (IL)-2 concentration produced by CD16+ reporter cells in serial dilution experiments. Serum and synovial fluid from non-RA control diseases did not lead to statistically different CD16 activation, while two-way repeated measures analysis of variance confirmed significant differences between RA serum and RA synovial fluid (p=0.035) and also between dilution steps (p=0.007).
As reported above, we observed no correlation between CD16 activation index in serum and clinical parameters in 106 patients with RA. In contrast, CD16 activation index determined in synovial fluid correlated with serum titres of RF (Spearman’s r=0.61, p<0.05) and anti-CCP antibodies (r=0.66, p<0.05), while no correlation with age (r=0.20) and synovial cell number (r=0.26) could be detected.
Together, these data show high CD16 bioactivity in synovial fluid from RA, with significant differences compared with serum and patients with non-RA arthritis. The data further suggest that CD16 bioactivity may be used to distinguish different forms of arthritis.
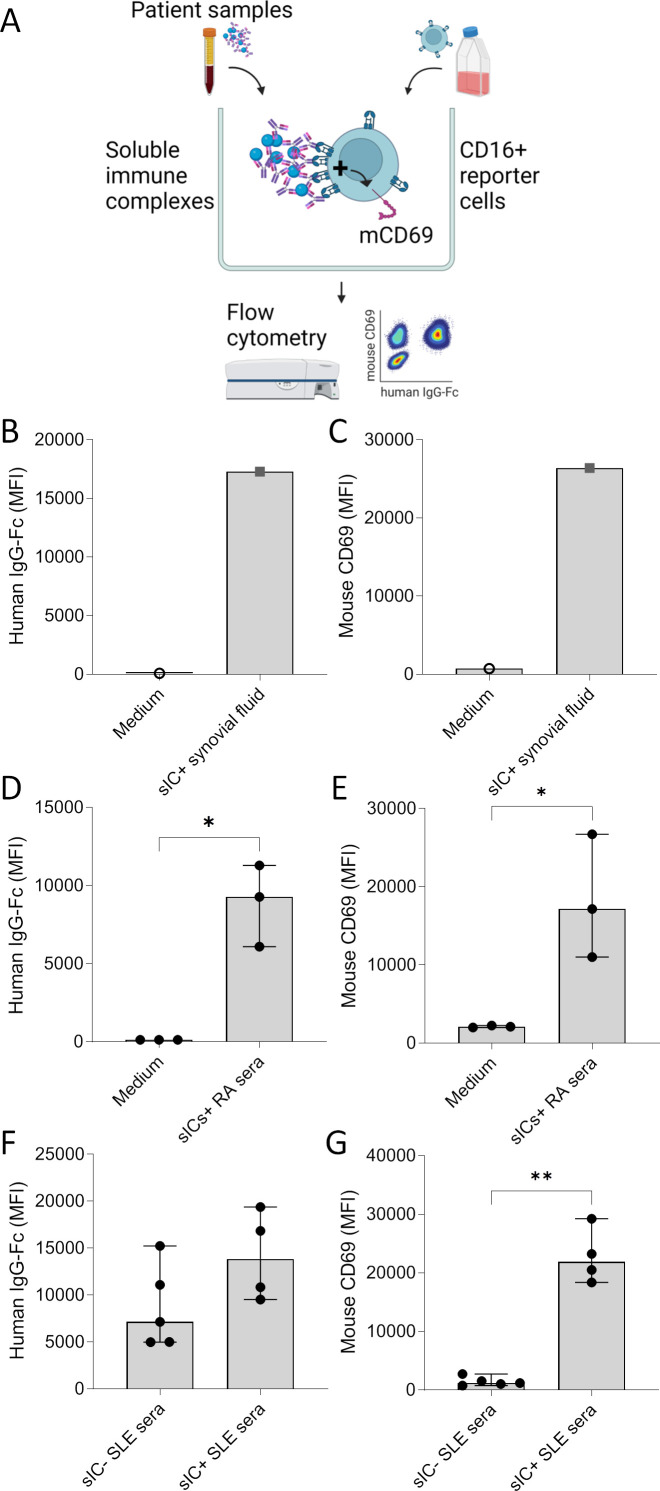
Confirmation of sIC-induced activation of CD16+ reporter cells and loading with human IgG-Fc by flow cytometry
The reporter cell model demonstrated the bioactivity of immune complexes in serum and in synovial fluid. While these results were based on the secretion of IL-2 by activated reporter cells, we next intended to confirm the reporter cell activation and the binding of human IgG by flow cytometry (figure 5A). As previously established, FcγR reporter cell activation can also be measured via upregulation of surface mouse CD69.17 Incubation of synovial fluid or RA serum containing sICs with reporter cells (in 1:90 and 1:30 dilutions in culture medium, respectively) shows significant CD69 upregulation (figure 5C and E) paired with loading of reporter cells with human IgG (figure 5B and D).
Figure 5. Confirmation of soluble immune complex (sIC)-induced activation and loading with human IgG-Fc on CD16+ reporter cells by flow cytometry. (A) Graphic experimental design. CD16+ reporter cells were incubated for 16 hours with medium and synovial fluid or serum and analysed by flow cytometry. (B/D/F) Mean fluorescence intensity (MFI) of reporter cells stained with a PE-labelled antihuman IgG Fc antibody after incubation with soluble immune complex-containing (sIC+) synovial fluid from a seropositive RF+ACPA+ patient with rheumatoid arthritis (RA) (n=1, figure 1D) and medium as negative control (n=1) (B), with sIC+ serum from patients with RA (n=3, selected based on CD16 bioactivity as shown in figure 2), and medium as negative control (n=3) (D), and with sera from patients with systemic lupus erythematosus (SLE). SLE sera were selected based on their CD16 bioactivity (figure 2) to contain sICs (sIC+, n=4) or not (sIC−, n=5) (F). (C/E/G) MFI of reporter cells stained with an APC-labelled antimouse CD69 antibody. The experiments were identical to the B/D/F, respectively, with C corresponding to B, E to D and G to F. Statistical analysis performed with one-tailed Mann-Whitney U tests. *P≤0.05, **p≤0.01.
Using our model, we also addressed the question if pro-inflammatory IgG modifications in SLE such as de-sialylation are sufficient to activate CD16 even in the absence of sIC formation. To this end, we compared sera from patients with active SLE with a low sIC index (sIC−) with sera from patients with a high sIC index (sIC+) with regard to overall IgG binding to the reporter cells. As shown in figure 5F,G, both sIC− and sIC+ SLE sera provided IgG for efficient binding to CD16+ reporter cells. While there was a tendency of higher binding of IgG in sIC+ SLE sera, the patient number was too low to confirm mathematical significance. Importantly, despite IgG loading of reporter cells by both sIC− and sIC+ SLE sera, only sIC+ sera induced significant CD69 upregulation. This confirms that monomeric SLE IgG can bind to CD16 without inducing CD16 activation
Taken together, flow cytometry confirmed the binding of human IgG to the reporter cells as well as the activation of reporter cells by sICs. It also confirmed the non-functional binding of monomeric IgG to CD16 and highlighted the importance of complexed forms of IgG in inducing cell activation through CD16 signalling.
Discussion
The functional proof of circulating and synovial sICs in RA is the key finding of our study. This provides a missing piece in the chain of events in RA pathophysiology, linking the serological products resulting from adaptive immunity to FcɣR(IIIA)-dependent innate immunity.
The highly sensitive reporter cell model we applied17 defined sICs via the functional engagement by CD16 and allowed the direct measurement of sICs in patient samples. This enabled a unique approach to the global characterisation of sICs in RA, integrating previously neglected but relevant factors such as complex size and IgG glycan profile. Another advantage of this method is that it only detects multimeric soluble ICs and is not confounded by monomeric IgGs in clinical samples. A different assay measuring CD16 bioactivity has recently been introduced, but not used to test serum or plasma from patients.36
We find that serial dilution of patient serum samples results in non-linear response curves with decreasing CD16 bioactivity in low dilutions (figures1D 2B). While proof is missing, we hypothesise that these curves—resembling the Heidelberg-Kendall precipitation curve describing IC size depending on antigen/antibody ratios—are a surrogate of varying sizes of the ‘super-complexes’ composed of sICs and FcRs. Given the stable expression of FcγRs on the reporter cell surface, increasingly low dilutions potentially surpass an activation plateau after which sICs saturate the FcγRs, preventing receptor cross-linking. To the best of our knowledge, we describe this finding for the first time, but further control experiments, for example, by using serum-free media, are warranted. Alternatively, we hypothesise that reporter cells experience activation-induced exhaustion or inhibition by other serum factors which may cross-react with the mouse thymoma reporter cell line. The latter is, however, less likely as peak mouse IL-2 secretion was found at the second lowest dilution in RA (figure 2B). Undefined serum factors may also explain the patient-dependent background IL-2-production by parental reporter cells devoid of FcγRs. We conclude that patient samples should therefore be measured in graded amounts to detect sICs across a wide range of concentrations.
We find sICs with high CD16 bioactivity in RA serum and synovial fluid. This finding is of high clinical relevance given the role of CD16 in RA suggested by genetic risk association studies that were strengthened by mechanistic animal models.11 12 We confirm the accumulation of sICs in joints in RA.19 In this study, we expanded on this using a functional model. Beyond that, the comparison of RA with non-RA synovial fluid samples including osteoarthritis, psoriatic arthritis, HLA-B27-positive spondyloarthritis, immune-related adverse event-associated arthritis showed intriguing interdisease differences in the CD16 bioactivity of synovial sICs. Non-RA diseases lacked detection of sICs in our assay. This finding is in line with previous knowledge on the response to B cell-targeting therapies such as rituximab, an anti-CD20 antibody with efficacy in RA and SLE but not in PsA or other non-RA arthritides.37 However, the non-RA group in which synovial fluid was analysed was heterogeneous, precluding general statements of local immune complexes in individual diseases within this group. Whether a CD16-sICs-based categorisation of arthritis could assist in clinical decision-making needs to be investigated in the future.
In total, sera from over 150 patients with RA were analysed. We further compared the CD16 bioactivity in sera of healthy individuals with patients with RA, PsA and SLE. The high sIC bioactivity in the serum of patients with SLE was in line with the results from our previous study,17 and thus confirmed this finding in an independent cohort. We also found a significantly higher sIC bioactivity in RA compared with HCs and PsA, making sIC bioactivity a viable option for differential diagnosis. However, while novel in its discovery, the degree of interpatient variance in circulating sIC bioactivity speaks against sIC quantification as a general RA biomarker. We were also unable to link sIC bioactivity to clinical parameters including a retrospective analysis of medication, disease activity, CRP levels, age and gender. The only meaningful clinical correlation was between CD16 bioactivity in synovial fluid and RF or anti-CCP serum titres, in line with IgG immune complex formation. Future longitudinal measurements will likely reveal possible therapeutic effects on serum CD16 bioactivity. In a patient with systemic sclerosis, we have recently described the disappearance of serum CD16 bioactivity during a combination immunosuppression therapy including CD19.CAR-T cells.38 39
There are limitations intrinsic to this study type, originating from its retrospective character and cryopreservation of serum. Cryopreservation may impact the integrity of immune complexes. Repeated freezing-thawing cycles were avoided to limit the procedural damage of sICs. The focus on CD16 was hypothesis-driven and prevents general statements regarding other FcγRs.
Notably, several healthy donors also showed a low level of circulating sICs or CD16 bioactivity in the serum. This indicates that sICs per se are not exclusively associated with clinically apparent disease, in contrast, it is rather likely that sICs may play a yet ill-defined role in physiological immune responses such as infections.34 The latter does not per se contradict the hypothesis that high levels and chronic persistence of bioactive circulating sICs in RA and especially SLE contribute to autoimmunity, but raises the obvious question about the causes of their occurrence and its consequences. Nota bene, the reporter assay applied in this study only provides information on the principle binding to, and activation of CD16. The CD16 bioactivity measured in the reporter assay does not directly imply the capacity to activate human immune cells in vivo bearing this receptor. As described in the introduction, we are not aware of studies that reliably proved the direct activation of CD16-bearing cells by native sICs. Given the large amounts of studies dealing with autoantibodies and also with sICs in the past, it is likely that attempts to measure direct effects on cells were not successful.
Risk-associated CD16 single nucleotide polymorphisms suggest a pathophysiological role of this FcγR in RA. With CD16 bioactivity of sICs in RA being shown here, previous reports of functional relationships between CD16 activation and pathophysiological hallmarks are substantiated. For example, recent insights on the effects of CD16 on osteoclastogenesis and bone loss which had been investigated using heat aggregation-induced sICs26 now seem to be more likely to happen in vivo. This supports the hypothesis that sICs regulate bone homeostasis.40 In this regard, sICs with CD16 bioactivity are an important missing piece to the puzzle in understanding CD16 biology in RA.
CD16-expressing cells in RA joints include natural killer (NK) cells and monocytes/macrophages. How and whether NK cells contribute to arthritis is not clear. Interestingly, these cells have also been associated with erosiveness41 and may facilitate bone loss by triggering osteoclastogenesis.42 NK cells may act pro-inflammatory by secreting cytokines such as interferon-γ.35 41 Synovial monocytes have been recently shown to upregulate genes involved in Fc-receptor signalling in active RA.43 Single cell analyses with ultra-high resolution will help to more precisely characterise CD16-positive synovial cells and their functions in heath and disease.44 We suggest to add information on sICs for clinical classification and stratification, in order to incorporate this recently neglected but highly important dimension of autoimmunity.45 46 Our model may also be helpful in trials targeting immune complex-mediated immune responses.47 48
Aside from the unclear pathogenicity of circulating immune complexes, further important questions regarding sICs cannot be answered by the reporter assay and remain unsolved: What is their size? Are they homogenous or heterogeneous? What are their constituents? In RA, the presence of citrullinated peptides has been suggested, complement is involved and mass spectrometry indicated abundant proteins within enriched proteins aggregated with IgG.21 49 50 Also RNA may be incorporated in immune complexes, at least in connective tissue diseases.51 However, studies on the biochemical characterisation of immune complexes with proven bioactivity at Fc receptors are still pending and will be the next step towards a better understanding of autoimmunity in RA.
Conclusion
RA is characterised by the presence of circulating and local sICs that can engage and activate CD16.
supplementary material
Acknowledgements
We thank Stefan Krienke, Anja Funkert and further staff of the Medizinische Klinik V, Heidelberg, and all physicians, involved in the treatment of patients and the sampling of materials or data. We also thank Haizhang Chen for his great initial work with reporter cells.
Footnotes
Funding: This study was funded by Deutsche Gesellschaft für Innere Medizin e.V.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not applicable.
Ethics approval: This study was approved by the local institutional review board (University of Heidelberg, S-272/2021). Participants gave informed consent to participate in the study before taking part.
Contributor Information
Ivana Andreeva, Email: ivana.andreeva1998@gmail.com.
Philipp Kolb, Email: philipp.kolb@uniklinik-freiburg.de.
Lea Rodon, Email: lea@rodon.de.
Norbert Blank, Email: norbert.blank@med.uni-heidelberg.de.
Hanns-Martin Lorenz, Email: hanns-martin.lorenz@med.uni-heidelberg.de.
Wolfgang Merkt, Email: wolfgang.merkt@med.uni-heidelberg.de.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
References
- 1.Alivernini S, Firestein GS, McInnes IB. The pathogenesis of rheumatoid arthritis. Immunity. 2022;55:2255–70. doi: 10.1016/j.immuni.2022.11.009. [DOI] [PubMed] [Google Scholar]
- 2.Chang MH, Nigrovic PA. Antibody-dependent and -independent mechanisms of inflammatory arthritis. JCI Insight. 2019;4:e125278. doi: 10.1172/jci.insight.125278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nielen MMJ, van Schaardenburg D, Reesink HW, et al. Specific autoantibodies precede the symptoms of rheumatoid arthritis: a study of serial measurements in blood donors. Arthritis Rheum. 2004;50:380–6. doi: 10.1002/art.20018. [DOI] [PubMed] [Google Scholar]
- 4.Rantapää-Dahlqvist S, de Jong BAW, Berglin E, et al. Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum. 2003;48:2741–9. doi: 10.1002/art.11223. [DOI] [PubMed] [Google Scholar]
- 5.Malmström V, Catrina AI, Klareskog L. The immunopathogenesis of seropositive rheumatoid arthritis: from triggering to targeting. Nat Rev Immunol. 2017;17:60–75. doi: 10.1038/nri.2016.124. [DOI] [PubMed] [Google Scholar]
- 6.Mori S, Koga Y, Sugimoto M. Different risk factors between interstitial lung disease and airway disease in rheumatoid arthritis. Respir Med. 2012;106:1591–9. doi: 10.1016/j.rmed.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 7.Harris ML, Darrah E, Lam GK, et al. Association of autoimmunity to peptidyl arginine deiminase type 4 with genotype and disease severity in rheumatoid arthritis. Arthritis Rheum. 2008;58:1958–67. doi: 10.1002/art.23596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee YH, Bae SC, Song GG. FCGR2A, FCGR3A, FCGR3B polymorphisms and susceptibility to rheumatoid arthritis: a meta-analysis. Clin Exp Rheumatol. 2015;33:647–54. [PubMed] [Google Scholar]
- 9.Morgan AW, Robinson JI, Barrett JH, et al. Association of FCGR2A and FCGR2A-FCGR3A haplotypes with susceptibility to giant cell arteritis. Arthritis Res Ther. 2006;8:R109. doi: 10.1186/ar1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Albert H, Collin M, Dudziak D, et al. In vivo enzymatic modulation of IgG glycosylation inhibits autoimmune disease in an IgG subclass-dependent manner. Proc Natl Acad Sci U S A. 2008;105:15005–9. doi: 10.1073/pnas.0808248105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ji H, Ohmura K, Mahmood U, et al. Arthritis Critically Dependent on Innate Immune System Players. Immunity. 2002;16:157–68. doi: 10.1016/S1074-7613(02)00275-3. [DOI] [PubMed] [Google Scholar]
- 12.Thabet MM, Huizinga TWJ, Marques RB, et al. Contribution of Fcreceptor IIIA gene 158V/F polymorphism and copy number variation to the risk of ACPA-positive rheumatoid arthritis. Ann Rheum Dis. 2009;68:1775–80. doi: 10.1136/ard.2008.099309. [DOI] [PubMed] [Google Scholar]
- 13.Aguilar OA, Fong LK, Ishiyama K, et al. The CD3ζ adaptor structure determines functional differences between human and mouse CD16 Fc receptor signaling. J Exp Med. 2022;219 doi: 10.1084/jem.20220022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nimmerjahn F, Ravetch JV. Fcgamma receptors as regulators of immune responses. Nat Rev Immunol. 2008;8:34–47. doi: 10.1038/nri2206. [DOI] [PubMed] [Google Scholar]
- 15.Bruhns P, Iannascoli B, England P, et al. Specificity and affinity of human Fcγ receptors and their polymorphic variants for human IgG subclasses. Blood. 2009;113:3716–25. doi: 10.1182/blood-2008-09-179754. [DOI] [PubMed] [Google Scholar]
- 16.Merkt W, Lorenz HM, Watzl C. Rituximab induces phenotypical and functional changes of NK cells in a non-malignant experimental setting. Arthritis Res Ther. 2016;18:206. doi: 10.1186/s13075-016-1101-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen H, Maul-Pavicic A, Holzer M, et al. Detection and functional resolution of soluble immune complexes by an FcγR reporter cell panel. EMBO Mol Med. 2022;14:e14182. doi: 10.15252/emmm.202114182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lock RJ, Unsworth DJ. Measurement of immune complexes is not useful in routine clinical practice. Ann Clin Biochem. 2000;37 (Pt 3):253–61. doi: 10.1258/0004563001899393. [DOI] [PubMed] [Google Scholar]
- 19.Halla JT, Volanakis JE, Schrohenloher RE. Immune complexes in rheumatoid arthritis sera and synovial fluids: a comparison of three methods. Arthritis Rheum. 1979;22:440–8. doi: 10.1002/art.1780220502. [DOI] [PubMed] [Google Scholar]
- 20.Van Steendam K, Tilleman K, De Ceuleneer M, et al. Citrullinated vimentin as an important antigen in immune complexes from synovial fluid of rheumatoid arthritis patients with antibodies against citrullinated proteins. Arthritis Res Ther. 2010;12:R132. doi: 10.1186/ar3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao X, Okeke N, Sharpe O, et al. Circulating immune complexes contain citrullinated fibrinogen in rheumatoid arthritis. Arthritis Res Ther. 2008;10:R94. doi: 10.1186/ar2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mathsson L, Lampa J, Mullazehi M, et al. Immune complexes from rheumatoid arthritis synovial fluid induce FcgammaRIIa dependent and rheumatoid factor correlated production of tumour necrosis factor-alpha by peripheral blood mononuclear cells. Arthritis Res Ther. 2006;8:R64. doi: 10.1186/ar1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sokolove J, Zhao X, Chandra PE, et al. Immune complexes containing citrullinated fibrinogen costimulate macrophages via Toll‐like receptor 4 and Fcγ receptor. Arthritis & Rheumatism . 2011;63:53–62. doi: 10.1002/art.30081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wright HL, Lyon M, Chapman EA, et al. Rheumatoid Arthritis Synovial Fluid Neutrophils Drive Inflammation Through Production of Chemokines. Front Immunol. 2021 doi: 10.1101/2020.07.16.20155291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Westhrin M, Kovcic V, Zhang Z, et al. Monoclonal immunoglobulins promote bone loss in multiple myeloma. Blood. 2020;136:2656–66. doi: 10.1182/blood.2020006045. [DOI] [PubMed] [Google Scholar]
- 26.Harre U, Lang SC, Pfeifle R, et al. Glycosylation of immunoglobulin G determines osteoclast differentiation and bone loss. Nat Commun. 2015;6:6651. doi: 10.1038/ncomms7651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lambert PH, et al. WHO collaborative study for evaluation of 18 methods for detecting immune-complexes in serum. J Clin Lab Immunol. 1978;1:1–15. [Google Scholar]
- 28.Kissel T, Toes REM, Huizinga TWJ, et al. Glycobiology of rheumatic diseases. Nat Rev Rheumatol. 2023;19:28–43. doi: 10.1038/s41584-022-00867-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parekh RB, Dwek RA, Sutton BJ, et al. Association of rheumatoid arthritis and primary osteoarthritis with changes in the glycosylation pattern of total serum IgG. Nature New Biol. 1985;316:452–7. doi: 10.1038/316452a0. [DOI] [PubMed] [Google Scholar]
- 30.Seeling M, Brückner C, Nimmerjahn F. Differential antibody glycosylation in autoimmunity: sweet biomarker or modulator of disease activity? Nat Rev Rheumatol. 2017;13:621–30. doi: 10.1038/nrrheum.2017.146. [DOI] [PubMed] [Google Scholar]
- 31.Scherer HU, Huizinga TWJ, Krönke G, et al. The B cell response to citrullinated antigens in the development of rheumatoid arthritis. Nat Rev Rheumatol. 2018;14:157–69. doi: 10.1038/nrrheum.2018.10. [DOI] [PubMed] [Google Scholar]
- 32.Rombouts Y, Ewing E, van de Stadt LA, et al. Anti-citrullinated protein antibodies acquire a pro-inflammatory Fc glycosylation phenotype prior to the onset of rheumatoid arthritis. Ann Rheum Dis. 2015;74:234–41. doi: 10.1136/annrheumdis-2013-203565. [DOI] [PubMed] [Google Scholar]
- 33.Corrales-Aguilar E, Trilling M, Reinhard H, et al. A novel assay for detecting virus-specific antibodies triggering activation of Fcγ receptors. J Immunol Methods. 2013;387:21–35. doi: 10.1016/j.jim.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 34.Ankerhold J, Giese S, Kolb P, et al. Circulating multimeric immune complexes contribute to immunopathology in COVID-19. Nat Commun. 2022;13:5654. doi: 10.1038/s41467-022-32867-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao S, Grieshaber-Bouyer R, Rao DA, et al. Effect of JAK Inhibition on the Induction of Proinflammatory HLA-DR+CD90+ Rheumatoid Arthritis Synovial Fibroblasts by Interferon-γ. Arthritis Rheumatol. 2022;74:441–52. doi: 10.1002/art.41958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Naito R, Ohmura K, Higuchi S, et al. Positive and negative regulation of the Fcγ receptor-stimulating activity of RNA-containing immune complexes by RNase. JCI Insight. 2023;8:e167799. doi: 10.1172/jci.insight.167799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Edwards JCW, Szczepanski L, Szechinski J, et al. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N Engl J Med. 2004;350:2572–81. doi: 10.1056/NEJMoa032534. [DOI] [PubMed] [Google Scholar]
- 38.Merkt W, Lorenz HM, Schmitt M. CAR T-Cell Therapy in Autoimmune Disease. N Engl J Med. 2024;390:1628–9. doi: 10.1056/NEJMc2403705. [DOI] [PubMed] [Google Scholar]
- 39.Merkt W, Freitag M, Claus M, et al. Third-generation CD19.CAR-T cell-containing combination therapy in Scl70+ systemic sclerosis. Ann Rheum Dis. 2024;83:543–6. doi: 10.1136/ard-2023-225174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Onuora S. Osteoimmunology: IgG immune complexes directly regulate bone homeostasis. Nat Rev Rheumatol. 2015;11:257. doi: 10.1038/nrrheum.2015.51. [DOI] [PubMed] [Google Scholar]
- 41.Yamin R, Berhani O, Peleg H, et al. High percentages and activity of synovial fluid NK cells present in patients with advanced stage active Rheumatoid Arthritis. Sci Rep. 2019;9:1351. doi: 10.1038/s41598-018-37448-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Söderström K, Stein E, Colmenero P, et al. Natural killer cells trigger osteoclastogenesis and bone destruction in arthritis. Proc Natl Acad Sci U S A. 2010;107:13028–33. doi: 10.1073/pnas.1000546107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang F, Wei K, Slowikowski K, et al. Defining inflammatory cell states in rheumatoid arthritis joint synovial tissues by integrating single-cell transcriptomics and mass cytometry. Nat Immunol. 2019;20:928–42. doi: 10.1038/s41590-019-0378-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang F, Jonsson AH, Nathan A, et al. Deconstruction of rheumatoid arthritis synovium defines inflammatory subtypes. Nat New Biol. 2023;623:616–24. doi: 10.1038/s41586-023-06708-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rivellese F, Surace AEA, Goldmann K, et al. Rituximab versus tocilizumab in rheumatoid arthritis: synovial biopsy-based biomarker analysis of the phase 4 R4RA randomized trial. Nat Med. 2022;28:1256–68. doi: 10.1038/s41591-022-01789-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Humby F, Durez P, Buch MH, et al. Rituximab versus tocilizumab in anti-TNF inadequate responder patients with rheumatoid arthritis (R4RA): 16-week outcomes of a stratified, biopsy-driven, multicentre, open-label, phase 4 randomised controlled trial. Lancet. 2021;397:305–17. doi: 10.1016/S0140-6736(20)32341-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Blumberg LJ, Humphries JE, Jones SD, et al. Blocking FcRn in humans reduces circulating IgG levels and inhibits IgG immune complex-mediated immune responses. Sci Adv. 2019;5:eaax9586. doi: 10.1126/sciadv.aax9586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zuercher AW, Spirig R, Baz Morelli A, et al. Next-generation Fc receptor-targeting biologics for autoimmune diseases. Autoimmun Rev. 2019;18:102366. doi: 10.1016/j.autrev.2019.102366. [DOI] [PubMed] [Google Scholar]
- 49.Ohyama K, Ueki Y, Kawakami A, et al. Immune complexome analysis of serum and its application in screening for immune complex antigens in rheumatoid arthritis. Clin Chem. 2011;57:905–9. doi: 10.1373/clinchem.2010.157776. [DOI] [PubMed] [Google Scholar]
- 50.Monach PA, Hueber W, Kessler B, et al. A broad screen for targets of immune complexes decorating arthritic joints highlights deposition of nucleosomes in rheumatoid arthritis. Proc Natl Acad Sci U S A. 2009;106:15867–72. doi: 10.1073/pnas.0908032106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lande R, Lee EY, Palazzo R, et al. CXCL4 assembles DNA into liquid crystalline complexes to amplify TLR9-mediated interferon-α production in systemic sclerosis. Nat Commun. 2019;10:1731. doi: 10.1038/s41467-019-09683-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.