Abstract
AIM
To evaluate the clinical effect of a new surgery technique (covering corneal stromal lenticule, CSL) for macular hole (MH) in pathological myopia.
METHODS
This was a prospective non-randomized series case study. Fourteen eyes of 14 patients whose axial length were more than 29 mm and suffered from MH and macular hole retinal detachment (MHRD) were included in this study. All cases were treated with 25-gauge pars plana vitrectomy (PPV) with internal limiting membrane (ILM) peeling, covering CSL and C3F8 gas tamponade. These cases were followed for 6mo, and the best-corrected visual acuity (BCVA), healing status of MH, the reattached rate of retinal detachment (RD), and reoperation rate were analyzed.
RESULTS
All cases were successfully performed the surgery and the postoperative follow-up was completed. After surgery, MHs were healed in all 14 eyes (100%, 14/14) after assessed by optical coherence tomography. The reattachment of retina was achieved in all 6 eyes (100%, 6/6) with MHRD. BCVA was improved in 12 eyes (85.71%, 12/14), and had no significant change in 2 eyes (14.29%, 2/14). The overall mean BCVA was improved from 1.80±0.77 to 0.82±0.46 logMAR (F=10.46, P<0.01). No serious complications occurred in all cases.
CONCLUSION
The new surgery technique (covering CSL) has high reattached rate of RD and high healing rate of MH in pathological myopia in the preliminary study. And it can effectively improve the visual function of patients. This new technique offers meaningful new ideas for treating refractory MH in pathological myopia.
Keywords: corneal stromal lenticule, macular hole, pathological myopia
INTRODUCTION
Compared to the macular hole (MH) and macular hole retinal detachment (MHRD) with normal ocular axis, MH and MHRD in pathological myopia with super-long ocular axis are often accompanied by retinochoroidal atrophy, posterior scleral staphyloma, tight adhesion of vitreous body and abnormal peripheral retina and other characteristics[1]. Due to these abnormal characteristics of pathological myopia, the surgery to treat these kinds of diseases is much more difficult than the ordinary MH surgery. And due to the lack of sufficient internal limiting membrane (ILM) in the atrophied macular area to cover the MH, the healing rate of MH is also low. The healing rate of pathological myopia MH in reports from different countries and regions is between 60% and 70%[2]–[4].
To improve the success rate of surgery, the team of professor Tomaso Capoross invented a technique named human amniotic membrane (AM) tamponade to treat pathological myopia MH and had achieved perfect effect in clinical application[5]. However, the AM is not a completely transparent tissue and it can form a corresponding translucent area on the macular area. The translucent area can theoretically have an influence on the vision or visual field. Inspired by this study, we proposed a novel surgical method that covering fully transparent corneal stromal lenticule (CSL) on the MH to promote MH healing. And this new surgery technique had achieved satisfactory effect in clinical trials. Therefore, we have reported our study as follows.
SUBJECTS AND METHODS
Ethical Approval
This was a prospective non-randomized series case study. The case data were obtained from inpatient department at Mianyang Wanjiang Eye Hospital. All the subjects signed informed consent. And the trial protocol was approved by the Medical Ethics Committee of Mianyang Wanjiang Eye Hospital (the approval number: WJYK-04) and in accordance with the ethical requirements of clinical trials and the Declaration of Helsinki.
Inclusion Criteria
The primary disease of all cases was MH or MHRD with pathological myopia and the ocular axial length was more than 29 mm.
Exclusion Criteria
The ocular axial length was less than 29 mm. The cases had undergone the surgery of macular buckling, posterior scleral reinforcement or any other vitreoretinal surgery. The cases could not, or had no value for second surgery because of systemic disease, ocular inflammation, or already blindness.
Preoperative Examination
All subjects underwent comprehensive ophthalmologic examination before surgery, including best-corrected visual acuity (BCVA), intraocular pressure (IOP), slit lamp biomicroscopy examination, fundus examination, optical coherence tomography (OCT; Heidelberg Spectralis-OCT, Germany) examination of macular area, scanning laser ophthalmoscope (SLO; Optos, UK) examination. The BCVA was converted to logMAR visual acuity to facilitate statistical analysis.
Preparation of Corneal Stromal Lenticule
The CSL (between 40–90 µm thickness) was obtained from small incision lenticule extraction (SMILE) surgery by femtosecond laser and stored in glycerol anhydrous at ultra-low temperature. The CSL was taken out of glycerol during pars plana vitrectomy (PPV), washed with balanced salt solution and stained with indocyanine green to facilitate identification during intraocular operation. And the CSL was trimmed to 3×3 mm2 size with microscopic scissors to cover the MH (Figure 1).
Figure 1. Preparation of the graft.
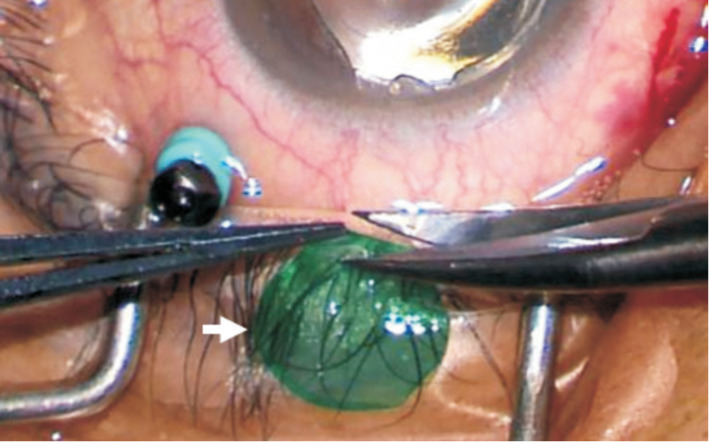
The CSL was obtained from SMILE surgery and stored in glycerol anhydrous at ultra-low temperature. During PPV, the CSL was taken out of glycerol and washed with balanced salt solution and stained with indocyanine green to facilitate identification during intraocular operation. And the CSL (white arrow) was trimmed to 3×3 mm2 size with microscopic scissors to cover the MH. CSL: Corneal stromal lenticule; SMILE: Small incision lenticule extraction; PPV: Pars plana vitrectomy; MH: Macular hole.
Surgical Methods
All subjects were treated with 25-gauge PPV with ILM peeling, covering CSL and C3F8 gas tamponade. The specific methods were as follows. The vitreous was excised through a 3-port, 25-gauge surgical incision. Triamcinolone acetonide was injected into vitreous cavity to completely remove the posterior vitreous cortex. The ILM of macular area was peeled after indocyanine green staining. The liquid under the retina was removed by fluid-air exchange. Retinal photocoagulation was performed if there were retinal holes or retinal degeneration areas in the surrounding retina. Then, a piece of 3×3 mm2 pre-processed CSL was passed the vitreous cavity through a 25-gauge valved trocar and laid flat on the surface of MH. The position of CSL should be in the middle of the MH, and the CSL should cover the edge of the MH. The residual liquid around the CSL was removed with a flute needle to keep the CSL tightly attaching retina and no longer moving (Figure 2). At last, 13% C3F8 gas was injected into vitreous cavity and the incision was sutured. After the surgery, the patients need to rest in face down or lateral position for 2 to 3wk, given anti-inflammatory and symptomatic treatment, monitored BCVA and IOP. All patients were reexamined with OCT and SLO in 1, 3, and 6mo after surgery.
Figure 2. Covering the CSL on the MH.
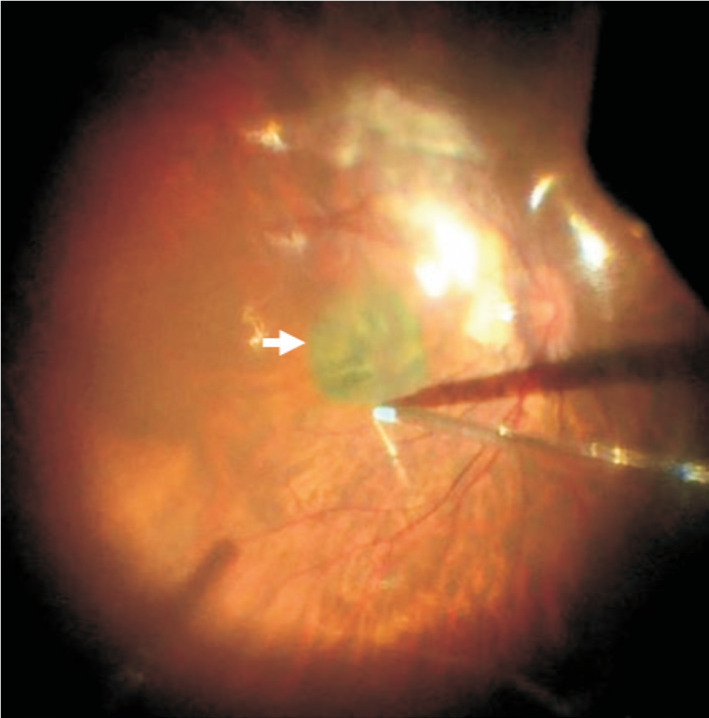
A piece of 3×3 mm2 pre-processed CSL (white arrow) was passed the vitreous cavity through a 25-gauge valved trocar and laid flat on the surface of MH. The position of CSL should be in the middle of the MH, and the CSL should cover the edge of the MH. The residual liquid around the CSL was removed with a flute needle to keep the CSL tightly attaching retina and no longer moving. CSL: Corneal stromal lenticule; MH: Macular hole.
Statistical Analysis
The SPSS 26.0 software was used for statistical analysis. The BCVA and IOP as measurement data were expressed by mean±standard deviation and analyzed by variance analysis with one-way repeated measures. The within-subject effect test was used for P>0.05 in Mochi sphericity test, α test. The multivariate test was used for P<0.05 in Mochi sphericity test, α test. The indicators for each time period were compared using the LSD-t test. The χ2 test was used to compare the count data between two groups. If 1≤theoretical frequency<5, Chi-square should be corrected. If theoretical frequency<1, the Fisher exact probability method was used.
RESULTS
A total of 14 eyes of 14 subjects met the inclusion criteria, and all subjects completed the treatment and half a year follow-up without missing halfway. The subjects were 3 males and 11 females with an average age of 55.8±11.6 (ranged 27 to 74)y. The mean preoperative BCVA was 1.80±0.77, and the mean ocular axial length was 30.3±0.9 (ranged 29.0 to 31.4) mm. The basic preoperative information of the subjects was shown in Table 1. The retina was reattached in all 6 eyes with MHRD. MHs were healed in all subjects (14 eyes). The typical cases of recovery of the macular area were shown in Figures 3 and 4. The mean BCVA of postoperative follow-up in 1, 3, and 6mo were 1.48±0.63, 1.06±0.47, and 0.82±0.46 logMAR.
Table 1. The basic information of the subjects before surgery.
| ID | Sex | Age (y) | Eye | Axial length (mm) | MH diameter (µm) | Combined with RD |
| 1 | F | 44 | OS | 31.4 | 518 | No |
| 2 | M | 74 | OS | 29.7 | 624 | Yes |
| 3 | F | 55 | OD | 30.0 | 627 | Yes |
| 4 | F | 53 | OS | 29.6 | 662 | Yes |
| 5 | F | 58 | OS | 29.3 | 660 | Yes |
| 6 | M | 72 | OS | 30.2 | 611 | Yes |
| 7 | M | 56 | OS | 30.5 | 643 | No |
| 8 | F | 62 | OD | 29.0 | 702 | No |
| 9 | F | 59 | OD | 30.2 | 1116 | No |
| 10 | F | 49 | OS | 30.5 | 1111 | No |
| 11 | F | 58 | OD | 32.6 | 762 | No |
| 12 | F | 51 | OS | 29.9 | 1325 | No |
| 13 | F | 63 | OD | 30.7 | 357 | Yes |
| 14 | F | 27 | OS | 31.2 | 807 | No |
OD: Oculus dexter; OS: Oculus sinister; MH: Macular hole; RD: Retinal detachment.
Figure 3. Information of the No.4 patient.
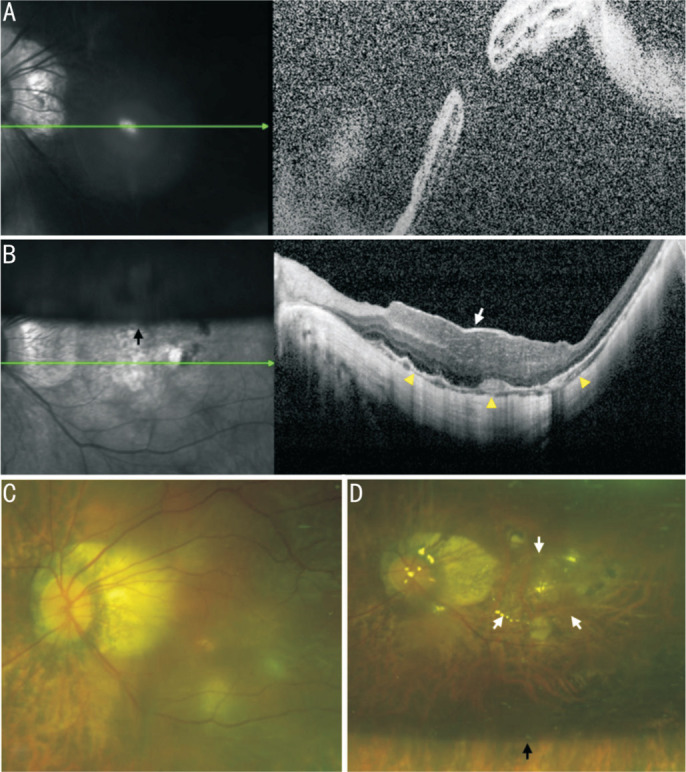
A, C: The OCT and SLO of macular area show an MHRD before PPV; B, D: The OCT and SLO of macular area show that RD was reattached and MH was healed by covering CSL (white arrow) after PPV. The yellow triangles point to ellipsoid zone of continuity healing. The black arrow points to the boundary of the C3F8 gas that had not yet been absorbed. OCT: Optical coherence tomography; SLO: Scanning laser ophthalmoscopy; PPV: Pars plana vitrectomy; RD: Retinal detachment; MH: Macular hole; MHRD: Macular hole retinal detachment; CSL: Corneal stromal lenticule.
Figure 4. Information of the No.8 patient.
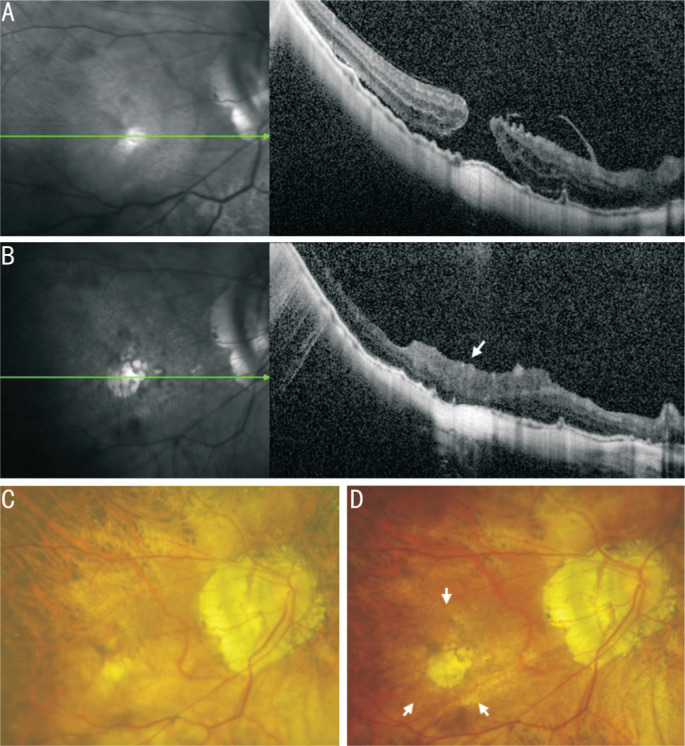
A, C: The OCT and SLO of macular area show an MH before PPV; B, D: The OCT and SLO of macular area show that MH was healed by covering CSL (white arrow) after PPV. Since the CSL is completely transparent, the CSL is almost invisible in SLO. OCT: Optical coherence tomography; SLO: Scanning laser ophthalmoscopy; PPV: Pars plana vitrectomy; MH: Macular hole; CSL: Corneal stromal lenticule.
At the last follow-up (6mo after surgery), the BCVA was improved in 12 eyes (85.71%, 12/14), had no significant change in 2 eyes (14.29%, 2/14). The overall mean BCVA was improved from 1.80±0.77 to 0.82±0.46 (F=10.46, P<0.01).
The IOP of all subjects was kept within normal limits. No intraocular rejection, infection and other serious complications occurred after surgery. The comparisons of preoperative and postoperative indicators are detailed in the Tables 2 and 3.
Table 2. Comparison of BCVA and IOP before and after surgery.
| Parameters | Preop. | Postop. 1mo | Postop. 3mo | Postop. 6mo | F | P | Partial Eta squared | Sphericity test |
| BCVA, logMAR | 1.80±0.77 | 1.48±0.63 | 1.06±0.47 | 0.82±0.46 | 10.46 | 0.00 | 0.74 | 0.00 |
| IOP, mm Hg | 16.64±3.69 | 17.55±4.75 | 17.08±3.29 | 17.34±2.28 | 0.44 | 0.73 | 0.11 | 0.02 |
BCVA: Best-corrected visual acuity; IOP: Intraocular pressure.
mean±SD
Table 3. Recovery situation of MH and RD.
| Parameters | MH |
RD |
Ellipsoid zone |
|||
| Healed | Unhealed | Detachment | In situ or reattachment | Continuity | Discontinuity | |
| Preop. | 0 | 14 | 6 | 8 | 0 | 14 |
| Postop. | 14 | 0 | 0 | 14 | 5 | 9 |
| P | 0.00 | 0.02 | 0.04 | |||
MH: Macular hole; RD: Retinal detachment.
n
DISCUSSION
Pathological myopia MH and its related complications (MHRD) seriously affect the visual function of patients. If the treatment is not timely done, the chance of secondary blindness and eyeball atrophy is extremely high. This disease mainly relies on surgery for treatment.
At present, the routine surgical method is PPV with ILM peeling on macular area and intraocular tamponade. And the healing rate of pathological myopia MH in reports from different countries and regions is between 60% and 70%[2]–[4]. In view of the inherent defects of the low success rate of routine surgery, scholars from all over the world have carried out a lot of technical improvement and innovation on pathological myopia MH surgery from inside and outside the eyeball. Among them, the internal surgical innovation techniques which represented by PPV include: ILM peeling[6], ILM translocation[7], ILM plug[8], inverted ILM flap[9], MH edge massage[10]–[11], autologous blood covering[12]–[13], lens capsule transplantation[14], autologous neurosensory retinal free flap[15], and the human AM plug[16], etc. The external surgical innovation techniques which represented by posterior scleral reinforcement[17] are derivated as posterior scleral shortening. Compared with routine surgery, although the above innovation techniques can significantly improve the healing rate of pathological myopia MH, there are still defects that cannot be ignored.
ILM related surgical techniques (ILM plug, ILM translocation, etc.) require extremely high skill of the operator. Even experienced ophthalmologists may not be able to perform the perfect surgical design for every patient. At the same time, the integrality of ILM of macular area is also the key factor which restricts the success of the surgery. In contrast, the techniques such as MH edge massage, autologous blood covering have low requirements on the operator. However, these techniques can only play a role in assisting MH healing, but have no substantial contribution to the overall clinical effect.
Autologous or allogeneic lens capsule transplantation, autologous neurosensory retinal free flap, and other innovation techniques are mostly used for recurrent, refractory MH surgery. Because these patients had already undergone one or more surgeries, there was no excess ILM to provide in macular area. These surgical techniques only are expedients. Autologous neurosensory retinal free flap in particular requires cutting a piece of retinal tissue in the peripheral retina, and this operation itself can cause a new harm to the patient. The external surgeries such as scleral shortening or posterior scleral reinforcement have long been faced with the problem of complex surgical methods and have a potential risk of compression ischemia in the macular area.
Our team has made technical improvements on the basis of human AM plug[5],[16] technique invented by professor Tomaso Capoross, and developed it into a new technique named AM covering for MH[18], and successfully applied it to repair of MH. Compared with the previous techniques of plugging, the advantages of our technique are as follows: the AM only covers the surfaces of the MH and does not damage the retinal pigment epithelium below the MH, the healing of MH is according to its own anatomic layers, which has no effect on the structure of the outer retina. This technique greatly improves the healing rate of MH without increasing iatrogenic damage. But due to the opacity of AM, it more or less affects the visual quality of patients. To solve this problem, we need to find an easily available, transparent, and biocompatible material to replace the ILM and AM, which can perfectly fit the surgical method of covering.
CSL is a kind of transparent material which can be obtained by SMILE surgery. The CSL, consisting of collagen fibrolamellar, is the anterior layer tissue of the stroma in the central corneal region. And the main cellular component between the fibrolamellar is the corneal stromal cells. Previously, the CSL removed by femtosecond laser was often discarded directly. In recent years, the clinical and fundamental investigations of CSL have gradually begun to treat hyperopia[19], presbyopia[20], corneal ulcer[21], corneal perforation[22], keratoconus[23] and so on. We consider that since the CSL itself is a natural tissue of the eye and its biocompatibility, permeability and comprehensive property are better than AM, if it can be applied to fundus or macular surgery, it can theoretically play a positive role in the recovery of patients' visual function. Based on this idea, our team innovatively proposed a new method that covering the CSL on MH to promote the healing of hole.
Therefore, we selected CSL with a thickness of 40–90 µm which extracted from SMILE surgery and stored in eye bank laboratory of Mianyang Wanjiang Eye Hospital. Since this surgery technique did not require the cellular activity of CSL, we chose the anhydrous glycerol to store the CSL, which method was both easy and cost-effective. Therefore, we aseptically processed this kind of CSL and stored it into anhydrous glycerol at ultra-low temperature. It had been shown that cell-free CSL could be stored in this way for up to 5y[24]. Each CSL was managed according to corneal transplantation specification and met the requirements of nosocomial infection.
In this study, all 14 eyes of 14 subjects achieved HM healing and retina reattached, and the forms of HM healing were as follow: the transparent CSL covered on the surface of the MH, the MH was healed according to its own anatomical hierarchy and had no iatrogenic damage on the outer retinal structure, 5 subjects achieved HM healing with ellipsoid zone continuity. The majority of the cases (12 cases) had BCVA improvement. No intraocular rejection or inflammatory reactions occurred in all cases. Limited to the current small sample numbers and short observation time, more clinical studies are still needed to verify and explore its mechanism and clinical significance.
Footnotes
Foundation: Supported by Medical Research Project of Sichuan (No.S23090).
Conflicts of Interest: Tang ZY, None; Qiao G, None; Zhang XJ, None; Xie LJ, None; Zou QX, None; He CM, None; Zhao L, None; Yang HQ, None; Quan Y, None; Cao K, None; Jiang H, None; He YK, None.
REFERENCES
- 1.Rizzo S, Caporossi T, Tartaro R, Finocchio L, Franco F, Barca F, Giansanti F. A human amniotic membrane plug to promote retinal breaks repair and recurrent macular hole closure. Retina. 2019;39(Suppl 1):S95–S103. doi: 10.1097/IAE.0000000000002320. [DOI] [PubMed] [Google Scholar]
- 2.Haritoglou C, Wolf A, Wachtlin J. Surgery of large and persistent macular holes. Ophthalmologe. 2019;116(11):1011–1019. doi: 10.1007/s00347-019-00949-x. [DOI] [PubMed] [Google Scholar]
- 3.Fotis K, Alexander P, Sax J, Reddie I, Kang CY, Chandra A. Macular detachment for the treatment of persistent full-thickness macular holes. Retina. 2019;39(Suppl 1):S104–S107. doi: 10.1097/IAE.0000000000002370. [DOI] [PubMed] [Google Scholar]
- 4.Wu TT, Kung YH, Chang CY, Chang SP. Surgical outcomes in eyes with extremely high myopia for macular hole without retinal detachment. Retina. 2018;38(10):2051–2055. doi: 10.1097/IAE.0000000000001806. [DOI] [PubMed] [Google Scholar]
- 5.Caporossi T, Pacini B, De Angelis L, Barca F, Peiretti E, Rizzo S. Human amniotic membrane to close recurrent, high myopic macular holes in pathologic myopia with axial length of ≥30 mm. Retina. 2020;40(10):1946–1954. doi: 10.1097/IAE.0000000000002699. [DOI] [PubMed] [Google Scholar]
- 6.Pradhan D, Agarwal L, Joshi I, Kushwaha A, Aditya K, Kumari A. Internal limiting membrane peeling in macular hole surgery. Ger Med Sci. 2022;20:Doc07. doi: 10.3205/000309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pires J, Nadal J, Gomes NL. Internal limiting membrane translocation for refractory macular holes. Br J Ophthalmol. 2017;101(3):377–382. doi: 10.1136/bjophthalmol-2015-308299. [DOI] [PubMed] [Google Scholar]
- 8.Giansanti F, Tartaro R, Caporossi T, Bacherini D, Savastano A, Barca F, Rizzo S. An internal limiting membrane plug and gas endotamponade for recurrent or persistent macular hole. J Ophthalmol. 2019;2019:6051724. doi: 10.1155/2019/6051724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen GH, Tzekov R, Jiang FZ, Mao SH, Tong YH, Li WS. Inverted ILM flap technique versus conventional ILM peeling for idiopathic large macular holes: a meta-analysis of randomized controlled trials. PLoS One. 2020;15(7):e0236431. doi: 10.1371/journal.pone.0236431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar A, Tinwala SI, Gogia V, Sehra SV. Tapping of macular hole edges: the outcomes of a novel technique for large macular holes. Asia Pac J Ophthalmol (Phila) 2013;2(5):305–309. doi: 10.1097/APO.0b013e31829a1919. [DOI] [PubMed] [Google Scholar]
- 11.Wang H, Ji M, Di R, Qi Y, Pei C, Gao S, Liu SW, Xie AM, Cheng YH. Parafoveal retinal massage combined with autologous blood cover in the management of giant, persistent or recurrent macular holes. Int J Ophthalmol. 2020;13(11):1773–1779. doi: 10.18240/ijo.2020.11.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lai CC, Chen YP, Wang NK, Chuang LH, Liu L, Chen KJ, Hwang YS, Wu WC, Chen TL. Vitrectomy with internal limiting membrane repositioning and autologous blood for macular hole retinal detachment in highly myopic eyes. Ophthalmology. 2015;122(9):1889–1898. doi: 10.1016/j.ophtha.2015.05.040. [DOI] [PubMed] [Google Scholar]
- 13.Wu AL, Chuang LH, Wang NK, Chen KJ, Liu L, Yeung L, Chen TL, Hwang YS, Wu WC, Lai CC. Refractory macular hole repaired by autologous retinal graft and blood clot. BMC Ophthalmol. 2018;18(1):213. doi: 10.1186/s12886-018-0898-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen SN, Yang CM. Lens capsular flap transplantation in the management of refractory macular hole from multiple etiologies. Retina. 2016;36(1):163–170. doi: 10.1097/IAE.0000000000000674. [DOI] [PubMed] [Google Scholar]
- 15.Chang YC, Liu PK, Kao TE, Chen KJ, Chen YH, Chiu WJ, Wu KY, Wu WC. Management of refractory large macular hole with autologous neurosensory retinal free flap transplantation. Retina. 2020;40(11):2134–2139. doi: 10.1097/IAE.0000000000002734. [DOI] [PubMed] [Google Scholar]
- 16.Caporossi T, de Angelis L, Pacini B, Tartaro R, Finocchio L, Barca F, Rizzo S. A human Amniotic Membrane plug to manage high myopic macular hole associated with retinal detachment. Acta Ophthalmol. 2020;98(2):e252–e256. doi: 10.1111/aos.14174. [DOI] [PubMed] [Google Scholar]
- 17.Qiao G, Zou QX, He CM, Lei XM, Zhang XJ, Dong WJ, Liao WY, Chen DB. Application of posterior scleral reinforcement in the treatment of retinal detachment due to macular hole with over-long axial length. Guoji Yanke Zazhi (Int Eye Sci) 2020;20(11):1971–1974. [Google Scholar]
- 18.Qiao G, Zhang XJ, Tang ZY, Zou QX, He CM, Lei XM, Zhao L, Quan Y, Yang HQ, Cao K, Dong WJ. Amniotic membrane for covering high myopic macular hole associated with retinal detachment following failed primary surgery. Int J Ophthalmol. 2022;15(5):760–765. doi: 10.18240/ijo.2022.05.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou YH, Zhang J, Zheng Y, Liu Q, Wei WB, Wang NL. The early clinical efficacy of allogeneic corneal lens inlays in correction of hyperopia. Zhonghua Yan Ke Za Zhi. 2015;51(9):683–688. [PubMed] [Google Scholar]
- 20.Liu YC, Teo EPW, Ang HP, Seah XY, Lwin NC, Yam GHF, Mehta JS. Biological corneal inlay for presbyopia derived from small incision lenticule extraction (SMILE) Sci Rep. 2018;8(1):1831. doi: 10.1038/s41598-018-20267-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Min Klimesova Y, Nemcokova M, Netukova M, Baxant AD, Hlavackova M, Kacerovska J, Studeny P. Corneal stromal lenticule transplantation for the treatment of corneal ulcers. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2024;168(1):55–61. doi: 10.5507/bp.2023.004. [DOI] [PubMed] [Google Scholar]
- 22.Abd Elaziz MS, Zaky AG, El SaebaySarhan AR. Stromal lenticule transplantation for management of corneal perforations; one year results. Graefes Arch Clin Exp Ophthalmol. 2017;255(6):1179–1184. doi: 10.1007/s00417-017-3645-6. [DOI] [PubMed] [Google Scholar]
- 23.Pedrotti E, Bonacci E, Fasolo A, Fraccaroli S, Anastasi M, Vinciguerra R, Vinciguerra P, Giorgio M. Meniscus-shaped stromal lenticule addition keratoplasty for corneal regularization and thickening in advanced keratoconus. Cornea. 2023;42(10):1221–1228. doi: 10.1097/ICO.0000000000003144. [DOI] [PubMed] [Google Scholar]
- 24.Cui H, Wang S, Liu L, Song ZW. Research status of the preservation of corneal stromal lenticule derived from SMILE. Journal of Aerospace Medicine. 2024;35(1):78–84. [Google Scholar]


