Dear Editor,
We write to present a 6-year-old boy with bilateral choroidal ganglioneuroma (CG) by comprehensive multimodal imaging (MMI) in a duration of 6-year follow-up. To our knowledge, this is the first case report with elaborate data to delineate the natural history of visual function and MMI in a boy with rare bilateral CG, confirmed by choroid biopsy. In this report, we summarize the 6-year follow-up (2017–2023) of best corrected visual acuity (BCVA), axial length (AL) and the spherical equivalent (SE) and MMI progress [especially ophthalmoscope and optical coherence tomography (OCT)] to illustrate how eyeball, retina and choroid evolve under this circumstance. Since there is no effective treatment for CG, our data may pave the way for future therapeutic development.
Ganglioneuromas are typically benign, slow-growing tumors arising from neural crest cells in the sympathetic nervous system, consisting of ganglion cells, Schwann cells, and fibrous tissue[1]. They have been reported in various locations, such as the orbit, vertebral spines, liver, and lung, but rarely occur in the choroid[2]. CG is an extremely rare tumor, commonly characterized by eye pain, severe vision loss, buphthalmos, high intraocular pressure (IOP)[3]. Some reports have associated CG with neurofibromatosis type 1 (NF-1), suggesting CG might be a rare manifestation of NF-1 syndrome[4]. However, the mechanism of ganglioneuroma remains unclear, and no effective treatment have been established due to the limited understanding of CG. Thus, its pathogenesis, natural history, and treatment remain elusive.
Most of reported CG cases described blindness and eye pain[5]–[8], with 14 of them co-occurring with NF-1. The CG were typically diagnosed histologically after ocular enucleation/evisceration, leaving an absence of imaging data for early diagnosis reference. Recently, our team reported a 6-year-old boy with bilateral CG and PTEN gene mutation[5]–[6], confirmed by choroidal biopsy. This report allowed us to observe this boy's natural history and changes through MMI. All procedures adhered to the tenets of the Declaration of Helsinki, and local approval was received from the Investigational Review Board of Zhongshan Ophthalmic Center, Sun Yat-sen University. Informed consent was obtained from the guardians of the subject after an explanation of the nature and possible consequences of the study.
A 6-year-old boy came to our clinic with a major complaint of gradual vision decline in both eyes for approximately two years. His medical history was unremarkable. At the first presentation at age 5y (year 2017), BCVA was 20/50 in his right eye (RE) and 20/25 in his left. and subsequent observations showed a gradual progressive decrease in vision. Ophthalmoscopy and OCT showed bilateral and extensive retinal detachments (Figures 1 and 2). The presence of a choroidal tumor was highly suspected based on the diffuse thickening of the eyewall detected by B-ultrasound (Figure 1). This suspicion was confirmed by a choroid biopsy, which revealed scattered ganglion and dense infiltrating spindle-shaped cells uniformly throughout the choroid, and neuronal markers including NeuN, CgA, SOX10, Syn, and Phox-2B[5]. Several other conditions were considered and ruled out over the following two years. Systemic work-up showed no obvious abnormalities and whole exome sequencing identified a novel de novo PTEN mutation[6].
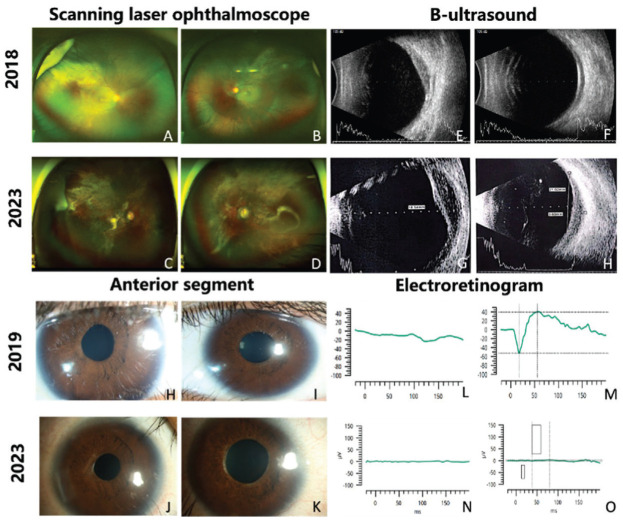
Figure 1. Comparison of scanning laser ophthalmoscope (A to D), B-ultrasound (E to H), anterior segment photos (H to K) and electroretinogram (L to O) of the patient with choroidal ganglioneuroma in 2018, 2019, and 2023.
A, B: Scanning laser ophthalmoscope in 2018 showed extensive superior-temporal and inferior-temporal retinal detachments in the right eye (RE, A) and superior-temporal retinal detachments in the left eye (LE, B); C, D: Scanning laser ophthalmoscope in 2023 showed that the expansion of retinal detachment and the presence of mottled pigmentation, retinal exudation, macular atrophy and pallor of optic nerve head; E, F: B-ultrasound in 2018 showed diffuse thickening of eye walls in both eyes and a raised mass in RE (E); H-K: Anterior segment photos in 2019 showed irregular pupils (H, I), while the iris at the margin of pupils became atrophic in 2023 (J, K); L-O: Electroretinography revealed significant impairment to photoreceptors, with severely decreased responses in LE and unrecordable responses in RE in 2019 (L, M), progressing to extinguishment in both eyes by 2023 (N, O).
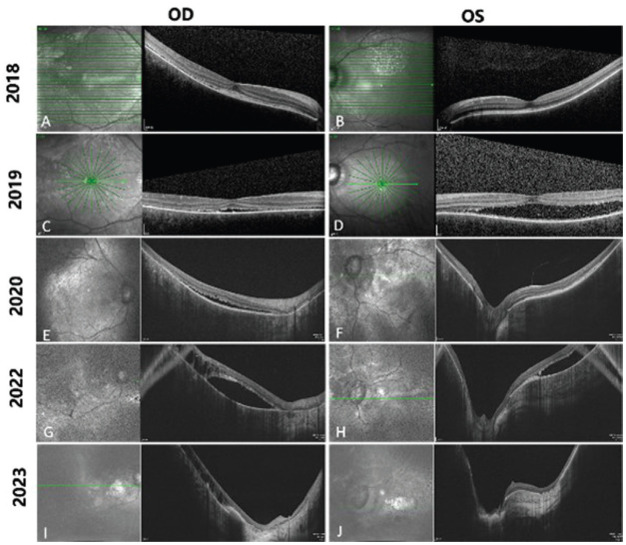
Figure 2. Consecutive optical coherence tomography images from 2018 to 2023.
Horizontal scan line length: 9.0 mm (A, B); 6.0 mm (C, D); 12 mm (E to H); 16 mm (I, J). In 2018, shallow retinal detachment with patchy subretinal hyperreflective foci and thinning of the outer nuclear layer (ONL) was noted in the right eye (RE, A), while the left eye (LE) showed thinning of ONL and intensively hyperreflective retinal pigment epithelium (RPE, B). In 2019, the subretinal fluid increased and the retina was thinned out in both eyes (C, D). In 2020, intraretinal cystic spaces appeared in the RE (E), and the subretinal hyperreflective materials was noted in the LE (F). In 2022, the outer plexiform layer was absent, intraretinal cystic spaces increased and the excavation of retinal tomography deepened in both eyes (G, H). At the last visit (2023), the excavation of retinal tomography further deepened, choroidal hyperreflectivity intensified, and the retina became much thinner in both eyes (I, J).
Visual Parameters and Anterior Segment
The patient's BCVA, AL and SE showed progressive changes during the 6-year follow-up period (Table 1). His BCVA declined rapidly in year 2017–2018 and slowly thereafter. His myopia rapidly advanced, with an annual average increase of 0.77 mm in AL in the right, 1.61 mm in the left, and 1.53 D in the SE. IOP fluctuated within the normal range over the past 2y, but increased to 40 mm Hg in the left eye (LE) during follow-up period, subsequently controlled with brinzolamide-timolol eyedrops. Other findings included transparent corneas and lenses, with a subsequent severe white cataract in the RE, treated with cataract surgery without lens implantation. Bilateral pupils were irregular and unresponsive to mydriatic agents, with extended hypopigmentation noted at the last visit (Figure 1). Anterior chamber angles were wide-open via gonioscopy.
Table 1. Natural history of visual parameters over time.
| Year | Age (y) | SE (D) | BCVA (Snellen) | IOP (mm Hg) | AL (mm) | Diagnosis | Treatment |
| 2017 | 5 | -2.5 | 20/50 | - | 25.15 | Myopia | Lens correction |
| -4.25 | 20/25 | 26.48 | |||||
| 2018 | 6 | -2 | 20/400 | 8 | - | Uveitis | Corticosteroids with CyA |
| -5.75 | 20/25 | 17 | |||||
| 2019 | 7 | -4 | CF/25 cm | 8.5 | 25.89 | CG | Maintenance BZ-TL |
| -8.25 | 20/63 | 23.9 | 28.99 | ||||
| 2020 | 8 | -3 | HM/50 cm | 10.5 | 26.23 | CG | Maintenance BZ-TL |
| -12 | 20/200 | 13.5 | 30.26 | ||||
| 2021 | 9 | -3.5 | 20/400 | 10.7 | 26.81 | CG | Maintenance BZ-TL |
| -10.5 | 20/100 | 14.7 | 30.42 | ||||
| 2022 | 10 | - | LP | 23.7 | 29.25 | CG with RE cataract | RE phacoemulsification; maintenance BZ-TL |
| 20/200 | 17.7 | 33.37 | |||||
| 2023 | 11 | - | HM/BE | 23.0 | 29.81 | CG | LE photocoagulation; maintenance BZ-TL |
| -15.00 | 20/333 | 14.0 | 36.14 |
SE: Spherical equivalent; BCVA: Best corrected visual acuity; IOP: Intraocular pressure; AL: Axial length; CyA: Cyclosporine A; RE: Right eye; LE: Left eye; CG: Choroidal ganglioneuroma; CF: Counting finger; LP: Light perception; HM: Hand motion; BE: Before eye; -: Not available; BZ-TL: Brinzolamide-timolol.
Ophthalmoscope, OCT, and B-ultrasound
Scanning laser ophthalmoscope and OCT recorded the retinal concomitant changes with CG. At the initial visit, extensive superior-temporal and inferior-temporal retinal detachments were noted in RE and only superior-temporal retinal detachments were in the LE (Figure 1). However, his cup-to-disc ratio had become 0.7:0.8 bilaterally under a normal IOP in 2018 (Figure 1). At the last visit, the area of retinal detachment expanded, and mottled pigmentation, retinal exudation, macular atrophy and pallor of optic nerve head were noted (Figure 1). As for OCT, in 2018, shallow retinal detachment with patchy subretinal hyperreflective foci and thinning of the outer nuclear layer (ONL) was observed in the RE, while in the LE was thinning of ONL and intensively hyperreflective retinal pigment epithelium (RPE; Figure 2). In 2019, the ONL was absent and disrupted, and the retina was thinned out in both eyes (Figure 2). In 2020, intraretinal cystic spaces appeared in the RE, ONL was completely absent in both eyes and retinal tomography demonstrated an obvious excavation (Figure 2). In 2022, the outer plexiform layer was absent, intraretinal cystic spaces increased and the excavation of retinal tomography deepened in both eyes (Figure 2). At the last visit (2023), the excavation of retinal tomography further deepened, choroidal hyperreflectivity intensified, and the retina became much thinner. Notably, each layer of the retina was indecipherable and became iso-reflectivity (Figure 2). In addition, an absence of choroidal vasculature was always evident throughout each phase. B-ultrasound showed a corresponding diffuse thickening of the eye walls and an enlarged choroidal mass (Figure 1).
Electrophysiological Features
The method for electroretinography recording utilizing RETeval® has been previously documented[9]. For each patient, the lower eyelids of both eyes were cleansed, and sensor strips were placed 2 mm below the lower eyelid margins. Tropicamide and phenylephrine hydrochloride (Mydrephrine P Ophthalmic Solution, Nitto Medic Co.) eye drops were instilled in both eyes. Following 20min of dark adaptation, rod and maximal responses were recorded. Subsequently, cone and flicker responses were recorded after 10min of light adaptation, using a flicker frequency of 28.3 Hz. Electrooculography (Arden ratio of 1:1 bilaterally) and electroretinography (2019 and 2023) revealed significant impairment to the RPE and photoreceptors, with notably abnormal responses in the LE and unrecordable responses in the RE in 2019, progressing to extinguishment in both eyes by 2023 (Figure 1).
DISCUSSION
Due to the rarity of CG, to date, extremely limited data are documented. Our team summarized the common features based on published reports[6], highlighting that CG usually co-occurs with NF-1 and often leads to blindness and eye pain, culminating in evisceration/enucleation. Unlike the published cases, our case was bilaterally involved and histopathologically diagnosed before enucleation, providing data that are of early diagnostic value and visualization of the complete progress. Due to no specific treatment strategy for CG, our data is crucial for finding a therapeutic window in the future.
The consecutive observation of the patient's fundus via ophthalmoscope and OCT has led us to a specific pathological hypothesis. In our patient diagnosed with CG, we observed a progressively dysfunctional RPE, which we believe is induced by an abnormal choroidal mass and the prolonged presence of subretinal fluid. This appears to be the underlying cause of the patient's irreversible vision loss. The presence of CG can lead to a decrease in the choroidal vascular structure. As a result, the clearing of nutrients and metabolic wastes from the RPE and the outer layers of the retina becomes impaired. This impairment can be observed through the accumulation of highly reflective material in the subretinal space, along with the thickening of the RPE, as detected in the early stages of OCT examination. These findings may partially explain the subsequent accumulation of subretinal fluid. Over time, the dysfunction of the outer layer affects the inner layer and even the entire retina. The later appearance of retinal atrophy, as well as the appearance of intraretinal cystic cavities, represent the dysfunction of the full-layer retina. The flat responses in electroretinography, ambiguous structure of the retinal layer and retinal thinning on OCT verify that CG eventually leads to the patient's whole retina dysfunction.
The AL and SE have shown a progressive increase in our patient, a pattern that has been observed in previously reported cases[10]. Apparently, CG has a very strong impact on eyeball development and refractive error. This phenomenon can be understood through three key factors. First, CG typically manifests at an early age, often affecting children during a crucial phase of eye growth and development. Second, the exophytic growth pattern of the tumor damages the scleral tissue, diminishing its supportive function. Third, CG components' extensive presence and proliferation hinder normal choroidal vessels. The choroid is known to secrete growth factors that regulate scleral growth during developmental stages[11]. In addition, choroidal thickness changes regulate the position of the retina and alter the focal plane of the optic cells, and long-term focal adjustment deficits can also affect eye development[11]. All these factors could exert impacts in eye development of our patient.
The report is limited by partial missing information at some follow-up visits, partly due to the COVID-19 pandemic. CG is a sporadic disease that most reported cases were not diagnosed until evisceration/enucleation, and it is tough to recruit enough cases for a case-series study. Nevertheless, our comprehensive observation of a bilateral CG patient provides vital insights that could guide future therapeutic development.
In conclusion, we have detailed the natural history of bilateral CG, providing an exhaustive characterization through visual parameters and MMI. It is characterized by exudative retinal detachment on the ophthalmoscope, a rapid increase in AL, diffuse choroidal thickening, and progressive retinal dysfunction with loss of choroidal vasculature on OCT and the enlarged mass of eyewalls on B-ultrasound. The absence of inflammation in the anterior chamber and vitreous is helpful for the exclusion of uveitis. Inherited retinal diseases, especially bestrophinopathy, can be excluded by the presence of a more extensive retinal detachment and enlarged mass on B-ultrasound. This comprehensive examination aids in identifying potential future therapeutic windows for treatment. Our pathological hypothesis, drawn from a series of consecutive observations, posits that the progressively dysfunctional RPE is induced by an abnormal choroidal mass and persistent subretinal fluid. This chain of events may ultimately lead to irreversible vision loss in our patient with CG.
Footnotes
We would like to thank Dr. Chi-Chao Chan from National Eye Institute, USA, for her professional opinion on the pathological diagnosis, Prof. Xiaoyan Peng from Beijing Tongren Hospital, China, for her great help in imaging analysis.
Authors' contributions: Ding XY conceived and designed the study. Cheng YZ, Sun LM, Yuan ME, Zhang LY. and Ke SY participated in data collection and interpretation. Cheng YZ analyzed the data and wrote the first draft of the manuscript. Ding XY and Sun LM critically reviewed the manuscript. All authors approved the submitted version.
Foundations: Supported by the Construction Project of High-Level Hospitals in Guangdong Province (No.303020107; No.303010303058); National Natural Science Foundation of China (No.82271092); Guangdong Basic and Applied Basic Research Foundation (No.2023A1515010430); Guangzhou Municipal Science and Technology Key Project (No.2024A03J0171).
Conflicts of Interest: Cheng YZ, None; Sun LM, None; Yuan ME, None; Zhang LY, None; Ke SY, None; Ding XY, None.
REFERENCES
- 1.Geoerger B, Hero B, Harms D, Grebe J, Scheidhauer K, Berthold F. Metabolic activity and clinical features of primary ganglioneuromas. Cancer. 2001;91(10):1905–1913. doi: 10.1002/1097-0142(20010515)91:10<1905::aid-cncr1213>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 2.Dai X, Zhang R, Li Y, Wu G. Multiple ganglioneuromas: a report of a case and review of the ganglioneuromas. Clin Neuropathol. 2009;28(3):193–196. doi: 10.5414/npp28193. [DOI] [PubMed] [Google Scholar]
- 3.Goyal S, Park A, Zeglam A, Brown H, Pemberton JD. Choroidal ganglioneuroma and orbital plexiform neurofibroma presenting as buphthalmos in an infant with neurofibromatosis type 1. Ophthalmic Plast Reconstr Surg. 2016;32(4):e87–e89. doi: 10.1097/IOP.0000000000000266. [DOI] [PubMed] [Google Scholar]
- 4.Ozgun G, Adim SB, Ugras N, Yazici B. Co-occurrence of choroidal pigmented ganglioneuroma and plexiform neurofibroma in a patient with neurofibromatosis 1. Kaohsiung J Med Sci. 2014;30(4):215–216. doi: 10.1016/j.kjms.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Jiang ZX, Zhang T, Liu X, Liang D, Zhong YM, Chan CC, Ding XY. Multimodal imaging features of bilateral choroidal ganglioneuroma. J Ophthalmol. 2020;2020:6231269. doi: 10.1155/2020/6231269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang ZX, Zhang T, Chen CL, Sun LM, Li SS, Ding XY. New PTEN mutation identified in a patient with rare bilateral choroidal ganglioneuroma. BMC Ophthalmol. 2020;20(1):487. doi: 10.1186/s12886-020-01760-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gupta H, Kandalkar B. Choroidal ganglioneuromas in Francois variant neurofibromatosis-1: a rare retinoblastoma mimic. Indian J Ophthalmol. 2022;70(7):2602–2604. doi: 10.4103/ijo.IJO_3109_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Srivastava AC, Shenoy V. Choroidal ganglioneuroma: an unexpected diagnosis. Am J Clin Pathol. 2021;156(Supplement_1):S87–S88. [Google Scholar]
- 9.Zhang T, Lu JL, Jiang ZX, Huang L, Zeng J, Cao LM, Luo XL, Yu BL, Ding XY. The development of electroretinographic oscillatory potentials in healthy young children. J Clin Med. 2022;11(19):5967. doi: 10.3390/jcm11195967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeParis SW, Bloomer M, Han Y, Vagefi MR, Shieh JTC, Solomon DA, Grenert J, de Alba Campomanes AG. Uveal ganglioneuroma due to germline PTEN mutation (cowden syndrome) presenting as unilateral infantile glaucoma. Ocul Oncol Pathol. 2017;3(2):122–128. doi: 10.1159/000450552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nickla DL, Wallman J. The multifunctional choroid. Prog Retin Eye Res. 2010;29(2):144–168. doi: 10.1016/j.preteyeres.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]