Summary
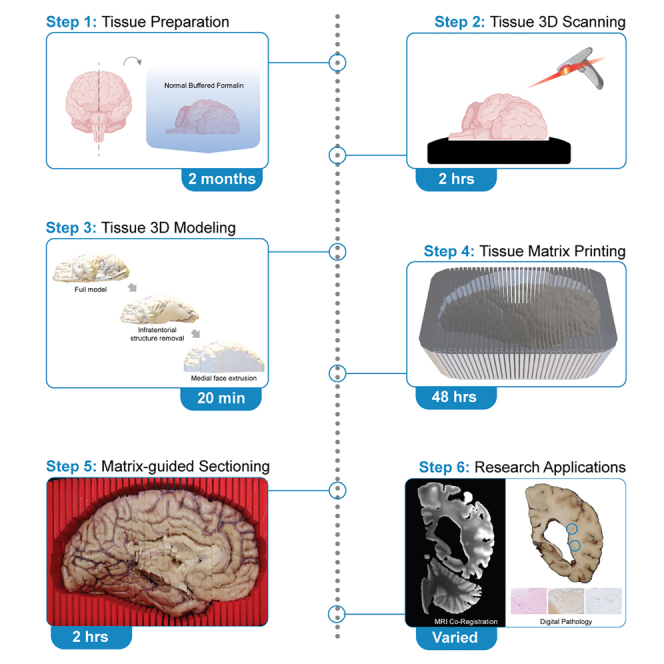
Despite numerous neuropathological criteria for the evaluation of microscopic tissue slides, there are no systemic standards for sectioning postmortem human brain tissue. Here, we present a protocol for postmortem brain hybrid structured-light scanning to facilitate 3D printing of sectioning matrices. We describe steps for tissue preparation, 3D scanning and printing, and matrix-guided sectioning. This protocol can be used for streamlining tissue histopathology as well as in brain morphology volumetric analyses and applications in medical education.
For complete details on the use and execution of this protocol, please refer to Barannikov et al.1
Subject areas: Health Sciences, Neuroscience, Systems biology, Biotechnology and bioengineering
Graphical abstract
Highlights
-
•
Hybrid structured-light three-dimensional (3D) scanning of human brain tissue
-
•
Refinement of 3D brain model for sectioning matrix generation
-
•
Generation of 3D-printed matrix based on modified 3D brain model
-
•
Matrix-guided tissue sectioning of hemisected supratentorial tissue
Publisher’s note: Undertaking any experimental protocol requires adherence to local institutional guidelines for laboratory safety and ethics.
Despite numerous neuropathological criteria for the evaluation of microscopic tissue slides, there are no systemic standards for sectioning postmortem human brain tissue. Here, we present a protocol for postmortem brain hybrid structured-light scanning to facilitate 3D printing of sectioning matrices. We describe steps for tissue preparation, 3D scanning and printing, and matrix-guided sectioning. This protocol can be used for streamlining tissue histopathology as well as in brain morphology volumetric analyses and applications in medical education.
Before you begin
In this protocol, a pipeline for the generation of three-dimensional (3D) printed tissue sectioning matrices based on hybrid structured-light scanning of postmortem human brain tissue is described. There are numerous neuropathological consensus criteria guidelines for the diagnosis of neurological disorders based on microscopic slides.2 However, there are limited guidelines and standardization for the processing and sectioning of autopsied tissue prior to histopathology. Sectioning autopsied brains along the appropriate plane and at the correct interval and thickness to capture discrete neuroanatomical structures is dependent on the experience and skill of the pathologist performing the procedure. With a standard tissue cassette depth of 4 mm, sectioning too thin can result in incomplete and misaligned tissue slabs, whereas sectioning at too thick of an interval requires further facing and dissection of the tissue slab to remove the desired region of interest for blocking. Procedural adaptations that maximize sampling of the procured section, minimize tissue waste, standardize workflow between different cases, and ensure the safety of the practitioner are highly desirable.
Tissue sectioning matrices are structures customized to different tissue types, which securely hold the specimen and allow the user to precisely cut along the plane of the sectioning guide. Metal and acrylic tissue matrices are commercially available for the dissection of organs of several different model organisms. Rodents, nonhuman primates, cats, dogs, rabbits, and pigs have uniformity in shape and size of organs at distinct age timepoints. In contrast, the morphology of human organs varies from individual to individual. There are differences in the human brain with respect to size, gyral pattern, and structural atrophy, a common feature of neurodegeneration. This variance of the human brain presents a current limitation to pathological methods and necessitates the ability to generate sectioning matrices specific to individual autopsied brain tissue. To date, matrices have been developed from 3D model renderings based on magnetic resonance imaging (MRI) of postmortem human, mouse, and nonhuman primate brains.3,4,5,6 MRI-based methods are ideal for the customization of the sample and analytical protocols of tissue derivatives. However, MRI technology in a research setting can be costly, have limited accessibility relative to clinical use, and depending on the setting require radiological expertise for both use of the system and interpretation of imaging results. Further, antemortem MRI is intermittently available for donated brain tissue and depending on the disease progression and severity, may not accurately represent the cross-sectional state of the tissue at autopsy.
As an alternative, 3D scanning and printing technologies are increasingly accessible, affordable, and adaptable. To meet the need for standardized and efficient preparation of postmortem human brain tissue for pathological study, a 3D scanning and printing workflow for over 140 postmortem brain autopsies was developed. Custom 3D sectioning matrices were created by scanning the left hemibrain of formalin-fixed postmortem human brain tissue. Using the 3D printed sectioning matrix, brain tissue was sectioned and sampled for routine histopathology by the University of Texas Health Science Center at San Antonio Neuropathology core and Brain Bank, with adapted coding segments used for visualization and analysis. This pipeline is cost-effective with each sectioning matrix costing approximately $12.50, requires minimal space and equipment, and standardizes brain processing such that clinical and research trainees can aid in sectioning brain tissue. Furthermore, hybrid structured-light generated 3D brain models have the potential application in downstream neuropathology and neuroanatomy medical education. Printed matrices based on hybrid structured-light 3D scans provide a precise sectioning plane and interval that allow for stereotactic co-registration of the tissue to other imaging modalities, and further enables targeted microscopic lesion detection and sampling.7 While in this protocol scanning and sectioning of postmortem human brain tissue are the primary focus, the adaptation of this pipeline for use with other animal models and tissue types is encouraged.
Institutional permissions
All experiments conducted using postmortem human brain tissue were approved by the University of Texas Health Science Center at San Antonio Institutional Review Board. Sheep brain tissue was acquired from a publicly available commercial source/vendor and was exempt from institutional permissions. To complete scanning and sectioning on postmortem human brain or other tissue types, be sure to seek approval from the respective institutional IRB/IACUC or similar governing body.
Tissue preparation for scanning
Timing: 1–2 months
Prior to scanning, brain tissue needs to be fixed in 10% normal buffered formalin (NBF). Fixation time can range from 36 h to two months dependent on tissue type and size for full formalin penetration. Tissue can remain in sealed containers of 10% NBF for long-term storage. Tissue is rinsed in running water prior to the imaging session for 3 h to limit noxious fumes. When ready to section the tissue, ensure that all materials and reagents are tempered to individual manufacturer recommendation, and complete all sectioning steps in within BSL-2 safety cabinet, on a downdraft table, or in a workspace compliant with regulatory biosafety requirements. Observe universal safety precautions to prevent exposure to potential pathogens.
Note: Harmful chemicals should be handled carefully and disposed of appropriately.
Starting with fresh, unfixed, and unfrozen brain tissue.
-
1.
Use a Mopec disposable knife to hemi-sect postmortem tissue into equal left and right halves along the midline or longitudinal fissure (transecting the corpus callosum).
-
2.
Weigh both halves of the hemisected tissue and record values.
-
3.
If fresh frozen tissue is desired, prepare the right hemisected brain for preservation at −80°C.
-
4.
Fix the left hemibrain by submerging the tissue in a solution of 10% NBF.
Note: To avoid deformation of fresh tissue, hemibrains are placed in a hair net suspended across the opening of the container with the lateral surface facing downward. Allow tissue to fully be infiltrated by the formalin solution in suspension prior to imaging and sectioning. It is recommended to incubate tissue for a minimum of two months at room temperature.
-
5.
Following fixation in 10% NBF, the tissue is now ready for 3D scanning.
Note: The right hemibrain can be frozen for molecular analysis and research studies.
-
6.
Section tissue into approximately 5 mm-thick coronal slabs, with notation of any macroscopic/structural pathological features.
-
7.
Catalog the sectioned tissue slabs photographically.
-
8.
Store the slabs in heat-sealable bags at −80°C for future use.
Scanning studio setup
Timing: Varied
A detailed overview of how to set up a 3D scanning studio is presented in the materials and equipment section of this protocol. Required for this protocol are a 3D scanner, dedicated scanning area, accompanying computer software, and 3D printer. The key resources table provides a full list of the reagents, materials, software, and other resources needed for completing this protocol.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Biological samples | ||
| Postmortem human brain | Varies | Each sample has a unique identifier correlated with patient information. |
| Chemicals, peptides, and recombinant proteins | ||
| Neutral-buffered formalin (https://shop.leicabiosystems.com/us/histology-consumables/reagents-solutions/pid-10-neutral-buffered-formalin-fixatives) | Leica | Cat#: 3800598 |
| Ethanol, 100% | Fisher | Cat#: BP2818500 |
| CaviCide disinfectant spray | Fisher | Cat#: 50-209-1727 |
| Sodium azide | Fisher | Cat#: S2002 |
| 1× phosphate-buffered saline pH 7.4 (https://www.fishersci.com/shop/products/pbs-phosphate-buffered-saline-1x-solution-ph-7.4-fisher-bioreagents/BP243820) | Fisher | Cat#: BP661-50 |
| Deposited data | ||
| Brain box tissue matrix templates | Zenodo | Zenodo: https://doi.org/10.5281/zenodo.12745266 |
| Software and algorithms | ||
| Anaconda | Anaconda | RRID: SCR_018317 |
| Python version 3.11.4 | Python Software Foundation | RRID: SCR_008394; https://www.python.org/downloads/release/python-3114/ |
| Jupyter core package | Project Jupyter | RRID: SCR_018416; https://www.jupyter.org/install |
| Jupyter Notebook | Project Jupyter | RRID: SCR_023339; https://www.jupyter.org/install |
| 3D Builder application | Microsoft Corporation | https://apps.microsoft.com/detail/9WZDNCRFJ3T6?launch=true&mode=full&referrer=bingwebsearch&ocid=bingwebsearch&hl=en-us&gl=US |
| ideaMaker application | Raise 3D | https://www.ideamaker.io/download.html |
| EXScanner application | Shining 3D | https://www.einscan.com/einscan-hx/ |
| Blender application | Microsoft Corporation | RRID: SCR_008606 |
| Other | ||
| Dell 27″ monitor | Dell | Cat#: P2722H |
| Smart uninterrupted power supply Tripp Lite | Office Depot | Cat#: 581578 |
| Muslin background 10 × 12 | Impact Studio | Cat#: BG-B-1012 |
| WD 2TB SSD external hard drive | Western Digital | Cat#: WDBAYN0020BBK-WESN |
| Dell OptiPlex 9020 | Dell | Cat#: D07S |
| NVIDIA Quadro RTX A200 graphics card | NVIDIA | Cat#: VCNRTXA200012GB-PB |
| LED light kit | Bescor | Cat#: XT160KB |
| EinScan H scanner | Shining 3D | Cat#: AFEINSCANH2 |
| JAYEGT motorized turntable | Amazon | Cat#: B09C5WFRVT |
| Raise3D Pro2 dual extruder 3D printer | Raise 3D | Cat#: 10132134060 |
| Raise3D premium 3D printer filament 1.75 mm | Raise 3D | Cat#: [S]5.11.00106 |
| 2.4 L plastic containers | Walmart | Cat#: 1-44-307 |
| Samsung T7 shield 2 TB SSD | Samsung | Cat#: MU-PE2T0S/AM |
| Fisherbrand cutting boards | Fisher | Cat#: 09-002-24A |
| Mopec disposable autopsy and pathology grossing prep knives | Mopec | Cat#: AH028 |
| Keystone safety heavyweight hairnets | Fisher | Cat#: 19-812-908 |
| Dremel Lite – sanding, engraving 25,000 RPM – battery powered | Dremel | Cat#: DML7760N10 |
| Parafilm | Fisher | Cat#: PM992 |
| Scalpel 179 style | Fisher | Cat#: PL63 |
| Smith-Victor 42″ pro-duty copy stand kit | Smith-Victor | Cat#: 402183 |
| Canon EOS M6 Mark II | Canon | Cat#: 3612C079 |
| Smith-Victor SlimPanel daylight LED 2-light kit with battery power | Smith-Victor | Cat#: 401619 |
| 0.125 general purpose HDPE sheets | Grainger | Cat#: 1ZAG6 |
| Glossy permanent vinyl 12 × 6 | Silhouette | Cat#: V12-GP-BLK-C |
| WypAll extra absorbent towels L40 | Fisher | Cat#: 06-666-132 |
| Kimtech Science Kimwipes | Fisher | Cat#: 06-666 |
Materials and equipment
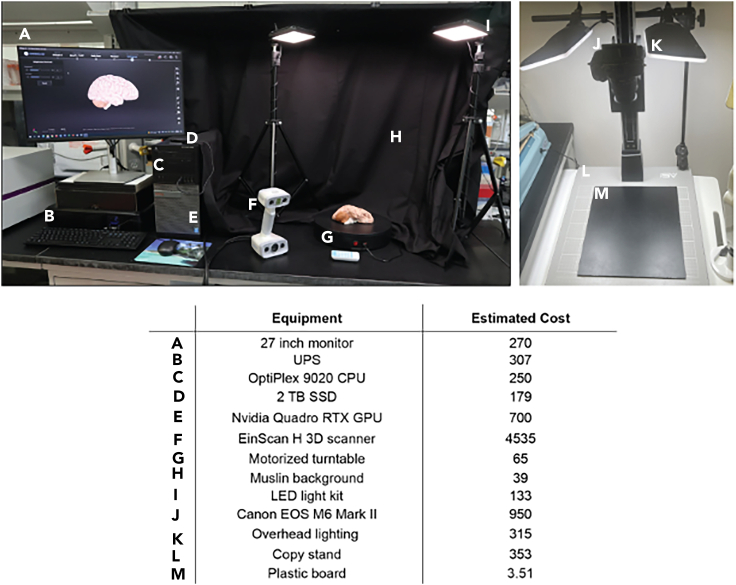
An example based on the custom studio used in this pipeline, along with its estimated cost has been provided (Figures 1A–1I). Scanning studios are customizable to meet both fiscal and space requirements and are assembled either prior to or concomitantly with tissue fixation. 3D scanning and instrument calibration necessitates consistent, uniform lighting for model acquisition (Figures 1A–1I). Use of a motorized turntable improves scan coverage of the brain surfaces (Figure 1G), while a muslin background limits exposure of flashing lights to others in the laboratory (Figure 1H). The important components that facilitate faster scanning and higher model quality include the graphics processing unit and the scanner instrument. Smaller or larger motorized turntable stages work with this set up, as do smaller and larger scanners and scanner support arms. This protocol is designed to work with a wide variety of 3D printer types. However, for printing of 3D sectioning matrices for human brains, the selected 3D printer must have a build surface area of at least 12 × 12 × 12 inches. Follow manufacturer instructions for the installation of each studio component.
Figure 1.
Equipment setup for 3D scanning and tissue imaging
(A–I) An example of an assembled 3D scanning studio including necessary computers, scanners, and peripherals.
(J–M) Equipment and materials needed for tissue photography. Component specifics and estimated cost are detailed in the table.
Step-by-step method details
Part 1: Tissue scanning
Timing: 3–4 h
In this step, formalin-fixed tissue is rinsed in running water, cleaned, placed on a motorized stage, and scanned using a EinScan H Structured Light Scanner via EXScanner software. Tissue scanning is conducted in two phases to capture the medial and lateral orientations of one hemisected brain. Resulting scans are aligned and meshed to generate a complete 3D brain model.
-
1.Prepare to scan formalin fixed tissue:
-
a.Remove the fixed hemibrain from 10% NBF and rinse in a continuous flow of running water for at least 3 h at room temperature.
-
a.
-
2.
Dab away excess water from the surface of the tissue using absorbent towels.
Note: Removal of water reduces sheen and glare during scanning. If needed, periodically wet the tissue or cover with a moistened towel to prevent excessive drying of the tissue (which may impact histopathology).
-
3.Ready the EXScanner application for scanning.
-
a.Open the EXScanner Application.
-
b.Select “White Light Mode”.
-
c.Select “New Project Group” and name the file.
-
d.Select the scanning properties applicable to the scan, including:
-
i.“Texture Alignment”. This property captures model geometry and texture.
-
ii.0.5 (M) Resolution. This property optimizes resolution with file size. Higher resolutions can be used but will increase file size.
-
iii.“Texture Scan”
-
i.
-
a.
-
4.Prepare the scanning area and sample for image capture of the lateral surface:
-
a.Turn on LED lights to a setting of 974300 K.
-
b.Place the rinsed postmortem tissue on a clean black surface board with the medial surface flat on the board and position the black surface board onto the motorized turntable.
-
a.
-
5.Scan the tissue to capture the lateral surface:
-
a.Position the EinScan H Scanner above the brain tissue so that the LED emitted light illuminates the tissue and no shadows are present.
-
a.
CRITICAL: The flashing light emitted from the EinScan H Scanner may have adverse effects on photosensitive persons.
-
6.
Press the “Play” button on the back of the EinScan H Scanner to begin “preview mode”.
-
7.Adjust brightness and zoom using buttons on the scanner or in EXScanner.
-
a.Aim to maximize brightness with exposure.
-
a.
Note: EXScanner indicates overexposed surfaces in red.
-
8.
Press the “Play” button again to begin scanning.
-
9.Slowly rotate the scanner around the tissue, using the image tracking feature in EXScanner as a guide.
-
a.Capture at least 600 frames and register approximately 16,000 points before proceeding to step 10 (Methods video S1).
-
a.
Note: EXScanner utilizes a gradient bar to indicate tracking quality status, changing color based on the distance from the tissue and overall tracking ability. Color changes are also indicated on the backside of the scanner. A red light indicates that the scanner is too close to the tissue or can no longer track, while a blue light indicates that the scanner is too far away. When the gradient bar or scanner light is green, the scanner is within range of the tissue and tracking is optimal.
-
10.Turn on the motorized table.
-
a.Keep the scanner maintained in one position so that new scan data collected uses previously scanned areas to maintain tracking.
-
b.When the turntable has completed one full rotation of the tissue, proceed to step 11 (Methods video S1).
-
a.
-
11.Slowly adjust the scanner position so that the green alignment status remains coordinated with the rotating tissue.
-
a.When a total of 1500 frames and 30,000 points are captured, proceed to step 12 (Methods video S1).
-
a.
Note: This motion increases scanning of curvatures and other small surface deviations.
-
12.
End the scan by pressing the “Play” button on the scanner or clicking “Pause” in EXScanner.
-
13.
Pre-process the lateral scan by selecting “Generate Point Clouds”.
CRITICAL: Delete extra noise around the scan image by holding the “shift” key and using the cursor to select unwanted pixels in black. Click “delete” to remove unwanted pixels from the scan. To deselect pixels, hold the “ctrl” key while selecting with the cursor.
-
14.Each phase of scanning corresponds to a project group in the EXScanner application. To name the previous lateral scan project group:
-
a.Select “Project Group” and enter “Lateral” as the name for the current lateral surface scan.
-
b.Click “+” to add a new project group for the medial surface and enter “Medial” in the “Name” field.
-
c.Apply the same scanning properties as those used in the lateral surface scan.
-
a.
-
15.Scan the tissue to capture the medial surface:
-
a.Reposition the brain on the black surface board by arranging the tissue so that the medial side is facing up. Maintain the brain tissue in the same anterior-posterior orientation relative to the lateral side.
-
a.
CRITICAL: Disparities in positioning between the lateral and medial sides can cause model distortion in later steps.
-
16.Repeat the scanning process as described in steps 5 through 14.
-
a.Capture frames and points at the surface edges where the lateral and medial sides meet. If all surfaces are scanned for alignment, proceed to step 17.
-
a.
-
17.
Store tissue submerged in diH2O or in 10% NBF until sectioning.
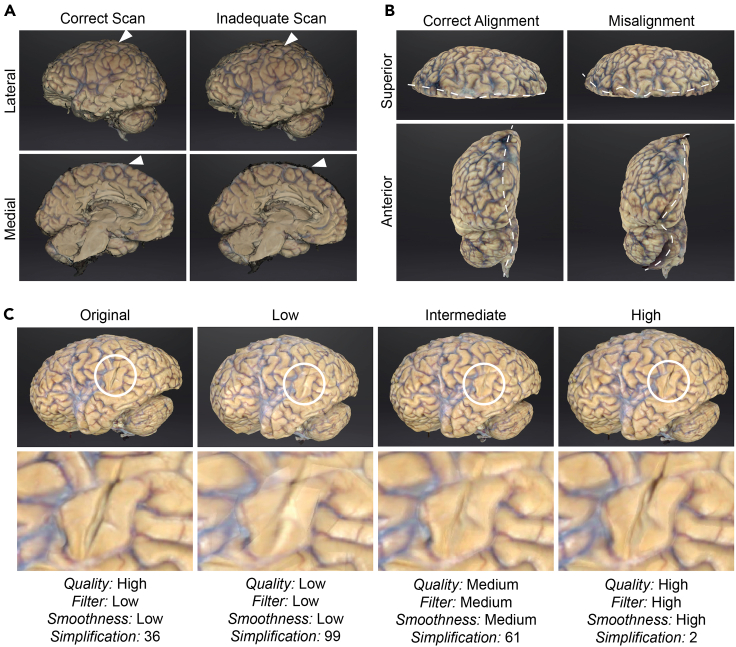
Note: Alignment heavily utilizes lateral and medial surface edge points. Poor scanning of surface edges can cause model distortion (Figures 2A and 2B) as the scans feature insufficient data for proper alignment.
-
18.Align the Lateral and Medial project group scans:
-
a.Select “Align” after the completion of the last scan and add the “Lateral” and “Medial” groups to the “Floating” and “Fixed” windows.
-
a.
Note: The order of selection is not important for alignment.
-
19.Select “Manual” alignment to align the scans into a raw 3D brain model.
-
a.Hold the “shift” key while using the cursor to select alignment points.
-
b.Choose at least three corresponding points on the “Lateral” and “Medial” scans.
-
a.
CRITICAL: It is not recommended to use the “automatic” alignment method for image captures, since often there are few structures easily identified on both the lateral and medial scans.
-
20.
Click “Apply” to complete the alignment. Click “Next” and “Exit”. The resulting alignment is now a raw 3D model.
-
21.
Use the “Edit” features to remove any artifacts or distortions such as overlapping pixels of mismatched anatomy (Figure 2B).
Note: If the alignment steps above result in a mismatched or anatomically incorrect model, see problem 1: registration and tracking errors during scanning in the troubleshooting section.
-
22.Generate the 3D model from the aligned scans:
-
a.Access the alignment attributes menu by clicking “Mesh Model”.
-
a.
Note: After alignment, the appearance of the raw 3D model is refined via meshing, a function that unifies alignments and other attribute properties into a complete 3D brain model. Shadows or other artifacts not removed from the raw 3D model during alignment are either smoothed into the three-dimensional surface or deleted. Further adjustments are made to improve texture’s accuracy and to remove duplicated tissue or anatomical inaccuracies.
CRITICAL: Steps 22–24 are required to export a complete 3D brain model for further processing.
-
23.
Chose attributes for refining the raw 3D model using the meshing function. Apply the chosen mesh attributes by clicking “Apply” followed by “Confirm”. The mesh attributes detailed in Table 1 are the recommended parameters.
-
24.
Click “Save” to save the meshed 3D model and name the file in the popup window. This model is now a complete and full 3D model of the scanned brain.
Note: It is recommended to save files in the .obj type for use in later steps.
-
25.
Measure the length of the complete 3D brain model. Measurements are provided upon clicking “Save” in EXScanner. This measurement will be used in Part 2.
Note: Use of the mesh function to refine the raw 3D model is a subjective process dependent on scan, alignment, and tissue quality. There are options to continue editing the mesh that include Texture, Simplification, Mesh Optimization, Smooth, Remove Small Floating Parts, Auto Hole Filling, Manual Hole Filling, Texture Remapping, Flip Normal, Cutting Plane Tool, and Mirror. Visit https://support.einscan.com/en/support/solutions/articles/60000813012-exscan-post-processing-tools for more information about these options. Additionally, a complete 3D brain model can be saved in one of three file types. The .3mf, .stl, and .obj file types are roughly 20,000 KB, 25,000 KB, and 35,000 KB in size respectively. As larger file types, the .3mf and .obj types preserve the original texture colors from 3D scanning, whereas the .stl file type does not.
Figure 2.
Scan, alignment, and mesh directly affect 3D brain model quality
(A) Comparison of correct compared to inadequate scans as seen from the medial and lateral surfaces in EXScanner for the same scanned brain. Scanning artifacts (white arrow) are indicated.
(B) Comparison of correct and misaligned scans from the same scanned brain as seen from the superior and anterior surface. Plane of alignment indicated by white dashed line.
(C) Comparison of original, low, intermediate, and high-quality raw 3D brain models of the same scanned brain. Encircled regions represent a magnified comparison across the same region with differing applications of mesh attributes to improve model quality. Attributes are unique to EXScanner software.
Table 1.
Recommended attributes for meshing the raw 3D model that produce consistent and clear models
| Attribute | Selection |
|---|---|
| Mesh type | airtight mesh |
| Quality | high |
| Filter | low |
| Smoothness | low |
| Remove small floating parts | 35 |
| Simplification | 50 |
| Maximum triangles | checked |
| Remove spike | checked |
Part 2: Refinement of 3D brain model for sectioning matrix generation
Timing: 20 min
In this step, the complete full 3D brain model from Part 1 is further processed including removal of the infratentorial structures, balance verification, and extrusion. The result is an extruded supratentorial 3D brain model ready for use in creating the 3D sectioning matrix.
Note: If returning to the protocol from a pause, open the EXScanner program and click “Post-Processing”. Open the .obj file containing the full complete 3D brain model from step 25. If continuing directly from Part 1, proceed to step 26 below.
-
26.Remove the infratentorial brainstem and cerebellar structures from the full 3D model:
-
a.Use the “Lasso” tool to remove the infratentorial brainstem and cerebellar structures.
-
i.Click the “Through” layer option.
-
ii.While holding the “shift” key lasso the brainstem and cerebellum starting across the midbrain from the superior colliculus to the mammillary body.
-
i.
-
a.
Note: Do not include the mammillary body in the lasso selection.
-
27.
Rotate the 3D brain to verify the correct selection of the brainstem and cerebellar structures for removal.
Note: To evaluate if the correct regions are selected, interchange between the “Visible” and “Through” layer options. The “Visible” option will select areas for deletion on only one surface, while holding the “ctrl” key will enable deselection of supratentorial structures.
-
28.
Click “Delete” when the correct selection of the infratentorial structures is complete.
-
29.Fill holes in the raw 3D model.
-
a.Click “Apply Edit”, then “Auto Hole Filling”.
-
i.Select holes to fill and click “Apply”.
-
i.
-
b.Fill any remaining holes in the mesh by clicking “Manual Hole Filling” and selecting the outlined areas.
-
a.
CRITICAL: Proceed to step 30 only when all holes are filled, otherwise the 3D brain model will feature surface gaps.
-
30.
Click “Confirm” and then “Save”.
Note: This action will generate a supratentorial 3D brain model of only the supratentorial structure for use in creating the 3D sectioning matrix. It is recommended to save the supratentorial 3D brain model with a unique file name in the .obj format.
-
31.Extrude the supratentorial 3D brain model for matrix building:
-
a.Open the 3D Builder application and select “Open”.
-
b.Navigate to the supratentorial 3D brain model .obj file and click “Load Object”.
-
a.
-
32.Click the 3D object and use the rotation tool to rotate the medial surface of the 3D model to sit parallel with the Z plane.
-
a.Click “Settle” and then “Apply”.
-
a.
-
33.
Perform balance verification on the 3D model by adjusting the yaw, pitch, and roll to further align the medial surface as parallel as possible with Z plane.
-
34.Prepare the supratentorial 3D brain model for extrusion.
-
a.Add 5 mm to the extrusion space in the “Z plane” tab.
-
a.
Note: Extrusion adds both cradling depth for the tissue to sit in the matrix and creates sufficient space to position the knife on the matrix during sectioning.
-
35.
Extrude the supratentorial 3D brain model. Navigate to the “Edit” option and select “Extrude down”.
Note: A green plane will appear over the 3D brain model.
-
36.Adjust the position of the supratentorial 3D brain model so that the top of the entire supratentorial structure sits beneath the green plane. Select “Extrude down”.
-
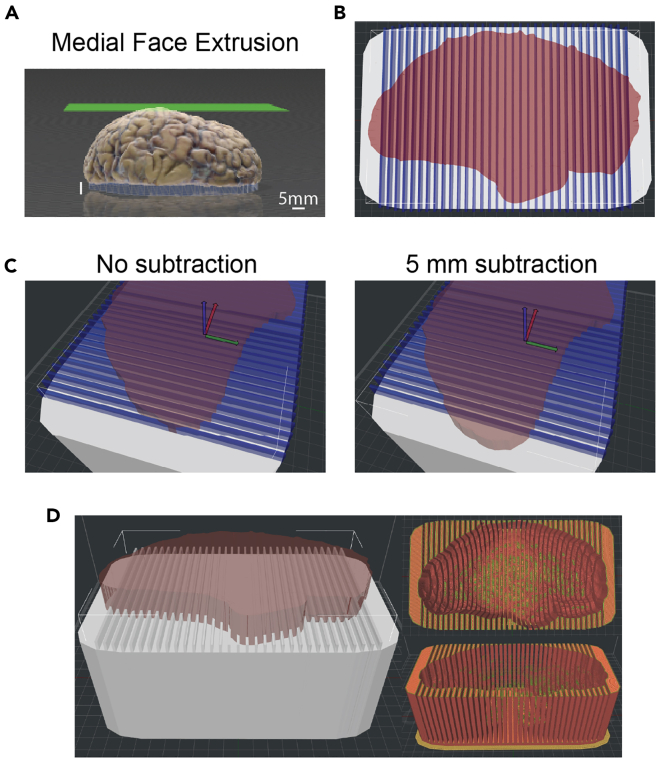
a.Successful extrusion results in a 5 mm white lengthening of the medial surface that sits underneath the model (Figure 3A). See problem 4: extrusion in troubleshooting to navigate issues with extrusion.
-
a.
-
37.
Save the extruded 3D brain model with a unique file name in the .obj format.
Figure 3.
3D sectioning matrix model generation using 3DBuilder and IdeaMaker
(A) Extrusion of the medial face of the supratentorial 3D brain model in 3DBuilder. Extrusion results in the medial face flush with the Z plane and positioned below the extrusion surface (green).
(B) Top-down view of a 3D matrix Brain Box template (white) modified with an extruded 3D brain scan (red) and with vertical modifiers that print out as the knife guides (blue).
(C) 5 mm subtraction in the X plane of the frontal pole of the extruded 3D brain model modifier in the Brain Box template. Prior to subtraction, the edge of the frontal pole meets the vertical knife guide modifier. After subtraction, 5 mm of the frontal pole is positioned anterior to the knife guide.
(D) Side and top down print previews for the completed 3D sectioning matrix.
Part 3: Matrix 3D printing
Timing: 48 h
In Part 3, IdeaMaker is used to apply the extruded 3D brain model created in Part 2 as a modifier to a pre-designed Brain Box matrix template (Available for download from Zenodo: https://doi.org/10.5281/zenodo.12745266), completing the generation of a 3D sectioning matrix for printing. A Gcode file of the resulting matrix is generated for 3D printing on a Raise3D Pro2 printer (Methods video S2).
-
38.Create the sectioning matrix:
-
a.Open the IdeaMaker program and choose “Open Idea File”.
-
i.Choose the Brain Box template file that best fits the size of the 3D brain model previously measured in step 25.
-
i.
-
a.
Note: Matrix templates are available for brains ranging in sizes from 90 mm to 190 mm in 10 mm increments and feature vertical modifiers (Figure 3B) that create knife guides at 5 mm intervals.
-
39.Add the extruded 3D brain model as a modifier to the Brain Box matrix template by selecting the Brain Box, navigating to “Modifier”, and clicking on “+”.
-
a.Navigate and load the extruded 3D brain model .obj file. A shadow-like image of the brain model will appear in the field.
-
b.Click on the Brain Box, go to “Modifier”, and in the modifier menu select the extruded 3D brain model.
-
c.Change the “Type” to “Remove the overlap regions”.
-
a.
-
40.Adjust the extruded 3D model in the Brain Box:
-
a.Adjust the position of the 3D model to the top of the Brain Box and center.
-
b.Rotate the extruded 3D brain model 180 degrees to true coronal orientation.
-
c.Move the 3D brain model along the X axis to the first knife guide at the frontal pole (Figure 3C).
-
d.Scale the 3D brain model using the uniform scaling option. In the “scale” field adjust the scale value to 101% so that the brain tissue will fit in the printed sectioning matrix.
-
e.Rotate the 3D brain model so that the medial portion is facing upwards and the brain itself is as level as possible.
-
i.Select the 3D brain model, move it on to the Brain Box and center (Figures 3B and 3C).
-
ii.Once the “X, Y, and Z values” are equal to 0mm, adjust the “Z value” to 6mm.
-
i.
-
a.
Note: If the 3D brain model is too big or too small for the premade Brain Box matrix template, use the “scale” tool to adjust the box size. Adjust the “X scale” to change the length of the box and the “Y scale” to change the width. Changing the “Z scale” or using the “uniform scaling” tool when adjusting Brain Box dimensions is not recommended. The ends of the knife guide modifiers extend to 4 mm above the edge of the Brain Box, leaving 2 mm for the knife to cut completely through the slice.
-
41.Line up the position of the extruded 3D brain model with the knife guides.
-
a.Position the first 5 mm knife guide by moving the 3D brain model along the X axis in small increments until the frontal pole just passes through the first guide (Figure 3C).
-
b.Subtract 5 mm from the X position so that the first guide creates a 5 mm section through the frontal pole.
-
c.Remove extra 5 mm slices from the posterior pole of the Brain Box so that excessive knife guides are eliminated.
Optional: Navigate to “Save Idea file as” and save the modified Brain Box as a .idea file. -
a.
-
42.Package, export and 3D print the sectioning matrix:
-
a.Review the modified Brain Box. Choose “Start Slicing”, and in the template pop-up select “Standard-Pro2-PLA” for standard print speed, printer model and printer material respectively.
-
a.
Note: See problem 7: 3D Matrix instability or costly/delayed printing time in troubleshooting for more details about speed, infill, and other print options.
-
43.
Move the “Layers” cursor back and forth to run through the matrix layers to preview.
Note: Make final adjustments by choosing “close preview” to return to the editor screen in IdeaMaker.
-
44.Export the completed sectioning matrix by clicking “Export to local disk”.
-
a.Save as a GCode file (gcode) to a USB or similar external storage device for transfer to the 3D printer.
-
a.
-
45.
Insert the USB into the 3D printer and begin 3D print.
Note: Consult the operating manual for the specific printer being utilized to adjust any printing settings and start the 3D print.
-
46.
Cool the 3D sectioning matrix on the printing platform when the print is finished (Methods video S3).
CRITICAL: Do not attempt to move the matrix prior to cooling, as the matrix may warp.
-
47.
Use a flat spatula or similar tool to pry the 3D sectioning matrix off the build plate. Gently remove the raft from the printed matrix and clean the build plate with 99% isopropyl alcohol.
Note: Depending on the printer type, infill density, and print speed, 3D printing of the sectioning matrix can take anywhere from several hours to two days. In this setup, the average printing time is 48 h using PLA filament at a thickness of 1.75 mm, infill density of 15%, 80.0 mm/s infill speed, layer height of 0.25 mm, and medium quality. Periodic monitoring may be necessary to prevent warm filament from accumulating on the printed 3D sectioning matrix. See problem 7: 3D Matrix instability or costly/delayed printing time in troubleshooting for more information.
Part 4: Matrix-guided tissue sectioning
Timing: 2 h
In the final step, postmortem human hemibrain is placed into the sample-specific 3D printed sectioning matrix for sectioning into 4 mm-thick coronal slabs. After sectioning, digital photographs are taken of each slab, targeted regions of interest are sampled, and tissue is placed into cassettes for downstream histology. In accordance with the National Institute on Aging-Alzheimer’s Association (NIA-AA) guidelines for completing histological neuropathological evaluations of Alzheimer’s disease on postmortem human brain,2 this protocol includes an additional histopathological workflow to evaluate macroscopic pathologic features for disease diagnosis. As tissue is sectioned, it is prudent to orient slabs to reflect true left or right directionality.
-
48.Prepare the 3D sectioning matrix for use during sectioning:
-
a.Smooth sharp or protruding surfaces and excess filament build-up on the inside of the matrix with sandpaper or a Dremel filing tool.
Optional: If using the sectioning matrix for tissue storage, drill holes on the anterior and posterior ends to prevent the matrix from floating in storage solution. -
a.
-
49.Separate, section, and photograph the infratentorial structures:
-
a.Prepare the sectioning area with a sterile Mopec disposable knife, scalpel, cutting board, paper towels, and pre-labeled histology cassettes and storage container filled with 1.5L 1× PBS 0.02% sodium azide (Methods video S4).
-
a.
Note: Harmful chemicals should be handled with care and disposed of properly.
-
50.Remove the infratentorial brainstem and cerebellum from the formalin-fixed hemibrain by using a scalpel to cut from the superior colliculus to the mammillary body.
-
a.Set the supratentorial tissue aside, temporarily covering with a dampened disposable cloth soaked in 1× PBS 0.02% sodium azide or water to mitigate drying (Methods video S4).
-
a.
-
51.Section the infratentorial tissue in the rostral-caudal plane at 4 mm intervals.
-
a.Separate the medulla and spinal cord when sectioning has reached the inferior cerebellar peduncle.
-
a.
-
52.
Photograph tissue (photography station Figures 1J–1M) and note any macroscopic neuroanatomical features necessary for pathological studies.
Note: It is recommended to use a solid-colored photography-specific surface to arrange tissue, clearing any debris from slabs, and dabbing with an absorbent towel to avoid glare/sheen.
-
53.Follow the NIA-AA2 guidelines for neuropathology to sample tissue from the brainstem and cerebellum.
-
a.Place samples for further studies in histological cassettes and submerge in 10% NBF.
-
b.Temporarily store the infratentorial tissue under a damp cloth.
-
a.
Note: Do not cut into the temporal lobe when removing the infratentorial tissue. Unlike the axis of the supratentorial cerebrum, structures of the infratentorial brainstem and cerebellum are oriented along a rostral-caudal axis. As such, sectioning the supratentorial and infratentorial portions along the same axis would inaccurately survey brainstem and cerebellar structures. A separate matrix may be generated for infratentorial tissue to cut the brainstem and cerebellum in the rostral-caudal plane. At any point during sectioning or after, tissue slabs can be returned to or stored in 1× PBS with 0.02% sodium azide or a strong 10% NBF solution with antibacterial and preservative properties. General purpose HDPE boards covered in black vinyl are used for photographing tissue sections. This provides a distinct and consistent background that can be removed in post-imaging processing. The cutting boards are solely used for sectioning tissue and not for photography. It is recommended to arrange two to three slabs on the photography board at a time for the best resolution and efficiency. Keep the board surface dry during photography and take photographs quickly to avoid drying out of the tissue.
-
54.Section the hemisected supratentorial tissue (see problem 8: sectioning technique and method troubleshooting in troubleshooting for suggestions on dealing with sectioning and inconsistencies):
-
a.Examine the tissue for malformation, edema, herniation, cerebrovascular lesions, atrophy, and other macroscopic features pertinent to downstream pathological assessments.
-
i.If the olfactory bulb, pituitary gland, and/or pineal gland are present, remove the structures prior to inserting the tissue into the sectioning matrix.
-
ii.Remove any small structures that could be deformed during tissue cutting including vascular and meningeal tissues.
-
i.
-
a.
-
55.
Gently place the hemisected supratentorial tissue in the sectioning matrix with the medial side facing upwards (Methods video S5).
CRITICAL: Avoid damaging the tissue on any pronounced support structures inside of the matrix.
-
56.
Position a Mopec disposable knife at the top of the frontal pole of the matrix so that the knife handle contacts the matrix (Methods video S5).
CRITICAL: Check the alignment of the knife before placing into the guide, keeping that the knife parallel.
-
57.Section the supratentorial tissue.
-
a.Apply downward pressure in a fluid motion drawing the knife towards oneself.
-
b.Use the length of the knife blade to section through as much of the tissue as possible.
-
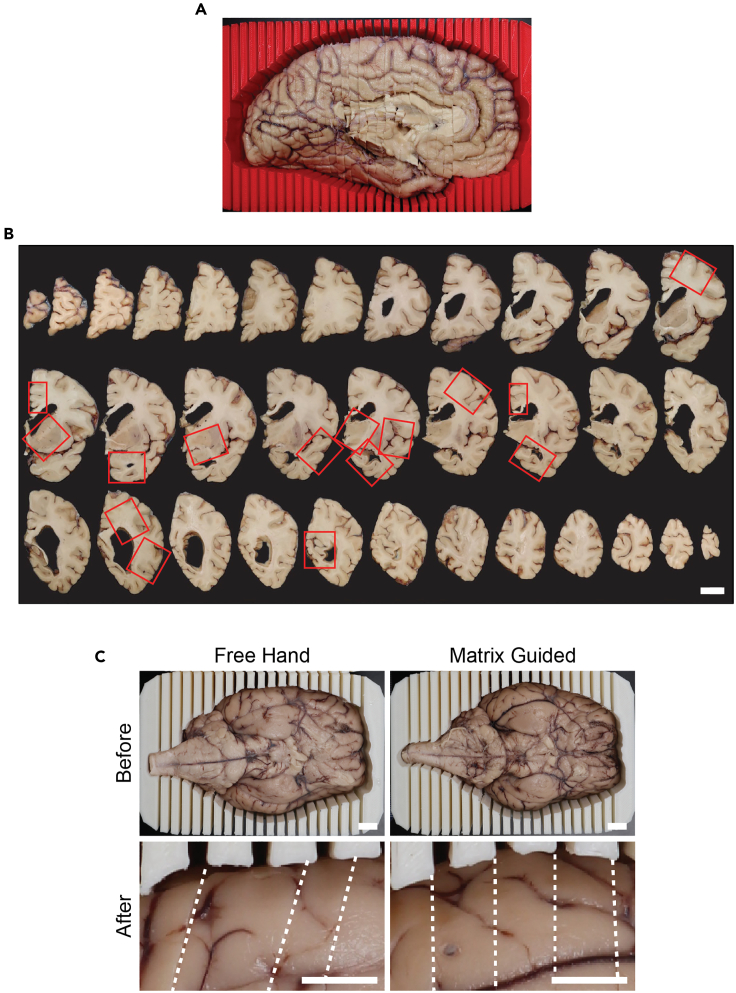
c.Section the tissue into 4 mm slabs moving from the frontal pole of the tissue to the occipital pole (Figure 4A and Methods video S5).
-
a.
Note: The total number of slabs will vary based on individual brain size.
-
58.Remove sectioned tissue slabs from the sectioning matrix by placing one hand over the medial surface of the matrix and inverting the matrix over the cutting board.
-
a.Tissue will gently shift out of the matrix and into the hand (Methods video S5).
-
a.
-
59.
Arrange the tissue slabs on the cutting board in order from frontal pole to occipital pole in true coronal orientation (Figure 4B and Methods video S5).
-
60.Photograph and document macroscopic anatomical features of the supratentorial tissue slabs:
-
a.Assess and note macroscopic features of the coronal slabs, including degree of ventricular enlargement, thickness of the cortical ribbon, hippocampal volume, and other atrophy.
-
a.
-
61.Photograph tissue slabs on a photography-specific surface, removing any debris from tissue slabs as needed.
-
a.Use an absorbent cloth to dab residual blood or water off the tissue slab. Return photographed slabs back to the cutting board, laying them in their previous location.
-
a.
CRITICAL: Cover with a damp paper towel to avoid drying until photography is complete.
- 62.
-
63.Store sectioned and sampled slabs from both the infratentorial and supratentorial tissue in either 10% NBF or 1× PBS with 0.02% sodium azide.
-
a.See the optional step below for storing tissue in the 3D sectioning matrix.
Optional: Store matrix-guided brain tissue slabs in the 3D sectioning matrix: Arrange sampled slabs in the correct anatomical order, stack the tissue slabs in small groups, and gently place the stacks back into the 3D sectioning matrix. Place the 3D sectioning matrix with the sectioned tissue nested inside into a plastic storage container with either 10% NBF or 1× PBS 0.02% sodium azide. Gently press the matrix into the bottom of the plastic storage container to submerge. The storage solution should flow into the pre-drilled holes. Arrange the infratentorial tissue along the sides of the 3D sectioning matrix in the plastic storage container. Other tissues, such as dura matter, can also be stored along the sides. Place an absorbent cloth over the top of the tissue-filled matrix to prevent tissue from floating and solution evaporation during storage. Secure the lid on the storage container. Wrap the lid and container with Parafilm to further prevent spills, leakage, and evaporation. Store the sealed plastic storage container at 4°C or room temperature for long-term storage. -
a.
Figure 4.
Postmortem brain tissue is reproducibly sectioned and sampled using a 3D sectioning matrix
(A) Printed 3D sectioning matrix with corresponding postmortem human hemibrain brain following removal of the infratentorial tissue and sectioning.
(B) Matrix-guided sections of postmortem human supratentorial brain tissue, oriented anterior-posterior from left to right, top to bottom. Sampling from matrix-guided slabs for histological neuropathological evaluations of Alzheimer’s disease based on NIA-AA guidelines (red outline), scale bar = 2 cm.
(C) Comparison of thickness consistency between postmortem sheep brain tissue before and after (magnified) sectioned by free hand versus guided sectioning in a sample-specific 3D matrix. Sections and mismatches indicated with a dotted white line, scale bar = 1 cm.
Expected outcomes
In this protocol, a pipeline is detailed for the systematic sectioning and sampling of postmortem human brain tissue utilizing 3D hybrid structure light scanning and 3D printing to generate customized sectioning matrices. While the aim of this pipeline is to facilitate consistent sampling and storage of donated postmortem human brain tissue, the application of this workflow, as well as adaptation for research model systems or tissue types, is also encouraged. The use of 3D printed sectioning matrices has demonstrated improved accuracy and consistency for the University of Texas Health Science Center at San Antonio research neuropathology practice. By using the sectioning matrix, the pipeline more closely replicates the suggested NIA-AA guidelines2 for Alzheimer’s disease histopathological diagnosis between samples while also limiting unnecessary tissue waste. This pipeline maintains biological safety by limiting the need to physically hold the brain tissue while sectioning. Considering the sensitive nature of brain banking and tissue biorepositories, use of 3D scanning and printing technologies facilitates a respectful and dignified way to process and fully utilize tissue for pathology and research.
Successful implementation of the final 3D sectioning matrix is contingent on adequate scanning and processing of the 3D model. Poor scan coverage and misalignment can result in an inaccurate 3D model and matrix (Figures 2A and 2B). The meshing attributes chosen during 3D model rendering also impact the final printed matrix. While selecting low resolution options may seem to save time or decrease file size, lower resolution removes small surface details from the printed matrix, resulting in difficulty fitting the tissue to the matrix (Figure 2C). Following matrix-guided tissue sectioning, the sampled postmortem tissue is placed in histology cassettes for paraffin wax processing. The use of a 3D printed sectioning matrix facilitates consistent sectioning of 4 mm tissue slabs (Figures 4A–4C) that match the thickness of histology cassettes, reducing the need to further face and dissect the tissue slab to sample regions of interest (Figure 4B).
Using a matrix-guided approach to generate samples for pathological-based diagnostics ensures rigorous and reproducible adherence to established guidelines for the diagnosis of neurological disorders based on microscopic slides. With the option to store tissue slabs in their respective anatomical orientation inside the sectioning matrix, histotechnologists or laboratory technicians proficient in tissue preparation can easily revisit tissue for additional sampling and assays. Routine neuropathological molecular phenotyping for neurodegenerative diagnoses is easily performed, and the consistent nature of section thickness and region sampling decreases potential diagnostic variability from case to case. Overall, diagnostic assessment of tissue for neuropathological molecular phenotyping is streamlined, improving the accuracy and proficiency of diagnostic assessments by pathologists. Recent reports find success using machine-learning and artificial intelligence (AI) to quickly assess pathology in samples from multiple organs.8 The implementation of this pipeline and the resulting reproducibility in pathological methods is suitable to complement current machine-learning methods and will improve AI-based assessment of pathologies in the human brain.
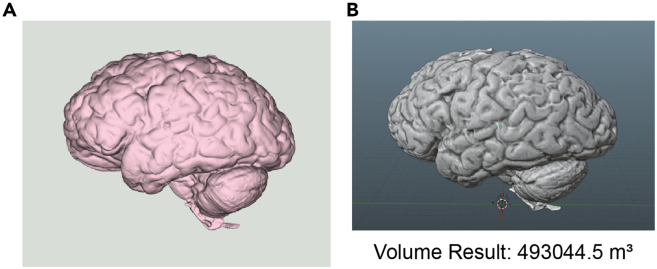
Complete 3D brain models featuring both the supra- and infratentorial structures are useful in other downstream applications beside use in creating sectioning matrices. EXScanner measures length, width, height, and other physical features of a hybrid structured light 3D-scanned brain. To demonstrate the use of 3D brain models in other applications, the volumes were visualized and measured using both Blender and Python. Using a .stl file, the modified Python coding segment in the quantification and statistical analysis section of this protocol generated two models with a brain volume of 49,3044.5 m³ (Figure 5).
Figure 5.
Rendered 3D brain models visualized and measured via Blender or Python
(A) Visualization Toolkit generated 3D brain model via Python-based rendering. 3D model is visualized as a mesh in Python to obtain measurements.
(B) Blender-rendered and measured volume of a hybrid structured light 3D-scanned brain. The volume and area of 3D brain model from Blender can be verified using Python. Full code segments detailed in the quantification and statistical analysis section.
Currently, MRI imaging serves as the gold standard for measuring indicators of atrophy, including decreased brain volume and decreased cortical thickness. While MRI provides these and other measures, the frequency in which cases donated to brain repositories having records of antemortem MRI imaging is low. Discrepancy between antemortem neuroimaging and postmortem tissue structure is likely, as atrophy is a progressive feature of neurodegeneration. Further, MRI accessibility and technical proficiency may be limited in different pathology settings. Despite these limitations, MRI remains a valuable tool that can be complemented with hybrid structured light scanning. In this regard, co-registering hybrid structured light scan-rendered 3D brain models with those from postmortem ex vivo MRI verifies the anatomical accuracy of the hybrid structured light scans and aids in evaluating disease progression and trajectories across different imaging modalities. Using advanced analysis methods such as mesh segmentation provides a proxy measurement of atrophy in distinct brain regions, while the use of model manipulation results in model segmentation and verification of deviations from standardized human brain dimensions.
In addition to serving as a cost-effective diagnostic and research modality, 3D brain modeling via hybrid structured light scanning has added value for medical education, especially considering that virtual and augmented reality-based teaching tools are increasingly prevalent. The integration of hybrid structured light scan-rendered 3D brain models in holographic capturing technology and surgical simulations currently in use is a beneficial tool for medical education, fostering real-world simulations for students training in anatomy or pathology. Taken together, the integration of hybrid structured light 3D scanning in neuropathology practice and education serves as both an accessible quality control measure and as a cost-effective solution for enhanced efficiency and reproducibility.
Quantification and statistical analysis
An added benefit of hybrid structured light scanning of postmortem human brain for the creation of custom sectioning matrices is the generation of 3D brain models that are compatible with a range of statistical analysis and visualization methods. In the following code segments, a .stl file is used to visualize and measure volume either via Python or Blender. Though other coding and modeling applications are suitable for visualization and measurements, Python allows for simple modification of the physical appearance of the 3D brain model. Python interprets a wide variety of 3D model files including .obj, .stl, and .ply files., and the use of Blender easily visualizes and measures model attributes.
-
1.Model Visualization using Python:
-
a.Open Anaconda Navigator.
-
a.
-
2.
Open Anaconda Prompt.
-
3.
Install the necessary modules into Anaconda Prompt.
> pip install vtk
> pip install vtkplotlib
> pip install mesh
> pip install meshlib.mrmeshpy
> pip install pymeshlab
-
4.
Import the necessary packages into JupyterLab.
> import vtk
> import vtkplotlib as vpl
> import pymeshlab as ml
> import meshlib
> import meshlib.mrmeshpy
> from stl.mesh import Mesh
-
5.
Define the path to the desired file.
> path = (r"C:∖Users∖userID∖Location/Folder/filename.stl")
-
6.
Define the path to retrieve it from the file.
> mesh = Mesh.from_file(path)
-
7.
Utilize vtk attributes to visualize the path.
> vpl.mesh_plot(mesh)
> vpl.mesh_plot(mesh, color = "light pink")
> vpl.show()
-
8.
Define a secondary component to load the 3D mesh.
> mesh2 = mr.loadMesh(mr.Path(r"C:∖Users∖userID∖Location/ Folder/filename.stl"))
-
9.
Define “MeshSet” attribute.
> ms = ml.MeshSet()
-
10.
Utilize “MeshSet” attribute.
> ms.load_new_mesh(r"C:∖Users∖userID∖Location/Folder/filename.stl")
-
11.
Utilize “meshlib.mrmeshpy” to save generated mesh under a new filename.
> mr.saveMesh(mesh2, mr.Path("brain.stl"))
-
12.Calculating volume using Python:
-
a.Open Anaconda Navigator.
-
a.
-
13.
Open Anaconda Prompt.
-
14.
Install the necessary packages into Anaconda Prompt.
> pip install numpy
-
15.
Import the necessary packages into JupyterLab.
> import numpy as np
> from stl.mesh import Mesh
-
16.
Define a path to the desired .stl file.
> your_mesh = Mesh.from_file (r"C:∖Users∖userID∖Location∖Folder∖filename.stl")
-
17.
Find the mass properties of the desired 3D mesh.
> Volume, cog, inertia = your_mesh.get_mass_properties()
-
18.
Print the obtained volume of the desired 3D mesh.
> print("Volume = {0}".format(volume))
-
19.Calculating volume using Blender:
-
a.Open Blender Application.
-
a.
-
20.
Click anywhere on the grid outside of the welcome box.
-
21.
Right click the gray cube in the middle of the screen and then “Delete”.
-
22.Import the desired file to Blender.
-
a.Click “File” in the upper left corner of the screen and select “Import”.
-
b.Select “.stl”.
-
c.Locate and click on the desired file.
-
d.Use the scroll button on the mouse to zoom out from imported mesh.
-
a.
-
23.Analyze the 3D mesh.
-
a.Click “Edit” in the upper left corner of the screen and select “Preferences”.
-
b.Click “Add-ons” and select the checkbox on “Mesh: 3D-Print Toolbox”.
-
c.Exit Preferences.
-
d.Locate the “Transform” tab.
-
i.The table may appear as a small arrow next to the “filename” of the mesh under “Collection”.
-
ii.You may need to click this arrow for the 3D Print option to become available.
-
i.
-
e.Click the “3D-Print” subheading and locate “Statistics” and select “Volume”.
-
f.Data on volume is under “Result”.
-
a.
-
24.
Exit the Blender Application.
Limitations
While this protocol aims to streamline the process of evaluating postmortem human brain tissue for neuropathology and brain banking, additional time and labor commitment is required by this workflow. It is recommended that individuals are familiar with neuroanatomy and well trained in neuropathology and histology prior to implementing the pipeline. Though the use of bioinformatic skills is becoming more prevalent, some may find using EXScanner, 3DBuilder, IdeaMaker, Python or Blender unfamiliar. The following resource, https://www.python.org/about/gettingstarted/, is recommended for those who are interested in more training prior to implementing the pipeline.
Troubleshooting
Problem 1 (Registration and tracking errors during scanning - Part 1)
The unique nature of the human brain means that the scanning angle and speed of scanning will vary from sample to sample. EXScanner retains scans based on the number of points and frames collected. Acquiring the minimum number of frames and points limits the likelihood that EXScanner will fail to register a complete model due to insufficient image capture. Poor tracking, scan rate, and coverage often result in failure to acquire sufficient frames and points.
Potential solutions
-
•
Reset the scanner in EXScanner software.
-
•
Reposition the scanner or the tissue to minimize vibration artifact or movement that can disrupt tracking.
-
•
Adjust the position of the hemibrain. Use non-adhesive supports, such as folded KimWipes, to maintain the tissue in a set position.
-
•
Adjust the brightness/contrast and zoom settings in EXScanner software.
-
•
Adjust zoom by hand via moving the scanner closer or further away from the sample.
-
•
Slow the rate of tissue scanning either by moving the handheld scanner more slowly, or by turning down the speed on the motorized turntable. Consider an adjustable arm or boom to support the scanner.
-
•
Do not end a scan until the acquisition of at minimum 2,000 frames and 35,000 points for successful use of “Generate point clouds” function.
Problem 2 (Inconsistencies in anatomical features of the 3D model – Part 2)
Model scan alignment greatly impacts the ability for the EXScanner program to correctly generate a raw 3D brain model. Further, lack of scan coverage contributes to alignment issues that then make removal of the infratentorial tissues and model extrusion inaccurate.
Potential solutions
-
•
Sufficient coverage of the adjoining edges of both halves coupled with manual verification of alignment points can mitigate this issue.
Problem 3 (Region selection and deletion errors – Part 2)
The exact location of the superior colliculi and the mammillary body on the medial surface can vary from brain to brain. Consequently, improper selection of the supra and infratentorial structures for removal is possible. When switching between the “Select Through” and the “Select Visible” functions in EXScanner portions of brain tissue may be deleted. Restoring deleted regions is not always achievable without removing other changes that have been made. For example, it is possible to select and delete portions of the temporal lobe when attempting to delete the cerebellum using the “Select Through” function.
Potential solutions
-
•
Save an unmodified 3D scan file prior to creating a model with the brainstem removed. If errors occur during the removal of the brainstem, reopen the unmodified 3D scan to attempt modification again.
-
•
Check selections before deleting them and utilize “ctrl + select” to remove desired areas. Reserve using the “Select Visible” option to only select areas on the medial side of the brain
-
•
Do not select the mammillary body when highlighting the brainstem region for removal in the EXScanner application. Make removal selections starting at the level of the superior colliculi up to the mammillary body.
-
•
Switch between options for “Select Through”, “Select Visible” and “ctrl + select” to accurately select the brainstem region for removal.
-
•
If a mammillary body structure is not easy to identify or is missing, use the colliculi and the midbrain to guide modification.
Problem 4 (Extrusion – Part 2)
Downstream processing of the Brain Box using the extruded 3D brain model is easily affected by poor processing of anatomical features and by aberrant extrusion. Erroneous removal of portions of the superior or inferior surfaces during processing generates an inaccurately extruded 3D brain model. Misalignment of the lateral and medial orientations with the extrusion plane results in mis-extrusion of the medial surface and an anatomically incorrect 3D sectioning matrix. The goal of using an extruded 3D brain model as a modifier of the Brain Box is to introduce a sample-specific mold of the scanned 3D brain that sits inside of the matrix with sufficient clearance for the sectioning knife to align within the knife guides prior to making the cut. If the applied modifier is not aligned parallel to the extrusion surface, then the resulting extruded brain 3D model sits angled relative to the sectioning plane. Consequentially, knife clearance is inconsistent through the printed matrix.
Potential solutions
-
•
The green line in the extrusion step must be located above the brain tissue. Alter the position of the green line using the mouse and by rotating the brain to verify that there are not any portions of the brain above the green line.
-
•
If alignment is anatomically incorrect, add additional project groups in other anatomical directions. Manually align “Lateral” and “Medial” groups and save as “Group 1”. Use project scans from other anatomical directions to align with “Group 1” for a more accurate model.
Problem 5 (3D matrix design and 3D printing – Part 3)
Mispairing of “Brain Box” template size to brain tissue size can result in a model that hinders tissue sectioning by either being too small, too large, or off-center.
Potential solutions
-
•
When choosing a box model for the brain, select a template with dimensions that are larger than that of the postmortem tissue. If the brain is slightly larger or smaller than the box model, use the “Scale” function to adjust the dimensions of the box model.
-
•
To avoid issues with creating a poor-fit, it is crucial that the brain tissue is positioned as flat as possible on the medial surface before extrusion during model generation. It is also essential to ensure that the brain model is properly aligned inside of the box model in step 40 of Part 3 Matrix 3D Printing section.
Problem 6 (PLA filament buildup during printing – Part 3)
Given that there are temperature changes during 3D printing, heated filament can leak out of the print head nozzles and collect onto the build plate. Aggregation of filament can create irregular bumps on the surface of the sectioning matrix and cause the brain tissue to not fit or the sectioning matrix to be warped.
Potential solutions
-
•
Remove the excess filament from the printing surface just before the print begins to avoid irregularities in the printed mold. It is recommended to consistently monitor printing to prevent buildup and to facilitate any corrective measures necessary during printing.
-
•
When creating the extruded model without the brainstem, ensure that the medial surface of the brain is as flat as possible and above the bottom of the extruded surface before attempting to 3D print.
Problem 7 (3D matrix instability or costly/delayed printing time – Part 3)
The sectioning matrix print time is dependent on print settings selected during packaging of the sectioning matrix file for printing. Internal structural support to the matrix is based on the infill setting, which provides stability and rigidity to the printed matrix. Modification of the infill settings alters the density and printing time of the matrix. While higher infill densities increase internal structural integrity, the use of higher infill also greatly increases material use and print time.
Potential solutions
-
•Adjust the infill settings:
-
○Select the “Brain Box”, go to “Modifier”, click on the “+”, and navigate to the “0–70 mm infill change.stl” file (download available from https://github.com/bienieklab/STAR-Protocols-). A shadowy cross section will be added to the field that will change the infill at that specific position on the box.
-
○The first layer is 2.5 mm high and subsequent layers are only 0.5 mm.
-
○Each layer is 10 mm apart, and the bottom most layer begins at the junction of the cutting guides and the box (4 mm).
-
○Go to “Modifier” and navigate to the infill file. Change “Type” to “Change settings of overlap with parent model”.
-
○Select the “+” underneath the “Type” section and navigate to “Infill”. Change “Infill density” and “Infill pattern type” to 60% and select the “Triangles” option.
-
○Select “Move” and center the infill template inside of the brain box.
-
○Review the “Brain Box” by choosing the “Start slicing” option at the top. This will populate template selection. Choose “Speed – Pro2 – PLA” and then click “Slice”. The “Speed-Pro2-PLA” option has an infill speed of 90 mm/s compared to the “Standard-Pro2-PLA” and the “High Quality-Pro2-PLA” settings that operate at 70 mm/s “Pro2” defines the printer model and “PLA” defines the printing material.
-
○If printing with multiple extruders or filaments, you can view the model in coordinating colors by choosing “Extruder color” in the drop-down menu next to the “Only current layer” box. Adjust the slicing parameters on the 3D printer prior to export to get an accurate time and cost measurement for the model. With the correct slicing parameters assigned, the model is then ready for export to the 3D printer. Consult the operating manual for the specific printer being utilized if these instructions vary.
-
○Lowering the “Infill Density” will result in a faster print time and less filament will be used.
-
○
Problem 8 (Sectioning technique and method troubleshooting – Part 4)
Variable positioning of the dissecting knife, inadequate space for knife placement, and incorrect matrix are all potential causes of variability in tissue slabs. In this protocol, a separate sectioning matrix is not used to section the brainstem and cerebellum. As such, the freehand cut of these regions increases the likelihood of sections with varying thickness.
Potential solution
-
•
Use of a bowing motion akin to playing a violin rather than a sawing motion to section tissue limits artifacts from the dissection knife.
-
•
Place a paper towel on top of the brain mold and floating tissue following its placement into the storage container.
-
•
Gently hold the infratentorial tissue between the thumb and index finger while using the middle finger to stabilize grip.
Problem 9 (Storage of tissue in the 3D sectioning matrix – Part 4)
The placement of sectioned tissue and its corresponding 3D sectioning matrix into a storage container can present its own technical difficulties. The sectioning matrix is susceptible to floating in its storage solution, especially when a low infill print setting is used that creates pockets of air within the matrix structure. The hollow nature causes the matrix to float, and sectioned tissue housed inside the matrix or free-floating in the storage container can shift.
Potential solution
-
•
Drill holes into the hollow portions along the top and bottom sides of the 3D sectioning matrix. A minimum of three to four holes per side is recommended.
-
•
Create a separate sectioning matrix for the infratentorial tissues in the rostral-caudal plane using the steps in this pipeline as a guide.
Note: The original Brain Box template has 196 mm (L) × 106 mm (w) × 75 mm (h) dimensions and is designed to fit inside plastic brain storage containers with a minimal clearance of 203 mm × 108 mm x 89 mm. Brain Box templates can be modified to accommodate a wide range of storage container sizes.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Kevin F. Bieniek (bieniek@uthscsa.edu).
Technical contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Kevin F. Bieniek (bieniek@uthscsa.edu).
Materials availability
This study did not generate new unique reagents. The Biggs Brain Bank catalogues and stores all 3D-printed matrices used.
Data and code availability
The coding segments utilized in this protocol can be found at the GitHub repository: https://github.com/bwoodsend/vtkplotlib.git and https://github.com/WoLpH/numpy-stl. Details of the utilization of the coding segments are listed in the quantification and statistical analysis portion of this protocol. This study did not generate any novel code. “Brain Box” templates and infill files are deposited and available for download at the Bieniek Lab GitHub repository (https://doi.org/10.5281/zenodo.12745266).
Acknowledgments
Support for K.F.B. and the Biggs Brain Bank Core at Univeristy of Texas Health Science Center at San Antonio is provided by the National Institute on Aging (P30AG066546), the Texas Alzheimer’s Research and Care Consortium, the Bill and Rebecca Reed Precision Medicine Center, and the Bartell and Mollie Zachry Endowment for Alzheimer Research and Patient Care. E.O. receives support via the Pathobiology of Occlusive Vascular Disease T32 (HL04776) from the National Heart, Lung, and Blood Institute and the SABER – Institutional Research and Academic Career Development Award (K12 GM111726) from the National Institute of General Medical Sciences.
Author contributions
K.F.B., M.M.S., and S.B. developed the protocol. M.K. prepared and maintained human postmortem brain tissue for scanning. M.M.S. scanned brain tissue and amended the Python code to 3D view scans. E.O. created figures and diagrams. K.F.B., M.M.S., and E.O. refined the protocol and wrote the manuscript. M.E.F., S.S., and K.F.B. established and supervised brain bank protocols and neuropathology operations.
Declaration of interests
The authors declare no competing interests.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xpro.2024.103246.
References
- 1.Barannikov S., Keating M., Seshadri S., Bieniek K.F. Applications of hybrid structure light three-dimensional scanning in neurodegenerative disease autopsy brain banking. Alzheimers Dement. 2023;19 [Google Scholar]
- 2.Montine T.J., Phelps C.H., Beach T.G., Bigio E.H., Cairns N.J., Dickson D.W., Duyckaerts C., Frosch M.P., Masliah E., Mirra S.S., et al. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: A practical approach. Acta Neuropathol. 2012;123:1–11. doi: 10.1007/s00401-011-0910-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yushkevich P.A., Muñoz López M., Iñiguez de Onzoño Martin M.M., Ittyerah R., Lim S., Ravikumar S., Bedard M.L., Pickup S., Liu W., Wang J., et al. Three-dimensional mapping of neurofibrillary tangle burden in the human medial temporal lobe. Brain. 2021;144:2784–2797. doi: 10.1093/brain/awab262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guy J.R., Sati P., Leibovitch E., Jacobson S., Silva A.C., Reich D.S. Custom fit 3D-printed brain holders for comparison of histology with MRI in marmosets. J. Neurosci. Methods. 2016;257:55–63. doi: 10.1016/j.jneumeth.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tyson A.L., Hilton S.T., Andreae L.C. Rapid, simple and inexpensive production of custom 3D printed equipment for large-volume fluorescence microscopy. Int. J. Pharm. 2015;494:651–656. doi: 10.1016/j.ijpharm.2015.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Absinta M., Nair G., Filippi M., Ray-Chaudhury A., Reyes-Mantilla M.I., Pardo C.A., Reich D.S. Postmortem Magnetic Resonance Imaging to Guide the Pathologic Cut: Individualized, 3-Dimensionally Printed Cutting Boxes for Fixed Brains. J. Neuropathol. Exp. Neurol. 2014;73:780–788. doi: 10.1097/NEN.0000000000000096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Charisis S., Rashid T., Liu H., Ware J.B., Jensen P.N., Austin T.R., Li K., Fadaee E., Hilal S., Chen C., et al. Assessment of Risk Factors and Clinical Importance of Enlarged Perivascular Spaces by Whole-Brain Investigation in the Multi-Ethnic Study of Atherosclerosis. JAMA Netw. Open. 2023;6 doi: 10.1001/jamanetworkopen.2023.9196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geaney A., O'Reilly P., Maxwell P., James J.A., McArt D., Salto-Tellez M. Translation of tissue-based artificial intelligence into clinical practice: from discovery to adoption. Oncogene. 2023;42:3545–3555. doi: 10.1038/s41388-023-02857-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The coding segments utilized in this protocol can be found at the GitHub repository: https://github.com/bwoodsend/vtkplotlib.git and https://github.com/WoLpH/numpy-stl. Details of the utilization of the coding segments are listed in the quantification and statistical analysis portion of this protocol. This study did not generate any novel code. “Brain Box” templates and infill files are deposited and available for download at the Bieniek Lab GitHub repository (https://doi.org/10.5281/zenodo.12745266).