Abstract
1-Aminocyclopropane-1-carboxylate (ACC) oxidase catalyses the final step in the biosynthesis of the plant hormone ethylene. The successful overexpression and characterization of active ACC oxidase from tomato has been achieved. PCR was used to insert the corrected cDNA coding for the tomato ACC oxidase into the pET-11a expression vector. Cloning of the resultant construct in Escherichia coli BL21(DE3)pLysE gave transformants which expressed ACC oxidase at levels greater than 30% of soluble protein under optimized conditions. When induced by addition of isopropyl-beta-D-thiogalactopyranoside (IPTG) at 37 degrees C the ACC oxidase expressed was less soluble and less active than when induced at 27 degrees C. The enzyme was purified to near homogeneity by a three-step chromatographic procedure. The specific activity of the purified recombinant ACC oxidase was typically 1.3-1.9 mol of ethylene/mol of enzyme per min, higher than values reported for native enzyme. Like the native enzyme it displayed a requirement for ferrous iron and ascorbate, and CO2 was an activator. The ability to discriminate between racemic diastereomers of 1-amino-2-ethyl cyclopropane-1-carboxylic acid was demonstrated. The enzyme was found to have a loose specificity for ascorbate, showing apparent preference for D-ascorbate and 5,6-O-isopropylidene L-ascorbate rather than L-ascorbate. The addition of catalase, dithiothreitol and BSA to incubation mixtures all resulted in significant increases in activity. When treated with diethylpyrocarbonate (DEPC) under mildly acidic conditions, the enzyme rapidly lost activity. Comparison of the rate of inactivation with the increase in absorbance at 240 nm gave results consistent with the modification of two to three histidine residues at the active site, although the possibility of additional modification of other nucleophilic residues cannot be excluded. Inactivation was largely prevented by the addition of substrates and ferrous iron, implying that DEPC treatment results in the modification of active-site histidines, which act as ligands for ferrous iron. CO2 offered no protection against DEPC inactivation, either in the absence or presence of substrates and/or ferrous iron.
Full text
PDF

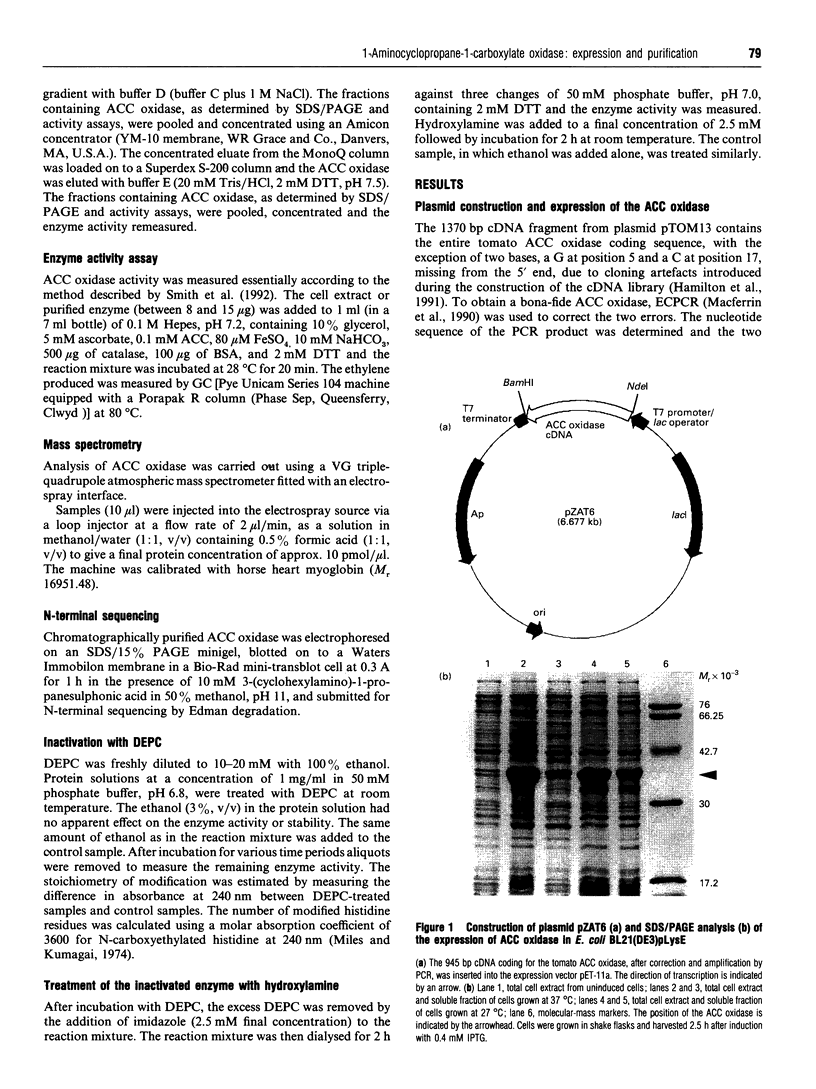






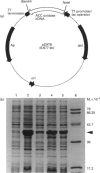
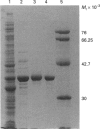
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. O., Yang S. F. Ethylene biosynthesis: Identification of 1-aminocyclopropane-1-carboxylic acid as an intermediate in the conversion of methionine to ethylene. Proc Natl Acad Sci U S A. 1979 Jan;76(1):170–174. doi: 10.1073/pnas.76.1.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Dong J. G., Fernández-Maculet J. C., Yang S. F. Purification and characterization of 1-aminocyclopropane-1-carboxylate oxidase from apple fruit. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9789–9793. doi: 10.1073/pnas.89.20.9789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J. G., Olson D., Silverstone A., Yang S. F. Sequence of a cDNA coding for a 1-aminocyclopropane-1-carboxylate oxidase homolog from apple fruit. Plant Physiol. 1992 Apr;98(4):1530–1531. doi: 10.1104/pp.98.4.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupille E., Rombaldi C., Lelièvre J. M., Cleyet-Marel J. C., Pech J. C., Latché A. Purification, properties and partial amino-acid sequence of 1-aminocyclopropane-1-carboxylic acid oxidase from apple fruits. Planta. 1993;190(1):65–70. doi: 10.1007/BF00195676. [DOI] [PubMed] [Google Scholar]
- Fernandez-Maculet J. C., Dong J. G., Yang S. F. Activation of 1-aminocyclopropane-1-carboxylate oxidase by carbon dioxide. Biochem Biophys Res Commun. 1993 Jun 30;193(3):1168–1173. doi: 10.1006/bbrc.1993.1748. [DOI] [PubMed] [Google Scholar]
- Hamilton A. J., Bouzayen M., Grierson D. Identification of a tomato gene for the ethylene-forming enzyme by expression in yeast. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7434–7437. doi: 10.1073/pnas.88.16.7434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman N. E., Yang S. F., Ichihara A., Sakamura S. Stereospecific conversion of 1-aminocyclopropanecarboxylic Acid to ethylene by plant tissues : conversion of stereoisomers of 1-amino-2-ethylcyclopropanecarboxylic Acid to 1-butene. Plant Physiol. 1982 Jul;70(1):195–199. doi: 10.1104/pp.70.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdsworth M. J., Bird C. R., Ray J., Schuch W., Grierson D. Structure and expression of an ethylene-related mRNA from tomato. Nucleic Acids Res. 1987 Jan 26;15(2):731–739. doi: 10.1093/nar/15.2.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köck M., Hamilton A., Grierson D. eth1, a gene involved in ethylene synthesis in tomato. Plant Mol Biol. 1991 Jul;17(1):141–142. doi: 10.1007/BF00036816. [DOI] [PubMed] [Google Scholar]
- MacFerrin K. D., Terranova M. P., Schreiber S. L., Verdine G. L. Overproduction and dissection of proteins by the expression-cassette polymerase chain reaction. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1937–1941. doi: 10.1073/pnas.87.5.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majamaa K., Günzler V., Hanauske-Abel H. M., Myllylä R., Kivirikko K. I. Partial identity of the 2-oxoglutarate and ascorbate binding sites of prolyl 4-hydroxylase. J Biol Chem. 1986 Jun 15;261(17):7819–7823. [PubMed] [Google Scholar]
- Matsuda J., Okabe S., Hashimoto T., Yamada Y. Molecular cloning of hyoscyamine 6 beta-hydroxylase, a 2-oxoglutarate-dependent dioxygenase, from cultured roots of Hyoscyamus niger. J Biol Chem. 1991 May 25;266(15):9460–9464. [PubMed] [Google Scholar]
- McGarvey D. J., Christoffersen R. E. Characterization and kinetic parameters of ethylene-forming enzyme from avocado fruit. J Biol Chem. 1992 Mar 25;267(9):5964–5967. [PubMed] [Google Scholar]
- McGarvey D. J., Yu H., Christoffersen R. E. Nucleotide sequence of a ripening-related cDNA from avocado fruit. Plant Mol Biol. 1990 Jul;15(1):165–167. doi: 10.1007/BF00017736. [DOI] [PubMed] [Google Scholar]
- McRae D. G., Coker J. A., Legge R. L., Thompson J. E. Bicarbonate/CO(2)-Facilitated Conversion of 1-Amino-cyclopropane-1-carboxylic Acid to Ethylene in Model Systems and Intact Tissues. Plant Physiol. 1983 Nov;73(3):784–790. doi: 10.1104/pp.73.3.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles E. W., Kumagai H. Modification of essential histidyl residues of the beta 2 subunit of tryptophan synthetase by photo-oxidation in the presence of pyridoxal 5'-phosphate and L-serine and by diethylpyrocarbonate. J Biol Chem. 1974 May 10;249(9):2843–2851. [PubMed] [Google Scholar]
- Miles E. W. Modification of histidyl residues in proteins by diethylpyrocarbonate. Methods Enzymol. 1977;47:431–442. doi: 10.1016/0076-6879(77)47043-5. [DOI] [PubMed] [Google Scholar]
- Myllylä R., Günzler V., Kivirikko K. I., Kaska D. D. Modification of vertebrate and algal prolyl 4-hydroxylases and vertebrate lysyl hydroxylase by diethyl pyrocarbonate. Evidence for histidine residues in the catalytic site of 2-oxoglutarate-coupled dioxygenases. Biochem J. 1992 Sep 15;286(Pt 3):923–927. doi: 10.1042/bj2860923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirrung M. C., Kaiser L. M., Chen J. Purification and properties of the apple fruit ethylene-forming enzyme. Biochemistry. 1993 Jul 27;32(29):7445–7450. doi: 10.1021/bi00080a015. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott R. A., Wang S., Eidsness M. K., Kriauciunas A., Frolik C. A., Chen V. J. X-ray absorption spectroscopic studies of the high-spin iron(II) active site of isopenicillin N synthase: evidence for Fe-S interaction in the enzyme-substrate complex. Biochemistry. 1992 May 19;31(19):4596–4601. doi: 10.1021/bi00134a009. [DOI] [PubMed] [Google Scholar]
- Spanu P., Reinhardt D., Boller T. Analysis and cloning of the ethylene-forming enzyme from tomato by functional expression of its mRNA in Xenopus laevis oocytes. EMBO J. 1991 Aug;10(8):2007–2013. doi: 10.1002/j.1460-2075.1991.tb07730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H., Dunn J. J., Dubendorff J. W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- Tschank G., Sanders J., Baringhaus K. H., Dallacker F., Kivirikko K. I., Günzler V. Structural requirements for the utilization of ascorbate analogues in the prolyl 4-hydroxylase reaction. Biochem J. 1994 May 15;300(Pt 1):75–79. doi: 10.1042/bj3000075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Woodson W. R. A Flower Senescence-Related mRNA from Carnation Shares Sequence Similarity with Fruit Ripening-Related mRNAs Involved in Ethylene Biosynthesis. Plant Physiol. 1991 Jul;96(3):1000–1001. doi: 10.1104/pp.96.3.1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson I. D., Zhu Y., Burmeister D. M., Dilley D. R. Apple ripening-related cDNA clone pAP4 confers ethylene-forming ability in transformed Saccharomyces cerevisiae. Plant Physiol. 1993 Jul;102(3):783–788. doi: 10.1104/pp.102.3.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip W. K., Dong J. G., Yang S. F. Purification and characterization of 1-aminocyclopropane-1-carboxylate synthase from apple fruits. Plant Physiol. 1991 Jan;95(1):251–257. doi: 10.1104/pp.95.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]