Abstract
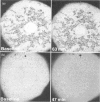
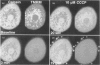
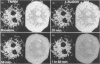
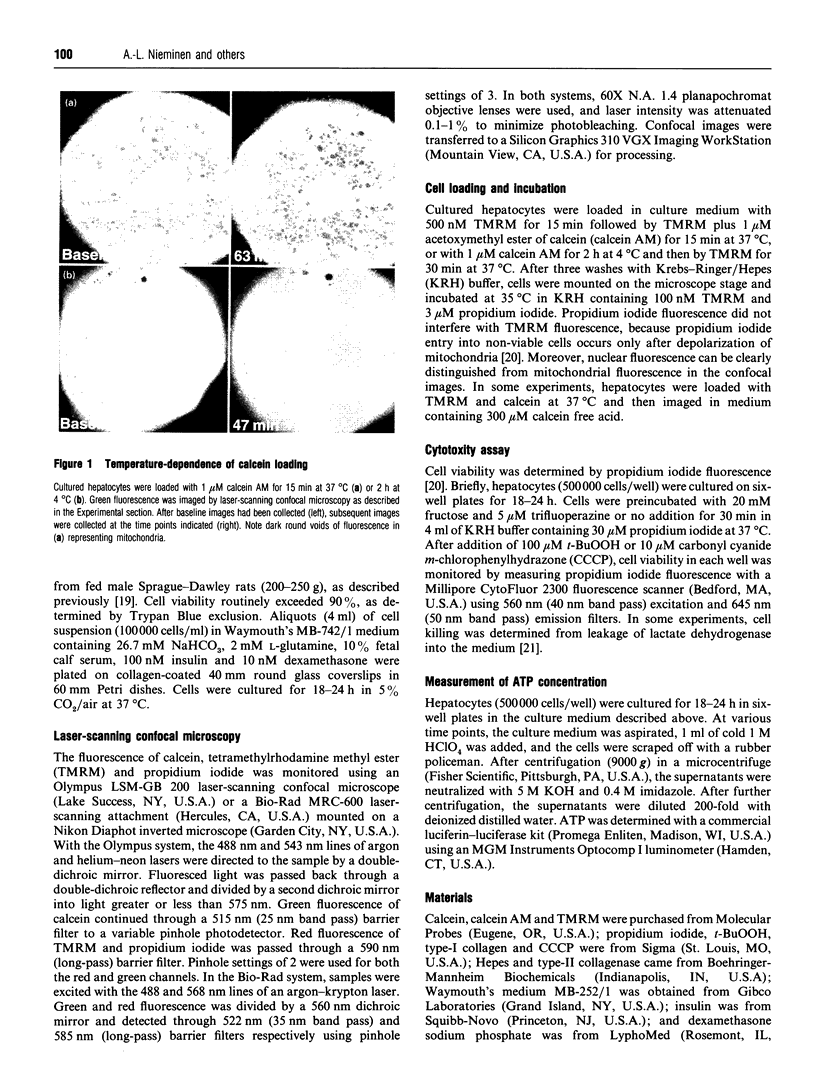
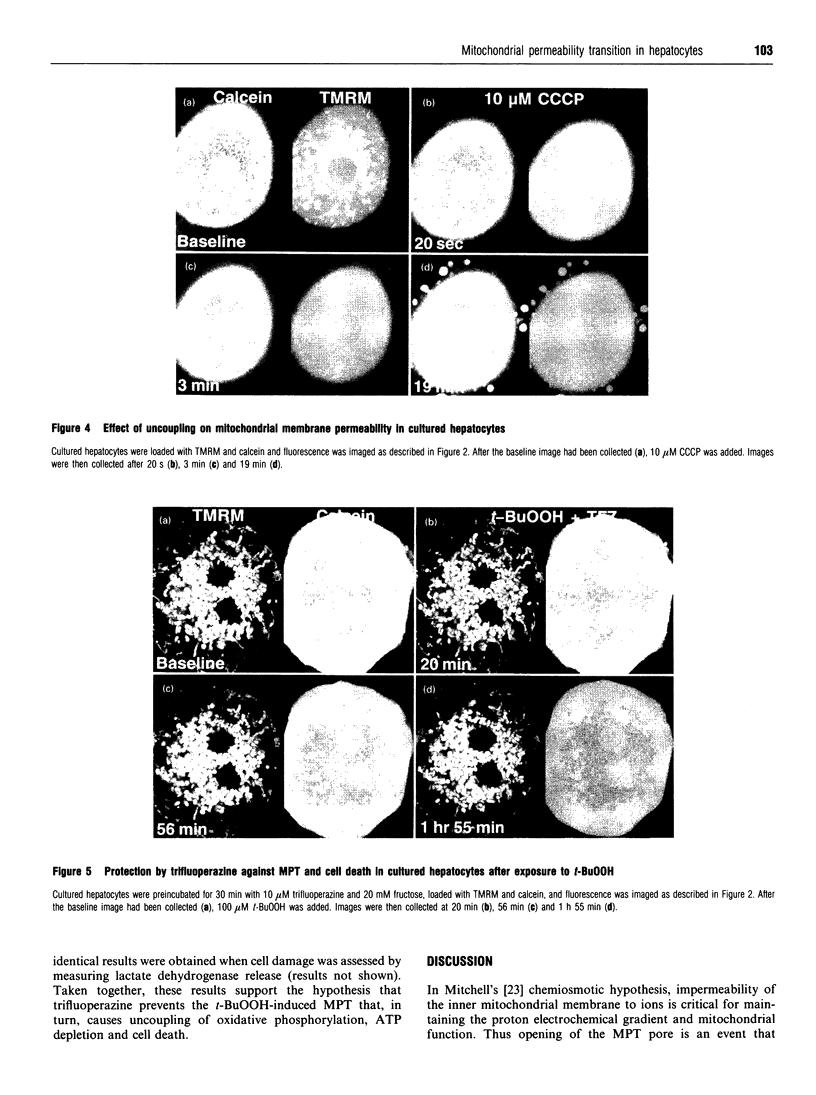
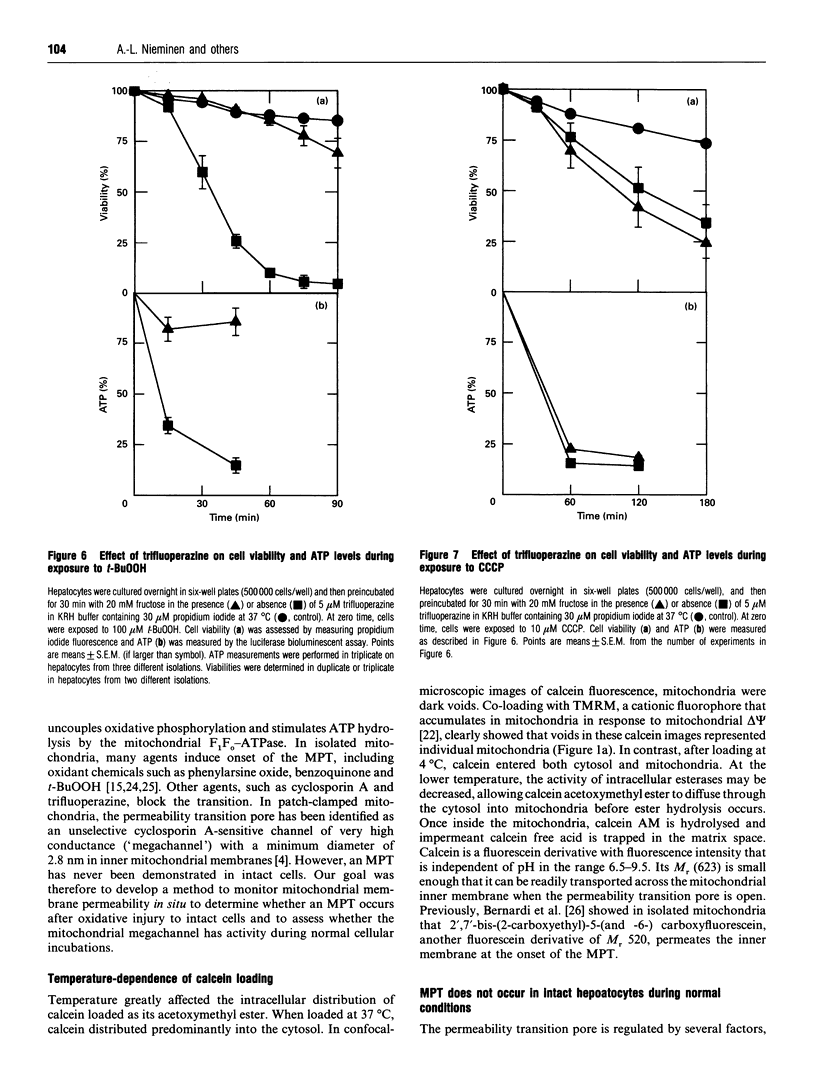
We have developed a novel method for monitoring the mitochondrial permeability transition in single intact hepatocytes during injury with t-butylhydroperoxide (t-BuOOH). Cultured hepatocytes were loaded with the fluorescence probes, calcein and tetramethylrhodamine methyl ester (TMRM). Depending on loading conditions, calcein labelled the cytosolic space exclusively and did not enter mitochondria or it stained both cytosol and mitochondria. TMRM labelled mitochondria as an indicator of mitochondrial polarization. Fluorescence of two probes was imaged simultaneously using laser-scanning confocal microscopy. During normal incubations, TMRM labelled mitochondria indefinitely (longer than 63 min), and calcein did not redistribute between cytosol and mitochondria. These findings indicate that the mitochondrial permeability transition pore ('megachannel') remained closed continuously. After addition of 100 microM t-BuOOH, mitochondria filled quickly with calcein, indicating the onset of mitochondrial permeability transition. This event was accompanied by mitochondrial depolarization, as shown by loss of TMRM. Subsequently, the concentration of ATP declined and cells lost viability. Trifluoperazine, a phospholipase inhibitor that inhibits the permeability transition in isolated mitochondria, prevented calcein redistribution into mitochondria, mitochondrial depolarization, ATP depletion and cell death. Carbonyl cyanide m-chlorophenylhydrazone (CCCP), a mitochondrial uncoupler, also rapidly depolarized mitochondria of intact hepatocytes but did not alone induce a permeability transition. Trifluoperazine did not prevent ATP depletion and cell death after the addition of CCCP. In conclusion, the permeability transition pore does not 'flicker' open during normal incubation of hepatocytes but remains continuously closed. Moreover, mitochondrial depolarization per se does not cause the permeability transition in intact cells. During oxidative stress, however, a permeability transition occurs quickly which leads to mitochondrial depolarization and cell death.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anundi I., King J., Owen D. A., Schneider H., Lemasters J. J., Thurman R. G. Fructose prevents hypoxic cell death in liver. Am J Physiol. 1987 Sep;253(3 Pt 1):G390–G396. doi: 10.1152/ajpgi.1987.253.3.G390. [DOI] [PubMed] [Google Scholar]
- Bernardi P. Modulation of the mitochondrial cyclosporin A-sensitive permeability transition pore by the proton electrochemical gradient. Evidence that the pore can be opened by membrane depolarization. J Biol Chem. 1992 May 5;267(13):8834–8839. [PubMed] [Google Scholar]
- Bernardi P., Vassanelli S., Veronese P., Colonna R., Szabó I., Zoratti M. Modulation of the mitochondrial permeability transition pore. Effect of protons and divalent cations. J Biol Chem. 1992 Feb 15;267(5):2934–2939. [PubMed] [Google Scholar]
- Bernardi P., Veronese P., Petronilli V. Modulation of the mitochondrial cyclosporin A-sensitive permeability transition pore. I. Evidence for two separate Me2+ binding sites with opposing effects on the pore open probability. J Biol Chem. 1993 Jan 15;268(2):1005–1010. [PubMed] [Google Scholar]
- Bond J. M., Chacon E., Herman B., Lemasters J. J. Intracellular pH and Ca2+ homeostasis in the pH paradox of reperfusion injury to neonatal rat cardiac myocytes. Am J Physiol. 1993 Jul;265(1 Pt 1):C129–C137. doi: 10.1152/ajpcell.1993.265.1.C129. [DOI] [PubMed] [Google Scholar]
- Broekemeier K. M., Carpenter-Deyo L., Reed D. J., Pfeiffer D. R. Cyclosporin A protects hepatocytes subjected to high Ca2+ and oxidative stress. FEBS Lett. 1992 Jun 15;304(2-3):192–194. doi: 10.1016/0014-5793(92)80616-o. [DOI] [PubMed] [Google Scholar]
- Broekemeier K. M., Dempsey M. E., Pfeiffer D. R. Cyclosporin A is a potent inhibitor of the inner membrane permeability transition in liver mitochondria. J Biol Chem. 1989 May 15;264(14):7826–7830. [PubMed] [Google Scholar]
- Broekemeier K. M., Pfeiffer D. R. Cyclosporin A-sensitive and insensitive mechanisms produce the permeability transition in mitochondria. Biochem Biophys Res Commun. 1989 Aug 30;163(1):561–566. doi: 10.1016/0006-291x(89)92174-8. [DOI] [PubMed] [Google Scholar]
- Broekemeier K. M., Schmid P. C., Schmid H. H., Pfeiffer D. R. Effects of phospholipase A2 inhibitors on ruthenium red-induced Ca2+ release from mitochondria. J Biol Chem. 1985 Jan 10;260(1):105–113. [PubMed] [Google Scholar]
- Connern C. P., Halestrap A. P. Recruitment of mitochondrial cyclophilin to the mitochondrial inner membrane under conditions of oxidative stress that enhance the opening of a calcium-sensitive non-specific channel. Biochem J. 1994 Sep 1;302(Pt 2):321–324. doi: 10.1042/bj3020321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crompton M., Costi A. Kinetic evidence for a heart mitochondrial pore activated by Ca2+, inorganic phosphate and oxidative stress. A potential mechanism for mitochondrial dysfunction during cellular Ca2+ overload. Eur J Biochem. 1988 Dec 15;178(2):489–501. doi: 10.1111/j.1432-1033.1988.tb14475.x. [DOI] [PubMed] [Google Scholar]
- Crompton M., Ellinger H., Costi A. Inhibition by cyclosporin A of a Ca2+-dependent pore in heart mitochondria activated by inorganic phosphate and oxidative stress. Biochem J. 1988 Oct 1;255(1):357–360. [PMC free article] [PubMed] [Google Scholar]
- Currin R. T., Gores G. J., Thurman R. G., Lemasters J. J. Protection by acidotic pH against anoxic cell killing in perfused rat liver: evidence for a pH paradox. FASEB J. 1991 Feb;5(2):207–210. doi: 10.1096/fasebj.5.2.2004664. [DOI] [PubMed] [Google Scholar]
- Ehrenberg B., Montana V., Wei M. D., Wuskell J. P., Loew L. M. Membrane potential can be determined in individual cells from the nernstian distribution of cationic dyes. Biophys J. 1988 May;53(5):785–794. doi: 10.1016/S0006-3495(88)83158-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier N., Ducet G., Crevat A. Action of cyclosporine on mitochondrial calcium fluxes. J Bioenerg Biomembr. 1987 Jun;19(3):297–303. doi: 10.1007/BF00762419. [DOI] [PubMed] [Google Scholar]
- Gores G. J., Flarsheim C. E., Dawson T. L., Nieminen A. L., Herman B., Lemasters J. J. Swelling, reductive stress, and cell death during chemical hypoxia in hepatocytes. Am J Physiol. 1989 Aug;257(2 Pt 1):C347–C354. doi: 10.1152/ajpcell.1989.257.2.C347. [DOI] [PubMed] [Google Scholar]
- Griffiths E. J., Halestrap A. P. Protection by Cyclosporin A of ischemia/reperfusion-induced damage in isolated rat hearts. J Mol Cell Cardiol. 1993 Dec;25(12):1461–1469. doi: 10.1006/jmcc.1993.1162. [DOI] [PubMed] [Google Scholar]
- Gunter T. E., Pfeiffer D. R. Mechanisms by which mitochondria transport calcium. Am J Physiol. 1990 May;258(5 Pt 1):C755–C786. doi: 10.1152/ajpcell.1990.258.5.C755. [DOI] [PubMed] [Google Scholar]
- Halestrap A. P. Calcium-dependent opening of a non-specific pore in the mitochondrial inner membrane is inhibited at pH values below 7. Implications for the protective effect of low pH against chemical and hypoxic cell damage. Biochem J. 1991 Sep 15;278(Pt 3):715–719. doi: 10.1042/bj2780715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman B., Nieminen A. L., Gores G. J., Lemasters J. J. Irreversible injury in anoxic hepatocytes precipitated by an abrupt increase in plasma membrane permeability. FASEB J. 1988 Feb;2(2):146–151. doi: 10.1096/fasebj.2.2.3342967. [DOI] [PubMed] [Google Scholar]
- Imberti R., Nieminen A. L., Herman B., Lemasters J. J. Mitochondrial and glycolytic dysfunction in lethal injury to hepatocytes by t-butylhydroperoxide: protection by fructose, cyclosporin A and trifluoperazine. J Pharmacol Exp Ther. 1993 Apr;265(1):392–400. [PubMed] [Google Scholar]
- Imberti R., Nieminen A. L., Herman B., Lemasters J. J. Synergism of cyclosporin A and phospholipase inhibitors in protection against lethal injury to rat hepatocytes from oxidant chemicals. Res Commun Chem Pathol Pharmacol. 1992 Oct;78(1):27–38. [PubMed] [Google Scholar]
- Lenartowicz E., Bernardi P., Azzone G. F. Phenylarsine oxide induces the cyclosporin A-sensitive membrane permeability transition in rat liver mitochondria. J Bioenerg Biomembr. 1991 Aug;23(4):679–688. doi: 10.1007/BF00785817. [DOI] [PubMed] [Google Scholar]
- Loud A. V. A quantitative stereological description of the ultrastructure of normal rat liver parenchymal cells. J Cell Biol. 1968 Apr;37(1):27–46. doi: 10.1083/jcb.37.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massari S., Azzone G. F. The equivalent pore radius of intact and damaged mitochondria and the mechanism of active shrinkage. Biochim Biophys Acta. 1972;283(1):23–29. doi: 10.1016/0005-2728(72)90094-1. [DOI] [PubMed] [Google Scholar]
- Mitchell P. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biol Rev Camb Philos Soc. 1966 Aug;41(3):445–502. doi: 10.1111/j.1469-185x.1966.tb01501.x. [DOI] [PubMed] [Google Scholar]
- Nieminen A. L., Dawson T. L., Gores G. J., Kawanishi T., Herman B., Lemasters J. J. Protection by acidotic pH and fructose against lethal injury to rat hepatocytes from mitochondrial inhibitors, ionophores and oxidant chemicals. Biochem Biophys Res Commun. 1990 Mar 16;167(2):600–606. doi: 10.1016/0006-291x(90)92067-a. [DOI] [PubMed] [Google Scholar]
- Nieminen A. L., Saylor A. K., Herman B., Lemasters J. J. ATP depletion rather than mitochondrial depolarization mediates hepatocyte killing after metabolic inhibition. Am J Physiol. 1994 Jul;267(1 Pt 1):C67–C74. doi: 10.1152/ajpcell.1994.267.1.C67. [DOI] [PubMed] [Google Scholar]
- Pastorino J. G., Snyder J. W., Serroni A., Hoek J. B., Farber J. L. Cyclosporin and carnitine prevent the anoxic death of cultured hepatocytes by inhibiting the mitochondrial permeability transition. J Biol Chem. 1993 Jul 5;268(19):13791–13798. [PubMed] [Google Scholar]
- Petronilli V., Costantini P., Scorrano L., Colonna R., Passamonti S., Bernardi P. The voltage sensor of the mitochondrial permeability transition pore is tuned by the oxidation-reduction state of vicinal thiols. Increase of the gating potential by oxidants and its reversal by reducing agents. J Biol Chem. 1994 Jun 17;269(24):16638–16642. [PubMed] [Google Scholar]
- Richter C., Schlegel J., Schweizer M. Prooxidant-induced Ca2+ release from liver mitochondria. Specific versus nonspecific pathways. Ann N Y Acad Sci. 1992 Nov 21;663:262–268. doi: 10.1111/j.1749-6632.1992.tb38669.x. [DOI] [PubMed] [Google Scholar]
- Szabó I., Zoratti M. The giant channel of the inner mitochondrial membrane is inhibited by cyclosporin A. J Biol Chem. 1991 Feb 25;266(6):3376–3379. [PubMed] [Google Scholar]
- Szabó I., Zoratti M. The mitochondrial megachannel is the permeability transition pore. J Bioenerg Biomembr. 1992 Feb;24(1):111–117. doi: 10.1007/BF00769537. [DOI] [PubMed] [Google Scholar]
- Weis M., Kass G. E., Orrenius S., Moldéus P. N-acetyl-p-benzoquinone imine induces Ca2+ release from mitochondria by stimulating pyridine nucleotide hydrolysis. J Biol Chem. 1992 Jan 15;267(2):804–809. [PubMed] [Google Scholar]