Abstract
Purpose
To investigate the clinical outcomes of micropulse transscleral laser therapy (MP-TLT) in a cohort of glaucoma patients, including safety profile, post-operative transient intraocular pressure (IOP) spikes, long-term efficacy and prognostic factors in terms of IOP-lowering.
Methods
This was a retrospective observational cohort study. Medical records of all patients who consecutively underwent MP-TLT between May 2019 and February 2023 at a tertiary referral centre were scrutinised and relevant data were retrospectively analysed.
Results
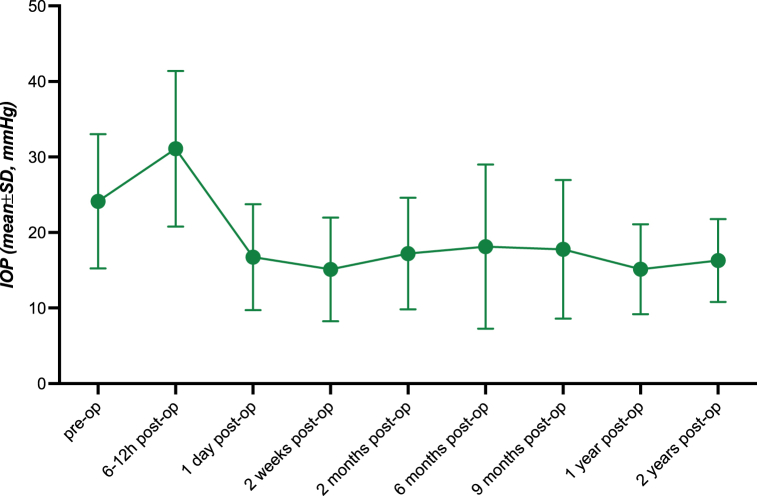
A total of 131 patients (138 eyes) with a mean age of 73.2 ± 14.2 years were included. Mean pre-interventional IOP was 24.1 ± 9.1 mmHg. Within 6–12 h following the intervention on the same day, an IOP spike was regularly observed, reaching on average 31.7 ± 10.3 mmHg (p < 0.001 to baseline). Two years after the intervention, mean IOP was 16.1 ± 5.6 mmHg (p < 0.005 to baseline). In 18 eyes, the treatment was repeated, and the IOP lowering effect was more durable after the second intervention compared to the first one (Cox-Mantel test, p=<0.005). Apart from the transient post-interventional IOP spikes, no severe complications were observed.
Conclusions
MP-TLT is associated with significant IOP spikes in the first post-operative hours. Thus, close post-interventional IOP monitoring or even preventive (additional) IOP-lowering treatment may be considered. In the long term, the procedure yields favourable outcomes in terms of safety and IOP reduction. Repeated MP-TLT treatment, if necessary, seems to achieve more sustained IOP reduction than the initial treatment.
Keywords: Glaucoma, Micropulse transscleral laser therapy
1. Introduction
Glaucoma is among the most prevalent blinding disorders worldwide and is characterized by the progressive, irreversible degeneration of the retinal ganglion cells, the output neurons of the retina. Clinically, the disease presents with deterioration of visual sensitivity, progressive visual field deficits and ultimately blindness [[1], [2], [3]]. Intraocular pressure (IOP) is determined by aqueous humour production by the ciliary body and aqueous humour outflow through the trabecular meshwork and the uveoscleral pathway. Increased IOP, which is typically the consequence of diminished aqueous humour outflow capacity, is the most significant glaucoma risk factor and the only disease-contributing factor that is readily modifiable with current treatment strategies [4,5].
Transscleral Cyclophotocoagulation (TS-CPC) uses laser energy to destroy the tissue of the ciliary body in order to inhibit aqueous humour production and consequently lower IOP. Traditionally, TS-CPC was applied using continuous laser energy and was reserved as a treatment option of last resort for refractory, advanced stage glaucoma, given the destructive and drastic effect on the ciliary body and aqueous humour dynamics [6,7]. Micropulse transscleral laser therapy (MP-TLT) is a relatively novel, emerging CPC technique that applies only short laser energy pulses to the ciliary body, and thus is believed to only partially inhibit its aqueous humour production. As such, the technique is increasingly proposed as a first-line or second-line treatment, also in milder glaucoma cases [[8], [9], [10], [11], [12], [13]]. However, evidence on the safety and efficacy of MP-TLT is still limited, and little is known on possible risk factors for treatment failure. Moreover, based on anecdotal reports, MP-TLT is believed to be associated with short-term post-operative, transient IOP spikes perhaps due to the intraocular inflammatory processes triggered by the tissue damage. Such IOP spikes would be an important parameter in the procedure's safety profile and may significantly influence the general choice of treatment but also the follow-up protocol, especially in patients with advanced glaucoma, in which transient IOP spikes could overstress the last remaining retinal ganglion cell axons. Yet, to the very best of our knowledge, no study has been published that systematically investigated the immediate IOP course in the first post-interventional hours and days.
Despite the paramount clinical importance of the intraocular mechanisms that regulate IOP, they are still only poorly understood [[14], [15], [16], [17], [18], [19]]. Furthermore, systemic influences and even interactions with the fellow eye have been proposed to contribute to IOP regulation. Mounting evidence supports the concept of so-called “consensual ophthalmotonic reactions” that occur in contralateral eyes after monocular IOP-reducing therapies or procedures of the fellow eyes, and a number of possible mechanisms of action have been proposed, e.g., hormonal, neuronal, and cytokine regulation of aqueous humour flow dynamics, as well as systemic pharmaceutical effects [[20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40]]. In a previous study, we conducted the first analysis of the effect of unilateral MP-TLT (and thus a procedure that primarily targets aqueous humour production) on IOP in untreated contralateral eyes and found a consensual IOP decrease, but this pilot study was limited by the small sample size and thus these preliminary findings are yet to be definitely tested [41].
The aim of this study is to investigate the clinical outcomes of MP-TLT in a cohort of glaucoma patients, including a comprehensive safety evaluation, an assessment of possible transient IOP spikes in the first post-interventional hours and days, possible consensual ophthalmotonic reactions of untreated fellow eyes, and an evaluation of the long-term efficacy and prognostic factors in terms of IOP-lowering.
2. Methods
2.1. Study design and ethical consideration
This present investigation was planned as a retrospective observational cohort study. The study was approved by the competent ethics committee (Ethikkommission Nordwest-und Zentralschweiz EKNZ; Project-ID 2022-00469; March 30, 2022, and May 2, 2022) and was conducted in accordance with the Declaration of Helsinki. Written informed consent has been obtained from all participants.
2.2. Micropulse transscleral laser therapy
All treatments were conducted by the same doctor (KG) in the surgery room under systemic sedation and topical anaesthesia (tetracaine drops and subconjunctival mepivacaine 1 % injection). The inferior and superior perilimbal perimeters were treated with the MP3 probe (CycloG6 Laser, Iridex, Mountain View, CA, USA). The temporal and nasal segments were not treated. According to the manufacturer's recommendation, in both the inferior and superior perimeters a total of 3 Watts of energy was delivered over a time period of 90 s at an application speed/fluency of 20 s per-sweep-per-hemisphere. The pre-operative therapy was first continued unchanged after the laser intervention.
2.3. Study subjects and review of medical records
Our clinical information system Medisight/Heyex EMR-1 (Heidelberg Engineering, Heidelberg, Germany) was used to identify all patients who consecutively underwent MP-TLT at the University Hospital Basel during the time period from May 2019 to February 2023. All patients who received the treatment on an in-patient basis were included. The indications for MP-TLT were insufficiently controlled IOP under the current maximal medical therapy or intolerance to the medical therapy. Medical records were investigated for patients’ sex, age, type of glaucoma, lens status, IOP in both eyes before and after MP-TLT (immediately after the intervention, on the first post-operative day, after two weeks, two months, six months, nine months, one year and two years), topical and systemic IOP lowering medication, previous IOP-reducing interventions, and intra-/post-operative complications.
2.4. Statistical analysis
Differences between IOP before MP-TLT and IOP after the procedure on the interventional day, first post-interventional day, after two weeks, two months, six months, nine months, one year and two years, in treated and contralateral eyes, as independent samples, were chosen as primary outcomes. All statistical analysis was conducted using the Statistica software, version 13.5.0.17 (TIBCO Software Inc., Palo Alto, CA, USA). Descriptive analysis was performed, including calculation of mean values, standard deviation, median values, and ranges. Data was tested for normality using Kolmogorov–Smirnov and Shapiro–Wilk tests. Given that the data was normally distributed, comparisons using non-paired and paired Student's t-test were performed.
Holm-Bonferroni correction for multiple comparisons was performed, and p-value remaining <0.05 after this correction was considered statistically significant. A survival analysis, Cox-Mantel test, was performed for the purposes of coarse numerical evaluation of the factors that could have possibly influenced the treatment outcome (age, gender, lens status, glaucoma type, pretreatment history and repeated treatment). This was a retrospective observational, hence non-interventional non-randomized study, and survival success was defined as the time in weeks from the MP-TLT intervention until the next intervention, laser or surgical - decided at the discretion of the treating physician - was performed. Censored observations were defined as a loss-to-follow-up within the first 52 weeks. Cox-Mantel p-value is reported separately for each factor listed above. In addition, correlation analysis was performed between the post-interventional IOP spikes after the first and repeated MP-TLT treatment in the same eye.
3. Results
3.1. Patients’ characteristics
A total of 138 eyes of 131 patients were included in this study and a total of 162 interventions were performed. In 66 and 60 patients, MP-TLT was performed only on the right eyes or left eyes, respectively. In 5 patients, the procedure was performed on both eyes. Mean age of the patients was 73.2 ± 14.2 years. A total of 68 patients were female. Eighty eyes were primary open-angle glaucoma (57.6 %), 30 eyes were pseudoexfoliation glaucoma (22.9 %), and the rest were other types of glaucoma (rubeotic secondary glaucoma n = 7, post-operative secondary glaucoma n = 5, uveitic secondary glaucoma n = 3, pigment dispersion glaucoma n = 1, juvenile glaucoma n = 5, angle-closure glaucoma n = 4, normal tension glaucoma n = 4, in total 19.5 %). Thirty-one eyes were phakic at the time of the intervention (22.3 %), while 105 eyes were pseudophakic (75.5 %) and 3 eyes were aphakic (2.2 %). Sixty-five patients had no history of previous IOP-lowering interventions (46.8 %), 27 patients had previous laser treatments only (19.4 %; specifically selective laser trabeculoplasty), 23 patients had previous filtering surgery only (16.5 %; including trabeculectomy, XEN gel stent implant, Ex-Press shunt, Preserflo MicroShunt). Twenty-four patients had previous laser (selective laser trabeculoplasty) and filtering interventions (17.3 %). Mean number of current IOP-lowering pharmaceuticals in the interventional eyes was 3.0 (range 0–5), whereas every drug of every substance group was counted as 1 (carbonic anhydrase inhibitors [topical and systemic application counted separately], α-adrenergic agonists, β-adrenergic antagonists, and prostaglandin analogs). Patients’ characteristics are shown in Table 1.
Table 1.
Patients’ characteristics.
| PATIENTS | 131 | |
| males | 63 | |
| females | 68 | |
| patients with intervention only on right eye | 66 | |
| patients with intervention only on left eye | 60 | |
| patients with intervention on both eyes | 5 | |
| AGE (in years) | ||
| mean | 73.2 | |
| median | 77 | |
| range | 29–97 | |
| INTERVENTIONS | 162 | |
| intervention on right eyes | 82 | |
| intervention on left eyes | 80 | |
| GLAUCOMA TYPE | 138 | |
| interventional eyes with POAG | 80 | |
| interventional eyes with PEXG | 30 | |
| interventional eyes with other type 1 | 29 | |
| LENS STATUS | ||
| phakic interventional eyes | 31 | |
| pseudophakic interventional eyes | 105 | |
| aphakic interventional eyes | 3 | |
| PREVIOUS IOP-LOWERING INTERVENTIONS | ||
| none | 65 | |
| laser | 27 | |
| filtering | 23 | |
| laser + filtering | 24 | |
Other glaucoma types: rubeotic secondary glaucoma n = 7, post-operative secondary glaucoma n = 5, uveitic secondary glaucoma n = 3, pigment dispersion glaucoma n = 1, juvenile glaucoma n = 5, angle-closure glaucoma n = 4, normal tension glaucoma n = 4.
Intraocular pressure, Intraocular pressure lowering medication, complications, and possible risk factors for treatment failure.
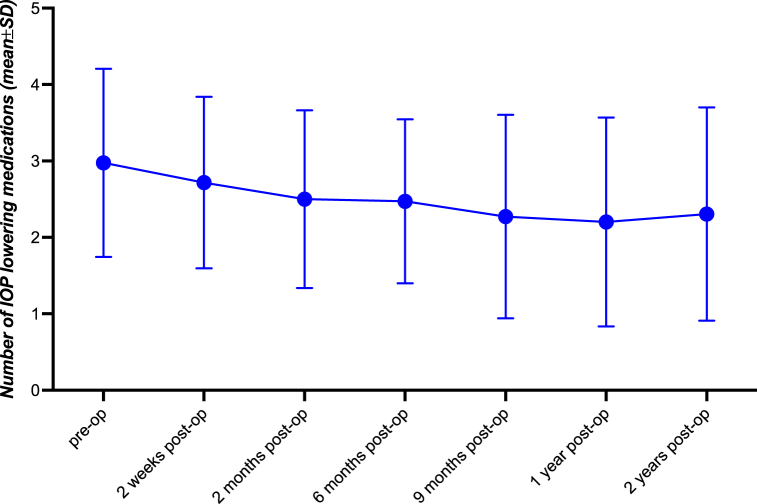
Before MP-TLT, mean IOP was significantly increased in interventional eyes (24.13 ± 9.09 mmHg) while it was within normal limits (10–21 mmHg) in the fellow eyes (15.89 ± 5.02 mmHg). Within the first 6–12 h after the intervention, mean IOP was significantly elevated in both the interventional eyes (31.67 ± 10.26 mmHg; p < 0.001) and the fellow eyes (17.03 ± 6.46 mmHg; p < 0.05). Starting from post-operative day 1 until two years post-operatively, mean IOP was significantly lowered continuously in interventional eyes (post-operative day 1: 17.02 ± 7.08 mmHg, p < 0.001; two years post-operatively: 16.1 ± 5.65 mmHg, p < 0.005). In the fellow eyes, mean IOP was significantly decreased on post-operative day 1 compared to baseline (13.4 ± 4.4 mmHg), yet rose close to baseline values until two weeks post-operatively (15.4 ± 5.4 mmHg; p > 0.5). Before MP-TLT, mean number of IOP lowering medication was 2.99 ± 1.28 mmHg in the interventional eyes and 1.9 ± 1.44 in the fellow eyes. Two years post-operatively, mean number of IOP lowering medications was 2.4 ± 1.31 in the interventional eyes. Complete data are shown in Table 2, Table 3 and displayed in Fig. 1, Fig. 2.
Table 2.
Intraocular pressure and intraocular pressure reducing medication in the treated eyes before and following intervention.
| Intraocular Pressure | Number of Intraocular Pressure Reducing Medications | ||
|---|---|---|---|
| PRE-OPERATIVE | |||
| available data for n eyes | 138 | ||
| mean | 24.1 mmHg | 3 | |
| standard deviation | 9.1 mmHg | 1.3 | |
| POST-OPERATIVE, 6–12 HOURS | |||
| available data for n eyes | 138 | ||
| mean | 31.7 mmHg | 3 | |
| standard deviation | 10.3 mmHg | 1.3 | |
| P-value of comparison with pre-operative | <0.001 | ||
| POST-OPERATIVE; 1 DAY | |||
| available data for n eyes | 138 | ||
| mean | 17.0 mmHg | 3 | |
| standard deviation | 7.1 mmHg | 1.3 | |
| P-value of comparison with pre-operative | <0.001 | ||
| POST-OPERATIVE; 2 WEEKS | |||
| available data for n eyes | 120 | ||
| mean | 14.8 mmHg | 2.7 | |
| standard deviation | 6.9 mmHg | 1.2 | |
| P-value of comparison with pre-operative | <0.001 | ||
| POST-OPERATIVE; 2 MONTHS | |||
| available data for n eyes | 98 | ||
| mean | 17.2 mmHg | 2.5 | |
| standard deviation | 6.9 mmHg | 1.2 | |
| P-value of comparison with pre-operative | <0.001 | ||
| POST-OPERATIVE; SIX MONTHS | |||
| available data for n eyes | 63 | ||
| mean | 17.7 mmHg | 2.45 | |
| standard deviation | 10.5 mmHg | 1.1 | |
| P-value of comparison with pre-operative | <0.001 | ||
| POST-OPERATIVE; NINE MONTHS | |||
| available data for n eyes | 47 | ||
| mean | 18.1 mmHg | 2.2 | |
| standard deviation | 9.5 mmHg | 1.3 | |
| P-value of comparison with pre-operative | <0.001 | ||
| POST-OPERATIVE; 1 YEAR | |||
| available data for n eyes | 31 | 31 | |
| mean | 14.9 mmHg | 2.2 | |
| standard deviation | 5.7 mmHg | 1.3 | |
| P-value of comparison with pre-operative | <0.001 | ||
| POST-OPERATIVE; 2 YEARS | |||
| available data for n eyes | 20 | ||
| mean | 16.1 mmHg | 2.4 | |
| standard deviation | 5.6 mmHg | 1.3 | |
| P-value of comparison with pre-operative | <0.005 | ||
Table 3.
Intraocular pressure in the fellow eyes before and until 2 weeks following intervention.
| Intraocular Pressure | ||
|---|---|---|
| PRE-OPERATIVE | ||
| available data for n eyes | 138 | |
| mean | 15.9 mmHg | |
| standard deviation | 5 mmHg | |
| POST-OPERATIVE, 6–12 HOURS | ||
| available data for n patients | 138 | |
| mean | 17 mmHg | |
| standard deviation | 6.5 mmHg | |
| P-value of comparison with pre-operative | <0.05 | |
| POST-OPERATIVE; 1 DAY | ||
| available data for n eyes | 138 | |
| mean | 13.4 mmHg | |
| standard deviation | 4.4 mmHg | |
| P-value of comparison with pre-operative | <0.01 | |
| POST-OPERATIVE; 2 WEEKS | ||
| available data for n eyes | 120 | |
| mean | 15.4 mmHg | |
| standard deviation | 5.4 mmHg | |
| P-value of comparison with pre-operative | >0.5 | |
1) Overall mean number of IOP lowering medication in fellow eyes was 1.9 ± 1.4; 29 eyes at this point had no medications.
Fig. 1.
Intraocular Pressure in Treated Eyes. Graph diagram displaying intraocular pressure in treated eyes before and after (6–12 h, 1 day, 2 weeks, 2 months, 6 months, 9 months, 1 year, 2 years) micropulse transscleral laser therapy.
Fig. 2.
Number of Intraocular Pressure reducing Compounds in Treated Eyes. Graph diagram showing number of IOP lowering medications in treated eyes before and following (6–12 h, 1 day, 2 weeks, 2 months, 6 months, 9 months, 1 year, 2 years) micropulse transscleral laser therapy.
Eighteen eyes required repetition of MP-TLT. Mean time to the second treatment (at the physician's discretion) was 17.8 ± 13.0 weeks (range 5–53 weeks). After the second MP-TLT, only 6 required another intervention (at an average of 36 weeks), while the remaining 12 did not for a mean observation period (prior to loss-to-follow-up) of 25.5 weeks, (range 1 day–84 weeks). Thus, the second MP-TLT may not have reached target IOP levels in all cases, but the IOP-lowering effect was more durable. Moreover, the immediate post-interventional IOP peaks-relative-to-baseline after the first and the second MP-CPC correlated significantly with each other (Pearson R = 0.55, p = 0.018), indicating an individually reproducible mechanism of post-interventional IOP spike.
After 24 interventions (15 %), we observed transient anterior chamber irritation, which was completely regressed within 5–7 days in all cases under local steroid treatment. Transient macular oedema was observed after 4 interventions (2 %), which was successfully treated with local steroid and COX-2 inhibitor therapy in 3 cases and dexamethasone intravitreal implant in one case.
In order to identify possible risk factors for treatment failure, the cohort was divided into groups of patients of younger versus older age, phakic versus pseudophakic patients, patients with primary open-angle glaucoma versus pseudoexfoliation glaucoma, patients with a history of previous filtering surgery with or without laser treatment versus other type of IOP lowering surgery, and female versus male patients. An observation period of 12 months was arbitrarily taken. Survival was defined as time in weeks until the next intervention was performed. If no intervention was necessary within 12 months, the eye ”survived“ for the whole 52 weeks and was not deemed censored. Censored observations were defined as a loss-to-follow-up within 52 weeks. For each possible risk factor mentioned above, Cox-Mantel analysis was performed. No statistically significant differences were found for any of the above-mentioned possible risk factors. P-values are shown in Table 4.
Table 4.
Cox-Mantel analysis of possible risk factors for treatment failure.
| Age1 | p = 0.085 |
|---|---|
| Lens status2 | p = 0.16 |
| Glaucoma type3 | p = 0.16 |
| Previous IOP-lowering procedures4 | p = 0.27 |
| Sex5 | p = 0.52 |
<77 years of age (n = 77) versus >77 years of age (n = 60).
Phakic (n = 31) versus pseudophakic (n = 104); aphakic patients (n = 3) were excluded in this analysis.
Primary open-angle glaucoma (n = 83) versus pseudoexfoliation glaucoma (n = 30); others (n = 25) were excluded in this analysis.
None (n = 64) versus filtering surgery with or without laser treatment (n = 47); others (n = 27) were excluded in this analysis.
Female (n = 73) versus male (n = 65) patients.
4. Discussion
TS-CPC was first described in the 1970s by Beckman and colleagues as an approach to lower IOP in glaucoma patients by ciliary body destruction using energy emitted by a ruby laser or a Neodymium:Yttrium–Aluminum-Garnet (Nd:YAG) laser [42,43]. The laser energy is absorbed by the melanin in the ciliary processes, leading to coagulative necrosis of the ciliary body. Given the drastic effect on the ciliary body physiology and since dissipated laser energy may damage surrounding ocular tissues, the procedure is associated with a significant risk of post-operative complications, such as intraocular hypotony, anterior chamber inflammation, macular oedema, or neurotrophic keratopathy. Over the decades, the technique evolved into what is today referred to as continuous wave TS-CPC, in which a semiconductor diode laser is used to continuously apply energy to the ciliary body for a certain period of time. Although the safety profile improved, continuous wave TS-CPC is still widely considered a last resort for severe, refractory glaucoma [6,7]. MP-TLT represents the new laser technology and became increasingly popular in recent years. In contrast to continuous wave TS-CPC, MP-TLT applies short pulses of energy separated by periods of rest, allowing for a more targeted and restrained treatment of the ciliary body [10]. This novel procedure has been associated with a much more favourable safety profile and, thus, has been proposed as a first-line or second-line treatment, also in milder glaucoma cases, so too at our institution [9]. However, in our patients, we repeatedly noted significant IOP spikes in the immediate hours following the intervention, which has not yet been discussed in the literature. To investigate this, we systematically analysed the post-interventional IOP course of all patients who consecutively underwent MP-TLT at our institution in recent years. Our analysis showed a statistically significant and clinically relevant transient increase in IOP within 6–12 h following MP-TLT and hence confirms our previous observations. Suggestively, transient post-interventional IOP spikes are a previously overlooked complication of this procedure. This is important because in many of our patients, the IOP spikes reached an extent that is possibly detrimental. Especially in patients with advanced glaucoma, such IOP spikes may overstress the last remaining retinal ganglion cell axons. Consequently, caution is to be exercised when indicating this procedure, and how the post-operative follow-up protocol is conducted. Interestingly, the extent of the post-interventional IOP spike seems to be an individual property. The immediate post-interventional IOP course after the first MP-CPC correlated with the one after the second MP-CPC. Post-interventional IOP-monitoring and/or preventive (additional temporary) pharmacological and/or short surgical (paracentesis) IOP-lowering may hence appear recommendable, particularly in patients who undergo a second MP-CPC and showed significant IOP spikes after the first treatment. As a possible underlying mechanism of these IOP spikes of 12–24 h duration, we hypothesize a rapid intraocular inflammatory reaction and initial swelling in the outflow relevant ocular segments, both trabecular meshwork and uveal tract, which then gradually gives way to aqueous suppression - but this is yet to be investigated.
Regarding the further course, we found a persistent IOP-lowering by 25–41 % and lowering of number of IOP-lowering medication by 10–28 % between two weeks and two years post-interventionally. Only 18 eyes required repetition of the MP-TLT because target IOP was not reached. In some patients, even with the second intervention the target IOP was not reached in the long term, but the IOP-lowering effect was more sustained, hence a certain cumulative effect can be assumed. We did not analyse the data from any third MP-TLT because too little data is available. Apart from the above mentioned transient IOP spikes in the first post-interventional hours, no other serious complications were observed, particularly no ocular hypotony. Transient anterior chamber inflammation and macular oedema were observed in a small number of patients and completely resolved either spontaneously or with treatment. This is consistent with previous studies und further supports MP-TLT as an efficient and generally safe procedure to lower IOP and number of IOP-lowering medications in glaucoma patients, that may be considered as a therapeutic approach not only in uncontrolled, refractory glaucoma [[11], [12], [13]]. Little is known about possible risk factors for treatment failure [10]. In our cohort, we found no evidence indicating that sex, lens status, or glaucoma type could serve as prognostic factors for treatment success, which is in line with the limited existing literature [[44], [45], [46]]. Put another way, it seems that MP-TLT performs well in terms of IOP lowering across a wide spectrum of glaucoma types and stages. Continuous wave TS-CPC has been found to be more successful in older age, presumably because an increase in the pigmentation of the nonpigmented epithelium of the ciliary body that occurs with aging renders it more susceptible to destruction by laser energy [47,48]. Chen et al. were the first to screen for age as a prognostic factor in MP-TLT and found better success rates in older patients as well. In our study, however, we found no statistically significant effect of age on the clinical outcome. Published evidence on the relationship between previous IOP-lowering surgery and the outcome of MP-TLT is controversial as well. While the study by Garcia and associates found higher success rates in patients who had a history of previous filtering surgery [49], Chen and colleagues reported a statistically insignificant trend towards the opposite [44]. In our study, we found no evidence supporting that previous IOP-lowering affects success rates of MP-TLT positively or negatively. Further research is necessary to determine the precise effect of age and previous IOP-lowering surgery on the outcome of MP-TLT.
The term “consensual ophthalmotonic reaction” was first coined in 1924 by Weekers and refers to the concept of a mutual regulation of IOP in the fellow eyes by an unknown mechanism, that manifests with reciprocal IOP responses in one eye after provoking IOP-changes in the contralateral eye [20]. Mounting evidence supports the existence of such an underlying IOP-regulating mechanism, although it could not yet be identified [[20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40]]. In a previous pilot-study, we found evidence for short-lasting consensual ophthalmotonic reactions in untreated fellow eyes following unilateral MP-TLT [41]. The findings of this present study are consistent and hence confirm the preliminary findings of our previous study. Unlike in other procedures that aim at lowering IOP pharmaceutically or by enhancing aqueous humour outflow capacity, in MP-TLT systemic pharmacological effects can be excluded and mechanisms triggered by alterations in aqueous humour flow across the trabecular meshwork appear implausible as well. Thus, our findings strongly point towards a systemic mechanism that guides consensual ophthalmotonic reactions. This mechanism might be an important target for future IOP-lowering treatment approaches.
This study is limited by the retrospective nature, the single-centre observational design, and the lack of controls. We included only patients who received MP-TLT during the study period on an in-patient basis. We chose not to include the patients who received MP-TLT without being hospitalized because those patients were not routinely examined in the immediate hours following the procedure. This limitation is, however, inherent to the retrospective nature of the study. Additionally, our study cannot offer further evidence on the possible mechanism of action of the post-interventional IOP spikes as well as the consensual ophthalmotonic reactions following MP-TLT. Further research is warranted to clarify the details of the IOP spikes triggered by MP-TLT and to describe strategies to handle them, as well as identify the mechanisms that guide consensual ophthalmotonic reactions. A better understanding of these mechanisms may advance our comprehension of IOP dynamics in health and disease and may finally lead to better treatment modalities in ocular hypertension and glaucoma.
In conclusion, our findings indicate that MP-TLT is an efficient, well tolerated and “one size fits all” method for reducing IOP. Caution is warranted regarding the individual pattern of IOP spikes in the immediate hours following the procedure, potentially jeopardising the last remaining retinal ganglion cell axons. Close post-interventional IOP monitoring or even preventive (additional) IOP-lowering treatment may be considered, especially in patients with advanced glaucoma who experienced IOP spikes after a previous MP-TLT. In addition, our findings further support the concept of consensual ophthalmotonic reactions and point towards a systemic mechanism of, at least short-term, IOP regulation.
Ethics and consent
This study was reviewed and approved by the competent ethics committee (Ethikkommission Nordwest-und Zentralschweiz EKNZ) with the approval number: 2022-00469; March 30, 2022, and May 2, 2022. This study was conducted in compliance with the Declaration of Helsinki. All patients (or their proxies/legal guardians) provided written informed consent to participate in the study and for their data to be published.
Funding
Hendrik Scholl is supported by the Swiss National Science Foundation (SNSF) (Project funding: “Developing novel outcomes for clinical trials in Stargardt disease using structure/function relationship and deep learning” #310030_201165, and National Centre of Competence in Research Molecular Systems Engineering: “NCCR MSE: Molecular Systems Engineering (phase II)” #51NF40-182895), the Wellcome Trust (PINNACLE study), and the Foundation Fighting Blindness Clinical Research Institute (ProgStar study).
Authorship
All named authors take responsibility for the integrity of the work as a whole and have given their approval for this version of the manuscript to be published.
Data availability statement
No data associated with our study has been deposited into a publicly available repository. All the data supporting the findings of this study are available or referenced within the article.
CRediT authorship contribution statement
Thomas Dervos: Writing – review & editing, Methodology, Investigation, Conceptualization. Laura L. Fortuna: Writing – review & editing, Methodology, Investigation, Conceptualization. Konstantin Gugleta: Writing – review & editing, Supervision, Project administration, Methodology, Investigation, Formal analysis, Conceptualization. Hendrik P.N. Scholl: Supervision, Resources. Zisis Gatzioufas: Writing – review & editing, Software, Methodology. Pascal W. Hasler: Writing – review & editing, Validation, Software. Valentin Arabin: Writing – review & editing, Data curation. Tim J. Enz: Writing – original draft, Validation, Supervision, Project administration, Methodology, Formal analysis, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
None.
References
- 1.Jonas J.B., Aung T., Bourne R.R., Bron A.M., Ritch R., Panda-Jonas S. Glaucoma. Lancet. Nov 11 2017;390(10108):2183–2193. doi: 10.1016/S0140-6736(17)31469-1. [DOI] [PubMed] [Google Scholar]
- 2.Stein J.D., Khawaja A.P., Weizer J.S. Glaucoma in adults-screening, diagnosis, and management: a review. JAMA, J. Am. Med. Assoc. 2021;325(2):164–174. doi: 10.1001/jama.2020.21899. [DOI] [PubMed] [Google Scholar]
- 3.Tham Y.C., Li X., Wong T.Y., Quigley H.A., Aung T., Cheng C.Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. Nov 2014;121(11):2081–2090. doi: 10.1016/j.ophtha.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 4.Tan J.C., Peters D.M., Kaufman P.L. Recent developments in understanding the pathophysiology of elevated intraocular pressure. Curr. Opin. Ophthalmol. Apr 2006;17(2):168–174. doi: 10.1097/01.icu.0000193079.55240.18. [DOI] [PubMed] [Google Scholar]
- 5.Conlon R., Saheb H., Ahmed I.I. Glaucoma treatment trends: a review. Can. J. Ophthalmol. Feb 2017;52(1):114–124. doi: 10.1016/j.jcjo.2016.07.013. [DOI] [PubMed] [Google Scholar]
- 6.Chen M.F., Kim C.H., Coleman A.L. Cyclodestructive procedures for refractory glaucoma. Cochrane Database Syst. Rev. 2019;3:CD012223. doi: 10.1002/14651858.CD012223.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anand N., Klug E., Nirappel A., Solá-Del Valle D. A review of cyclodestructive procedures for the treatment of glaucoma. Semin. Ophthalmol. Aug 17 2020;35(5–6):261–275. doi: 10.1080/08820538.2020.1810711. [DOI] [PubMed] [Google Scholar]
- 8.Ma A., Yu S.W.Y., Wong J.K.W. Micropulse laser for the treatment of glaucoma: a literature review. Surv. Ophthalmol. 2019;64(4):486–497. doi: 10.1016/j.survophthal.2019.01.001. [DOI] [PubMed] [Google Scholar]
- 9.Dastiridou A.I., Katsanos A., Denis P., et al. Cyclodestructive procedures in glaucoma: a review of current and emerging options. Adv. Ther. Dec 2018;35(12):2103–2127. doi: 10.1007/s12325-018-0837-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanchez F.G., Peirano-Bonomi J.C., Brossard Barbosa N., Khoueir Z., Grippo T.M. Update on micropulse transscleral cyclophotocoagulation. J. Glaucoma. Jul 2020;29(7):598–603. doi: 10.1097/IJG.0000000000001539. [DOI] [PubMed] [Google Scholar]
- 11.de Vries V.A., Pals J., Poelman H.J., Rostamzad P., Wolfs R.C.W., Ramdas W.D. Efficacy and safety of micropulse transscleral cyclophotocoagulation. J. Clin. Med. Jun 15 2022;11(12) doi: 10.3390/jcm11123447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ling Q., Cai Z., Zhang X., Duan X. The efficacy and safety of micropulse transscleral laser treatment in glaucoma: a systematic review and meta-analysis. BMC Ophthalmol. Jun 12 2023;23(1):263. doi: 10.1186/s12886-023-03017-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Souissi S., Le Mer Y., Metge F., et al. An update on continuous-wave cyclophotocoagulation (CW-CPC) and micropulse transscleral laser treatment (MP-TLT) for adult and paediatric refractory glaucoma. Acta Ophthalmol. Aug 2021;99(5):e621–e653. doi: 10.1111/aos.14661. [DOI] [PubMed] [Google Scholar]
- 14.Johnstone M.A. The aqueous outflow system as a mechanical pump: evidence from examination of tissue and aqueous movement in human and non-human primates. J. Glaucoma. Oct 2004;13(5):421–438. doi: 10.1097/01.ijg.0000131757.63542.24. [DOI] [PubMed] [Google Scholar]
- 15.Johnstone M., Martin E., Jamil A. Pulsatile flow into the aqueous veins: manifestations in normal and glaucomatous eyes. Exp. Eye Res. May 2011;92(5):318–327. doi: 10.1016/j.exer.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gonzalez J.M., Ko M.K., Hong Y.K., Weigert R., Tan J.C.H. Deep tissue analysis of distal aqueous drainage structures and contractile features. Sci. Rep. Dec 06 2017;7(1) doi: 10.1038/s41598-017-16897-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnstone M.A. Intraocular pressure regulation: findings of pulse-dependent trabecular meshwork motion lead to unifying concepts of intraocular pressure homeostasis. J Ocul Pharmacol Ther. 2014;30(2–3):88–93. doi: 10.1089/jop.2013.0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carreon T., van der Merwe E., Fellman R.L., Johnstone M., Bhattacharya S.K. Aqueous outflow - a continuum from trabecular meshwork to episcleral veins. Prog. Retin. Eye Res. Mar 2017;57:108–133. doi: 10.1016/j.preteyeres.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lusthaus J.A., Khatib T.Z., Meyer P.A.R., McCluskey P., Martin K.R. Aqueous outflow imaging techniques and what they tell us about intraocular pressure regulation. Eye (Lond). Jan 2021;35(1):216–235. doi: 10.1038/s41433-020-01136-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weekers L. Reaction Ophtalmotonique Consensuelle. Arch Opthalmol; Paris: 1924. Modification expérimentales de l’ophtalmotonous; pp. 641–658. [Google Scholar]
- 21.Piltz J., Gross R., Shin D.H., et al. Contralateral effect of topical beta-adrenergic antagonists in initial one-eyed trials in the ocular hypertension treatment study. Am. J. Ophthalmol. Oct 2000;130(4):441–453. doi: 10.1016/s0002-9394(00)00527-4. [DOI] [PubMed] [Google Scholar]
- 22.Kwitko G.M., Shin D.H., Ahn B.H., Hong Y.J. Bilateral effects of long-term monocular timolol therapy. Am. J. Ophthalmol. Dec 15 1987;104(6):591–594. doi: 10.1016/0002-9394(87)90169-3. [DOI] [PubMed] [Google Scholar]
- 23.Martin X.D., Rabineau P.A. Intraocular pressure effects of timolol after unilateral instillation. Ophthalmology. Dec 1988;95(12):1620–1623. doi: 10.1016/s0161-6420(88)32966-0. [DOI] [PubMed] [Google Scholar]
- 24.Rhodes K.M., Weinstein R., Saltzmann R.M., et al. Intraocular pressure reduction in the untreated fellow eye after selective laser trabeculoplasty. Curr. Med. Res. Opin. Mar 2009;25(3):787–796. doi: 10.1185/03007990902728316. [DOI] [PubMed] [Google Scholar]
- 25.Gibbens M.V. The consensual ophthalmotonic reaction. Br. J. Ophthalmol. Oct 1988;72(10):746–749. doi: 10.1136/bjo.72.10.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Newman H., Kurtz S., David R. Intraocular pressure changes in the contralateral eye after topical treatment: does an "ophthalmotonic consensual reaction" exist? Isr. Med. Assoc. J. Sep 2010;12(9):568–571. [PubMed] [Google Scholar]
- 27.Aghayeva F.A., Chronopoulos P., Schuster A.K., Pfeiffer N., Hoffmann E.M. Inter-eye relationship of intraocular pressure change after unilateral trabeculectomy, filtering canaloplasty, or PreserFlo™ microshunt implantation. Graefes Arch. Clin. Exp. Ophthalmol. Oct 2021;259(10):3045–3053. doi: 10.1007/s00417-021-05188-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Y., Fan X., Wu L. Selective laser trabeculoplasty lowered the untreated fellow eye long-term intraocular pressure: a 3-year observational study. Lasers Med Sci. Apr 2022;37(3):1487–1493. doi: 10.1007/s10103-021-03253-w. [DOI] [PubMed] [Google Scholar]
- 29.G. L . Ann Oculist; Paris: 1924. Etude de quelques reactions dans les yeux par une contusion oculaire unilateral; recherches experimentales et cliniques; pp. 87–106. [Google Scholar]
- 30.Diestelhorst M., Krieglstein G. The effect of trabeculectomy on the aqueous humor flow of the unoperated fellow eye. Graefes Arch. Clin. Exp. Ophthalmol. 1991;229(3):274–276. doi: 10.1007/BF00167883. [DOI] [PubMed] [Google Scholar]
- 31.Schmerl E., Steinberg B. Central control of intraocular pressure by active principles. Am. J. Ophthalmol. Sep 1948;31(9):1097–1101. doi: 10.1016/0002-9394(48)92440-4. [DOI] [PubMed] [Google Scholar]
- 32.Gloster J., Greaves D.P. Effect of diencephalic stimulation upon intra-ocular pressure. Br. J. Ophthalmol. Sep 1957;41(9):513–532. doi: 10.1136/bjo.41.9.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cox C.E., Fitzgerald C.R., King R.L. A preliminary report on the supraoptic nucleus and control of intraocular pressure. Invest. Ophthalmol. Jan 1975;14(1):26–28. [PubMed] [Google Scholar]
- 34.Gibbens M.V. Sympathetic influences on the consensual ophthalmotonic reaction. Br. J. Ophthalmol. Oct 1988;72(10):750–753. doi: 10.1136/bjo.72.10.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yarangümeli A., Köz O.G., Kural G. The effect of trabeculectomy on the intraocular pressure of the unoperated fellow eye. J. Glaucoma. Apr 2003;12(2):108–113. doi: 10.1097/00061198-200304000-00004. [DOI] [PubMed] [Google Scholar]
- 36.Vysniauskiene I., Shaarawy T., Flammer J., Haefliger I.O. Intraocular pressure changes in the contralateral eye after trabeculectomy with mitomycin C. Br. J. Ophthalmol. Jul 2005;89(7):809–811. doi: 10.1136/bjo.2004.050294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Al-Ghadyan A., Mead A., Sears M. Increased pressure after paracentesis of the rabbit eye is completely accounted for by prostaglandin synthesis and release plus pupillary block. Invest. Ophthalmol. Vis. Sci. Apr 1979;18(4):361–365. [PubMed] [Google Scholar]
- 38.Detorakis E.T., Tsiklis N., Pallikaris I.G., Tsilimbaris M.K. Changes in the intraocular pressure of fellow untreated eyes following uncomplicated trabeculectomy. Ophthalmic Surg Lasers Imaging. 2011;42(2):138–143. doi: 10.3928/15428877-20110125-02. [DOI] [PubMed] [Google Scholar]
- 39.Shum J.W.H., Choy B.N.K., Ho W.L., Chan J.C.H., Lai J.S.M. Consensual ophthalmotonic reaction in Chinese patients following augmented trabeculectomy or ExPRESS shunt implantation. Medicine (Baltim.) Jul 2016;95(29) doi: 10.1097/MD.0000000000004190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaushik S., Agarwal A., Kaur S., Lomi N., Raj S., Pandav S.S. Change in intraocular pressure in the fellow eye after glaucoma surgery in 1 eye. J. Glaucoma. Mar 2016;25(3):324–329. doi: 10.1097/IJG.0000000000000217. [DOI] [PubMed] [Google Scholar]
- 41.Fortuna L.L., Dervos T., Gatzioufas Z., Scholl H.P.N., Gugleta K., Enz T.J. Short-term effect of micropulse transscleral laser therapy on intraocular pressure in untreated fellow eyes of glaucoma patients: preliminary results. J. Clin. Med. May 26 2023;12(11) doi: 10.3390/jcm12113680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beckman H., Kinoshita A., Rota A.N., Sugar H.S. Transscleral ruby laser irradiation of the ciliary body in the treatment of intractable glaucoma. Trans. Am. Acad. Ophthalmol. Otolaryngol. 1972;76(2):423–436. [PubMed] [Google Scholar]
- 43.Beckman H., Sugar H.S. Neodymium laser cyclocoagulation. Arch. Ophthalmol. Jul 1973;90(1):27–28. doi: 10.1001/archopht.1973.01000050029006. [DOI] [PubMed] [Google Scholar]
- 44.Chen H.S., Yeh P.H., Yeh C.T., et al. Micropulse transscleral cyclophotocoagulation in a Taiwanese population: 2-year clinical outcomes and prognostic factors. Graefes Arch. Clin. Exp. Ophthalmol. Apr 2022;260(4):1265–1273. doi: 10.1007/s00417-021-05468-7. [DOI] [PubMed] [Google Scholar]
- 45.Zanutigh V., Perrone L.D., Gómez-Caride G., Perrone F., Valvecchia G., Logioco C. Success rate in micropulse diode laser treatment with regard to lens status, refractive errors, and glaucoma subtypes. Int. Ophthalmol. Jul 2023;43(7):2407–2417. doi: 10.1007/s10792-023-02640-2. [DOI] [PubMed] [Google Scholar]
- 46.Jammal A.A., Costa D.C., Vasconcellos J.P.C., Costa V.P. Prospective evaluation of micropulse transscleral diode cyclophotocoagulation in refractory glaucoma: 1 year results. Arq. Bras. Oftalmol. Jun 27 2019;82(5):381–388. doi: 10.5935/0004-2749.20190076. [DOI] [PubMed] [Google Scholar]
- 47.Schlote T., Derse M., Rassmann K., Nicaeus T., Dietz K., Thiel H.J. Efficacy and safety of contact transscleral diode laser cyclophotocoagulation for advanced glaucoma. J. Glaucoma. Aug 2001;10(4):294–301. doi: 10.1097/00061198-200108000-00009. [DOI] [PubMed] [Google Scholar]
- 48.Gärtner J. Electron microscopic observations on the cilio-zonular border area of the human eye with particular reference to the aging changes. Z. Anat. Entwicklungsgesch. 1970;131(3):263–273. doi: 10.1007/BF00520969. [DOI] [PubMed] [Google Scholar]
- 49.Garcia G.A., Nguyen C.V., Yelenskiy A., et al. Micropulse transscleral diode laser cyclophotocoagulation in refractory glaucoma: short-term efficacy, safety, and impact of surgical history on outcomes. Ophthalmol Glaucoma. 2019;2(6):402–412. doi: 10.1016/j.ogla.2019.08.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data associated with our study has been deposited into a publicly available repository. All the data supporting the findings of this study are available or referenced within the article.