Abstract
Species composition and densities of wild ungulate communities in Europe have changed over the last decades. As ungulates play an important role in the life-cycle of the tick species Ixodes ricinus, these changes could affect both the life-cycle of I. ricinus and the transmission of tick-borne pathogens like Borrelia burgdorferi (s.l.) and Anaplasma phagocytophilum. Due to morphological and behavioural differences among the ungulate species, these species might have different effects on the densities of questing I. ricinus, either directly through a bloodmeal or indirectly via the impact of ungulates on rodent numbers via the vegetation. In this study, we aimed to investigate these direct and indirect effects of five different ungulate species, fallow deer (Dama dama), roe deer (Capreolus capreolus), red deer (Cervus elaphus), moose (Alces alces), and wild boar (Sus scrofa), on the presence and abundance of I. ricinus ticks. In the summer of 2019, on 20 1 × 1 km transects in south-central Sweden that differed in ungulate community composition, we collected data on tick presence and abundance (by dragging a cloth), ungulate community composition (using camera traps), vegetation height (using the drop-disc method), temperature above field layer and rodent abundance (by snap-trapping). Using generalized linear mixed models we did not find any associations between vegetation height and tick presence/abundance or ungulate visitation frequencies, or between ungulate visitation frequencies and the presence/abundance of questing I. ricinus. The power of our analyses was, however, low due to very low tick and rodent numbers. We did find a negative association between adult ticks and air temperature, where we were more likely to find adult ticks if temperature in the field layer was lower. We conclude that more elaborate long-term studies are needed to elucidate the investigated associations. Such future studies should differentiate among the potential impacts of different ungulate species instead of treating all ungulate species as one group.
Keywords: Ixodes ricinus, Ungulate, Tick-borne pathogen, Tick abundance, Vegetation, Air temperature
Graphical abstract
Highlights
-
•
A study exploring associations between ungulate community composition and Ixodes ricinus abundance.
-
•
Data on ungulate and tick abundance, but also vegetation, temperature, and small mammals, were collected in Sweden.
-
•
No associations were found between ungulate visitation frequencies, field layer height, and abundance of I. ricinus.
-
•
The study indicates that it is important to differentiate among ungulate species instead of treating them as one group.
1. Introduction
During the past decades, species composition and densities of ungulate communities have profoundly changed across different parts of Europe (Apollonio et al., 2010; Spitzer, 2019; Linell et al., 2020). As ungulates play a central role in the life-cycle of the tick species Ixodes ricinus, these changes in ungulates communities can have allowed I. ricinus to increase in densities and expand its range (Jaenson et al., 2012). Wild ungulates – both via their presence and abundance – may affect the number of questing I. ricinus (Gilbert et al., 2012; Hofmeester et al., 2017a), which results in ungulates playing a major role in the transmission of tick-borne pathogens such as Borrelia burgdorferi (s.l.) and Anaplasma phagocytophilum. Ungulates may affect the number of questing ticks through two main mechanisms: directly, by providing a blood meal for I. ricinus; and indirectly, by shaping the field layer vegetation, consisting of grasses, forbs, dwarf shrubs, mosses, and ferns. Ungulates shape the height, cover, structural density, and species composition of the field layer through grazing, browsing, trampling, fraying, stripping, uprooting, defecating, and seed dispersal (Gill and Beardall, 2001; Ramirez et al., 2018). Such changes in the field layer can strongly shape the microclimatic (temperature and humidity) conditions for questing ticks (Daniel et al., 1977). By affecting the field layer vegetation, ungulates do not only affect microclimatic conditions for I. ricinus but also the abundance of other vertebrate host species for I. ricinus, especially small mammals. Small mammals select for habitats with a higher and denser field layer, which provides food and protection against avian predators (Ecke et al., 2002). Several studies have indicated that small mammal densities increase after the exclusion of ungulates (e.g. Keesing et al., 1998; McCauley et al., 2006; Gandy et al., 2021). Such increasing density of small mammals will likely influence the density of I. ricinus, since larvae mainly feed on these hosts (Hofmeester et al., 2016).
While high densities of ungulates can have strong impacts on the field layer, this impact likely varies among ungulate species due to these species having different morphological and behavioural characteristics. Fabri et al. (2021) suggested that this variation in traits among ungulate species leads to these species contributing differently to the life-cycle of I. ricinus and to the transmission of tick-borne pathogens. For example, species such as fallow deer (Dama dama) that live in larger herds may more strongly affect vegetation than solitary species, such as moose. As another example, the rooting and wallowing behaviour of wild boar (Sus scrofa) may explain their generally very low I. ricinus burden, while fallow deer concentrating their grazing with their heads down in the field layer may drive their high tick burdens. Importantly, variation in feeding types will also influence the impact of ungulate species on the field layer. Red deer (Cervus elaphus) and fallow deer, both intermediate feeders, include large amounts of grasses and forbs in their diets during large parts of the year (Spitzer et al., 2020). These grasses and forbs, but also mosses, become more dominant under high grazing pressure, possibly because herbaceous species regrow more easily after defoliation than woody plant species (reviewed by Kirby, 2001). Such an herbaceous-dominated field layer produces optimal conditions for I. ricinus, creating an optimal microclimate and vegetation height for questing (Leal et al., 2020). High densities of fallow or red deer, however, may strongly reduce the height of the field layer (Gandy et al., 2021), leading to less optimal conditions for questing ticks. Moose (Alces alces) and roe deer (Capreolus capreolus) are more selective feeders (Spitzer et al., 2020) and should therefore have less strong effects on the field layer. Wild boar have a more diverse diet than the deer species, containing grasses, fruits, seeds, and crops (Spitzer et al., 2020). During the growing season, they predominantly graze, with potentially strong effects on the field layer. In addition, their extensive rooting can temporarily remove the field layer across large areas (Wirthner et al., 2012), strongly limiting questing opportunities for I. ricinus.
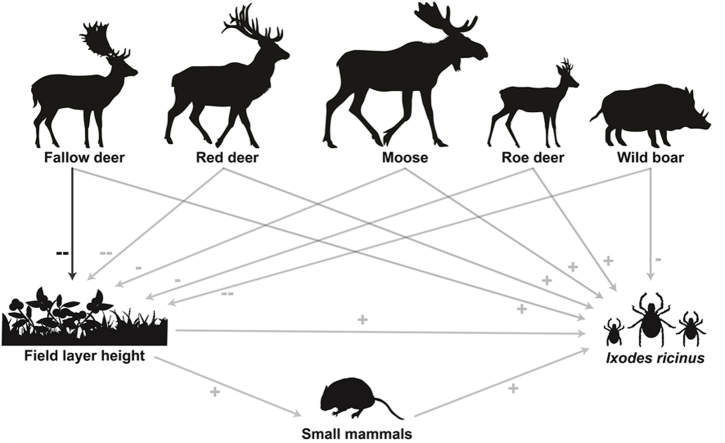
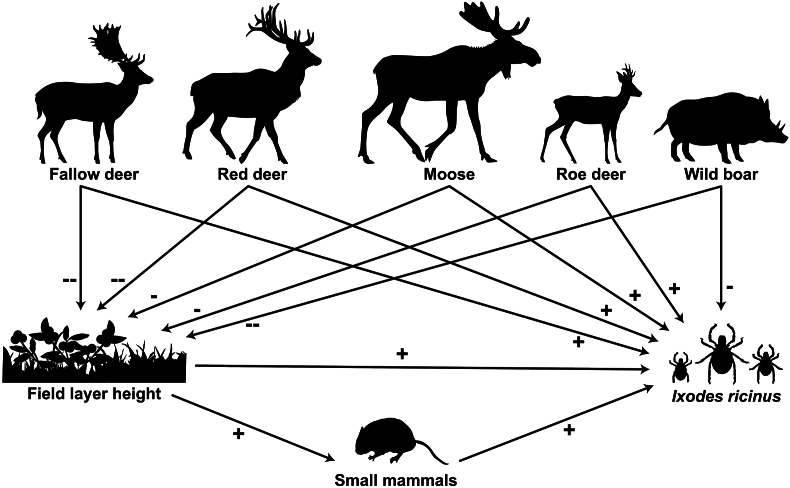
In the context of ticks and tick-borne pathogens, the effects of ungulates have been frequently studied for individual ungulate species (e.g. Michalik et al., 2012; Overzier et al., 2013; Sgroi et al., 2021) or by treating multiple ungulate species as one group (e.g. Hofmeester et al., 2017a). However, previous studies have rarely studied them as different species within the same community, which could imply that important information is lost (Fabri et al., 2021). In this study, performed in south-central Sweden, we used naturally occurring strong contrasts in the densities of four different deer species and wild boar to investigate the associations between ungulate community composition and density, field layer height, air temperature, small mammal abundance and abundances of I. ricinus in the vegetation. We designed our study to explore whether associations with field layer height, small mammal abundance and I. ricinus densities differed along density gradients of the different ungulate species, and whether we could find evidence for the following hypotheses (Fig. 1).
-
•
H1: Higher densities of fallow deer, red deer and/or wild boar are associated with (i) more reduced field layer height, (ii) lower abundance of small mammals and/or (iii) lower tick abundance.
-
•
H2: Higher moose and/or roe deer densities will not be strongly associated with (i) field layer height and temperature and/or (ii) abundance of small mammals and/or (iii) are, either neutrally or positively, linked with tick abundance.
Fig. 1.
Schematic representation on how we hypothesize that the different ungulate species are associated with the field layer height, small mammal abundance and Ixodes ricinus abundance.
Furthermore, the study design aimed to explore the relations between the ungulate community composition and the number of questing I. ricinus infected with the following tick-borne pathogens: Borrelia burgdorferi (s.l.); Borrelia miyamotoi; Anaplasma phagocytophilum; Babesia microti; and Babesia clade-X. Fabri et al. (2021) showed that ungulate species differ in their contribution to the transmission of tick-borne pathogens. Based on the results of Fabri et al. (2021) we hypothesize that higher densities of fallow deer and/or red deer are associated with a lower prevalence of B. burgdorferi (s.l.), while higher densities of moose and/or roe deer are not strongly associated with this pathogen in ticks. In terms of A. phagocytophilum, we hypothesized that a higher density of either of the deer species is associated with a higher infection prevalence.
2. Materials and methods
2.1. Study area and selection of sampling points
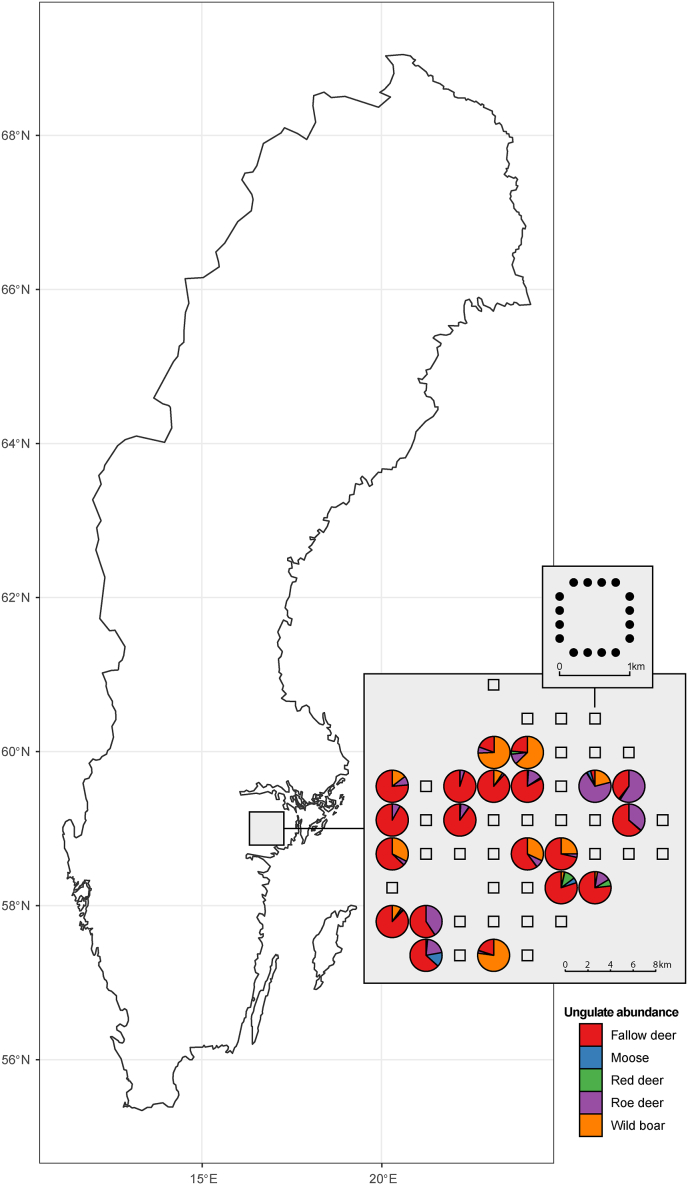
The study area, located in Södermanland County in south-central Sweden (Fig. 2), hosts the most diverse and abundant ungulate populations in Sweden (Spitzer et al., 2019). Furthermore, ticks of the species I. ricinus are common in this area (Jaenson et al., 2012; Kjær et al., 2019). The area has a hemi-boreal climate with average monthly rainfall of 49 mm and average July and January temperature of 15 °C and −2 °C, respectively (SMHI, 2022). The habitat is characterized by forests dominated by Scots pine (Pinus sylvestris), Norway spruce (Picea abies), birch (Betula spp.) and European oak (Quercus robur) interspersed with agricultural lands with various crops (Spitzer et al., 2019). Across this area, we established a sampling grid of 50 evenly distributed square transects of 1 × 1 km each, with 3 km between the centres of each square (Fig. 2). On each transect, from 2012 onwards, yearly pellet group counts has been performed on 16 sampling points evenly distributed along the four sides of the transect (four sampling points along each site, situated 250 m apart), as described in detail by Spitzer et al. (2021). These pellet counts were performed just after snowmelt (March–April) to establish the presence and abundance of five ungulate species, i.e. fallow deer, roe deer, red deer, moose and wild boar. Based on the pellet counts from 2016, 2017 and 2018, we selected 20 transects that represented as strong as possible gradients of densities of the different ungulate species (see Supplementary file S1: Text A.1 for details). On each of those 20 transects, we selected 8 of the 16 count sampling points located in forest (see Supplementary file S1: Text A.1). On 156 of these 160 selected sampling points, we collected data on both ungulate and small mammal abundance (see Section 2.2 Data collection). We aimed to collect data on tick abundance, height of field layer and temperature above the field layer for the same sampling points. However, due to logistic and geophysical challenges (e.g. recent clear-cutting or too much woody debris on the ground), we only managed to collect data on tick abundance, height of field layer and temperature above the field layer for 116 of the 156 sampling points. For the other 40 sampling points, we aimed to select another sampling point along the side of the transect as close as possible to the original sampling points (see Supplementary file S1: Text A.1). We succeeded for 37 sampling points and collected data on tick abundance, air temperature and height of field layer for those additional 37 points as well. Thus, we collected data on ticks, air temperature and vegetation for 153 out of the 156 sampling points for which we collected ungulate and small mammal data.
Fig. 2.
Location of the study site in Sweden and the sampling grid of square transects. For one transect the layout of the 16 sampling points is shown. For the 20 transects where we collected data on the abundance of ungulate species with the aid of camera traps, the ratio of the five ungulate species is indicated by pie charts.
2.2. Data collection
During the late spring and summer of 2019 (May–August), we collected ticks on the sampling points by dragging a 1 m2 white cloth over the field layer vegetation. We only dragged for ticks when the field layer was dry, and when the temperature was above 8 °C. A total of 100 m was dragged on each sampling point, divided into 10 transects of 10 m (see Supplementary file S1: Text A.2 for details). The number of dragged ticks was counted per life stage: larvae, nymphs, female adults and male adults. All nymphs and adults were collected individually in Eppendorf tubes, including those found crawling on the field staff. All collected ticks were stored at −18 °C until further analyses. For each 10-m transect, we recorded the temperature with a standard thermometer with 1 decimal accuracy at approximately 1 m height above the field layer. Furthermore, we measured the height of the field layer (max. 50 cm) every 2.5 m along the 10-m transects (i.e. on 5 points for each transect and 50 points per sampling point), using the drop disc method (Stewart et al., 2001) (see Supplementary file S1: Text A.2 for details). We aimed to visit each sampling point twice during the growing season to collect data on tick abundance, temperature and vegetation height. However, due to bad weather in the field this was only possible for 102 sampling points of the total of 153. The other 51 were only visited once. All collected ticks were morphologically identified to species following Arthur (1963) and Hillyard (1996). We confirmed our initial morphological identification microscopically for 40% of the ticks (n = 291).
To estimate local variation in use by ungulate we deployed camera traps (HC500, Reconyx Inc., Holmen, WI) with passive infrared sensors to determine the visitation frequencies of the five ungulate species on each sampling point during a period of 11 days per sampling point between June and August 2019. This method is characterized by high accuracy in terms of detection, allows for data collection during both day and night, and minimizes human disturbance (Hofmeester et al., 2017b). Camera traps were placed at a height of 40 cm, facing north and parallel to the ground. Vegetation that obstructed the view of the camera trap was pruned (Hofmeester et al., 2017b). The sampling effort totalled 1793 camera-trap days across all sampling points. The application TRAPPER (Bubnicki et al., 2016) was used to process the collected images. Multiple detections of individuals of the same species that were observed less than 5 min apart were grouped into sequences. We then estimated the total number of individuals per species in each sequence. We used the method described by Hofmeester et al. (2017b) to estimate the effective detection distance per species (Supplementary file S1: Table A.1). To measure the impact that ungulates might have on vegetation and ticks, we determined the total amount of time that all individuals of each of the five species spent in front of the camera traps. For this, we multiplied the number of animals with total sequence time (difference in time between first and last image of a sequence). We corrected this measure of time spent in front of the cameras for the total amount of time that camera traps were active in a given location, as well as the effective detection distance. This resulted in a measure of the number of minutes a species spent in front of the camera standardized per 10 m of effective detection distance per 100 days, which we will refer to as visitation frequency.
Small mammals were snap-trapped during July 2019 to estimate their abundance, after obtaining permission from land owners or the country administrative board. At each sampling point, 6 snap traps were placed within a radius of 2 m. Traps were baited with cotton strips (wetted with vegetable oil) and a piece of dried apple. The traps were checked for three consecutive days. Due to bad weather and untraceable traps, our trapping effort totalled 2673 trap nights (out of a potential maximum total of 2862 nights). All trapped small mammals were morphologically identified to species. Furthermore, we collected all ticks and a spleen sample from each trapped animal. All ticks were morphologically identified with the aid of a microscope to species following Arthur (1963) and Hillyard (1996).
2.3. DNA extraction and pathogen detection
The procedures for the hydrolysis of questing ticks, the DNA extraction of feeding ticks and spleen from small mammals, as well as for the pathogen detection are described in detail in Fabri et al. (2021). In short, DNA was obtained from the ticks with ammonium hydroxide as described in Wielinga et al. (2006). The DNA from the spleens was extracted using the Qiagen DNeasy Blood & Tissue Kit according to the manufacturer’s protocol (Qiagen, Hilden, Germany). Multiplex real-time PCR was used for the detection of gene fragments of A. phagocytophilum msp2 (Stigum et al., 2019), B. burgdorferi (s.l.) ospA/flab (Heylen et al., 2013), B. miyamotoi flagellin (Hovius et al., 2013), B. microti ITS (Krawczyk et al., 2020) and Babesia clade-X 18S rDNA (Øines et al., 2012). In addition, to identify the ecotype of A. phagocytophilum we amplified all but two (i.e. one questing nymph and one larva from a small mammal) ticks that were positive for A. phagocytophilum with conventional PCR targeting a fragment of the GroEL gene followed by sequencing (Stigum et al., 2019). To identify the genospecies of the samples that were positive for B. burgdorferi (s.l.), we amplified a fragment of the IGS region of Borrelia from 95 questing ticks (out of 98 positives), 57 ticks from small mammals (out of 58 positives) and 7 spleens (out of 7 positives) with conventional PCR followed by sequencing (Heylen et al., 2013). We did the same for all questing ticks positive for Babesia clade-X to identify the species based on a fragment of 18S rRNA gene (Casati et al., 2006).
2.4. Statistical analyses
As only two-thirds of the sampling points were sampled twice for tick abundance, field layer height and temperature (see Supplementary file S1: Text A.2), we randomly selected one of the sampling moments for these points for further analyses. Furthermore, we only included the 153 sampling points (out of the 156 original points) where all data were collected (i.e. tick densities, temperature, height of field layer, small mammals and visitation frequencies of ungulates). We log10-transformed the visitation frequencies of the five ungulates species to adjust for overdispersion in the data. To adjust for the large number of zeroes, we added 0.01, i.e. the lowest visitation frequency, to all values. With these log10-transformed data we performed a principal components analysis (PCA) to determine if the visitation frequency of the ungulate species were correlated to each other (Supplementary file S2: Figure B.1). According to the broken-stick model (reviewed in Cangelosi and Goriely, 2007) this was not the case. To explore whether ungulates or deer can be treated as one group, or that the different ungulate species should be treated differently, we performed all further statistical analyses in three-fold: once with the visitation frequency of each ungulate species separately, once with the visitation frequencies of all deer species combined, and once with the visitation frequencies of all ungulates species (i.e. all deer plus wild boar) combined.
To test if there were any direct effects of ungulate visitation frequencies on (i) the mean height of the field layer and (ii) the variation in height as the coefficient of the variance of the field layer height, we used generalized linear mixed models (GLMMs) with a Gaussian distribution, using the glmmTMB package in R (Brooks et al., 2022). These GLMMs included the log10-transformed visitation frequencies of the ungulate species and sampling month as fixed effects, and transect ID, to correct for clustering of sampling points within transects, as random intercept. To adjust for overdispersion in the data, we log10-transformed both the mean height of the field layer and the coefficient of the variance of the height of the field layer. Furthermore, we tested if there were any effects of ungulate visitation frequencies, vegetation height or temperature on the presence and abundance of I. ricinus. With the presence of ticks as a response variable, we performed GLMMs with a binomial distribution, which included as fixed effects the log10-transformed ungulate visitation frequencies, the mean height of the field layer (log10-transformed), the coefficient of the variance of the height of the field layer (log10-transformed), the temperature squared and the sampling month. As a random intercept, we included the transect ID where the data was collected. Furthermore, we performed GLMMs with a negative binomial distribution with tick abundance (number of ticks) as a response variable, with the same fixed and random effects. All plots were sampled equally, and therefore the number of ticks did not need to be corrected for the amount of meters dragged. We ran models for the presence and abundance of all tick life stages together and for nymphs and adults together. Furthermore, we ran one model for the presence of each tick life stage separately. For the separate tick life stages, we did not use abundance as a response variable because we found few ticks in general, and when accounting for tick life stages separately, the value at most of our points was zero. For all GLMMs we performed model selection (including the null model), starting with the full models with all parameters but without interactions, using the dredge function of the MuMIn package in R (Barton and Barton, 2015). We selected the best fitting model based on the principle of Occam’s razor, i.e. from all models with differences in Akaike’s information criterion (ΔAIC) < 4, we selected the models with the lowest number of variables (Burnham and Anderson, 2004). We assessed whether the residuals of our models were normally distributed using a normal Q-Q plot, aided by a Shapiro-Wilk normality test. Based on these plots and tests, the residuals of all presented models were deemed to be normally distributed.
Due to a low trapping success for small mammals (see Section 3. Results) and consequently a low variance among the sampling points, we decided not to run models to investigate the associations between the abundance of the different ungulate species and the abundance of small mammals. All analyses and visualizations were performed in R version 3.6.0 (R Core Team, 2019).
3. Results
On the 153 sampling points we visited, of which 102 were visited twice, we found a total of 2376 questing I. ricinus ticks of different life stages (Table 1) and one questing Haemaphysalis punctata male adult. On 21% of the sampling points no ticks were found. We tested 676 I. ricinus and the one H. punctata for the presence of tick-borne pathogens (Table 1). Furthermore, we tested 54 I. ricinus found crawling on field staff during fieldwork (Table 1). All questing I. ricinus nymphs and adults in this study are the same as the questing nymphs and adults from 2019 included in Fabri et al. (2021). Their pathogen-prevalence is described in more detail in Table 1 compared to Fabri et al. (2021). The male H. punctata was negative for all pathogens tested. We found all pathogens that we tested for in questing I. ricinus, albeit with varying degrees of infection prevalence (Table 1). Due to a relatively low number of I. ricinus found (average of 0.89 per 10 m2, which varied per sampling point between 0.00 and 15.20 per 10 m2), and consequently an even lower number of ticks positive for tick-borne pathogens (Table 1), we decided not to run models to look at the effect of ungulate community composition on tick-borne pathogens.
Table 1.
Numbers of Ixodes ricinus counted, collected and tested for tick-borne pathogens. The number of positive ticks is given per tick-borne pathogen, including the infection prevalence (in parentheses) followed by a breakdown of the sequencing results.
| Larvae | Nymphs | Adults |
Total | ||||
|---|---|---|---|---|---|---|---|
| Female | Male | Total | |||||
| No. dragged | 1684 | 628 | 34 | 28 | 62 | 2376 | |
| No. collected while crawling on field staff | 0 | 51 | 1 | 2 | 3 | 54 | |
| No. tested | 0 | 665 | 35 | 30 | 65 | 730 | |
| Anaplasma phagocytophilum | NA | 31 (4.7%) | 2 (5.7%) | 5(16.7%) | 7 (10.8%) | 38 (5.2%) | |
| Ecotype 1 | 7 | 1 | 0 | 1 | 8 | ||
| Ecotype 2 | 1 | 0 | 0 | 0 | 1 | ||
| Unknown ecotype | 23 | 1 | 5 | 6 | 29 | ||
| Borrelia burgdorferi (s.l.) | NA | 85 (12.8%) | 5 (14.3%) | 8 (26.7%) | 13 (20.0%) | 98 (13.4%) | |
| B. afzelii | 20 | 1 | 0 | 1 | 21 | ||
| B. burgdorferi (s.s.) | 3 | 0 | 0 | 0 | 3 | ||
| B. garinii | 8 | 1 | 0 | 1 | 9 | ||
| B. valaisiana | 3 | 0 | 0 | 0 | 3 | ||
| Unknown genospecies | 51 | 3 | 8 | 11 | 62 | ||
| Borrelia miyamotoi | NA | 6 (0.6%) | 1 (2.9%) | 0 (0%) | 1 (1.5%) | 7 (1.0%) | |
| Babesia microti | NA | 0 (0%) | 0 (0%) | 2 (6.7%) | 2 (3.1%) | 2 (0.3%) | |
| Babesia clade-X | NA | 4 (0.6) | 0 (0%) | 0 (0%) | 0 (0%) | 4 (0.5%) | |
| B. venatorum | 2 | 0 | 0 | 0 | 2 | ||
| Unknown species | 2 | 0 | 0 | 0 | 2 | ||
We caught 56 small mammals in total, giving a trapping result of 2 individuals per 100 trap-nights. These included 25 Apodemus flavicollis, 10 Apodemus sylvaticus, 17 Myodes glareolus, three Sorex araneus and one Microtus agrestis. None of the small mammals was positive for A. phagocytophilum, B. microti or Babesia clade-X. Seven were positive for B. burgdorferi (s.l.) and three for B. miyamotoi (Table 2). From all individuals, we collected 497 I. ricinus larvae, 11 I. ricinus nymphs, two I. ricinus males, two Ixodes trianguliceps nymphs and one I. trianguliceps male (Table 3). None of the ticks were positive for B. microti, while we found the other tick-borne pathogens only in a small number of ticks (Table 3).
Table 2.
Numbers of small mammals caught per species and the number of positive individuals per tick-borne pathogen followed by a breakdown of the sequencing results.
| Apodemus flavicollis | Apodemus sylvaticus | Myodes glareolus | Sorex araneus | Microtus agrestis | ||
|---|---|---|---|---|---|---|
| No. caught | 25 | 10 | 17 | 3 | 1 | |
| Anaplasma phagocytophilum | 0 | 0 | 0 | 0 | 0 | |
| Borrelia burgdorferi (s.l.) | 2 | 0 | 4 | 0 | 1 | |
| B. afzelii | 0 | 0 | 1 | 0 | 0 | |
| Unknown genospecies | 2 | 0 | 3 | 0 | 1 | |
| Borrelia miyamotoi | 2 | 0 | 1 | 0 | 0 | |
| Babesia microti | 0 | 0 | 0 | 0 | 0 | |
| Babesia clade-X | 0 | 0 | 0 | 0 | 0 | |
Table 3.
Numbers of ticks collected from small mammals per life stage and species. The number of positive ticks is given per tick-borne pathogen followed by a breakdown of the sequencing results.
|
Apodemus flavicollis |
Apodemus sylvaticus |
Myodes glareolus |
Sorex araneus |
Microtus agrestis |
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IR |
IT |
IR |
IT |
IR |
IT |
IR |
IT |
IR |
IT |
|||||||||||||||||
| L | N | M | N | M | L | N | M | N | M | L | N | M | N | M | L | N | M | N | M | L | N | M | N | M | ||
| No. collected | 229 | 3 | 1 | 0 | 0 | 38 | 3 | 1 | 1 | 0 | 116 | 4 | 0 | 1 | 1 | 107 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Anaplasma phagocytophilum | 7 | 1 | 0 | NA | NA | 0 | 0 | 0 | 0 | NA | 2 | 0 | NA | 0 | 0 | 2 | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
| Unknown ecotype | 7 | 1 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 2 | ||||||||||||||
| Borrelia burgdorferi (s.l.) | 9 | 1 | 0 | NA | NA | 1 | 1 | 0 | 0 | NA | 33 | 1 | NA | 0 | 0 | 3 | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
| B. afzelii | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 6 | 0 | 0 | 0 | 0 | ||||||||||||||
| B. burgdorferi (s.s.) | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | ||||||||||||||
| B. garinii | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 0 | 0 | 1 | ||||||||||||||
| B. lusitaniae | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 0 | ||||||||||||||
| Unknown genospecies | 7 | 0 | 0 | 1 | 1 | 0 | 0 | 18 | 0 | 0 | 0 | 2 | ||||||||||||||
| Borrelia miyamotoi | 1 | 0 | 0 | NA | NA | 0 | 0 | 0 | 0 | NA | 0 | 0 | NA | 0 | 0 | 1 | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
| Babesia microti | 0 | 0 | 0 | NA | NA | 0 | 0 | 0 | 0 | NA | 0 | 0 | NA | 0 | 0 | 0 | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
| Babesia clade-X | 4 | 0 | 0 | NA | NA | 0 | 0 | 0 | 0 | NA | 7 | 0 | NA | 0 | 0 | 0 | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
Abbreviations: IR, Ixodes ricinus; IT, Ixodes trianguliceps; L, larvae; N, nymphs; M, males.
The camera traps caught all five ungulate species (i.e. fallow deer, roe deer, red deer, moose, and wild boar), although not all species occurred on all transects (Supplementary file S2: Figure B.2). The selected models (Supplementary file S2: Table B.1) showed that a lower mean height of the field layer was not associated with the visitation frequencies of the five ungulate species, regardless of whether and how we grouped visitation frequencies of the ungulate species. We found that mean vegetation height was lower during the sampling month of May compared to the other sampling months (Supplementary file S2: Table B.1). None of the selected models (Supplementary file S2: Table B.2) showed any association between the coefficient of the variance of the mean field layer height and the visitation frequencies of any of the ungulate species or the visitation frequencies of all deer or all ungulate species combined.
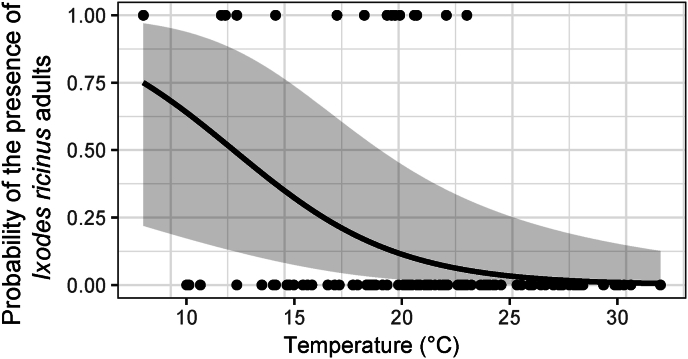
None of the selected models (Supplementary file S2: Tables B.3 and B.4) suggested any association of the larval presence and nymphal presence with any of the predictor variables (ungulate visitation frequencies, vegetation height or temperature), regardless of whether and how we grouped visitation frequencies of the ungulate species. This was also the case for the presence and abundance of all tick stages together (Supplementary file S2: Tables B.6 and B.8) and the presence of nymphs and adults together (Supplementary file S2: Table B.7). The selected models for adult I. ricinus presence (Supplementary file S2: Table B.5) and abundance of nymphs and adults together (Supplementary file S2: Table B.9), suggested that I. ricinus were more likely to be present at lower temperatures (Fig. 3). We did not find any effect of vegetation height and ungulate visitation frequencies on tick presence for any tick life stage.
Fig. 3.
The presence of Ixodes ricinus adults as a function of the temperature above the field layer.
4. Discussion
Ungulates may play an important role in the life-cycle of I. ricinus, either by providing a blood meal or by shaping the vegetation and hereby affecting microclimatic conditions for I. ricinus and/or affecting the habitat of rodents as other important tick hosts. Fabri et al. (2021) showed that ungulate species differ in their contribution to feeding I. ricinus and to the transmission of tick-borne pathogens. In this study, we aimed to test whether the ungulate community composition contributes to the number of questing I. ricinus. A priori, when designing the study, we had several hypotheses about the relation between deer and small mammals, and small mammals and ticks that, following data collection, we could not test due to a low number of small mammals and ticks found. Furthermore, we hypothesized that high densities of fallow and/or red deer and wild boar should lead to strongly reduced field layer height and reduced tick densities, whereas such patterns should not be observed for moose and roe deer. We did not find any association of the height of the field layer with the ungulate visitation frequencies, or with the presence/abundance of I. ricinus in this study.
Gandy et al. (2021) proposed several mechanisms for how ungulates affect the density of questing I. ricinus: (i) a higher ungulate density feeds more adult ticks leading to a higher density of questing larvae which, assuming there are enough hosts to provide blood meals, will translate into high nymph densities; and (ii) ungulate grazing pressure results in shorter vegetation and fewer rodents, resulting in lower densities of I. ricinus. Both these mechanisms are, however, not mutually exclusive and potentially cancel each other out, which might explain why we did not find any associations between the presence of questing I. ricinus and the density of the ungulate species, as expressed by the visitation frequencies, in our study area. There may also be other explanations for not finding support for either of the hypotheses proposed by Gandy et al. (2021). One of them could be the fact that we found a very low abundance of questing I. ricinus, lower than other studies in Europe (e.g. Dobson et al., 2011; Heylen et al., 2019; van Gestel et al., 2021). In addition to low densities, ticks were totally absent from most of our sampling points. We hypothesize that this could be due to the geographical location of our study site and/or due to extreme temperatures during the summer and winter before our study season (SMHI, 2022). Also, for some of the ungulate species, especially red deer and moose, we found relatively low visitation frequencies, and they were absent from the large majority of our sampling points. Due to these low densities, our trapping effort might not have been enough for these species. The very low abundance of I. ricinus and low visitation frequencies of some of the ungulate species, and especially the very large number of sampling points with zero tick- and ungulate data, might explain the lack of associations between ungulate species and questing I. ricinus. This large number of zeros strongly influenced the power of our statistical models. We hypothesized that a higher density of wild boar would lead to a lower abundance of questing ticks, due to the behaviour of rooting. Our study did not support this hypothesis either, which might be explained by us collecting ticks on the field layer vegetation. We did not consider that due to rooting, the field layer vegetation could be destroyed, and by only collecting ticks on the vegetation we did not take the rooting behaviour into account. The height of the field layer could also have influenced the success of catching ticks using the dragging technique. For example, when the vegetation is very high, ticks might not be within reach of the dragging cloth. Only a few sampling points had a relatively high field layer, and we did not find any association between the number of ticks and the height of the field layer. We did, however, hypothesize that the number of ticks should increase with the height of the field layer, and if at the same time our sampling efficiency decreases with the height of the field layer, these effects might have cancelled each other out. Therefore, not finding an association between the number of ticks and the height of the field layer, might have been a result of a reduced efficiency of our sampling method combined with the increased field layer height. In our study, we did not measure the relative humidity, even though relative humidity is an important factor affecting the number of questing ticks (Li et al., 2012). However, since the relative humidity likely was correlated with the temperature above the field layer (Li et al., 2012), we believe that adding relative humidity to our models would not have resulted in different conclusions in our study.
Exclosure studies have shown that the absence of ungulates leads to a reduced I. ricinus density (e.g. Hofmeester et al., 2017a; Gandy et al., 2021). These kinds of studies, however, look at the difference between presence and absence of all ungulate species. Hofmeester et al. (2017a) found that when deer were present, the density of ticks did not change with the density of deer. In our study area, we had no locations without ungulates, and we could therefore only compare the different densities of the ungulate species. If indeed the densities of ungulate species do not drive the densities of questing I. ricinus, as proposed by Hofmeester et al. (2017a), this could also explain why we did not find any associations between the abundance of I. ricinus and ungulates. There are however some studies that did find a correlation between ticks and deer density (e.g. Gilbert et al., 2012), although these studies were generally performed at relatively low deer densities. The densities of especially fallow deer in our study area are high, and it could be that these are already too high to detect any effect on the presence of I. ricinus, even on transects with relatively low fallow deer visitation frequencies.
We found that none of the ungulate species were associated with shorter or higher field layer vegetation in the study area. Moose, red deer and roe deer have a relatively smaller proportion of field layer vegetation in their diet (Spitzer et al., 2020) and aggregate in smaller groups, compared to fallow deer in our study area. This would explain why the visitation rates of these species were not associated with the field layer height. Furthermore, in our study area, we found that fallow deer occurred in much higher densities than the other ungulate species (Supplementary file S2: Figure B.2). This could explain why, in our study, moose, red deer and roe deer did not have an effect on the field layer, but not why we did not find any association between the field layer height and fallow deer visitation frequencies. We hypothesized that due to their rooting behaviour, wild boar would have a great impact on the field layer vegetation. Our data did not suggest such an association between wild boar and the field layer height, which could again be explained by us conducting the study on field layer vegetation and not on areas where the field layer vegetation is absent due to rooting behaviour.
In the questing nymphs and adults, we found a B. burgdorferi (s.l.) infection prevalence of 13% and 20%, respectively. Ixodes ricinus is becoming infected with B. burgdorferi (s.l.) through a single blood meal per life stage, and a higher prevalence of B. burgdorferi (s.l.) in adults compared to nymphs is therefore as expected (Gray, 1998). This is in line with previous studies in Denmark, Norway and Sweden, reporting prevalence ranges of 0–44% in nymphs and 0–71% in adults (Paulauskas et al., 2008; Vennestrom et al., 2008; Kjelland et al., 2010, 2018; Soleng et al., 2013; Hvidsten et al., 2015; Mysterud et al., 2018). In our study, A. phagocytophilum occurred at lower prevalence than B. burgdorferi (s.l.), namely 5% in nymphs and 11% in adults. This is higher than in most previous studies in Scandinavia which reported a prevalence of 1% in nymphs and 5% in adults (Soleng et al., 2013; Kjelland et al., 2018; Mysterud et al., 2018), although Kjær et al. (2019) reported a prevalence of 14% in I. ricinus nymphs.
Small mammal populations in the study area were in a natural low phase of the rodent cycle (Ecke and Hörnfeldt, 2024). Due to the resulting and unfortunate low number of small mammals caught in our study, we could not investigate how different ungulate species affect the small mammal abundance. The low number of small mammals we caught is in line with the cyclicity of small mammal density that is observed in large parts of Europe (Andreassen et al., 2021) and the rodent populations in our study area likely being at a low phase of the cycle. We found that none of the small mammals was positive for A. phagocytophilum, while approximately 10% were positive for B. burgdorferi (s.l.). In other studies performed in Fennoscandia, the infection prevalence of B. burgdorferi (s.l.) in small mammals varied between 0 and 59% (Frandsen et al., 1995; Hellgren et al., 2011; Råberg, 2012; Andersson et al., 2013; Cayol et al., 2018; Mysterud et al., 2019; Zhong et al., 2019). Few have studied A. phagocytophilum in small mammals in Fennoscandia, but infection prevalence of 22% and 35% has been observed in Finland (Kallio et al., 2014; Cayol et al., 2018). We found a lower number of ticks collected from small mammals infected with B. burgdorferi (s.l.) than previously reported in Sweden (Tälleklint and Jaenson, 1994). Furthermore, we found few ticks from small mammals infected with A. phagocytophilum, which is in line with other studies from Europe (Bown et al., 2006; Silaghi et al., 2012; Blaňarová et al., 2014; Jahfari et al., 2014).
5. Conclusions
In this study, we aimed to better understand the association between different ungulate species and the abundance of I. ricinus, either directly or via vegetation and/or small mammals. The density of ungulate species was not associated with the height of the vegetation. When treating all ungulate species as one group, we did not find an association either. Our results did not find any association between ungulate species and the abundance of I. ricinus or between field layer height and I. ricinus abundance. A long-term study that can accommodate for variation in tick and rodent numbers may be necessary to investigate this. However, the data from this study does indicate that it is important to differentiate among the ungulate species in such a study instead of treating all ungulate species as one group.
Funding
This study was largely financed by Grant 2018.4.3–4511 of the Future Animals, Nature and Health platform at the Swedish University of Agricultural Sciences. H. Sprong was supported by a Grant of the European Interreg North Sea Region programme, as part of the NorthTick project. The pathogen detection was financially supported by the Dutch Ministry of Health, Welfare and Sports. N.D. Fabri was supported by a Grant of the Dutch Research Council (NWO. 022.005.021) and J.P.G.M. Cromsigt was supported by the Swedish Environmental Protection Agency (Naturvårdsverket, NV-01337-15/NV-03047/NV-08503-18).
Ethical approval
An ethical permit for snap-trapping of small mammals was obtained from the Swedish Board of Agriculture (Dnr A 18-2019).
CRediT authorship contribution statement
Nannet D. Fabri: Conceptualization, Methodology, Resources, Formal analysis, Writing – original draft, Writing – review & editing, Visualization, Funding acquisition, Project administration. Tim R. Hofmeester: Conceptualization, Methodology, Resources, Formal analysis, Writing – original draft, Writing – review & editing, Funding acquisition. Frauke Ecke: Methodology, Resources, Writing – review & editing. Hein Sprong: Methodology, Resources, Writing – review & editing, Funding acquisition. Jordi Timmermans: Resources, Formal analysis, Writing – review & editing. Hans Heesterbeek: Writing – review & editing. Joris P.G.M. Cromsigt: Conceptualization, Methodology, Formal analysis, Writing – original draft, Writing – review & editing, Funding acquisition.
Declaration of competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank all landowners on whose land we could perform our study. We thank Manoj Fonville, Ryanne Jaarsma and Björn Donnars for their support in the laboratory preparations and molecular analyses of the samples, and Wouter Krigee and Michael Wentzel for their contributions in the data collections. We also thank Sonya Juthberg, Annika Holmgren, Åke Nordström, Sabine Pfeffer and all other field technicians and students who performed the pellet counts from 2012 onwards. We also thank Sander Vink for drawing the silhouettes of ungulates used in this paper. The other silhouettes are drawn by the authors.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.crpvbd.2024.100206.
Appendix A. Supplementary data
The following are the supplementary data to this article:
Data availability
The data supporting the conclusions of this article are included within the article and its supplementary files. The datasets used and/or analysed during the present study are available from the corresponding author upon request.
References
- Andersson M., Scherman K., Råberg L. Multiple-strain infections of Borrelia afzelii: A role for within-host interactions in the maintenance of antigenic diversity? Am. Nat. 2013;181:545–554. doi: 10.1086/669905. [DOI] [PubMed] [Google Scholar]
- Andreassen H.P., Sundell J., Ecke F., Halle S., Haapakoski M., Henttonen H., et al. Population cycles and outbreaks of small rodents: Ten essential questions we still need to solve. Oecologia. 2021;195:601–622. doi: 10.1007/s00442-020-04810-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apollonio M., Andersen R., Putman R. Cambridge University Press; Cambridge, UK: 2010. European Ungulates and Their Management in the 21st Century. [Google Scholar]
- Arthur D.R. Butterworths; London, UK: 1963. British Ticks. [Google Scholar]
- Barton K., Barton M.K. Package ‘MuMIn’. 2015. https://cran.r-project.org/web/packages/MuMIn/index.html
- Blaňarová L., Stanko M., Carpi G., Miklisová D., Víchová B., Mošanský L., et al. Distinct Anaplasma phagocytophilum genotypes associated with Ixodes trianguliceps ticks and rodents in Central Europe. Ticks Tick Borne Dis. 2014;5:928–938. doi: 10.1016/j.ttbdis.2014.07.012. [DOI] [PubMed] [Google Scholar]
- Bown K.J., Begon M., Bennett M., Birtles R.J., Burthe S., Lambin X., et al. Sympatric Ixodes trianguliceps and Ixodes ricinus ticks feeding on field voles (Microtus agrestis): Potential for increased risk of Anaplasma phagocytophilum in the United Kingdom? Vector Borne Zoonot. Diss. 2006;6:404–410. doi: 10.1089/vbz.2006.6.404. [DOI] [PubMed] [Google Scholar]
- Brooks M., Bolker M., Kristensen K., Maechler M., Magnusson A., McGillycuddy M., et al. Package ‘glmmTMB’. 2022. https://github.com/glmmTMB/glmmTMB
- Bubnicki J.W., Churski M., Kuijper D.P.J. TRAPPER: An open source web‐based application to manage camera trapping projects. Methods Ecol. Evol. 2016;7:1209–1216. [Google Scholar]
- Burnham K., Anderson D. Multimodel inference: Understanding AIC and BIC in model selection. Socio. Methods Res. 2004;33:261–304. [Google Scholar]
- Cangelosi R., Goriely A. Component retention in principal component analysis with application to cDNA microarray data. Biol. Direct. 2007;2:2. doi: 10.1186/1745-6150-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casati S., Sager H., Gern L., Piffaretti J.-C. Presence of potentially pathogenic Babesia sp. for human in Ixodes ricinus in Switzerland. Ann. Agric. Environ. Med. 2006;13:65–70. [PubMed] [Google Scholar]
- Cayol C., Jääskeläinen A., Koskela E., Kyröläinen S., Mappes T., Siukkola A., Kallio E.R. Sympatric transmission within wild rodent populations. Sci. Rep. 2018;8 doi: 10.1186/s13071-017-2112-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel M., Cerny V., Dusbabek F., Honzakova E., Olejnicek J. Influence of microclimate on the life cycle of the common tick Ixodes ricinus (L.) in an open area in comparison with forest habitats. Folia Parasitol. 1977;24:149–160. [PubMed] [Google Scholar]
- Dobson A.D.M., Taylor J.L., Randolph S.E. Tick (Ixodes ricinus) abundance and seasonality at recreational sites in the UK: Hazards in relation to fine-scale habitat types revealed by complementary sampling methods. Ticks Tick-Borne Dis. 2011;2:67–74. doi: 10.1016/j.ttbdis.2011.03.002. [DOI] [PubMed] [Google Scholar]
- Ecke F., Hörnfeldt B. [Miljöövervakning av smågnagare] Environmental monitoring of small rodents. 2024. http://www.slu.se/mo-smagnagare
- Ecke F., Löfgren O., Sörlin D. Population dynamics of small mammals in relation to forest age and structural habitat factors in northern Sweden. J. Appl. Ecol. 2002;39:781–792. [Google Scholar]
- Fabri N.D., Sprong H., Hofmeester T.R., Heesterbeek H., Donnars B.F., Widemo F., et al. Wild ungulate species differ in their contribution to the transmission of Ixodes ricinus-borne pathogens. Parasites Vectors. 2021;14:360. doi: 10.1186/s13071-021-04860-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frandsen F., Bresciani J., Hansen H. Prevalence of antibodies to Borrelia burgdorferi in Danish rodents. APMIS. 1995;103:247–253. [PubMed] [Google Scholar]
- Gandy S., Kilbride E., Biek R., Millins C., Gilbert L. Experimental evidence for opposing effects of high deer density on tick-borne pathogen prevalence and hazard. Parasites Vectors. 2021;14:509. doi: 10.1186/s13071-021-05000-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert L., Maffey G.L., Ramsay S.L., Hester A.J. The effect of deer management on the abundance of Ixodes ricinus in Scotland. Ecol. Appl. 2012;22:658–667. doi: 10.1890/11-0458.1. [DOI] [PubMed] [Google Scholar]
- Gill R.M.A., Beardall V. The impact of deer on woodlands: The effects of browsing and seed dispersal on vegetation structure and composition. Forestry Int. J. For. Res. 2001;74:209–218. [Google Scholar]
- Gray J.S. The ecology of ticks transmitting Lyme borreliosis. Exp. Appl. Acarol. 1998;22:249–258. [Google Scholar]
- Hellgren O., Andersson M., Råberg L. The genetic structure of Borrelia afzelii varies with geographic but not ecological sampling scale. J. Evol. Biol. 2011;24:159–167. doi: 10.1111/j.1420-9101.2010.02148.x. [DOI] [PubMed] [Google Scholar]
- Heylen D., Lasters R., Adriaensen F., Fonville M., Sprong H., Matthysen E. Ticks and tick-borne diseases in the city: Role of landscape connectivity and green space characteristics in a metropolitan area. Sci. Total Environ. 2019;670:941–949. doi: 10.1016/j.scitotenv.2019.03.235. [DOI] [PubMed] [Google Scholar]
- Heylen D., Tijsse E., Fonville M., Matthysen E., Sprong H. Transmission dynamics of Borrelia burgdorferi s.l. in a bird tick community. Environ. Microbiol. 2013;15:663–673. doi: 10.1111/1462-2920.12059. [DOI] [PubMed] [Google Scholar]
- Hillyard P.D. Field Studies Council; Shrewsbury, UK: 1996. Ticks of North-West Europe. [Google Scholar]
- Hofmeester T.R., Coipan E.C., van Wieren S.E., Prins H.H.T., Takken W., Sprong H. Few vertebrate species dominate the Borrelia burgdorferi s.l. life cycle. Environ. Res. Lett. 2016;11 [Google Scholar]
- Hofmeester T.R., Rowcliffe J.M., Jansen P.A. A simple method for estimating the effective detection distance of camera traps. Remote Sens. Ecol. Conserv. 2017;3:81–89. [Google Scholar]
- Hofmeester T.R., Sprong H., Jansen P.A., Prins H.H.T., van Wieren S.E. Deer presence rather than abundance determines the population density of the sheep tick, Ixodes ricinus, in Dutch forests. Parasites Vectors. 2017;10:433. doi: 10.1186/s13071-017-2370-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovius J.W.R., de Wever B., Sohne M., Brouwer M.C., Coumou J., Wagemakers A., et al. A case of meningoencephalitis by the relapsing fever spirochaete Borrelia miyamotoi in Europe. Lancet. 2013;382:658. doi: 10.1016/S0140-6736(13)61644-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hvidsten D., Stordal F., Lager M., Rognerud B., Kristiansen B.-E., Matussek A., et al. Borrelia burgdorferi sensu lato-infected Ixodes ricinus collected from vegetation near the Arctic Circle. Ticks Tick Borne Dis. 2015;6:768–773. doi: 10.1016/j.ttbdis.2015.07.002. [DOI] [PubMed] [Google Scholar]
- Jaenson T.G.T., Jaenson D.G., Eisen L., Petersson E., Lindgren E. Changes in the geographical distribution and abundance of the tick Ixodes ricinus during the past 30 years in Sweden. Parasites Vectors. 2012;5:8. doi: 10.1186/1756-3305-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahfari S., Coipan E.C., Fonville M., van Leeuwen A.D., Hengeveld P., Heylen D., et al. Circulation of four Anaplasma phagocytophilum ecotypes in Europe. Parasites Vectors. 2014;7:365. doi: 10.1186/1756-3305-7-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallio E.R., Begon M., Birtles R.J., Bown K.J., Koskela E., Mappes T., Watts P.C. First report of Anaplasma phagocytophilum and Babesia microti in rodents in Finland. Vector Borne Zoonot. Dis. 2014;14:389–393. doi: 10.1089/vbz.2013.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keesing F. Impacts of ungulates on the demography and diversity of small mammals in central Kenya. Oecologia. 1998;116:381–389. doi: 10.1007/s004420050601. [DOI] [PubMed] [Google Scholar]
- Kirby K.J. The impact of deer on the ground flora of British broadleaved woodland. Forestry Int. J. For. Res. 2001;74:219–229. [Google Scholar]
- Kjær L.J., Soleng A., Edgar K.S., Lindstedt H.E.H., Paulsen K.M., Andreassen Å.K., et al. Predicting and mapping human risk of exposure to Ixodes ricinus nymphs using climatic and environmental data, Denmark, Norway and Sweden, 2016. Euro Surveill. 2019;24 doi: 10.2807/1560-7917.ES.2019.24.9.1800101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjelland V., Paulsen K.M., Rollum R., Jenkins A., Stuen S., Soleng A., et al. Tick-borne encephalitis virus, Borrelia burgdorferi sensu lato, Borrelia miyamotoi, Anaplasma phagocytophilum and “Candidatus Neoehrlichia mikurensis” in Ixodes ricinus ticks collected from recreational islands in southern Norway. Ticks Tick Borne Dis. 2018;9:1098–1102. doi: 10.1016/j.ttbdis.2018.04.005. [DOI] [PubMed] [Google Scholar]
- Kjelland V., Stuen S., Skarpaas T., Slettan A. Prevalence and genotypes of Borrelia burgdorferi sensu lato infection in Ixodes ricinus ticks in southern Norway. Scand. J. Infect. Dis. 2010;42:579–585. doi: 10.3109/00365541003716526. [DOI] [PubMed] [Google Scholar]
- Krawczyk A.I., van Duijvendijk G.L.A., Swart A., Heylen D., Jaarsma R.I., Jacobs F.H.H., et al. Effect of rodent density on tick and tick-borne pathogen populations: consequences for infectious disease risk. Parasites Vectors. 2020;13:34. doi: 10.1186/s13071-020-3902-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal B., Zamora E., Fuentes A., Thomas D.B., Dearth R.K. Questing by tick larvae (Acari: Ixodidae): A review of the influences that affect off-host survival. Ann. Entomol. Soc. Am. 2020;113:425–438. doi: 10.1093/aesa/saaa013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Heyman P., Cochez C., Simons L., Vanwambeke S.O. A multi-level analysis of the relationship between environmental factors and questing Ixodes ricinus dynamics in Belgium. Parasites Vectors. 2012;5:149. doi: 10.1186/1756-3305-5-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnell J.D.C., Cretois B., Nilsen E.B., Rolandsen C.M., Solberg E.J., Veiberg V., et al. The challenges and opportunities of coexisting with wild ungulates in the human-dominated landscapes of Europe's Anthropocene. Biol. Conserv. 2020;244 [Google Scholar]
- McCauley D.J., Keesing F., Young T.P., Allan B.F., Pringle R.M. Indirect effects of large herbivores on snakes in an African savanna. Ecology. 2006;87:2657–2663. doi: 10.1890/0012-9658(2006)87[2657:ieolho]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Michalik J., Stańczak J., Cieniuch S., Racewicz M., Sikora B., Dabert M. Wild boars as hosts of human-pathogenic Anaplasma phagocytophilum variants. Emerg. Infect. Dis. 2012;18:998. doi: 10.3201/eid1806.110997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mysterud A., Stigum V.M., Jaarsma R.I., Sprong H. Genospecies of Borrelia burgdorferisensu lato detected in 16 mammal species and questing ticks from northern Europe. Sci. Rep. 2019;9:5088. doi: 10.1038/s41598-019-41686-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mysterud A., Stigum V.M., Seland I.V., Herland A., Easterday W.R., Jore S., et al. Tick abundance, pathogen prevalence, and disease incidence in two contrasting regions at the northern distribution range of Europe. Parasites Vectors. 2018;11:309. doi: 10.1186/s13071-018-2890-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Øines Ø., Radzijevskaja J., Paulauskas A., Rosef O. Prevalence and diversity of Babesia spp. in questing Ixodes ricinus ticks from Norway. Parasites Vectors. 2012;5:156. doi: 10.1186/1756-3305-5-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overzier E., Pfister K., Herb I., Mahling M., Böck G., Silaghi C. Detection of tick-borne pathogens in roe deer (Capreolus capreolus), in questing ticks (Ixodes ricinus), and in ticks infesting roe deer in southern Germany. Ticks Tick Borne Dis. 2013;4:320–328. doi: 10.1016/j.ttbdis.2013.01.004. [DOI] [PubMed] [Google Scholar]
- Paulauskas A., Arnbrasiene D., Radzijevskaja J., Rosef O., Turcinaviciene J. Diversity in prevalence and genospecies of Borrelia burgdorferi sensu lato in Ixodes ricinus ticks and rodents in Lithuania and Norway. Int. J. Med. Microbiol. 2008;298:180–187. [Google Scholar]
- R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2019. R: A language and environment for statistical computing.https://www.R-project.org/ [Google Scholar]
- Råberg L. Infection intensity and infectivity of the tick-borne pathogen Borrelia afzelii. J. Evol. Biol. 2012;25:1448–1453. doi: 10.1111/j.1420-9101.2012.02515.x. [DOI] [PubMed] [Google Scholar]
- Ramirez J.I., Jansen P.A., Poorter L. Effects of wild ungulates on the regeneration, structure and functioning of temperate forests: A semi-quantitative review. For. Ecol. Manag. 2018;424:406–419. [Google Scholar]
- Sgroi G., Iatta R., Lia R.P., D'Alessio N., Manoj R.R.S., Veneziano V., Otranto D. Spotted fever group rickettsiae in Dermacentor marginatus from wild boars in Italy. Transbound. Emerg. Dis. 2021;68:2111–2120. doi: 10.1111/tbed.13859. [DOI] [PubMed] [Google Scholar]
- Silaghi C., Woll D., Hamel D., Pfister K., Mahling M., Pfeffer M. Babesia spp. and Anaplasma phagocytophilum in questing ticks, ticks parasitizing rodents and the parasitized rodents - analyzing the host-pathogen-vector interface in a metropolitan area. Parasites Vectors. 2012;5:191. doi: 10.1186/1756-3305-5-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMHI . 2022. Ladda Ner Meteorologiscka Observationer.http://www.smhi.se/data/meteorologi/ladda-ner-meteorologiska-observationer/#param=airtemperatureInstant,stations=all [Google Scholar]
- Soleng A., Kjelland V. Borrelia burgdorferi sensu lato and Anaplasma phagocytophilum in Ixodes ricinus ticks in Brønnøysund in northern Norway. Ticks Tick Borne Dis. 2013;4:218–221. doi: 10.1016/j.ttbdis.2012.11.006. [DOI] [PubMed] [Google Scholar]
- Spitzer R. Swedish University of Agricultural Sciences; Umeå, Sweden: 2019. Trophic resource use and partitioning in multispecies ungulate communities. PhD Thesis. [Google Scholar]
- Spitzer R., Churski M., Felton A., Heurich M., Kuijper D.P.J., Landman M., et al. Doubting dung: eDNA reveals high rates of misidentification in diverse European ungulate communities. Eur. J. Wildl. Res. 2019;65:28. [Google Scholar]
- Spitzer R., Coissac E., Felton A., Fohringer C., Juvany L., Landman M., et al. Small shrubs with large importance? Smaller deer may increase the moose-forestry conflict through feeding competition over Vaccinium shrubs in the field layer. For. Ecol. Manag. 2021;480 [Google Scholar]
- Spitzer R., Felton A., Landman M., Singh N.J., Widemo F., Cromsigt J.P.G.M. Fifty years of European ungulate dietary studies: A synthesis. Oikos. 2020;129:1668–1680. [Google Scholar]
- Stewart K.E.J., Bourn N.A.D., Thomas J.A. An evaluation of three quick methods commonly used to assess sward height in ecology. J. Appl. Ecol. 2001;38:1148–1154. [Google Scholar]
- Stigum V.M., Jaarsma R.I., Sprong H., Rolandsen C.M., Mysterud A. Infection prevalence and ecotypes of Anaplasma phagocytophilum in moose Alces alces, red deer Cervus elaphus, roe deer Capreolus capreolus and Ixodes ricinus ticks from Norway. Parasites Vectors. 2019;12:1. doi: 10.1186/s13071-018-3256-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tälleklint L., Jaenson T.G.T. Transmission of Borrelia burgdorferi s.l. from mammal reservoirs to the primary vector of Lyme borreliosis, Ixodes ricinus (Acari: Ixodidae), in Sweden. J. Med. Entomol. 1994;31:880–886. doi: 10.1093/jmedent/31.6.880. [DOI] [PubMed] [Google Scholar]
- van Gestel M., Verheyen K., Matthysen E., Heylen D. Danger on the track? Tick densities near recreation infrastructures in forests. Urban For. Urban Green. 2021;59 [Google Scholar]
- Vennestrom J., Egholm H., Jensen P.M. Occurrence of multiple infections with different Borrelia burgdorferi genospecies in Danish Ixodes ricinus nymphs. Parasitol. Int. 2008;57:32–37. doi: 10.1016/j.parint.2007.07.004. [DOI] [PubMed] [Google Scholar]
- Wielinga P.R., Gaasenbeek C., Fonville M., de Boer A., de Vries A., Dimmers W., et al. Longitudinal analysis of tick densities and Borrelia, Anaplasma, and Ehrlichia infections of Ixodes ricinus ticks in different habitat areas in The Netherlands. Appl. Environ. Microbiol. 2006;72:7594–7601. doi: 10.1128/AEM.01851-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirthner S., Schütz M., Page-Dumroese D.S., Busse M.D., Kirchner J.W., Risch A.C. Do changes in soil properties after rooting by wild boars (Sus scrofa) affect understory vegetation in Swiss hardwood forests? Can. J. For. Res. 2012;42:585–592. [Google Scholar]
- Zhong X., Nouri M., Råberg L. Colonization and pathology of Borrelia afzelii in its natural hosts. Ticks Tick Borne Dis. 2019;10:822–827. doi: 10.1016/j.ttbdis.2019.03.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the conclusions of this article are included within the article and its supplementary files. The datasets used and/or analysed during the present study are available from the corresponding author upon request.