Abstract
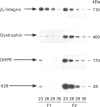
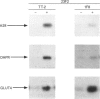
1. Several cell-surface domains of sarcolemma and T-tubule from skeletal-muscle fibre were isolated and characterized. 2. A protocol of subcellular fractionation was set up that involved the sequential low- and high-speed homogenization of rat skeletal muscle followed by KCl washing, Ca2+ loading and sucrose-density-gradient centrifugation. This protocol led to the separation of cell-surface membranes from membranes enriched in sarcoplasmic reticulum and intracellular GLUT4-containing vesicles. 3. Agglutination of cell-surface membranes using wheat-germ agglutinin allowed the isolation of three distinct cell-surface membrane domains: sarcolemmal fraction 1 (SM1), sarcolemmal fraction 2 (SM2) and a T-tubule fraction enriched in protein tt28 and the alpha 2-component of dihydropyridine receptor. 4. Fractions SM1 and SM2 represented distinct sarcolemmal subcompartments based on different compositions of biochemical markers: SM2 was characterized by high levels of beta 1-integrin and dystrophin, and SM1 was enriched in beta 1-integrin but lacked dystrophin. 5. The caveolae-associated molecule caveolin was very abundant in SM1, SM2 and T-tubules, suggesting the presence of caveolae or caveolin-rich domains in these cell-surface membrane domains. In contrast, clathrin heavy chain was abundant in SM1 and T-tubules, but only trace levels were detected in SM2. 6. Immunoadsorption of T-tubule vesicles with antibodies against protein tt28 and against GLUT4 revealed the presence of GLUT4 in T-tubules under basal conditions and it also allowed the identification of two distinct pools of T-tubules showing different contents of tt28 and dihydropyridine receptors. 7. Our data on distribution of clathrin and dystrophin reveal the existence of subcompartments in sarcolemma from muscle fibre, featuring selective mutually exclusive components. T-tubules contain caveolin and clathrin suggesting that they contain caveolin- and clathrin-rich domains. Furthermore, evidence for the heterogeneous distribution of membrane proteins in T-tubules is also presented.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arahata K., Ishiura S., Ishiguro T., Tsukahara T., Suhara Y., Eguchi C., Ishihara T., Nonaka I., Ozawa E., Sugita H. Immunostaining of skeletal and cardiac muscle surface membrane with antibody against Duchenne muscular dystrophy peptide. Nature. 1988 Jun 30;333(6176):861–863. doi: 10.1038/333861a0. [DOI] [PubMed] [Google Scholar]
- Bozyczko D., Decker C., Muschler J., Horwitz A. F. Integrin on developing and adult skeletal muscle. Exp Cell Res. 1989 Jul;183(1):72–91. doi: 10.1016/0014-4827(89)90419-9. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burdett E., Beeler T., Klip A. Distribution of glucose transporters and insulin receptors in the plasma membrane and transverse tubules of skeletal muscle. Arch Biochem Biophys. 1987 Feb 15;253(1):279–286. doi: 10.1016/0003-9861(87)90661-8. [DOI] [PubMed] [Google Scholar]
- Camps M., Castelló A., Muñoz P., Monfar M., Testar X., Palacín M., Zorzano A. Effect of diabetes and fasting on GLUT-4 (muscle/fat) glucose-transporter expression in insulin-sensitive tissues. Heterogeneous response in heart, red and white muscle. Biochem J. 1992 Mar 15;282(Pt 3):765–772. doi: 10.1042/bj2820765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caswell A. H., Baker S. P., Boyd H., Potter L. T., Garcia M. beta-adrenergic receptor and adenylate cyclase in transverse tubules of skeletal muscle. J Biol Chem. 1978 May 10;253(9):3049–3054. [PubMed] [Google Scholar]
- Charuk J. H., Howlett S., Michalak M. Subfractionation of cardiac sarcolemma with wheat-germ agglutinin. Biochem J. 1989 Dec 15;264(3):885–892. doi: 10.1042/bj2640885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudek R. W., Dohm G. L., Holman G. D., Cushman S. W., Wilson C. M. Glucose transporter localization in rat skeletal muscle. Autoradiographic study using ATB-[2-3H]BMPA photolabel. FEBS Lett. 1994 Feb 21;339(3):205–208. doi: 10.1016/0014-5793(94)80416-8. [DOI] [PubMed] [Google Scholar]
- Dunn S. M. Voltage-dependent calcium channels in skeletal muscle transverse tubules. Measurements of calcium efflux in membrane vesicles. J Biol Chem. 1989 Jul 5;264(19):11053–11060. [PubMed] [Google Scholar]
- Eastwood A. B., Franzini-Armstrong C., Peracchia C. Structure of membranes in crayfish muscle: comparison of phasic and tonic fibres. J Muscle Res Cell Motil. 1982 Sep;3(3):273–294. doi: 10.1007/BF00713038. [DOI] [PubMed] [Google Scholar]
- Eaton B. L., Pepe F. A. M band protein. Two components isolated from chicken breast muscle. J Cell Biol. 1972 Dec;55(3):681–695. doi: 10.1083/jcb.55.3.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fosset M., Jaimovich E., Delpont E., Lazdunski M. [3H]nitrendipine receptors in skeletal muscle. J Biol Chem. 1983 May 25;258(10):6086–6092. [PubMed] [Google Scholar]
- Friedman J. E., Dudek R. W., Whitehead D. S., Downes D. L., Frisell W. R., Caro J. F., Dohm G. L. Immunolocalization of glucose transporter GLUT4 within human skeletal muscle. Diabetes. 1991 Jan;40(1):150–154. doi: 10.2337/diab.40.1.150. [DOI] [PubMed] [Google Scholar]
- Glenney J. R., Jr The sequence of human caveolin reveals identity with VIP21, a component of transport vesicles. FEBS Lett. 1992 Dec 7;314(1):45–48. doi: 10.1016/0014-5793(92)81458-x. [DOI] [PubMed] [Google Scholar]
- Gumà A., Mora C., Santalucía T., Viñals F., Testar X., Palacín M., Zorzano A. System A transport activity is stimulated in skeletal muscle in response to diabetes. FEBS Lett. 1992 Sep 21;310(1):51–54. doi: 10.1016/0014-5793(92)81144-b. [DOI] [PubMed] [Google Scholar]
- Hidalgo C., Gonzalez M. E., Lagos R. Characterization of the Ca2+- or Mg2+-ATPase of transverse tubule membranes isolated from rabbit skeletal muscle. J Biol Chem. 1983 Nov 25;258(22):13937–13945. [PubMed] [Google Scholar]
- Jorgensen A. O., Arnold W., Shen A. C., Yuan S. H., Gaver M., Campbell K. P. Identification of novel proteins unique to either transverse tubules (TS28) or the sarcolemma (SL50) in rabbit skeletal muscle. J Cell Biol. 1990 Apr;110(4):1173–1185. doi: 10.1083/jcb.110.4.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen A. O., Shen A. C., Arnold W., Leung A. T., Campbell K. P. Subcellular distribution of the 1,4-dihydropyridine receptor in rabbit skeletal muscle in situ: an immunofluorescence and immunocolloidal gold-labeling study. J Cell Biol. 1989 Jul;109(1):135–147. doi: 10.1083/jcb.109.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidwai A. M., Radcliffe M. A., Lee E. Y., Daniel E. E. Isolation and properties of skeletal muscle plasma membrane. Biochim Biophys Acta. 1973 Mar 29;298(3):593–607. doi: 10.1016/0005-2736(73)90076-x. [DOI] [PubMed] [Google Scholar]
- Klip A., Marette A. Acute and chronic signals controlling glucose transport in skeletal muscle. J Cell Biochem. 1992 Jan;48(1):51–60. doi: 10.1002/jcb.240480109. [DOI] [PubMed] [Google Scholar]
- Klip A., Ramlal T., Young D. A., Holloszy J. O. Insulin-induced translocation of glucose transporters in rat hindlimb muscles. FEBS Lett. 1987 Nov 16;224(1):224–230. doi: 10.1016/0014-5793(87)80452-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leube R. E., Kaiser P., Seiter A., Zimbelmann R., Franke W. W., Rehm H., Knaus P., Prior P., Betz H., Reinke H. Synaptophysin: molecular organization and mRNA expression as determined from cloned cDNA. EMBO J. 1987 Nov;6(11):3261–3268. doi: 10.1002/j.1460-2075.1987.tb02644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisanti M. P., Scherer P. E., Tang Z., Sargiacomo M. Caveolae, caveolin and caveolin-rich membrane domains: a signalling hypothesis. Trends Cell Biol. 1994 Jul;4(7):231–235. doi: 10.1016/0962-8924(94)90114-7. [DOI] [PubMed] [Google Scholar]
- Marette A., Burdett E., Douen A., Vranic M., Klip A. Insulin induces the translocation of GLUT4 from a unique intracellular organelle to transverse tubules in rat skeletal muscle. Diabetes. 1992 Dec;41(12):1562–1569. doi: 10.2337/diab.41.12.1562. [DOI] [PubMed] [Google Scholar]
- Masuda T., Fujimaki N., Ozawa E., Ishikawa H. Confocal laser microscopy of dystrophin localization in guinea pig skeletal muscle fibers. J Cell Biol. 1992 Nov;119(3):543–548. doi: 10.1083/jcb.119.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura K., Ervasti J. M., Ohlendieck K., Kahl S. D., Campbell K. P. Association of dystrophin-related protein with dystrophin-associated proteins in mdx mouse muscle. Nature. 1992 Dec 10;360(6404):588–591. doi: 10.1038/360588a0. [DOI] [PubMed] [Google Scholar]
- North A. J., Galazkiewicz B., Byers T. J., Glenney J. R., Jr, Small J. V. Complementary distributions of vinculin and dystrophin define two distinct sarcolemma domains in smooth muscle. J Cell Biol. 1993 Mar;120(5):1159–1167. doi: 10.1083/jcb.120.5.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlendieck K., Ervasti J. M., Snook J. B., Campbell K. P. Dystrophin-glycoprotein complex is highly enriched in isolated skeletal muscle sarcolemma. J Cell Biol. 1991 Jan;112(1):135–148. doi: 10.1083/jcb.112.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pley U., Parham P. Clathrin: its role in receptor-mediated vesicular transport and specialized functions in neurons. Crit Rev Biochem Mol Biol. 1993;28(5):431–464. doi: 10.3109/10409239309078441. [DOI] [PubMed] [Google Scholar]
- Porter G. A., Dmytrenko G. M., Winkelmann J. C., Bloch R. J. Dystrophin colocalizes with beta-spectrin in distinct subsarcolemmal domains in mammalian skeletal muscle. J Cell Biol. 1992 Jun;117(5):997–1005. doi: 10.1083/jcb.117.5.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujades C., Forsberg E., Enrich C., Johansson S. Changes in cell surface expression of fibronectin and fibronectin receptor during liver regeneration. J Cell Sci. 1992 Aug;102(Pt 4):815–820. doi: 10.1242/jcs.102.4.815. [DOI] [PubMed] [Google Scholar]
- Rayns D. G., Simpson F. O., Bertaud W. S. Surface features of striated muscle. II. Guinea-pig skeletal muscle. J Cell Sci. 1968 Dec;3(4):475–482. doi: 10.1242/jcs.3.4.475. [DOI] [PubMed] [Google Scholar]
- Rosemblatt M. S., Scales D. J. Morphological, immunological and biochemical characterization of purified transverse tubule membranes isolated from rabbit skeletal muscle. Mol Cell Biochem. 1989 May 4;87(1):57–69. doi: 10.1007/BF00421083. [DOI] [PubMed] [Google Scholar]
- Rosemblatt M., Hidalgo C., Vergara C., Ikemoto N. Immunological and biochemical properties of transverse tubule membranes isolated from rabbit skeletal muscle. J Biol Chem. 1981 Aug 10;256(15):8140–8148. [PubMed] [Google Scholar]
- Rothberg K. G., Heuser J. E., Donzell W. C., Ying Y. S., Glenney J. R., Anderson R. G. Caveolin, a protein component of caveolae membrane coats. Cell. 1992 Feb 21;68(4):673–682. doi: 10.1016/0092-8674(92)90143-z. [DOI] [PubMed] [Google Scholar]
- Sabbadini R. A., Dahms A. S. Biochemical properties of isolated transverse tubular membranes. J Bioenerg Biomembr. 1989 Apr;21(2):163–213. doi: 10.1007/BF00812068. [DOI] [PubMed] [Google Scholar]
- Schmalbruch H., Hellhammer U. The number of satellite cells in normal human muscle. Anat Rec. 1976 Jul;185(3):279–287. doi: 10.1002/ar.1091850303. [DOI] [PubMed] [Google Scholar]
- Schmid A., Barhanin J., Coppola T., Borsotto M., Lazdunski M. Immunochemical analysis of subunit structures of 1,4-dihydropyridine receptors associated with voltage-dependent Ca2+ channels in skeletal, cardiac, and smooth muscles. Biochemistry. 1986 Jun 17;25(12):3492–3495. doi: 10.1021/bi00360a002. [DOI] [PubMed] [Google Scholar]
- Seiler S., Fleischer S. Isolation of plasma membrane vesicles from rabbit skeletal muscle and their use in ion transport studies. J Biol Chem. 1982 Nov 25;257(22):13862–13871. [PubMed] [Google Scholar]
- Straub V., Bittner R. E., Léger J. J., Voit T. Direct visualization of the dystrophin network on skeletal muscle fiber membrane. J Cell Biol. 1992 Dec;119(5):1183–1191. doi: 10.1083/jcb.119.5.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toutant M., Barhanin J., Bockaert J., Rouot B. G-proteins in skeletal muscle. Evidence for a 40 kDa pertussis-toxin substrate in purified transverse tubules. Biochem J. 1988 Sep 1;254(2):405–409. doi: 10.1042/bj2540405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toutant M., Gabrion J., Vandaele S., Peraldi-Roux S., Barhanin J., Bockaert J., Rouot B. Cellular distribution and biochemical characterization of G proteins in skeletal muscle: comparative location with voltage-dependent calcium channels. EMBO J. 1990 Feb;9(2):363–369. doi: 10.1002/j.1460-2075.1990.tb08119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treuheit M. J., Vaghy P. L., Kirley T. L. Mg(2+)-ATPase from rabbit skeletal muscle transverse tubules is 67-kilodalton glycoprotein. J Biol Chem. 1992 Jun 15;267(17):11777–11782. [PubMed] [Google Scholar]
- Watkins S. C., Hoffman E. P., Slayter H. S., Kunkel L. M. Immunoelectron microscopic localization of dystrophin in myofibres. Nature. 1988 Jun 30;333(6176):863–866. doi: 10.1038/333863a0. [DOI] [PubMed] [Google Scholar]
- Yuan S., Arnold W., Jorgensen A. O. Biogenesis of transverse tubules: immunocytochemical localization of a transverse tubular protein (TS28) and a sarcolemmal protein (SL50) in rabbit skeletal muscle developing in situ. J Cell Biol. 1990 Apr;110(4):1187–1198. doi: 10.1083/jcb.110.4.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zampighi G., Vergara J., Ramón F. On the connection between the transverse tubules and the plasma membrane in frog semitendinosus skeletal muscle. Are caveolae the mouths of the transverse tubule system? J Cell Biol. 1975 Mar;64(3):734–740. doi: 10.1083/jcb.64.3.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubrzycka-Gaarn E. E., Bulman D. E., Karpati G., Burghes A. H., Belfall B., Klamut H. J., Talbot J., Hodges R. S., Ray P. N., Worton R. G. The Duchenne muscular dystrophy gene product is localized in sarcolemma of human skeletal muscle. Nature. 1988 Jun 2;333(6172):466–469. doi: 10.1038/333466a0. [DOI] [PubMed] [Google Scholar]
- Zubrzycka-Gaarn E., MacDonald G., Phillips L., Jorgensen A. O., MacLennan D. H. Monoclonal antibodies to the Ca2+ + Mg2+-dependent ATPase of sarcoplasmic reticulum identify polymorphic forms of the enzyme and indicate the presence in the enzyme of a classical high-affinity Ca2+ binding site. J Bioenerg Biomembr. 1984 Dec;16(5-6):441–464. doi: 10.1007/BF00743238. [DOI] [PubMed] [Google Scholar]