Cutaneous squamous cell carcinoma (cSCC) is the second most common malignancy of the skin. Local cSCCs are treated surgically or by other locally destructive therapies. Some cSCC tumours arise from actinic keratoses (AKs), which are lesions of partial-thickness epidermal keratinocyte dysplasia. AKs are the most common precancerous skin lesions, affecting millions of people worldwide. When multiple and adjacent, AKs are termed “field cancerization” and are often treated with field therapy, such as topical fluorouracil, imiquimod, tirbanibulin, and physical modalities such as ablative laser, chemical peels, and photodynamic therapy. These therapies often cause inflammatory reactions including skin irritation, erythema, pain and discomfort, and peeling. Other side effects include blistering, scarring, and dyspigmentation (1). There is an unmet need for treatment of AKs with a reduced side effect profile, which may increase patient compliance with therapy. A better side effect profile may also reduce the effect on daily activities, and allow for treatment year-round with no need for “down time” from social and work-related activities.
Apart from their well-known lipid-lowering clinical use, statins have previously been shown to inhibit some types of cancer in vitro and in observational studies. Statins inhibit cancer cells in vitro in head and neck cancer (2) as well as small-cell lung cancer (3). In clinical studies, meta-analyses showed that statins reduce the risk of colorectal cancer (4) and prostate cancer (5). Statins are competitive inhibitors of 3-hydroxy-3-methylglutarylcoenzyme A reductase (HMGCR), a rate-limiting enzyme in the mevalonate pathway, which is important for the biosynthesis of cholesterol and other products that are essential for regulation of gene expression, cell growth and differentiation, cytoskeleton assembly, and post-translational modification of proteins involved in intracellular signalling (6). Cancer cells may be more sensitive to lower levels of cholesterol than healthy cells (7). This anti-neoplastic effect may also be attributed to the role statins have in reducing cell production of isoprenoids, which are intermediary products of the mevalonate pathway. The mevalonate pathway begins with the conversion of acetyl-CoA to mevalonate. Mevalonate is then converted to a series of isoprenoid lipid intermediates, including the 15-carbon farnesyl pyrophosphate (FPP) and 20-carbon geranylgeranyl pyrophosphate (GGPP). FPP and GGPP are prenyl moieties that are attached to proteins during prenylation. Prenylation is an essential post-translational modification that attaches an isoprenoid to a protein, providing it with a hydrophobic C-terminal, allowing greater interaction with cell membranes and the signalling proteins therein. This C terminal modification effects not only localization, but also has a role in protein–protein interactions and protein stability (8). Prenylation is key in the signalling activity of small GTPase proteins, such as the RAS, RHO, and RAC proteins, which have a role in tumorigenesis, proliferation, and migration of cancer cells (7).
We have previously demonstrated efficacy of topical statin therapy in porokeratosis, a disease associated with mevalonate pathways mutations. Patients with porokeratosis are at increased risk of developing cSCC, and topical statin therapy clears precancerous lesions and may reduce the risk of cSCC (9). There are no preclinical studies on statins in cSCC. We tested the in-vitro response of a cSCC cell line to statin exposure.
MATERIAL AND METHODS (see APPENDIX S1)
RESULTS
Statins inhibit cSCC cell growth through apoptosis
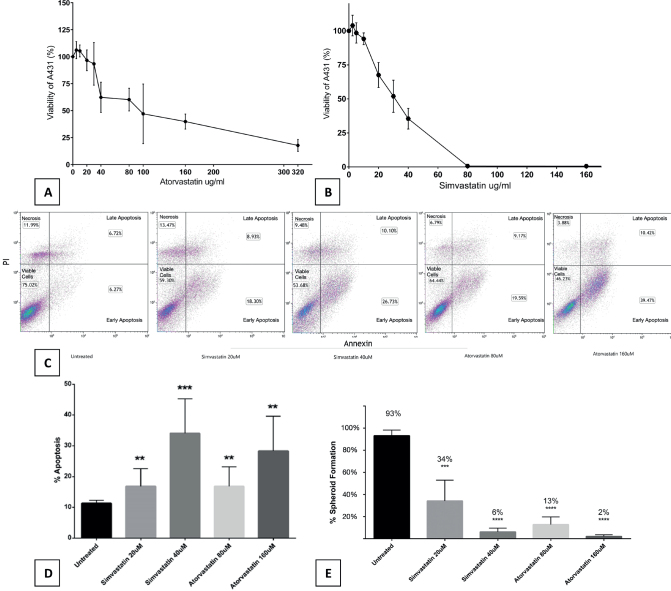
To evaluate the in-vitro effect of statins on cSCC cells, we incubated cells from a known cSCC cell line, A431, for 72 h with increasing concentrations of either atorvastatin or simvastatin vs control (supplemented with Dulbecco modified Eagle’s medium [DMEM]). A consistent, dose-dependent decrease in cell viability was observed with both atorvastatin and simvastatin when compared to control (Fig. 1 A–B).
Fig. 1.
Statins inhibit cSCC cell growth in a dose-dependent manner through apoptosis and reduce 3D spatial organization. A431 cells (5000 cells/well) were incubated with increasing doses of (A) atorvastatin and (B) simvastatin. Cells incubated with statins showed decreased viability in a dose-dependent manner when measured with ELISA. Each experiment was performed in triplicate and repeated at least 3 times. Statistical analyses were performed with repeated measures ANOVA, which showed significance for atorvastatin (p = 0.00050) and simvastatin (p = 0.00000000001293). IC50 estimated values were 90µM for atorvastatin and 30µM for simvastatin. Annexin V FACS showed a dose-dependent increase in apoptosis for cSCC (C) cells exposed to statins (25,000 cells/well). When compared with control, there was (D) a significant increase in overall apoptosis for 20µM simvastatin (p = 0.0046), 40µM simvastatin (p = 0.0005), 80µM atorvastatin (p = 0.007), and 160µM atorvastatin (p = 0.001). All viable droplets were measured (n = 1,283). All treatment groups showed (E) a significant decrease in spheroid formation when compared with control. Statistical analyses were performed with a two-tailed t-test. **p < 0.01, ***p < 0.001 and ****p < 0.0001.
We examined the cell death underlying mechanism using annexin V flow cytometry apoptosis assay. Fluorescence activated cell sorting (FACS) demonstrated a significant increase in apoptosis for all statin concentrations tested (Fig. 1 C–D).
Statins inhibit cSCC from forming 3-dimensional structures
To determine whether statins inhibit the ability of cSCC cells to form 3-dimensional structures we incubated A431 cells for 144 h with increasing doses of simvastatin and atorvastatin. During this time, we allowed the formation of 3-dimensional spheroids of cells against the surface of a hanging drop (Appendix S1; Fig. S1). All treatment groups showed statistically significant decrease in spheroid formation (Fig. 1E). Inhibition of spheroid creation reflects a reduced ability of cells to organize, orient, and travel.
DISCUSSION
In this cSCC model, statins increased apoptosis and inhibited growth of cells. Statins also inhibited 3-dimensional organization of cells. In-vitro anti-tumour effects were previously shown in models of head and neck SCC (2). In cell lines from the oro- and nasopharynx, statins resulted in reduced proliferation, increased apoptosis, and accumulation of cells in the G0/G1 phase (2). A large German retrospective cohort showed a decrease in mortality in head and neck cancer patients who were under statin therapy (10).
In a small-cell lung cancer cell line, statins were shown to induce oxidative stress accumulation and apoptosis to overcome treatment resistance through the geranylgeranyl diphosphate synthase 1 (GGPS1)–RAB7A–autophagy axis (11). RAB7A is a small GTPase Ras-related protein that has been shown to have an important role in the formation of the autophagosome (11).
To our knowledge, there are no preclinical studies investigating statins in cSCC. Population-based studies show conflicting results: while early studies showed increased risk of non-melanoma skin cancer among statin users (12), subsequent studies, including recent meta-analysis, showed no such association (13). Importantly, the first pass of statin metabolism by the liver decreases the bioavailability of statins in peripheral tissues including the skin when administered orally (14). Topical application of statins allows bypassing of the first-pass effect and this approach was applied successfully in treating porokeratosis, a group of keratinization disorders with increased risk of cSCC. Our group has shown that topical application of statins with or without cholesterol normalized the skin phenotype in patients with porokeratosis (9). This treatment may decrease the risk of developing cSCC in these patients.
We did not have a healthy skin cell line comparator, which might have shed light on whether cSCC cells are more susceptible to statins than healthy skin. However, from our limited clinical experience with topical statins, patients do not complain of topical side effects such as erythema and irritation, and of 87 patients in a trial of topical lovastatin 2% for treatment of porokeratosis, only minor and infrequent adverse events were reported (15). Given statins’ anti-tumour effect in cSCC as well as other cancers, they may have a clinical role when applied topically, either alone or in combination with another therapy, especially in precancerous lesions such as AK.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by the Davidoff Foundation.
Funding Statement
This work was supported by the Davidoff Foundation.
Footnotes
The authors have no conflicts of interest to declare.
REFERENCES
- 1.Eisen DB, Asgari MM, Bennett DD, Connolly SM, Dellavalle RP, Freeman EE, et al. Guidelines of care for the management of actinic keratosis. J Am Acad Dermatol 2021; 85: e209–233. 10.1016/j.jaad.2021.02.082 [DOI] [PubMed] [Google Scholar]
- 2.Pavan LMC, Rêgo DF, Elias ST, De Luca Canto G, Guerra ENS. In vitro anti-tumor effects of statins on head and neck squamous cell carcinoma: a systematic review. PLoS One 2015; 10: e0130476. 10.1371/journal.pone.0130476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guo C, Wan R, He Y, Lin SH, Cao J, Qiu Y, et al. Therapeutic targeting of the mevalonate-geranylgeranyl diphosphate pathway with statins overcomes chemotherapy resistance in small cell lung cancer. Nat Cancer 2022; 3: 614–628. 10.1038/s43018-022-00358-1 [DOI] [PubMed] [Google Scholar]
- 4.Liu Y, Tang W, Wang J, Xie L, Li T, He Y, et al. Association between statin use and colorectal cancer risk: a meta-analysis of 42 studies. Cancer Causes Control 2014; 25: 237–249. 10.1007/s10552-013-0326-6 [DOI] [PubMed] [Google Scholar]
- 5.Bansal D, Undela K, D’Cruz S, Schifano F. Statin use and risk of prostate cancer: a meta-analysis of observational studies. PLoS One 2012; 7: e46691. 10.1371/journal.pone.0046691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tamanoi F, Azizian M, Ashrafi M, Bathaie S. Mevalonate pathway and human cancers. Curr Mol Pharmacol 2017; 10: 77–85. 10.2174/1874467209666160112123205 [DOI] [PubMed] [Google Scholar]
- 7.Di Bello E, Zwergel C, Mai A, Valente S. The innovative potential of statins in cancer: new targets for new therapies. Front Chem 2020; 8: 516. 10.3389/fchem.2020.00516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang M, Casey PJ. Protein prenylation: unique fats make their mark on biology. Nat Rev Mol Cell Biol 2016; 17: 110–122. 10.1038/nrm.2015.11 [DOI] [PubMed] [Google Scholar]
- 9.Atzmony L, Lim YH, Hamilton C, Leventhal JS, Wagner A, Paller AS, et al. Topical cholesterol/lovastatin for the treatment of porokeratosis: a pathogenesis-directed therapy. J Am Acad Dermatol 2020; 82: 123–131. 10.1016/j.jaad.2019.08.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wüster J, Heiland M, Nahles S, Preissner R, Preissner S. Statin medication improves five-year survival rates in patients with head and neck cancer: a retrospective case-control study of about 100,000 patients. Cancers (Basel) 2023; 15: 3093. 10.3390/cancers15123093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo C, Wan R, He Y, Lin SH, Cao J, Qiu Y, et al. Therapeutic targeting of the mevalonate-geranylgeranyl diphosphate pathway with statins overcomes chemotherapy resistance in small cell lung cancer. Nat Cancer 2022; 3: 614–628. 10.1038/s43018-022-00358-1 [DOI] [PubMed] [Google Scholar]
- 12.Wang A, Stefanick ML, Kapphahn K, Hedlin H, Desai M, Manson JAE, et al. Relation of statin use with non-melanoma skin cancer: prospective results from the Women’s Health Initiative. Br J Cancer 2016; 114: 314–320. 10.1038/bjc.2015.376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang K, Marley A, Tang H, Song Y, Tang JY, Han J. Statin use and non-melanoma skin cancer risk: a meta-analysis of randomized controlled trials and observational studies. Oncotarget 2017; 8: 75411–75417. 10.18632/oncotarget.20034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schachter M. Chemical, pharmacokinetic and pharmacodynamic properties of statins: an update. Fundam Clin Pharmacol 2005; 19: 117–215. 10.1111/j.1472-8206.2004.00299.x [DOI] [PubMed] [Google Scholar]
- 15.Santa Lucia G, Snyder A, Lateef A, Drohan A, Gregoski MJ, Barton V, et al. Safety and efficacy of topical lovastatin plus cholesterol cream vs topical lovastatin cream alone for the treatment of disseminated superficial actinic porokeratosis. JAMA Dermatol 2023; 159: 488–495. 10.1001/jamadermatol.2023.0205 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.